Abstract
Multiple myeloma (MM) is an incurable B-cell malignancy, requiring new therapeutic strategies. We have found that synthetic alkyl-lysophospholipids (ALPs) edelfosine and perifosine induced apoptosis in MM cell lines and patient MM cells, whereas normal B and T lymphocytes were spared. ALPs induced recruitment of Fas/CD95 death receptor, Fas-associated death domain–containing protein, and procaspase-8 into lipid rafts, leading to the formation of the death-inducing signaling complex (DISC) and apoptosis. TNF-related apoptosis-inducing ligand receptor-1/death receptor 4 (TRAIL-R1/DR4) and TRAIL-R2/DR5, as well as Bid, were also recruited into lipid rafts, linking death receptor and mitochondrial signaling pathways. ALPs induced mitochondrial cytochrome c release. Bcl-XL overexpression prevented cytochrome c release and apoptosis. A Fas/CD95-deficient MM subline expressing DR4 and DR5 was resistant to edelfosine. Fas/CD95 retrovirus transduction bestowed edelfosine sensitivity in these cells. A Fas/CD95 mutant lacking part of the intracellular domain was ineffective. Lipid raft disruption prevented ALP-induced Fas/CD95 clustering, DISC formation, and apoptosis. ALP-induced apoptosis was Fas/CD95 ligand (FasL/CD95L) independent. ALP-induced recruitment of death receptors in lipid rafts potentiated MM cell killing by FasL/CD95L and TRAIL. These data uncover a novel lipid raft–mediated therapy in MM involving concentration of death receptors in membrane rafts, with Fas/CD95 playing a major role in ALP-mediated apoptosis.
Introduction
Multiple myeloma (MM) is a lymphoid cancer of terminally differentiated B-cell lineage or plasma cells that accounts for 10% of all hematologic cancers,1 ranking as the second most common blood cancer, after non-Hodgkin lymphoma.2 MM, currently an incurable disease, is characterized as a tumor composed of long-surviving rather than fast-growing malignant plasma cells.3,4 This implies that a therapeutic potential may lie in potentiating apoptosis.
Synthetic alkyl-lysophospholipids (ALPs) constitute a heterogeneous group of unnatural lipids with promising anticancer activity. Unlike most conventional chemotherapeutic drugs, ALPs do not target the DNA, but they act at the level of cell membranes affecting apoptotic signaling.5-7 The prototype of these ALPs is 1-O-octadecyl-2-O-methyl-rac-glycero-3-phosphocholine (ET-18-OCH3, edelfosine) that induces selective apoptosis in tumor cells, whereas normal cells are spared.8 Examples of clinically relevant oral anticancer ALPs include the prototypic compound edelfosine and the novel drug octadecyl-(1,1-dimethyl-piperidinio-4-yl)-phosphate (D-21266, perifosine).5,9 We have found that edelfosine induces selective apoptosis in leukemic cells by its preferential incorporation into cancer cells,8,10,11 leading to the intracellular activation of the death receptor Fas/CD95 by its recruitment together with downstream signaling molecules into clusters of lipid rafts.10-12 This edelfosine-induced Fas/CD95 intracellular activation and concentration in lipid rafts was independent of its ligand Fas/CD95 ligand (FasL/CD95L) and did not require sphingomyelinase activation.10,11 Edelfosine was the first antitumor drug reported to act through a novel mechanism involving Fas/CD95 translocation into lipid rafts,12 and recent findings further support that coclustering of Fas/CD95 with lipid rafts is a novel and efficient mode of inducing apoptosis in cancer chemotherapy.11-17
Fas/CD95 shares with other death receptors of the tumor necrosis factor (TNF) receptor superfamily, such as TNF-related apoptosis-inducing ligand (TRAIL) receptors, the capacity to transmit apoptosis signals through the presence of a death domain in its cytoplasmic portion after receptor engagement with its ligand or an agonistic antibody.18 Unlike the intracellular regions of other transmembrane receptors involved in signal transduction, the death domain does not possess enzymatic activity but mediates signaling through protein-protein interactions. Stimulation of Fas/CD95 results in receptor aggregation and recruitment of the adaptor molecule Fas-associated death domain–containing protein (FADD) through interaction between its own death domain and the clustered receptor death domains. FADD, in turn, contains a death effector domain that binds to an analogous domain repeated in tandem within the zymogen form of procaspase-8, forming the so-called death-inducing signaling complex (DISC), made up of aggregated Fas/CD95, FADD, and procaspase-8.19 On recruitment by FADD, procaspase-8 oligomerization drives its activation through self-cleavage, activating downstream effector caspases and leading to apoptosis. We and others have recently reported that Fas/CD95 is translocated into lipid raft clusters during Fas/CD95-mediated apoptosis.10-12,16,17,20-22 Thus, aggregation of membrane platforms where Fas/CD95 molecules are brought together is suggested to facilitate DISC formation and thereby potentiate Fas/CD95 signaling.10-12,16,17,20-23 Lipid rafts are membrane microdomains highly enriched in cholesterol and sphingolipids varying in size from 50 to 70 nm, and the proteins located in these microdomains are severely limited in their ability to freely diffuse over the plasma membrane.24 Thus, raft association tends to concentrate specific proteins within plasma membrane microdomains, affecting protein function.25
Because ALPs are potent apoptotic inducers in cancer cells and MM is a slowly proliferating tumor of long-lived plasma cells, we reasoned that this disease could be particularly suitable for the therapeutic use of ALPs. Thus, we investigated the action of ALPs on MM and analyzed the role of death receptor and lipid raft coclustering in their antimyeloma action.
Materials and methods
Reagents
Edelfosine was from INKEYSA (Barcelona, Spain). Perifosine was from Zentaris (Frankfurt, Germany). RhTRAIL and rhFasL were from Alexis Biochemicals (Lausen, Switzerland). All other chemicals were from Sigma Chemical (St Louis, MO), Roche Biochemicals (Mannheim, Germany), or Merck (Darmstadt, Germany).
Cell lines and primary cells
Human MM cell lines MM144, MM1S, MM1R, RPMI-8226, and OPM-2 (provided by A. Pandiella, Centro de Investigación del Cáncer, Salamanca, Spain), as well as Jurkat T-lymphoid and JY B-lymphoid leukemia cell lines (American Type Culture Collection, Manassas, VA), were cultured in RPMI-1640 medium containing 10% (vol/vol) heat-inactivated fetal bovine serum (FBS), 2 mM l-glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin at 37°C in humidified 95% air and 5% CO2.
Heparanized bone marrow aspirates, obtained from patients with newly diagnosed MM and after signing informed consent, were provided by the Hematology Department of the University Hospital (Salamanca, Spain). The study was approved by the Ethics Committee of the University Hospital. Mononuclear cells were isolated by Ficoll-Hypaque (Amersham Biosciences, Uppsala, Sweden) density gradient centrifugation and subjected to positive selection using magnetic cell sorting (MACS) CD138 MicroBeads and MACS LS separation columns (Miltenyi Biotec, Bergisch Gladbach, Germany). Purified CD138+ tumor cells (> 95% plasma cells) and CD138− nontransformed cells were resuspended in RPMI-1640/10% FBS and used immediately for experiments.
To isolate normal peripheral blood lymphocytes (PBLs), mononuclear cells were obtained from fresh human peripheral blood from healthy volunteers by centrifugation on Ficoll-Hypaque density gradients, washed with phosphate-buffered saline (PBS), and resuspended in RPMI-1640/10% FBS as described.26 Monocytes were depleted by culture dish adherence after overnight incubation. Nonadherent cells (lymphocytes) were washed with PBS and collected by centrifugation. PBL preparations were typically 69% to 74% CD3+, 25% to 28% CD19+, and less than 0.4% CD14+. To further purify T cells, nonadherent cells were washed with PBS and passed twice through a nylon wool column to deplete residual B cells and monocytes. Purified T-cell preparations were typically greater than 96% CD3+, less than 0.3% CD14+, and less than 4% CD25+. Normal PBL B cells were isolated by incubation of PBLs with CD19 magnetic beads, and then CD19+ B cells were removed from the beads using CD19 DETACHaBEAD (Dynal Biotech, Oslo, Norway) following the manufacturer's directions.
Human umbilical vein endothelial cells were obtained by collagenase digestion of umbilical cord veins as described.27
Immunofluorescence flow cytometry
Cell-surface expression of death receptors was analyzed by flow cytometry in 4 × 105 cells as described previously,10,28 measuring both the percentage of antigen-positive cells and the mean fluorescence intensity (MFI), in a Becton Dickinson (San Jose, CA) fluorescence-activated cell sorting (FACS)Calibur flow cytometer using an anti-Fas/CD95 SM1/1 IgG monoclonal antibody (Bender MedSystems, Vienna, Austria) and specific monoclonal antibodies against the extracellular domains of human DR4 and DR5 (R&D Systems, Minneapolis, MN). P3X63 myeloma culture supernatant, provided by F. Sánchez-Madrid (Hospital de La Princesa, Madrid, Spain), and an isotype-matched fluorescein isothiocyanate (FITC)–conjugated nonrelevant IgG monoclonal antibody (Becton Dickinson) were used as negative controls, leading to virtually identical background values.
Confocal microscopy
Cells were settled onto poly-l-lysine–coated slides and analyzed with a Zeiss LSM 510 laser scan confocal microscope (Oberkochen, Germany) for membrane raft and protein visualization as described.12 Colocalization assays were analyzed by excitation of the corresponding fluorochromes in the same section. Negative controls, lacking the primary antibody or using an irrelevant antibody, showed no staining. Images were viewed and acquired by using a Plan Apochromat 63×/1.40 NA oil-immersion objective lens and Zeiss LSM 510 software version 3.5.
Apoptosis assay
Quantitation of apoptotic cells was calculated by flow cytometry as the percentage of cells in the sub-G1 region (hypodiploidy) in cell-cycle analysis as previously described.29
The implication of Fas/CD95-FasL/CD95L interaction in ALP-induced apoptosis was evaluated as previously described.10 Cells (2.5 × 105/mL) were preincubated at 4°C for 30 minutes in PBS + 1% bovine serum albumin (BSA) in the absence (controls) and in the presence of 100 ng/mL blocking anti-Fas/CD95 SM1/23 IgG2b monoclonal antibody (Bender MedSystems). Then, cells were resuspended at 5 × 105/mL in complete RPMI-1640 culture medium in the absence or presence of the same amount of blocking anti-Fas/CD95 antibody and treated with 50 ng/mL cytotoxic Fas agonistic anti-Fas/CD95 CH-11 monoclonal antibody (Upstate Biotechnology, Lake Placid, NY), 10 μM edelfosine or 10 μM perifosine at 37°C for 24 hours. Then, cells were collected by centrifugation and analyzed for apoptosis by flow cytometry as described earlier in this section. Experiments performed with mock irrelevant isotype immunoglobulins had no effect.
Edelfosine uptake
Drug uptake was measured as described previously8 after incubating cells (106) with 10 nmol [3H]edelfosine (10 μM) for 6 hours in RPMI-1640/10% FBS and subsequent exhaustive washing (5 times) with PBS + 2% BSA. [3H]edelfosine (specific activity, 42 Ci [155.4 × 1010 Bq]/mmol) was synthesized by titration of the 9-octadecenyl derivative (Amersham Buchler, Braunschweig, Germany).
Lipid raft isolation and Western blotting
Lipid rafts were isolated by using lysis conditions and centrifugation on discontinuous sucrose gradients as previously reported.12 In brief, 108 cells were washed with ice-cold PBS and lysed for 60 minutes on ice with 1% Triton X-100 in TNEV buffer (10 mM Tris-HCl, pH 7.5, 150 mM NaCl, 5 mM EDTA, 1 mM Na3VO4) containing 1 mM phenylmethylsulfonyl fluoride (PMSF). Cells were then homogenized with 10 to 15 strokes in a Potter-Elvehjem tissue grinder. Nuclei and cellular debris were pelleted by centrifugation at 200g (1000 rpm) for 8 minutes. Then, 1 mL cleared supernatant was mixed with 1 mL 85% (wt/vol) sucrose in TNEV buffer and transferred to the bottom of a Beckman 14 × 95-mm centrifuge tube. The diluted lysate was overlaid with 6 mL 35% (wt/vol) sucrose in TNEV buffer and finally 3.5 mL 5% (wt/vol) sucrose in TNEV buffer. The samples were centrifuged in an SW40 rotor at 257 000g (38 000 rpm) at rmax for 18 hours at 4°C in a Beckman Optima LE-80K ultracentrifuge (Beckman Instruments, Palo Alto, CA), and then 1-mL fractions were collected from the top of the gradient. To determine the location of distinct proteins in the discontinuous sucrose gradient, 20 μL of the individual fractions were subjected to SDS–polyacrylamide gel electrophoresis (PAGE) and immunoblotted using corresponding specific monoclonal antibodies and an enhanced chemiluminescence detection system (Amersham, Buckinghamshire, United Kingdom). The location of GM1-containing lipid rafts was determined using cholera toxin (CTx) B subunit conjugated to horseradish peroxidase as described previously.30 Proteins were identified using specific antibodies: anti–48-kDa Fas/CD95 (C-20) rabbit polyclonal antibody, and anti–56-kDa DR4 (N-19), anti–48-kDa DR5 (N-19), and anti–22-kDa Bid (N-19) goat polyclonal antibodies (Santa Cruz Biotechnology, Santa Cruz, CA); anti–29-kDa FADD monoclonal antibody (clone-1) (BD Transduction Laboratories, Lexington, KY); and anti–55-kDa procaspase-8 (Ab-3) monoclonal antibody (Oncogene Research Products, Cambridge, MA).
Coimmunoprecipitation
MM144 cells (3 × 107–6 × 107) were lysed with 500 μL lysis buffer (20 mM Tris-HCl, pH 7.5, 100 mM KCl, 0.9% Triton X-100, 10% glycerol, 2 mM Na3VO4, 2 mM PMSF). Lysates were precleared with protein A-Sepharose and immunoprecipitated with anti-Fas/CD95 (C-20) rabbit polyclonal antibody precoupled to protein A-Sepharose as previously described.31 Samples were subjected to SDS-PAGE and immunoblotted with specific antibodies against Fas/CD95 FADD and caspase-8. To immunoprecipitate membrane raft proteins, fractions 4 to 6 (200 μL each) from the lipid raft isolation sucrose gradient were pooled and used for the coimmunoprecipitation assay as described earlier in this section. Lysates and membrane raft pools were also immunoprecipitated with P3X63 myeloma supernatant or with rabbit nonrevelant IgG that was isolated from pooled normal nonimmune rabbit serum, as negative controls, showing no signal.
Cholesterol depletion and sequestration
For cholesterol depletion, 2.5 × 105 cells/mL were pretreated with 2.5 mg/mL methyl-β-cyclodextrin (MCD) for 30 minutes at 37°C in serum-free medium. Cells were then washed 3 times with PBS and resuspended in complete culture medium before ALP addition. For cholesterol sequestration, cells were treated with 0.3 μg/mL filipin for 1 hour at 37°C in serum-free medium and then processed as described earlier in this section.
Retroviral gene expression of Fas/CD95
Construction of retroviral vectors.
Human Fas/CD95 and a 57 COOH-terminally truncated Fas/CD95 mutant (FasΔ57C) were cloned into the pLNCX2 retroviral vector (BD Biosciences Clontech, Palo Alto, CA) at the XhoI and SalI sites of the multiple cloning site. The resulting constructs were transformed to Escherichia coli DH5α cells. These constructs, as well as the empty vector, pLNCX2, were used in the subsequent transfection experiments.
Transfection of the packaging cell line.
On the day before transfection the retroviral packaging cell line PT67 was plated on 60-mm diameter plates (1 × 106–2 × 106 cells in 4 mL DMEM/10% FBS). Five minutes prior to transfection, 25 μM chloroquine was added to each plate. The transfection solution contained DNA (5-10 μg retroviral vector pLNCX2, pLNCX2-Fas, or pLNCX2-FasΔ57C), 31 μL 2 M CaCl2, 30 μL carrier DNA (200 ng/μL), and H2O to 250 μL. After mixing, 250 μL of 2 × HEPES-buffered saline solution (pH 7.0) was added by bubbling, and the resulting solution was immediately dropped onto cells. The cells were then returned to the 37°C incubator (5% CO2) for 24 hours. Subsequently, the medium was changed to 3 mL fresh culture medium containing 10% FBS, and 24 to 72 hours later, the supernatant from transfected cells was centrifuged at 1000g for 5 minutes in an Eppendorf 5810R centrifuge (Hamburg, Germany).
Infection of MM cells.
The target Fas/CD95-deficient OPM-2 cells were plated 12 to 18 hours before infection at a cell density of 1 to 2 × 105/60-mm diameter plate in RPMI-1640/10% FBS. For infection, the medium from the retroviral packaging PT67 cells was collected and filtered through a 0.45-μm cellulose acetate sterile filter. Target cells were infected with 1 mL viral supernatant containing Polybrene at 8 μg/mL for 1 hour. RPMI-1640/10% FBS medium (8 mL) was added, and cells were grown for 24 hours and then exposed to another round of infection. After the second round of infection, cells were grown for 2 to 3 days before selection with 400 μg/mL G418.
Cell transfection with BCL-XL
The human MM144 cells were transfected by electroporation with the SFFV-Neo expression vector containing the human BCL-XL open reading frame driven by the long terminal repeat of the splenic focus-forming virus (pSFFV-BCL-XL). As a control, transfection was performed with empty SFFV-Neo plasmid. MM144 cells (4 × 107) were subjected to electroporation at 500 V, 1700 μF, 72 ohm in a BTX electro cell manipulator 600 (Biotechnologies & Experimental Research, San Diego, CA) and selected by growth in the presence of 400 μg/mL G418.
Mitochondrial cytochrome c release
Release of cytochrome c from mitochondria to cytosol was analyzed by Western blot as described previously,32 using antibodies against cytochrome c (7H8.2C12; BD Pharmingen, San Diego, CA) and cytochrome oxidase subunit II (12C4-F12; Molecular Probes, Eugene, OR).
Results
Edelfosine and perifosine selectively induce apoptosis in MM cells
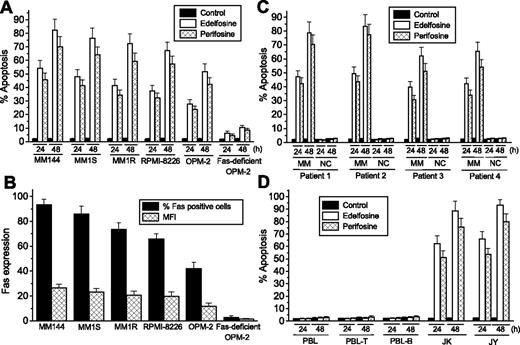
We found that the ALPs edelfosine and perifosine induced a potent apoptotic response in different MM cell lines (Figure 1A). There was a good correlation between the cell-surface Fas/CD95 content in distinct MM cell lines and their sensitivity to undergo ALP-induced apoptosis (Figure 1A,B). MM144 cells were more sensitive to ALPs than other cell lines, and they showed a higher cell-surface Fas/CD95 content. In addition, we generated a subline from the OPM-2 cell line that lacked Fas/CD95 (Figure 1B) after protracted cultures (> 7 months) of parental cells, and these Fas/CD95-deficient OPM-2 cells were rather ALP resistant (Figure 1A). The level of Fas cell-surface expression of the distinct MM cells was measured either by the percentage of Fas-positive cells or the mean fluorescence intensity, showing a good correspondence between both values (Figure 1B). Both dexamethasone-sensitive MM1S cells and dexamethasone-resistant MM1R cells showed similar ALP-induced rates of apoptotic cell death (Figure 1A), suggesting that these ALPs can circumvent dexamethasone resistance.
Selective induction of apoptosis in MM cells by edelfosine and perifosine and correlation with Fas/CD95 cell-surface expression. (A) MM cell lines were incubated for 24 and 48 hours with 10 μM edelfosine or perifosine, and apoptosis was then quantitated as the percentage of cells in the sub-G1 region in cell-cycle analysis by flow cytometry. (B) Cell-surface expression of Fas/CD95 in different MM cell lines was analyzed by flow cytometry. Percentage of Fas-positive cells and MFI values were estimated using the P3X63 myeloma supernatant and an isotype-matched FITC-conjugated nonrelevant IgG monoclonal antibody as negative controls. (C) Freshly isolated tumor MM cells and normal mononuclear cells (NCs) from patients with MM were incubated for the indicated times with 10 μM edelfosine or perifosine and analyzed for apoptosis as above. (D) Normal peripheral blood lymphocytes (PBLs), purified PBL-T cells, and purified PBL-B cells, as well as leukemic T-lymphoid Jurkat (JK) and B-lymphoid JY cells, were incubated for the indicated times with 10 μM edelfosine or perifosine and analyzed for apoptosis as described. Untreated control cells were run in parallel. Data shown are means ± SD of 4 independent determinations.
Selective induction of apoptosis in MM cells by edelfosine and perifosine and correlation with Fas/CD95 cell-surface expression. (A) MM cell lines were incubated for 24 and 48 hours with 10 μM edelfosine or perifosine, and apoptosis was then quantitated as the percentage of cells in the sub-G1 region in cell-cycle analysis by flow cytometry. (B) Cell-surface expression of Fas/CD95 in different MM cell lines was analyzed by flow cytometry. Percentage of Fas-positive cells and MFI values were estimated using the P3X63 myeloma supernatant and an isotype-matched FITC-conjugated nonrelevant IgG monoclonal antibody as negative controls. (C) Freshly isolated tumor MM cells and normal mononuclear cells (NCs) from patients with MM were incubated for the indicated times with 10 μM edelfosine or perifosine and analyzed for apoptosis as above. (D) Normal peripheral blood lymphocytes (PBLs), purified PBL-T cells, and purified PBL-B cells, as well as leukemic T-lymphoid Jurkat (JK) and B-lymphoid JY cells, were incubated for the indicated times with 10 μM edelfosine or perifosine and analyzed for apoptosis as described. Untreated control cells were run in parallel. Data shown are means ± SD of 4 independent determinations.
Both ALPs also promoted apoptosis in primary cultures of MM cells derived from patients (Figure 1C), which were Fas/CD95-positive (data not shown). Normal mononuclear cells from the same patients were spared (Figure 1C). Edelfosine behaved somewhat more potently than perifosine in its capacity to induce apoptosis in both MM cell lines and primary cells, but both drugs were selective in their action for MM cells. Normal cells, including normal PBLs as well as normal purified B and T cells from healthy volunteers, were not affected by these drugs (Figure 1D). However, T-lymphoid Jurkat and B-lymphoid JY leukemic cells were very sensitive to the apoptotic action of ALPs (Figure 1D). In addition, normal human umbilical vein endothelial cells were also spared by these drugs (data not shown).
Clustering of Fas/CD95 in lipid rafts is crucial to ALP-induced apoptosis in MM cells
Following a time-course analysis we found that edelfosine and perifosine induced an apoptotic response in MM144 cells after about 15 hours of incubation (Figure 2A). This effect was rather slow when compared with the rapid triggering of apoptosis following edelfosine treatment in T-lymphoid Jurkat cells (> 20% apoptosis after 6 hours of incubation).33 This timing in the induction of apoptosis was further corroborated by caspase-3 activation in edelfosine-treated MM144 cells, as assessed by cleavage of procaspase-3 into the p18 active form and cleavage of the typical caspase-3 substrate poly(ADP-ribose) polymerase (PARP), using a polyclonal anti–caspase-3 antibody that recognized the 18-kDa subunit of active caspase-3 and an anti-PARP monoclonal antibody that detected both the 116-kDa intact form and the 85-kDa cleaved form of PARP (Figure 2B).
Time-course of ALP-induced apoptosis and coclustering of membrane rafts and Fas/CD95 in edelfosine- and perifosine-treated MM cells. (A) MM144 cells were incubated with 10 μM edelfosine or perifosine for the indicated times, and the proportion of cells in the sub-G1 region, representing apoptotic cells, was quantitated by flow cytometry. Data shown are means ± SD of 3 independent determinations. (B) MM144 cells were treated with 10 μM edelfosine for the indicated times and analyzed by immunoblotting with an anti–caspase-3 antibody that recognized only the p18 subunit of active caspase-3 and with an anti-PARP antibody that detected both the 116-kDa intact form of PARP and its p85 cleaved form. Data shown are representative of 3 experiments performed. (C) MM144 cells were either untreated (control) or treated with 10 μM edelfosine or perifosine for 12 hours and then stained with FITC-CTx B subunit to identify lipid rafts (green fluorescence) and with a specific anti-Fas/CD95 monoclonal antibody, followed by CY3-conjugated anti–mouse Ig antibody (red fluorescence). Areas of colocalization between membrane rafts and Fas/CD95 in the merge panels are yellow. Images shown are representative of 3 independent experiments. Bar, 10 μm.
Time-course of ALP-induced apoptosis and coclustering of membrane rafts and Fas/CD95 in edelfosine- and perifosine-treated MM cells. (A) MM144 cells were incubated with 10 μM edelfosine or perifosine for the indicated times, and the proportion of cells in the sub-G1 region, representing apoptotic cells, was quantitated by flow cytometry. Data shown are means ± SD of 3 independent determinations. (B) MM144 cells were treated with 10 μM edelfosine for the indicated times and analyzed by immunoblotting with an anti–caspase-3 antibody that recognized only the p18 subunit of active caspase-3 and with an anti-PARP antibody that detected both the 116-kDa intact form of PARP and its p85 cleaved form. Data shown are representative of 3 experiments performed. (C) MM144 cells were either untreated (control) or treated with 10 μM edelfosine or perifosine for 12 hours and then stained with FITC-CTx B subunit to identify lipid rafts (green fluorescence) and with a specific anti-Fas/CD95 monoclonal antibody, followed by CY3-conjugated anti–mouse Ig antibody (red fluorescence). Areas of colocalization between membrane rafts and Fas/CD95 in the merge panels are yellow. Images shown are representative of 3 independent experiments. Bar, 10 μm.
Because we have recently shown that edelfosine triggers apoptosis in T-lymphoid and myeloid hematologic tumor cells through a novel mechanism of action involving coaggregation of Fas/CD95 and downstream signaling molecules with lipid rafts,11,12 we examined whether ALPs induced a similar apoptotic mechanism in MM cells that represent terminally differentiated B plasma cells. We found that edelfosine and perifosine promoted a potent coclustering of lipid rafts and Fas/CD95 in MM144 cells, as assessed by using the raft marker FITC-CTx B subunit that binds ganglioside GM1,34 mainly found in rafts35 (Figure 2C). We also found a remarkable coclustering of Fas/CD95 and lipid rafts in ALP-treated malignant cells derived from patients with MM (data not shown). Disruption of lipid rafts by MCD or filipin, which interferes with protein association with lipid rafts by cholesterol depletion or sequestration, respectively,12,25,36 inhibited ALP-induced formation of Fas/CD95 clusters (Figure 3A) and apoptosis (Figure 3B) in MM cells. These results indicate that Fas/CD95 clustering in lipid rafts plays an important role in ALP-induced apoptosis in MM cells.
Disruption of membrane rafts inhibits ALP-induced Fas/CD95 clustering and apoptosis. MM144 cells were untreated (control) or pretreated with MCD or filipin and then incubated with 10 μM edelfosine or perifosine for 12 hours and analyzed for Fas clustering by confocal microscopy (A), or for 24 hours, and examined for the percentage of apoptotic cells by flow cytometry (B). Images shown in panel A are representative of 3 independent experiments. Scale bar, 10 μm. Data shown in panel B are means ± SD of 3 independent determinations.
Disruption of membrane rafts inhibits ALP-induced Fas/CD95 clustering and apoptosis. MM144 cells were untreated (control) or pretreated with MCD or filipin and then incubated with 10 μM edelfosine or perifosine for 12 hours and analyzed for Fas clustering by confocal microscopy (A), or for 24 hours, and examined for the percentage of apoptotic cells by flow cytometry (B). Images shown in panel A are representative of 3 independent experiments. Scale bar, 10 μm. Data shown in panel B are means ± SD of 3 independent determinations.
ALP-induced translocation of death receptors and downstream signaling molecules into lipid rafts
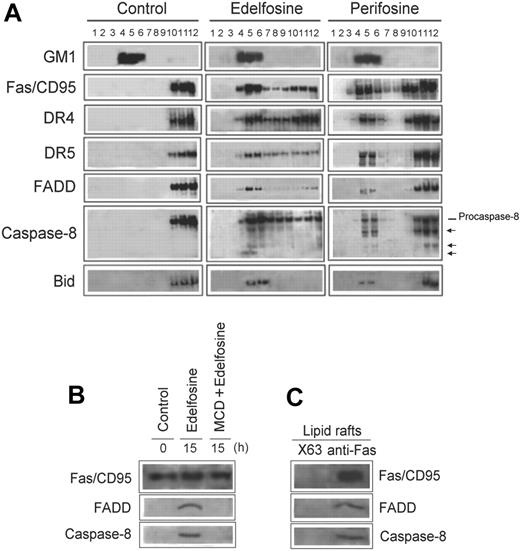
Fas/CD95 and lipid raft coclustering was further confirmed by isolation of membrane rafts from MM144 cells, untreated and treated with either edelfosine or perifosine (Figure 4A). Lipid rafts were isolated according to their insolubility in Triton X-100 lysis buffer at 4°C and fractionated by discontinuous sucrose gradient centrifugation.11,30 GM1-containing lipid rafts, at the upper part (fractions 4-6) of the sucrose gradient (Figure 4A), were located using CTx B subunit conjugated to horseradish peroxidase.30 In addition to Fas/CD95, we also found that TRAIL receptors, DR4 and DR5, were translocated into lipid rafts in both edelfosine- and perifosine-treated cells (Figure 4A). Death receptor downstream signaling molecules FADD and procaspase-8 were also translocated into membrane rafts (Figure 4A). Interestingly, a portion of the active cleaved caspase-8 forms were also found in lipid rafts following ALP treatment (Figure 4A). Our results indicate that edelfosine and perifosine induce a reorganization of the lipid raft protein content, translocating apoptotic proteins to these detergent-insoluble membrane domains. These data also show that the constituents of the DISC, the major apoptotic complex in death receptor signaling, are translocated into lipid rafts. This suggests that lipid rafts become enriched in apoptotic signaling molecules in ALP-treated MM cells, harboring and bringing together major components of the death receptor extrinsic pathway of apoptosis. We also found that Bid, which acts as a bridge between Fas/CD95 signaling and mitochondria,37,38 was translocated into lipid rafts following treatment of MM144 cells with edelfosine or perifosine (Figure 4A). A time-course analysis showed that the translocation of the proteins mentioned earlier in this section into lipid rafts occurred after 12 to 15 hours of incubation with the drugs (data not shown) (ie, by the time apoptosis was triggered) (Figure 2A). We observed that edelfosine was more potent than perifosine in its ability to recruit apoptotic molecules into membrane rafts (Figure 4A). This differential protein-translocating activity of the ALPs might explain the higher antitumor activity of edelfosine when compared with perifosine in MM cells (Figure 1A,C). Note the almost complete translocation of Fas/CD95, DR5, FADD, and Bid to lipid rafts following edelfosine treatment (Figure 4A).
Recruitment of death receptors and downstream signaling molecules and DISC formation in membrane rafts following ALP treatment of MM cells. (A) Untreated MM144 cells (control) and MM144 cells treated with 10 μM edelfosine or perifosine for 15 hours were lysed in 1% Triton X-100 and fractionated by centrifugation on a discontinuous sucrose density gradient. An equal volume of each collected fraction was subjected to SDS-PAGE before analysis of the indicated proteins using specific antibodies. The migration positions of the 55-kDa procaspase-8 as well as of the cleavage products (arrows) are denoted. Location of GM1-containing lipid rafts (fractions 4-6) was determined using CTx B subunit conjugated to horseradish peroxidase. (B) Fas/CD95 was immunoprecipitated from untreated control or edelfosine-treated (15 hours) MM144 cell extracts. Fas/CD95 was also immunoprecipitated from MM144 cells pretreated with MCD to disrupt lipid rafts and then treated for 15 hours with edelfosine. Immunoprecipitates were subjected to SDS-PAGE and immunoblotted with specific antibodies against Fas/CD95, FADD, and caspase-8. (C) Fas/CD95 was immunoprecipitated from a pool of membrane raft–enriched fractions 4 to 6 from sucrose gradients similar to those shown in Figure 4A of edelfosine-treated MM144 cells. Immunoprecipitates were subjected to SDS-PAGE and immunoblotted with Fas/CD95-, FADD-, and caspase-8–specific antibodies, respectively. Membrane raft–enriched fractions were also immunoprecipitated with P3X63 (X63) myeloma supernatant as a negative control, rendering no signal. Experiments shown are representative of 3 performed.
Recruitment of death receptors and downstream signaling molecules and DISC formation in membrane rafts following ALP treatment of MM cells. (A) Untreated MM144 cells (control) and MM144 cells treated with 10 μM edelfosine or perifosine for 15 hours were lysed in 1% Triton X-100 and fractionated by centrifugation on a discontinuous sucrose density gradient. An equal volume of each collected fraction was subjected to SDS-PAGE before analysis of the indicated proteins using specific antibodies. The migration positions of the 55-kDa procaspase-8 as well as of the cleavage products (arrows) are denoted. Location of GM1-containing lipid rafts (fractions 4-6) was determined using CTx B subunit conjugated to horseradish peroxidase. (B) Fas/CD95 was immunoprecipitated from untreated control or edelfosine-treated (15 hours) MM144 cell extracts. Fas/CD95 was also immunoprecipitated from MM144 cells pretreated with MCD to disrupt lipid rafts and then treated for 15 hours with edelfosine. Immunoprecipitates were subjected to SDS-PAGE and immunoblotted with specific antibodies against Fas/CD95, FADD, and caspase-8. (C) Fas/CD95 was immunoprecipitated from a pool of membrane raft–enriched fractions 4 to 6 from sucrose gradients similar to those shown in Figure 4A of edelfosine-treated MM144 cells. Immunoprecipitates were subjected to SDS-PAGE and immunoblotted with Fas/CD95-, FADD-, and caspase-8–specific antibodies, respectively. Membrane raft–enriched fractions were also immunoprecipitated with P3X63 (X63) myeloma supernatant as a negative control, rendering no signal. Experiments shown are representative of 3 performed.
Edelfosine induces DISC formation
Because edelfosine induced translocation of Fas/CD95, FADD, and caspase-8 into lipid rafts, we asked next whether edelfosine treatment of MM cells could lead to DISC formation. Following a time-course analysis, we found that, by the time (15-hour incubation) edelfosine induced translocation of Fas/CD95 and downstream molecules into lipid rafts, coimmunoprecipitation assays showed the formation of DISC containing Fas/CD95, FADD, and caspase-8 in edelfosine-treated MM144 cell extracts (Figure 4B). We also found DISC formation in membrane rafts isolated from edelfosine-treated MM144 cells (Figure 4C). Immunoprecipitation with either P3X63 myeloma supernatant (Figure 4C) or rabbit nonrelevant IgG (data not shown), used as negative controls, rendered no signal. Disruption of lipid rafts with MCD prevented DISC formation in edelfosine-treated cells (Figure 4B). DISC assembly was also detected in perifosine-treated cells, although to a lesser extent (data not shown). These data indicate that ALPs induce DISC formation through translocation and concentration of their constituents into lipid rafts.
Fas/CD95 is required for ALP-induced apoptosis
To further investigate the relevance of Fas/CD95 in ALP-mediated apoptosis in MM cells, we used an OPM-2 subline lacking Fas/CD95 (Figure 1A) that was generated after protracted cultures of parental OPM-2 cells. Fas/CD95-deficient OPM-2 cells expressed DR4 and DR5 at similar levels as the parental OPM-2 cells (Figure 5A) but were ALP resistant (Figure 1A). This resistance was not due to a deficiency in drug uptake, because Fas/CD95-deficient OPM-2 cells as well as the drug-sensitive MM144 cells and OPM-2 parental cells were able to take up the ether lipid at similar figures (Figure 5B). ALP treatment did not restore Fas/CD95 expression in Fas/CD95-deficient OPM-2 cells and did not increase Fas/CD95 cell-surface expression in Fas/CD95-positive MM144 cells (Figure 5C,D). These data are in agreement with our previous findings showing that edelfosine treatment did not up-regulate Fas/CD95 expression in leukemic cells.10,11 When Fas/CD95 was ectopically expressed in Fas/CD95-deficient OPM-2 cells through retroviral transduction, we found a high cell-surface expression of Fas/CD95 and cells turned ALP sensitive (Figure 5E,F) without affecting drug uptake (data not shown). Cells infected with empty virus behaved as uninfected cells (data not shown). Furthermore, we infected Fas/CD95-deficient OPM-2 cells with retroviruses containing a truncated version of human Fas/CD95 (Fas-deficient OPM-2-FasΔ57C), lacking the 57 COOH-terminal amino acids (amino acids 279-335) that included part of the Fas/CD95 death domain.39 These Fas-deficient OPM-2-FasΔ57C cells expressed high levels of cell-surface Fas/CD95 but were resistant to ALPs (Figure 5E,F). These data demonstrate that Fas/CD95 plays a critical role in edelfosine-induced apoptosis.
Requirement of Fas/CD95 for ALP-induced apoptosis. (A) Cell-surface expression of Fas/CD95, DR4, and DR5 in MM144, OPM-2, and Fas/CD95-deficient OPM-2 cells was determined by flow cytometry. Percentage of positive cells for each death receptor was estimated using the P3X63 myeloma culture supernatant and an isotype-matched FITC-conjugated nonrelevant IgG monoclonal antibody as negative controls. (B) Edelfosine uptake was determined after incubating MM144, OPM-2, and Fas/CD95-deficient OPM-2 cells with 10 nmol [3H]edelfosine for 6 hours. (C,D) Fas/CD95-deficient OPM-2 and MM144 cells were untreated (control) or treated with 10 μM edelfosine or perifosine for 12 hours, and then Fas/CD95 cell-surface expression was determined by flow cytometry as the percentage of Fas-positive cells (C) or MFI (D). (E) Cell-surface expression of Fas/CD95 was analyzed by immunofluorescence flow cytometry in Fas/CD95-deficient OPM-2 infected with empty virus (Fas-deficient OPM-2–vector), recombinant retrovirus containing human Fas/CD95 (Fas-deficient OPM-2–Fas), and recombinant retrovirus containing a truncated version of human Fas/CD95 (Fas-deficient OPM-2-FasΔ57C), lacking the 57 COOH-terminal amino acids. Percentage of Fas/CD95+ cells was estimated using the P3X63 myeloma supernatant and an isotype-matched FITC-conjugated nonrelevant IgG monoclonal antibody as negative controls. (F) Induction of apoptosis in the above retrovirus-infected cells was determined by flow cytometry after a 24-hour incubation with 10 μM edelfosine or perifosine. Untreated control cells were run in parallel. Data shown are means ± SD of 3 independent experiments.
Requirement of Fas/CD95 for ALP-induced apoptosis. (A) Cell-surface expression of Fas/CD95, DR4, and DR5 in MM144, OPM-2, and Fas/CD95-deficient OPM-2 cells was determined by flow cytometry. Percentage of positive cells for each death receptor was estimated using the P3X63 myeloma culture supernatant and an isotype-matched FITC-conjugated nonrelevant IgG monoclonal antibody as negative controls. (B) Edelfosine uptake was determined after incubating MM144, OPM-2, and Fas/CD95-deficient OPM-2 cells with 10 nmol [3H]edelfosine for 6 hours. (C,D) Fas/CD95-deficient OPM-2 and MM144 cells were untreated (control) or treated with 10 μM edelfosine or perifosine for 12 hours, and then Fas/CD95 cell-surface expression was determined by flow cytometry as the percentage of Fas-positive cells (C) or MFI (D). (E) Cell-surface expression of Fas/CD95 was analyzed by immunofluorescence flow cytometry in Fas/CD95-deficient OPM-2 infected with empty virus (Fas-deficient OPM-2–vector), recombinant retrovirus containing human Fas/CD95 (Fas-deficient OPM-2–Fas), and recombinant retrovirus containing a truncated version of human Fas/CD95 (Fas-deficient OPM-2-FasΔ57C), lacking the 57 COOH-terminal amino acids. Percentage of Fas/CD95+ cells was estimated using the P3X63 myeloma supernatant and an isotype-matched FITC-conjugated nonrelevant IgG monoclonal antibody as negative controls. (F) Induction of apoptosis in the above retrovirus-infected cells was determined by flow cytometry after a 24-hour incubation with 10 μM edelfosine or perifosine. Untreated control cells were run in parallel. Data shown are means ± SD of 3 independent experiments.
Involvement of mitochondria in ALP-induced apoptosis in MM cells
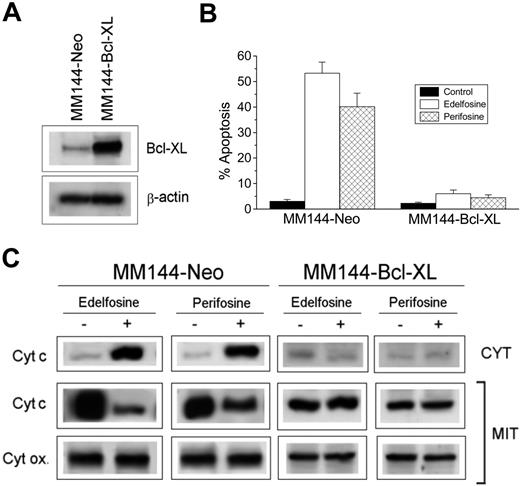
Because Bid was translocated into lipid rafts in ALP-treated cells and edelfosine-mediated apoptosis has been found to be dependent on mitochondrial signaling,29,40,41 we analyzed the putative implication of mitochondria in ALP-induced apoptosis in MM cells. Because Bcl-XL acts as a safeguard of mitochondria, preventing cytochrome c release and apoptosis,42 we examined the effect of overexpressing Bcl-XL in MM144 cells to further analyze the role of mitochondria in the ALP antitumor action on MM. We stably transfected MM144 cells with pSFFV-BCL-XL (MM144-Bcl-XL), containing the human BCL-XL open reading frame, or with control pSFFV-Neo plasmid (MM144-Neo). MM144-Neo cells behaved as nontransfected MM144 cells in all parameters studied. Western blot analysis showed that MM144-Neo cells expressed small levels of endogenous Bcl-XL, whereas a high expression of this protein was observed in MM144-Bcl-XL cells (Figure 6A). MM144-Neo cells underwent apoptosis after treatment with either edelfosine or perifosine. However, overexpression of Bcl-XL by gene transfer in MM144 cells prevented ALP-induced apoptosis (Figure 6B). Both edelfosine and perifosine induced the appearance of cytochrome c in the cytosolic fraction of MM144 cells and its disappearance from the mitochondrial fraction by the time Fas/CD95 and Bid were translocated into lipid rafts (Figure 6C). This indicates that ALPs induce mitochondrial cytochrome c release. However, cytochrome c release was averted in MM144-Bcl-XL cells (Figure 6C). These data show that mitochondria are involved in ALP-induced MM cell death.
Prevention of ALP-induced cytochrome c release and apoptosis by overexpression of Bcl-XL. (A) Western blot analysis for Bcl-XL. Cell lysates from Neo- (MM144-Neo) and Bcl-XL–(MM144-Bcl-XL) transfected MM144 cells were subjected to SDS-PAGE and immunoblotted with a Bcl-XL specific antibody to analyze Bcl-XL content. β-Actin was used as a loading control. Experiments shown are representative of 3 performed. (B) Neo- and Bcl-XL–transfected MM144 cells were incubated for 24 hours with 10 μM edelfosine or perifosine, and apoptosis was then quantitated as the percentage of cells in the sub-G1 region by flow cytometry. Untreated control cells were run in parallel. Data shown are means ± SD of 4 independent determinations. (C) Western blot analysis of cytochrome c in cytosolic (CYT) and mitochondrial (MIT) extracts from MM144-Neo and MM144-Bcl-XL cells untreated (−) or treated (+) for 15 hours with 10 μM edelfosine or perifosine. Cytochrome oxidase subunit II (Cyt ox) was also analyzed in the mitochondrial extracts as a control for mitochondrial protein loading. Experiments shown are representative of 3 performed.
Prevention of ALP-induced cytochrome c release and apoptosis by overexpression of Bcl-XL. (A) Western blot analysis for Bcl-XL. Cell lysates from Neo- (MM144-Neo) and Bcl-XL–(MM144-Bcl-XL) transfected MM144 cells were subjected to SDS-PAGE and immunoblotted with a Bcl-XL specific antibody to analyze Bcl-XL content. β-Actin was used as a loading control. Experiments shown are representative of 3 performed. (B) Neo- and Bcl-XL–transfected MM144 cells were incubated for 24 hours with 10 μM edelfosine or perifosine, and apoptosis was then quantitated as the percentage of cells in the sub-G1 region by flow cytometry. Untreated control cells were run in parallel. Data shown are means ± SD of 4 independent determinations. (C) Western blot analysis of cytochrome c in cytosolic (CYT) and mitochondrial (MIT) extracts from MM144-Neo and MM144-Bcl-XL cells untreated (−) or treated (+) for 15 hours with 10 μM edelfosine or perifosine. Cytochrome oxidase subunit II (Cyt ox) was also analyzed in the mitochondrial extracts as a control for mitochondrial protein loading. Experiments shown are representative of 3 performed.
ALP-induced apoptosis in MM cells is independent of FasL/CD95L
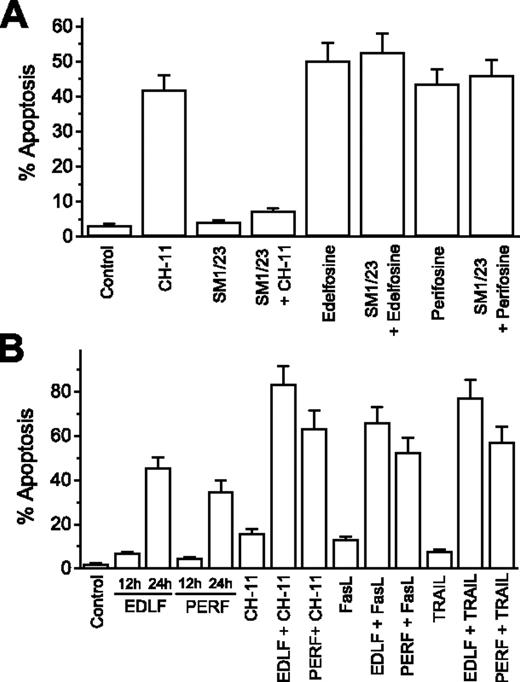
Because Fas/CD95 was critical for the apoptotic effect of edelfosine and perifosine, we asked whether this apoptotic response was dependent on Fas/CD95-FasL/CD95L interactions. To examine the potential role of receptor-ligand interactions in ALP-induced apoptosis, MM144 cells were preincubated with an anti-Fas/CD95 blocking monoclonal antibody (SM1/23), which abrogates Fas/CD95-FasL/CD95L-mediated killing. Preincubation with the blocking SM1/23 anti-Fas/CD95 antibody totally prevented apoptosis induced by the agonistic CH-11 anti-Fas/CD95 monoclonal antibody, but it did not affect edelfosine- and perifosine-induced apoptosis (Figure 7A). This suggests that Fas/CD95-FasL/CD95L interactions are not required for ALP-induced apoptosis in MM cells.
ALP-induced apoptosis is independent of Fas/CD95-FasL/CD95L interaction, and ALP treatment enhances cytotoxicity of death receptor ligands. (A) Fas/CD95-FasL/CD95L interaction is not required for ALP-induced apoptosis. MM144 cells were untreated or preincubated with blocking SM1/23 antibody before addition of 50 ng/mL cytotoxic anti-Fas/CD95 CH-11 monoclonal antibody, 10 μM edelfosine, or 10 μM perifosine. After 24 hours of incubation, apoptosis was quantitated by flow cytometry. (B) ALPs enhance cytotoxicity of Fas/CD95 and TRAIL ligands. MM144 cells were untreated or treated for 12 hours with 10 μM edelfosine (EDLF) or perifosine (PERF), followed by addition of 50 ng/mL cytotoxic anti-Fas/CD95 CH-11 monoclonal antibody, 100 ng/mL FasL/CD95L, or 50 ng/mL TRAIL for 12 hours. Untreated control cells and samples treated with 10 μM edelfosine or perifosine for 12 hours and 24 hours were also run in parallel. Apoptosis was then determined by flow cytometry. Data shown are means ± SD of 3 independent experiments.
ALP-induced apoptosis is independent of Fas/CD95-FasL/CD95L interaction, and ALP treatment enhances cytotoxicity of death receptor ligands. (A) Fas/CD95-FasL/CD95L interaction is not required for ALP-induced apoptosis. MM144 cells were untreated or preincubated with blocking SM1/23 antibody before addition of 50 ng/mL cytotoxic anti-Fas/CD95 CH-11 monoclonal antibody, 10 μM edelfosine, or 10 μM perifosine. After 24 hours of incubation, apoptosis was quantitated by flow cytometry. (B) ALPs enhance cytotoxicity of Fas/CD95 and TRAIL ligands. MM144 cells were untreated or treated for 12 hours with 10 μM edelfosine (EDLF) or perifosine (PERF), followed by addition of 50 ng/mL cytotoxic anti-Fas/CD95 CH-11 monoclonal antibody, 100 ng/mL FasL/CD95L, or 50 ng/mL TRAIL for 12 hours. Untreated control cells and samples treated with 10 μM edelfosine or perifosine for 12 hours and 24 hours were also run in parallel. Apoptosis was then determined by flow cytometry. Data shown are means ± SD of 3 independent experiments.
ALP-elicited recruitment of death receptors in lipid rafts potentiates apoptosis by death receptor ligands in MM cells
Because edelfosine and perifosine induced a potent recruitment of death receptors and downstream signaling molecules in aggregated lipid rafts, thus concentrating these molecules in specific domains of the cell surface, we next examine whether this could further sensitize MM cells to death receptor ligands. We found that pretreatment of MM144 cells with edelfosine and perifosine potentiated significantly the antitumor responses to the extracellular engagement of death receptors by the action of their ligands (FasL/CD95L and TRAIL) or the agonistic cytotoxic anti-Fas/CD95 CH-11 antibody (Figure 7B).
Discussion
The data reported here show that edelfosine and perifosine induce apoptosis in MM cells through the recruitment of the major death receptors Fas/CD95, DR4, and DR5, together with downstream signaling molecules, into lipid rafts. This represents a novel therapeutical approach for MM treatment. Our data show that this concentration of death receptors and downstream apoptotic molecules in lipid rafts leads to DISC formation, setting off a sequence of events from the cell surface, independently of FasL/CD95L, that results in apoptosis. A critical role for Fas/CD95 in ALP-mediated apoptosis of MM cells can be inferred from this study. MM cells lacking Fas/CD95, but expressing DR4 and DR5, were resistant to ALPs. However, Fas/CD95 retrovirus transduction turned Fas/CD95-deficient cells into ALP-sensitive cells. Partial deletion of the death domain of Fas/CD95 was ineffective in restoring ALP sensitivity. These data indicate that the presence of Fas/CD95, independently of DR4 or DR5, is essential for ALP-mediated apoptosis in MM cells, and that the Fas/CD95 death domain is required for the apoptotic response. In addition, we have found that mitochondrial signaling is also involved in ALP-mediated apoptosis in MM cells, as assessed by ALP-induced mitochondrial cytochrome c release and by prevention of ALP-induced mitochondrial cytochrome c release and apoptosis through Bcl-XL overexpression. The recruitment of Bid, a Fas/CD95-mitochondria linker,37,38 into lipid rafts following ALP treatment suggests a link between death receptor (extrinsic)– and mitochondrial (intrinsic)–signaling routes in ALP-induced apoptosis. These data, involving Fas/CD95 and mitochondria in the ALP-induced apoptotic response in MM cells, are in agreement with previous reports in distinct tumor cell types showing that FADD dominant negative-expressing cells and Fas-deficient cells 10,11,43 as well as Bcl-2-and Bcl-XL-overexpressing cells are ALP-resistant.8,29 Our data indicate that membrane rafts are critical for the action of edelfosine and perifosine as disruption of lipid rafts prevented Fas/CD95 clustering, DISC formation and apoptosis in ALP-treated MM cells. Thus, edelfosine and perifosine can be considered as efficient antitumor drugs acting through an unprecedented mechanism of action in the killing of MM cells by translocation and concentration of death receptors in lipid rafts.
Our original studies demonstrated that coclustering of Fas/CD95 and lipid rafts underlay the antitumor action of edelfosine in human leukemic cells,12 involving for the first time membrane rafts in Fas/CD95-mediated apoptosis and cancer chemotherapy. Subsequently, additional antitumor drugs, including resveratrol,13 cisplatinum,14 aplidin,15 and now the herein reported perifosine (this work), have been shown to induce similar coclustering of Fas/CD95 and lipid rafts in their respective antitumor actions. Overall, these evidences suggest that the concentration of death receptors in lipid rafts is a crucial event in the regulation of apoptosis as well as in the antitumor action of distinct anticancer drugs, even though the extent of death receptor recruitment in lipid rafts is highly dependent on both target cell and stimulus.11,15-17 Our data show that ALPs, especially edelfosine, exacerbate the recruitment of apoptotic molecules in lipid rafts in MM cells, rendering MM particularly suitable for ALP treatment. ALPs were able to recruit the 3 major death receptors, Fas/CD95, DR4, and DR5, together with adaptor molecules and downstream signaling molecules into lipid rafts in MM cells. These data square with recent findings showing redistribution of both Fas/CD95 and TRAIL receptors in lipid rafts following cancer chemotherapy.15,44
The concentration of death receptors in lipid rafts following ALP treatment rendered MM cells more sensitive to the action of death receptor ligands. This is of particular importance for TRAIL, because this ligand shows a promising and selective antitumor action in different cancer cells45 as well as antimyeloma activity.46,47 Thus, our findings indicate that edelfosine and perifosine are not only effective in the killing of MM cells, but also they might be valuable drugs in combination therapy. In addition, MM1R cells, which showed resistance to dexamethasone treatment, were readily killed by these ALPs, suggesting that these agents could circumvent drug resistance in MM. Edelfosine has been shown to induce cell killing in MM cells resistant to doxorubicin, melphalan, mitoxantrone, VP-16, cytoxan, and vincristine,48 and perifosine has been reported to be cytotoxic to MM cells resistant to dexamethasone and melphalan.49 In addition, ALPs have been shown to be effective antitumor drugs in mouse MM models.49,50
A remarkable finding of the current study is that ALPs killed malignant MM cells, sparing normal cells derived from the same patient. Normal B and T cells as well as vascular endothelial cells were also spared. This agrees with previous reports showing that edelfosine is not toxic to normal cells at concentrations that kill a broad range of tumor cells.8,10,51
The present findings further support the notion that ALPs are effective in the treatment of hematologic malignancies, and that the induction of apoptosis through coclustering of death receptors in lipid rafts is a promising target in cancer therapy.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Contribution: C.G. designed and performed the research and analyzed the data; and F.M. designed the research, analyzed the data, and wrote the paper.
Acknowledgments
This work was supported by grants from Fondo de Investigación Sanitaria and European Commission (FIS-FEDER 06/0813, 04/0843, 02/1199), Ministerio de Educación y Ciencia (SAF2005-04293), Fundación de Investigación Médica Mutua Madrileña (FMM), Fundación “la Caixa” (BM05-30-0), and Junta de Castilla y León (CSI04A05), and by the Ramón y Cajal Program from the Ministerio de Educación y Ciencia of Spain (C.G.).



![Figure 5. Requirement of Fas/CD95 for ALP-induced apoptosis. (A) Cell-surface expression of Fas/CD95, DR4, and DR5 in MM144, OPM-2, and Fas/CD95-deficient OPM-2 cells was determined by flow cytometry. Percentage of positive cells for each death receptor was estimated using the P3X63 myeloma culture supernatant and an isotype-matched FITC-conjugated nonrelevant IgG monoclonal antibody as negative controls. (B) Edelfosine uptake was determined after incubating MM144, OPM-2, and Fas/CD95-deficient OPM-2 cells with 10 nmol [3H]edelfosine for 6 hours. (C,D) Fas/CD95-deficient OPM-2 and MM144 cells were untreated (control) or treated with 10 μM edelfosine or perifosine for 12 hours, and then Fas/CD95 cell-surface expression was determined by flow cytometry as the percentage of Fas-positive cells (C) or MFI (D). (E) Cell-surface expression of Fas/CD95 was analyzed by immunofluorescence flow cytometry in Fas/CD95-deficient OPM-2 infected with empty virus (Fas-deficient OPM-2–vector), recombinant retrovirus containing human Fas/CD95 (Fas-deficient OPM-2–Fas), and recombinant retrovirus containing a truncated version of human Fas/CD95 (Fas-deficient OPM-2-FasΔ57C), lacking the 57 COOH-terminal amino acids. Percentage of Fas/CD95+ cells was estimated using the P3X63 myeloma supernatant and an isotype-matched FITC-conjugated nonrelevant IgG monoclonal antibody as negative controls. (F) Induction of apoptosis in the above retrovirus-infected cells was determined by flow cytometry after a 24-hour incubation with 10 μM edelfosine or perifosine. Untreated control cells were run in parallel. Data shown are means ± SD of 3 independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/109/2/10.1182_blood-2006-04-016824/4/m_zh80020706370005.jpeg?Expires=1765890445&Signature=Uqqa~pL6UdURS3NgrMmyJKMkdPZlfGVDd2wBS7oBGjeV4-cCp2VAUMXChuwLghGm83lQ3uavafYsFvkGqXmJi3wZ2zRI-h0Y1uENpo5c368e9WO0NQamJ00AzC5QqCBcsb3gNZbA5~yoHYi0GHsJwX3jfM2MqQfrNKynE~BMJkvH11U3Rc3qih-ZiDJhtq2PsYvvmobhYaxrLoUKrIRpCzOdNRyFDgj-iFHHjR2R1IJ420XuNPY9Z9YnIdpZSnSGskr-pRLOWHtb6AifBHOQJpGbzkoQosO-1TGs4bzDQ-iix9sg2dxV03gdPXtdBQEkM~vzQELtWXhOQzCnXnM3pw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal