Abstract
Chronic lymphocytic leukemia (CLL) B cells express BR3, the specific receptor for the B cell–activating factor of tumor necrosis factor family (BAFF). CLL cells also express 2 other receptors for BAFF, namely B-cell maturation antigen (BCMA) and the transmembrane activator and calcium modulator and cyclophilin ligand-interactor (TACI), which also bind a proliferation-inducing ligand (APRIL). We found that signaling through BR3, but not BCMA or TACI, activated the alternative nuclear factor of κ B (NF-κB) pathway in CLL cells, whereas signaling through BCMA/TACI induced activation of the canonical NF-κB pathway. Blocking BR3 did not inhibit the capacity of BAFF to support CLL cell survival in vitro. On the other hand, specifically blocking the canonical NF-κB pathway with UTC, an inhibitor of IκB kinase β (IKKβ), or transfection of CLL cells with the IκBα super-repressor, blocked the capacity of BAFF and APRIL to promote CLL cell survival in vitro. This contrasts what is found with normal blood B cells, which apparently depend on activation of the alternative NF-κB pathway for BAFF-enhanced survival. These findings suggest that inhibitors of protein kinase IKKβ, which is required for activation of the canonical NF-κB pathway, might have a therapeutic role in this disease.
Introduction
The B cell–activating factor of tumor necrosis factor (TNF) family (BAFF, also known as BlyS, TALL-1, zTNF4, and THANK) is a potent regulator of normal B-cell development and function.1-3 A proliferation-inducing ligand (APRIL, also termed TALL-2 and TRAD-1) also is a member of the TNF family and shares significant homology with BAFF. APRIL has been found to stimulate the growth of tumor cells and the proliferation of primary lymphocytes.3-5 BAFF and APRIL bind 2 receptors of the TNF superfamily, B-cell maturation antigen (BCMA) and transmembrane activator or the calcium modulator and cyclophilin ligand-interactor (TACI).6-8 BAFF, but not APRIL, binds a third receptor known as BAFF receptor (BAFF-R or BR3).9,10 BCMA, TACI, and BR3 are expressed on normal B lymphocytes.9,11,12
Neoplastic B cells in chronic lymphocytic leukemia (CLL) also express these receptors for BAFF and APRIL, which, when ligated, can promote CLL cell survival in vitro.13-18 Furthermore, nurselike cells (NLCs), which can protect CLL cells in vitro and presumably in vivo,19,20 express high levels of BAFF and APRIL, accounting in part for their capacity to promote CLL cell survival in a paracrine fashion.21 Kern et al14 found that CLL cells themselves might express BAFF or APRIL, suggesting that these factors also might function in an autocrine fashion to promote leukemia cell survival.14 Understanding the mechanisms whereby BAFF and APRIL support CLL survival could lead to the development of inhibitors to BAFF and APRIL signaling that may be therapeutic in patients with this disease.
Many members of the TNF superfamily trigger the activation of nuclear factor of κ B (NF-κB). Recent studies have revealed that 2 NF-κB pathways, the canonical pathway and the alternative pathway, regulate the activity of NF-κB.22,23 Activation of the canonical NF-κB pathway proceeds through the degradation of the inhibitor of NF-κBα (IκBα), which is induced after its phosphorylation by the β subunit of the IκB kinase (IKK) complex (IKKβ).24 Degradation of IκBα leads to the nuclear translocation of active NF-κB heterodimers (composed of p50, p65, and/or c-Rel), where they can affect changes in gene expression. Activation of the alternative NF-κB pathway results from processing of NF-κB2/p100 to p52, which is triggered by the phosphorylation of NF-κB2/p100 by the α subunit of the IKK complex (IKKα).25 This allows for nuclear translocation of p52 along with RelB, where together they can influence the expression of genes that are distinct from those regulated by the canonical NF-κB pathway.26 Studies suggest that CLL cells may have constitutively activated NF-κB, which appears to enhance leukemia cell survival.27,28 However, the relative contribution of each NF-κB pathway to leukemia cell survival has not been described. We examined what NF-κB pathways are stimulated in CLL cells by BAFF or APRIL and investigated the relative contribution of each pathway to BAFF- and APRIL-induced leukemia cell survival.
Materials and methods
Cell preparation
After informed consent was obtained in compliance with the Declaration of Helsinki, blood samples were collected from patients at the University of California at San Diego (UCSD) Medical Center who satisfied diagnostic and immunophenotypic criteria for common B-cell CLL.29 Approval was obtained from the UCSD institutional review board for these studies. Blood mononuclear cells were isolated through density-gradient centrifugation with Ficoll-Hypaque (Pharmacia, Uppsala, Sweden). Cells were suspended in fetal calf serum (FCS) containing 10% dimethyl sulfoxide (DMSO) for storage in liquid nitrogen. The viability of the CLL cells was at least 85% at the initiation of cell culture, as assessed by their capacity to exclude propidium iodide (PI; Molecular Probes, Eugene, OR). All CLL mononuclear cell samples contained more than 95% CD19+/CD5+/CD3− CLL B cells, as assessed by flow cytometry using fluorochrome-conjugated monoclonal antibodies (mAbs) specific for CD19, CD5, or CD3 (BD PharMingen, La Jolla, CA). CLL cells were cultured in RPMI-1640 (Gibco, Rockville, MD) supplemented with 10% FCS and penicillin-streptomycin-glutamine (culture media) in 5% CO2 in air at 37°C. CD19+ B cells of healthy donors were isolated from the buffy coats of blood samples collected from adult volunteers at the San Diego Blood Bank (San Diego, CA), as described.20,30
Cell isolation
Normal blood CD19+ B cells were isolated from the buffy coats of healthy donors using CD19 Dynabeads and Detach a Beads (Dynal AS, Oslo, Norway) according to the manufacturer's instructions. Purity of the isolated B cells was greater than 95%, as assessed by flow cytometry with a fluorochrome-conjugated anti-CD19 mAb that did not compete with the anti-CD19 mAb used for previous positive selection (data not shown).
Reagents
Recombinant human BAFF (rhBAFF) was a kind gift from Dr G. Zhang (National Jewish Medical and Research Center, Denver, CO). rhAPRIL was purchased from Alexis Biochemicals (San Diego, CA). We obtained humanized anti–human BR3 antibody (h9.1), recombinant human BR3-Fc, recombinant human BCMA-Fc, and control human IgG from Genentech (South San Francisco, CA) and Biogen-Idec (Cambridge, MA).
Antibodies
Rat anti-BCMA and anti-TACI mAbs were purchased from Alexis Biochemicals. The relevant isotype control mAbs were from BD PharMingen. Phycoerythrin (PE)–labeled goat anti–rat IgG was from Southern Biotech (Birmingham, AL). Biotinylated anti-BR3 antibody and mouse IgG2a isotype control were obtained from Genentech. Allophycocyanin-labeled streptavidin was purchased from BD PharMingen. Mouse mAb specific for IκBα was from Imgenex (San Diego, CA). Rabbit anti–phospho-IκBα(Ser32) antibody was from Cell Signaling Technology (Beverly, MA). Mouse anti-p52 and rabbit anti-p65 antibodies for immunoblot analysis were from Upstate Biotechnology (Lake Placid, NY). We used mouse anti-HA mAb from Roche Diagnostics (Indianapolis, IN), anti–SP-1 from Santa Cruz Biotechnology (Santa Cruz, CA), and anti–β-actin antibodies from Sigma Immunochemicals (St Louis, MO).
Synthesis of the IKKβ inhibitor
We synthesized an inhibitor of IKKβ, namely 5-(4-fluorophenyl)-2-ureido-thiophene-3 carboxylic acid amide (UTC).31 UTC was prepared in 3 steps according to the procedure described in the Patent Cooperation Treaty patent application WO 02/30353 A2 beginning with 2-(4-fluorophenyl)ethanol. Oxidation of this alcohol to the corresponding aldehyde using pyridinium chlorochromate followed by condensation with 2-cyanoacetamide and sulfur provided the substituted thiophene, 2-amino-5-(4-fluorophenyl)thiophene-3-carboxamide. Finally, the amino function of this thiophene was converted to the ureido group by reaction with trichloroacetylisocyanate followed by treatment with ammonia to yield the final product, UTC.
Flow cytometry
To analyze for surface membrane expression of BCMA, TACI, or BR3, the cells were stained with saturating amounts of primary antibodies for 30 minutes at 4°C in RPMI-1640 or phosphate-buffered saline (PBS) supplemented with 0.5% BSA (fluorescence-activated cell sorter [FACS] buffer), washed 2 times, and then counterstained with PE-labeled secondary antibody or allophycocyanin-labeled streptavidin for 30 minutes at 4°C. After 2 washes, the cells were analyzed with FACSCalibur (Becton Dickinson, Mountain View, CA). Flow cytometry data were analyzed with FlowJo software (Tree Star, San Carlos, CA).
Measurement of cell viability
Freshly thawed CLL B cells were cultured at the concentration of 1 × 106/mL under various conditions. Determination of CLL cell viability in this study was based on an analysis of mitochondrial transmembrane potential (ΔΨm) using 3,3′-dehexyloxacarbocyamine iodine (DiOC6) and cell membrane permeability to PI, as described.32 For viability assays, 100 μL cell culture was collected at the indicated time points and transferred to polypropylene tubes containing 100 μL of 80 nM DiOC6 (Molecular Probes) and 2 μg/mL PI in FACS buffer. The cells then were incubated at 37°C for 15 minutes and analyzed within 30 minutes by flow cytometry with a FACSCalibur (Becton Dickinson). Fluorescence was recorded at 525 nm (FL-1) for DiOC6 and at 600 nm (FL-3) for PI.
Immunoblot analysis
Cell lysates were prepared with radioimmunoprecipitation assay (RIPA) buffer (10 mM Tris [tris(hydroxymethyl)aminomethane], pH 7.4, 150 mM NaCl, 1% Triton X-100, 0.1% deoxycholate, 0.1% sodium dodecyl sulfate [SDS], 5 mM ethylenediaminetetraacetic acid [EDTA]), containing 1 × complete protease inhibitor cocktail (Roche Diagnostics), 1 mM sodium fluoride (NaF), and 1 mM sodium vanadate (Na3VO4). Lysates were normalized for total protein (25 μg) and subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE; 4%-15% gradient gels; Bio-Rad, Hercules, CA) and immunoblot assay. We incubated the blots with secondary antibodies that were conjugated with horseradish peroxidase. Blots then were prepared for enhanced chemiluminescence (ECL) detection (Amersham, Little Chalfont, United Kingdom) and subsequent autoradiography with Super RX film (Fuji, Tokyo, Japan). Defined sections of the film were scanned for density measurement and analyzed using image software from the National Institutes of Health (Bethesda, MD).
Subcellular fractionation and detection of cytoplasmic or nuclear NF-κB
For fractionation experiments, cells were collected by centrifugation and washed with PBS. The cell pellet containing 5 × 106 cells was suspended in 100 μL hypotonic buffer (50 mM Tris [pH 7.4], 5 mM EDTA, 10 mM NaCl, 0.05% Nonidet P-40 [NP-40]) containing 1 × complete protease inhibitor cocktail, 1 mM NaF, and 1 mM Na3VO4. After 10 minutes, the lysate was spun at 500g, and the supernatant was collected as cytoplasmic lysates. The pellet was washed 5 times in hypotonic buffer containing 0.1% NP-40, and the remaining pellet was suspended in 100 μL RIPA buffer containing protease and phosphatase inhibitors. After 10 minutes, the lysate was spun at 20 000g for 15 minutes, and the supernatants recovered as nuclear lysates.
Electrophoretic mobility shift assays
Nuclear proteins were extracted using a nuclear extraction kit (Pierce, Rockford, IL) in the presence of 1 × complete protease inhibitor cocktail (Roche Diagnostics). Total protein was measured using a modified Bradford test (Bio-Rad). Two micrograms nuclear protein extract was incubated on ice for 30 minutes with antibodies to p50 and p65 (Santa Cruz Biotechnology). Later, a radiolabeled double-stranded probe that encompassed the κB1 site was added, followed by incubation at room temperature for 30 minutes. Samples were loaded on a 6% acrylamide gel and run at 150 V for 3.5 hours.
Plasmid
A pcDNA3-based expression vector for hemagglutinin (HA)–tagged IκBα mutant (S32A/S36A), also referred to as IκBα super-repressor (IκBα-SR), was as described.33 Mutation of IκBα-SR was confirmed by DNA sequencing. pmaxGFP (green fluorescent protein) was obtained from Amaxa (Gaithersburg, MD).
Cell transfection
CLL cells were transfected with the use of nucleofection technology (Amaxa). Cells were suspended in solution from the human B cell nucleofector kit, also available as part of the Amaxa cell optimization kit, according to the manufacturer's instructions. Briefly, 100 μL of 5 × 106 cell suspension mixed with 5 μg cDNA was transferred to the provided cuvette and nucleofected with a Nucleofector apparatus (Amaxa). Cells were transfected with the U-15 pulsing parameter and were immediately transferred to wells containing culture medium prewarmed to 37°C in 12-well plates. After transfection, the cells were cultured before analysis. pmaxGFP was used to gauge transfection efficiency.
Statistical analysis
Results are shown as mean ± SD of at least 5 samples each. For statistical comparison between groups, the Bonferroni t test or the Student paired t test was used, as indicated. Analyses were performed with PRISM software version 3.0 (GraphPad Software, San Diego, CA).
Results
Expression of BCMA, TACI, and BR3 on CLL B cells
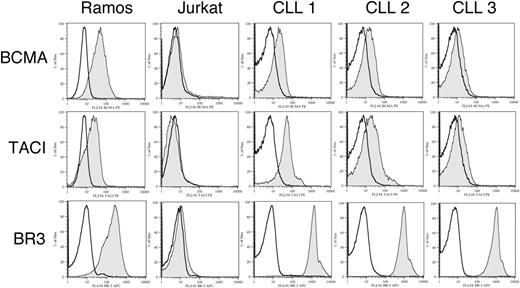
We examined for surface expression of BCMA, TACI, and BR3 on CLL B cells using flow cytometry. Of 11 samples tested, we found 8 expressed detectable BCMA, 9 expressed detectable TACI, and 11 expressed BR3, consistent with earlier findings.13,14,18,34 The average mean fluorescence intensity ratio (MFIR) for CLL cells stained with antibodies specific for BCMA, TACI, or BR3 on CLL B cells was 2.6 ± 2.1, 4.2 ± 3.0, or 24.1 ± 5.5, respectively. All CLL samples expressed at least one of the APRIL receptors, BCMA or TACI. Three representative samples are shown in Figure 1. Thus, CLL B cells typically express all 3 receptors for BAFF or APRIL.
Expression of BCMA, TACI, and BR3 on CLL B cells. B cells from CLL patients were examined by flow cytometry for surface expression of BCMA, TACI, and BR3 after labeling with specific primary and secondary antibodies (gray histogram) or isotype controls (open histograms). Representative histograms of 3 CLL patients are shown.
Expression of BCMA, TACI, and BR3 on CLL B cells. B cells from CLL patients were examined by flow cytometry for surface expression of BCMA, TACI, and BR3 after labeling with specific primary and secondary antibodies (gray histogram) or isotype controls (open histograms). Representative histograms of 3 CLL patients are shown.
Effects of rhBAFF and rhAPRIL on NF-κB signaling pathways in CLL B cells
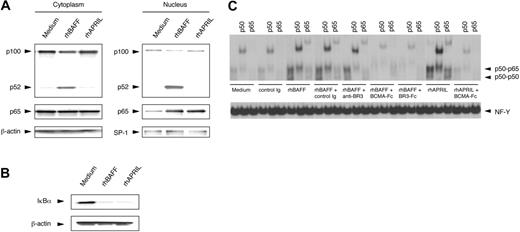
We examined for activation of NF-κB in CLL cells treated with rhBAFF or rhAPRIL at concentrations that could promote CLL B-cell survival in vitro. Studies indicate that BAFF could induce the activation of the NF-κB2/p100 in normal B cells.35,36 Such activation involves processing of p100 to p52 with subsequent translocation of p52 to the nucleus. We found that rhBAFF could also induce the translocation of p52 to the nucleus in CLL B cells (Figure 2A), demonstrating activation of the alternative NF-κB pathway. In contrast, we did not observe translocation of p52 to the nucleus in CLL cells treated with rhAPRIL, even at concentrations that could support CLL cell survival in vitro. rhBAFF and rhAPRIL, however, induced translocation of p65 to the nucleus and degradation of IκBα, indicating that each could activate the canonical NF-κB pathway in CLL cells (Figure 2A-B). Activation of the canonical NF-κB pathway by rhBAFF or rhAPRIL was verified with electrophoretic mobility shift assay (EMSA). Nuclear extracts prepared from CLL cells cultured with rhBAFF or rhAPRIL contained increased amounts of proteins capable of binding NF-κB consensus motifs, which experienced a supershift when preincubated with anti-p50 or anti-p65 antibodies (Figure 2C). Nuclear extracts of CLL cells treated with rhBAFF or rhAPRIL in the presence of soluble BCMA (BCMA-Fc), which can bind BAFF or APRIL and can preclude these factors from binding their receptors, had less NF-κB binding activity. Nuclear extracts of CLL cells treated with rhBAFF in the presence of soluble BR3 (BR3-Fc) also contained smaller amounts of NF-κB binding activity. However, nuclear extracts of CLL cells treated with rhBAFF and anti-BR3 antibody, which can bind to BR3 and block BAFF binding to BR3 but not to BCMA or TACI,52 contained amounts of NF-κB–binding factors similar to those found in extracts prepared from CLL cells treated with rhBAFF alone (Figure 2C). These results suggest that signaling through BR3, but not through BCMA or TACI, activated the alternative NF-κB pathway in CLL cells, whereas signaling through BCMA or TACI activated the canonical NF-κB pathway.
Activation of NF-κB in CLL B cells by rhBAFF or rhAPRIL. (A) Immunoblot analyses with anti-p100 or anti-p65 antibodies. CLL B cells were cultured with or without rhBAFF (50 ng/mL) or rhAPRIL (500 ng/mL) for 24 hours. Cytoplasmic and nuclear extracts were prepared as described in “Materials and methods.” We evaluated for equal loading in each lane by stripping the blot and probing it with antibodies specific for β-actin (for cytoplasmic extracts) or SP-1 (for nuclear extracts). The MFIR for each receptor on this CLL sample was 2.8, 3.6, or 31.3 for BCMA, TACI, or BR3, respectively. Translocation of p65 to the nucleus was seen in CLL cells treated with rhBAFF or rhAPRIL. In contrast, translocation of p52 was observed only in CLL cells treated with rhBAFF. (B) Immunoblot analysis with anti-IκBα antibodies. CLL B cells were cultured with or without rhBAFF (50 ng/mL) or rhAPRIL (500 ng/mL) for 30 minutes. Total cell lysates were prepared as described in “Materials and methods.” Degradation of IκBα was observed in CLL cells treated with rhBAFF or rhAPRIL. (C) EMSAs of nuclear extracts from CLL cells. CLL B cells were cultured with or without rhBAFF (50 ng/mL), rhAPRIL (500 ng/mL), BCMA-Fc (10 μg/mL), BR3-Fc (10 μg/mL), anti-BR3 (10 μg/mL), or control immunoglobulin (10 μg/mL) for 24 hours. We monitored for equal loading of protein in each lane by examining NF-Y binding to DNA. The MFIR of CLL cells stained for BCMA, TACI, or BR3 was 3.7, 8.8, or 24.3, respectively. Nuclear extracts prepared from CLL cells cultured with rhBAFF or rhAPRIL contained increased amounts of proteins capable of binding the NF-κB consensus motifs, which experienced a supershift when preincubated with anti-p50 or anti-p65 antibodies. Nuclear extracts of CLL cells treated with rhBAFF or rhAPRIL in the presence of BCMA-Fc or BR3-Fc had less NF-κB binding activity. However, nuclear extracts of CLL cells treated with rhBAFF and anti-BR3 antibody contained amounts of NF-κB binding factors similar to those of extracts prepared from CLL cells treated with rhBAFF alone.
Activation of NF-κB in CLL B cells by rhBAFF or rhAPRIL. (A) Immunoblot analyses with anti-p100 or anti-p65 antibodies. CLL B cells were cultured with or without rhBAFF (50 ng/mL) or rhAPRIL (500 ng/mL) for 24 hours. Cytoplasmic and nuclear extracts were prepared as described in “Materials and methods.” We evaluated for equal loading in each lane by stripping the blot and probing it with antibodies specific for β-actin (for cytoplasmic extracts) or SP-1 (for nuclear extracts). The MFIR for each receptor on this CLL sample was 2.8, 3.6, or 31.3 for BCMA, TACI, or BR3, respectively. Translocation of p65 to the nucleus was seen in CLL cells treated with rhBAFF or rhAPRIL. In contrast, translocation of p52 was observed only in CLL cells treated with rhBAFF. (B) Immunoblot analysis with anti-IκBα antibodies. CLL B cells were cultured with or without rhBAFF (50 ng/mL) or rhAPRIL (500 ng/mL) for 30 minutes. Total cell lysates were prepared as described in “Materials and methods.” Degradation of IκBα was observed in CLL cells treated with rhBAFF or rhAPRIL. (C) EMSAs of nuclear extracts from CLL cells. CLL B cells were cultured with or without rhBAFF (50 ng/mL), rhAPRIL (500 ng/mL), BCMA-Fc (10 μg/mL), BR3-Fc (10 μg/mL), anti-BR3 (10 μg/mL), or control immunoglobulin (10 μg/mL) for 24 hours. We monitored for equal loading of protein in each lane by examining NF-Y binding to DNA. The MFIR of CLL cells stained for BCMA, TACI, or BR3 was 3.7, 8.8, or 24.3, respectively. Nuclear extracts prepared from CLL cells cultured with rhBAFF or rhAPRIL contained increased amounts of proteins capable of binding the NF-κB consensus motifs, which experienced a supershift when preincubated with anti-p50 or anti-p65 antibodies. Nuclear extracts of CLL cells treated with rhBAFF or rhAPRIL in the presence of BCMA-Fc or BR3-Fc had less NF-κB binding activity. However, nuclear extracts of CLL cells treated with rhBAFF and anti-BR3 antibody contained amounts of NF-κB binding factors similar to those of extracts prepared from CLL cells treated with rhBAFF alone.
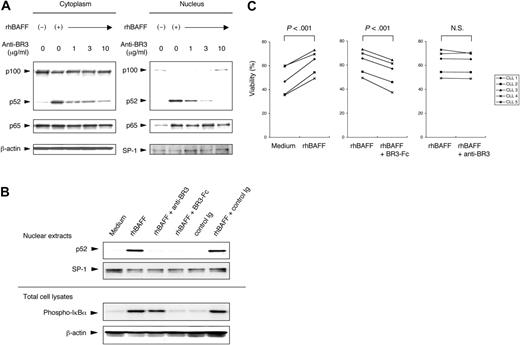
To verify the selective capacity of BR3 to activate the alternative NF-κB pathway, CLL cells were cultured with rhBAFF and increasing concentrations of anti-BR3 antibody. CLL cells cultured with rhBAFF without anti-BR3 were stimulated to effect nuclear translocation of p52 and p65. The addition of anti-BR3 inhibited BAFF from inducing activation of the alternative NF-κB pathway. Anti-BR3 at 10 μg/mL could completely inhibit BAFF-induced translocation of p52, but not p65 (Table 1; Figure 3A). BR3-Fc inhibited p52 translocation to the nucleus and phosphorylation of IκBα induced by rhBAFF. Anti-BR3, however, could not inhibit the phosphorylation of IκBα (Figure 3B). These data indicate that signaling through BR3 is necessary and sufficient to activate the alternative NF-κB pathway in CLL cells.
Densitometry analysis of nuclear p52 and p100
| . | Medium . | rhBAFF . | rhBAFF + anti-BR3, 1 μg/mL . | rhBAFF + anti-BR3, 3 μg/mL . | rhBAFF + anti-BR3, 10 μg/mL . |
|---|---|---|---|---|---|
| p52 | 1.00 ± 0.00 | 47.41 ± 7.20 | 27.45 ± 0.72 | 9.29 ± 2.94 | 1.73 ± 0.57 |
| p100 | 1.00 ± 0.00 | 0.13 ± 0.07 | 0.18 ± 0.09 | 0.38 ± 0.30 | 1.04 ± 0.47 |
| . | Medium . | rhBAFF . | rhBAFF + anti-BR3, 1 μg/mL . | rhBAFF + anti-BR3, 3 μg/mL . | rhBAFF + anti-BR3, 10 μg/mL . |
|---|---|---|---|---|---|
| p52 | 1.00 ± 0.00 | 47.41 ± 7.20 | 27.45 ± 0.72 | 9.29 ± 2.94 | 1.73 ± 0.57 |
| p100 | 1.00 ± 0.00 | 0.13 ± 0.07 | 0.18 ± 0.09 | 0.38 ± 0.30 | 1.04 ± 0.47 |
CLL B cells were cultured with or without rhBAFF (50 ng/mL) at the indicated concentration of anti-BR3 antibody for immunoblot analysis. Defined sections of the immunoblot film were analyzed with image analysis software from the National Institutes of Health. Results are presented as density of the specific bands using extracts of treated cells relative to that of bands from extracts of CLL cells cultured in medium alone. Data are shown as mean ± SD from experiments performed on leukemia samples from 3 patients. Anti-BR3 antibody at 10 μg/mL could completely inhibit BAFF-induced translocation of p52.
Blocking the alternative NF-κB pathway with anti-BR3 antibody. (A) CLL B cells were cultured for 24 hours with or without rhBAFF (50 ng/mL) and anti-BR3 at the indicated concentrations. Cytoplasmic and nuclear extracts were prepared as described in “Materials and methods” for immunoblot analysis. Protein content was normalized to 25 μg for the cytoplasmic fraction and 12.5 μg for the nuclear fraction. MFIR of CLL cells stained for BCMA, TACI, or BR3 was 3.7, 8.8, or 24.3, respectively. Translocation of p52 and p65 to the nucleus was seen in CLL cells treated with rhBAFF. Anti-BR3 at 10 μg/mL could completely inhibit nuclear translocation of p52 induced by rhBAFF. (B) CLL B cells were cultured with or without rhBAFF (50 ng/mL), anti-BR3 (10 μg/mL), BR3-Fc (10 μg/mL), or control immunoglobulin (10 μg/mL) for 24 hours. Nuclear extracts and total cell lysates were prepared as described in “Materials and methods.” BR3-Fc inhibited the rhBAFF-induced nuclear translocation of p52 and the phosphorylation of IκBα. Anti-BR3 could inhibit the nuclear translocation of p52 but not the phosphorylation of IκBα. (C) CLL B cells were cultured with or without rhBAFF (50 ng/mL) and anti-BR3 (10 μg/mL) or BR3-Fc (10 μg/mL) for 48 hours. Results are viability of samples from each of 5 patients. The viability of CLL cells cultured with rhBAFF and BR3-Fc was significantly lower than that of CLL cells cultured with rhBAFF alone (P < .001; Student paired t test). Anti-BR3 did not impair the survival of CLL cells cultured with rhBAFF.
Blocking the alternative NF-κB pathway with anti-BR3 antibody. (A) CLL B cells were cultured for 24 hours with or without rhBAFF (50 ng/mL) and anti-BR3 at the indicated concentrations. Cytoplasmic and nuclear extracts were prepared as described in “Materials and methods” for immunoblot analysis. Protein content was normalized to 25 μg for the cytoplasmic fraction and 12.5 μg for the nuclear fraction. MFIR of CLL cells stained for BCMA, TACI, or BR3 was 3.7, 8.8, or 24.3, respectively. Translocation of p52 and p65 to the nucleus was seen in CLL cells treated with rhBAFF. Anti-BR3 at 10 μg/mL could completely inhibit nuclear translocation of p52 induced by rhBAFF. (B) CLL B cells were cultured with or without rhBAFF (50 ng/mL), anti-BR3 (10 μg/mL), BR3-Fc (10 μg/mL), or control immunoglobulin (10 μg/mL) for 24 hours. Nuclear extracts and total cell lysates were prepared as described in “Materials and methods.” BR3-Fc inhibited the rhBAFF-induced nuclear translocation of p52 and the phosphorylation of IκBα. Anti-BR3 could inhibit the nuclear translocation of p52 but not the phosphorylation of IκBα. (C) CLL B cells were cultured with or without rhBAFF (50 ng/mL) and anti-BR3 (10 μg/mL) or BR3-Fc (10 μg/mL) for 48 hours. Results are viability of samples from each of 5 patients. The viability of CLL cells cultured with rhBAFF and BR3-Fc was significantly lower than that of CLL cells cultured with rhBAFF alone (P < .001; Student paired t test). Anti-BR3 did not impair the survival of CLL cells cultured with rhBAFF.
CLL cells were cultured with rhBAFF and anti-BR3 or BR3-Fc to examine the role of the alternative pathway of NF-κB in the survival of CLL cells. We examined the viability of CLL cells at 24 hours (data not shown) and at 48 hours (Figure 3C). The addition of BR3-Fc to CLL cells cultured with rhBAFF inhibited the antiapoptotic effect of rhBAFF at 24 hours (P < .005) and at 48 hours (P < .001). On the other hand, anti-BR3, at a concentration that could completely block activation of the alternative NF-κB pathway, did not impair the capacity of rhBAFF to enhance CLL cell survival at 24 hours and at 48 hours in vitro. These results suggest that signaling through the alternative NF-κB pathway does not contribute significantly to CLL cell survival.
Blocking activation of the canonical NF-κB pathway with inhibitors of IKKβ
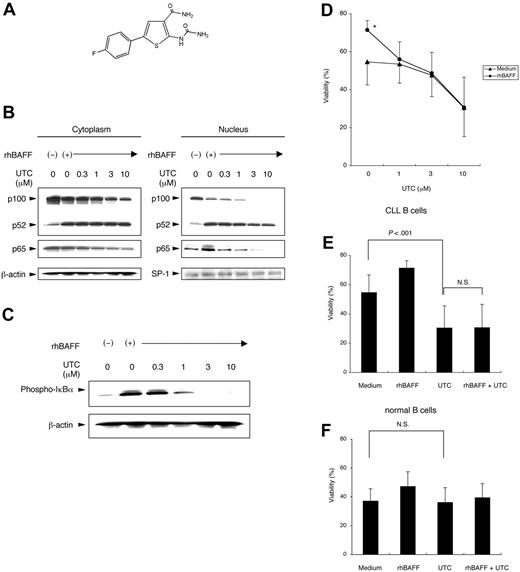
Activation of the canonical NF-κB pathway depends on IKKκ-dependent degradation of IκBα.37 Several compounds or natural products have been found to inhibit IKKβ, the subunit responsible for the phosphorylation of IκBα.31 We synthesized one such IKKβ inhibitor, 5-(4-fluorophenyl)-2-ureido-thiophene-3 carboxylic acid amide (UTC), to block the canonical NF-κB pathway in CLL cells (Figure 4A) (see “Materials and methods”).
Blocking the canonical NF-κB pathway with IKKβ inhibitor. (A) Chemical structure of the IKKβ inhibitor UTC. (B) CLL cells were preincubated with or without various concentrations of UTC for 1 hour. Cells were cultured with or without rhBAFF (50 ng/mL) for 24 hours, and cytoplasmic and nucleic cell lysates were prepared. Protein content was normalized to 25 μg for the cytoplasmic fraction and to 12.5 μg for the nuclear fraction. MFIR of CLL cells stained for BCMA, TACI, or BR3 was 1.6, 2.5, or 19.8, respectively. UTC inhibited the BAFF-induced nuclear translocation of p65 but not of p52. (C) Total cell lysates of CLL cells were prepared after the same treatment described. UTC inhibited the BAFF-induced phosphorylation of IκBα. (D) CLL cells were cultured with or without rhBAFF (50 ng/mL) and various concentrations of UTC for 48 hours. Results are from experiments performed on leukemia samples from each of 8 patients. The viability of CLL cells cultured with rhBAFF was significantly higher than that of CLL cells cultured in medium alone (*P < .01; Bonferroni t test). The protective effect of rhBAFF was inhibited by at least 1 μM UTC. (E) CLL cells were cultured with or without rhBAFF (50 ng/mL) and UTC (10 μM) for 48 hours. Results are from experiments performed on leukemia samples from each of 8 patients. The viability of CLL cells cultured with UTC was significantly lower than that of CLL cells cultured in medium alone (P < .001; Bonferroni t test). The capacity of BAFF to promote leukemia cell survival was not observed when the CLL cells were cultured with UTC. (F) Isolated normal B cells of healthy donors were cultured with or without rhBAFF (50 ng/mL) and UTC (10 μM) for 48 hours. Results are from studies performed on samples from each of 8 donors. No significant difference was observed in the viabilities of normal B cells cultured with or without UTC. (D-F) Error bars indicate SEM.
Blocking the canonical NF-κB pathway with IKKβ inhibitor. (A) Chemical structure of the IKKβ inhibitor UTC. (B) CLL cells were preincubated with or without various concentrations of UTC for 1 hour. Cells were cultured with or without rhBAFF (50 ng/mL) for 24 hours, and cytoplasmic and nucleic cell lysates were prepared. Protein content was normalized to 25 μg for the cytoplasmic fraction and to 12.5 μg for the nuclear fraction. MFIR of CLL cells stained for BCMA, TACI, or BR3 was 1.6, 2.5, or 19.8, respectively. UTC inhibited the BAFF-induced nuclear translocation of p65 but not of p52. (C) Total cell lysates of CLL cells were prepared after the same treatment described. UTC inhibited the BAFF-induced phosphorylation of IκBα. (D) CLL cells were cultured with or without rhBAFF (50 ng/mL) and various concentrations of UTC for 48 hours. Results are from experiments performed on leukemia samples from each of 8 patients. The viability of CLL cells cultured with rhBAFF was significantly higher than that of CLL cells cultured in medium alone (*P < .01; Bonferroni t test). The protective effect of rhBAFF was inhibited by at least 1 μM UTC. (E) CLL cells were cultured with or without rhBAFF (50 ng/mL) and UTC (10 μM) for 48 hours. Results are from experiments performed on leukemia samples from each of 8 patients. The viability of CLL cells cultured with UTC was significantly lower than that of CLL cells cultured in medium alone (P < .001; Bonferroni t test). The capacity of BAFF to promote leukemia cell survival was not observed when the CLL cells were cultured with UTC. (F) Isolated normal B cells of healthy donors were cultured with or without rhBAFF (50 ng/mL) and UTC (10 μM) for 48 hours. Results are from studies performed on samples from each of 8 donors. No significant difference was observed in the viabilities of normal B cells cultured with or without UTC. (D-F) Error bars indicate SEM.
First we examined whether UTC could block activation of the canonical NF-κB pathway in CLL cells. CLL cells were preincubated with or without varying concentrations of UTC for 1 hour. The treated cells then were cultured with or without rhBAFF for 24 hours. UTC inhibited BAFF-induced the nuclear translocation of p65 but not of p52 (Figure 4B). UTC also the inhibited phosphorylation of IκBα (Figure 4C). These data indicate that UTC can block BAFF-induced activation of the canonical NF-κB pathway but not of the alternative NF-κB pathway.
CLL cells were cultured with or without rhBAFF and UTC to determine whether blocking the canonical NF-κB pathway could impair the capacity of rhBAFF to enhance the survival of CLL cells in vitro. The viability of CLL cells cultured with rhBAFF was significantly higher than that of CLL cells cultured in medium alone (P < .01). The antiapoptotic effect of rhBAFF was inhibited by UTC at 1 μM, a concentration of UTC that did not impair the viability of CLL cells cultured without rhBAFF (Figure 4D). Treatment of CLL cells with UTC at 10 μM significantly decreased the viability of CLL cells compared with that of untreated cells (Figure 4E). However, UTC did not have any effect on the survival of isolated blood B cells of healthy donors in vitro (Figure 4F). These findings suggest that activation of the canonical NF-κB pathway may play a more important role in promoting the survival of CLL cells than of normal B cells.
Blocking activation of the canonical NF-κB pathway with IκBα-SR
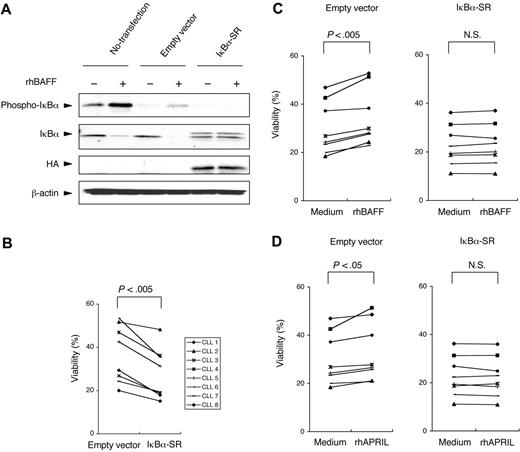
Conceivably, UTC also could affect signaling pathways other those leading to activation of the canonical NF-κB pathway. If so, then the capacity of UTC to inhibit the survival-promoting effects of rhBAFF on CLL cells may not be caused by its capacity to block activation of the canonical NF-κB pathway. To rule out this possibility, we transfected IκBα-SR into CLL cells with a plasmid expression vector. IκBα-SR encodes a mutant IκBα in which the serines at positions 32 and 36 are replaced by alanines.33 As such, this mutant form of IκBα can bind to p50 and p65 but cannot be phosphorylated on cellular activation and therefore resists proteolytic degradation. In control studies, we transfected CLL cells with a control plasmid expression vector (pcDNA3) or with IκBα-SR and then monitored the cells for degradation of IκBα after treatment with rhBAFF. Phosphorylation and degradation of IκBα were observed in rhBAFF-treated CLL cells that were not transfected or that were transfected with the control pcDNA3 vector. On the other hand, examination of CLL cells transfected with IκBα-SR revealed persistent, high-level expression of IκBα that was unaffected by treatment with rhBAFF (Figure 5A).
Blocking activation of the canonical NF-κB pathway through the transfection of IκBα-SR. (A) HA-tagged IκBα-SR (S32A/S36A) or empty pcDNA3 vector was transfected into CLL cells through nucleofection. Twenty-four hours after transfection, the cells were cultured in serum-free medium for 3 hours and then were stimulated with rhBAFF (50 ng/mL) for 30 minutes, and total cell lysates were prepared for immunoblot analysis. MFIR of CLL cells stained for BCMA, TACI, or BR3 was 8.2, 7.0, or 20.8, respectively. Phosphorylation and degradation of IκBα were seen in nontransfected CLL cells and empty vector–transfected CLL cells when these cells were stimulated with rhBAFF. On the other hand, phosphorylation of IκBα was not seen in IκBα-SR–transfected CLL cells. High levels of IκBα were seen in CLL cells transfected with IκBα-SR that were not affected by stimulation with rhBAFF. (B) Samples from each of 8 CLL patients were split and then transfected with an empty control vector or with IκBα-SR. This graph presents the viability of each CLL sample 24 hours after transfection. For each patient sample, the IκBα–SR–transfected CLL cells underwent apoptosis more readily than did control CLL cells transfected with the empty control vector 24 hours after transfection (P < .005; Student paired t test). (C-D) CLL cells were transfected with the empty control vector or with IκBα-SR. Four hours after transfection, the cells were cultured with or without rhBAFF (50 ng/mL) or rhAPRIL (500 ng/mL) for 24 hours. In empty control vector–transfected cells, the viability of CLL cells cultured in medium with rhBAFF or rhAPRIL was significantly higher than that of CLL cells cultured in medium alone (P < .005, P < .05, respectively; Student paired t test). The survival of IκBα–SR transfected cells could not be enhanced by rhBAFF or rhAPRIL.
Blocking activation of the canonical NF-κB pathway through the transfection of IκBα-SR. (A) HA-tagged IκBα-SR (S32A/S36A) or empty pcDNA3 vector was transfected into CLL cells through nucleofection. Twenty-four hours after transfection, the cells were cultured in serum-free medium for 3 hours and then were stimulated with rhBAFF (50 ng/mL) for 30 minutes, and total cell lysates were prepared for immunoblot analysis. MFIR of CLL cells stained for BCMA, TACI, or BR3 was 8.2, 7.0, or 20.8, respectively. Phosphorylation and degradation of IκBα were seen in nontransfected CLL cells and empty vector–transfected CLL cells when these cells were stimulated with rhBAFF. On the other hand, phosphorylation of IκBα was not seen in IκBα-SR–transfected CLL cells. High levels of IκBα were seen in CLL cells transfected with IκBα-SR that were not affected by stimulation with rhBAFF. (B) Samples from each of 8 CLL patients were split and then transfected with an empty control vector or with IκBα-SR. This graph presents the viability of each CLL sample 24 hours after transfection. For each patient sample, the IκBα–SR–transfected CLL cells underwent apoptosis more readily than did control CLL cells transfected with the empty control vector 24 hours after transfection (P < .005; Student paired t test). (C-D) CLL cells were transfected with the empty control vector or with IκBα-SR. Four hours after transfection, the cells were cultured with or without rhBAFF (50 ng/mL) or rhAPRIL (500 ng/mL) for 24 hours. In empty control vector–transfected cells, the viability of CLL cells cultured in medium with rhBAFF or rhAPRIL was significantly higher than that of CLL cells cultured in medium alone (P < .005, P < .05, respectively; Student paired t test). The survival of IκBα–SR transfected cells could not be enhanced by rhBAFF or rhAPRIL.
We transfected CLL cells from each of 8 patients with IκBα-SR, the control pcDNA3 vector, or the green fluorescent protein (GFP) expression plasmid. Transfection efficiencies ranged from 30% to 55%, as assessed by flow cytometry of cells transfected with the GFP expression plasmid. In all samples tested, the CLL cells transfected with IκBα-SR had lower viabilities after transfection than did control treated cells or CLL cells transfected with control vector 24 hours after transfection (Figure 5B). Moreover, treatment of CLL cells with rhBAFF or rhAPRIL after transfection significantly enhanced the viability of the cells transfected with the control expression plasmid but had no effect on CLL cells transfected with IκBα-SR (Figure 5C-D). These results support the notion that activation of the canonical NF-κB pathway plays a critical role in promoting CLL cell survival after treatment with BAFF or APRIL.
Discussion
BAFF and APRIL are potent regulators of B-cell development and survival. BAFF plays a critical role in promoting the survival of normal or neoplastic B cells, such as those causing CLL, lymphoma, or myeloma.13-18,21 APRIL reportedly can stimulate tumor cell growth and can induce the proliferation of primary B lymphocytes.3-5 Moreover, transgenic mice that constitutively overexpress APRIL develop clonal expansions of B1-type B lymphocytes that have characteristics similar to those of CLL cells.38 Nurselike cells (NLCs) that presumably reside in the leukemia cell microenvironment 19,20 also express high levels of BAFF and APRIL, which may promote CLL cell survival in a paracrine manner.21 Conceivably, strategies that can block the leukemia cell signaling pathways induced by BAFF or APRIL may disrupt the protective effect(s) of these factors on leukemia cells and prove effective in the treatment of this disease. Therefore, we examined the mechanisms whereby BAFF and APRIL support leukemia cell survival.
BAFF and APRIL can induce the activation NF-κB in normal B cells. However, relative contributions of the 2 NF-κB signaling pathways in CLL cells from their receptors—BCMA, TACI, and BR3—have not been described. In this study, we found that rhBAFF, but not rhAPRIL, could induce the degradation of p100 to p52 and the translocation of p52 to the nucleus, indicating that BAFF can activate the alternative NF-κB pathway in CLL cells (Figure 2A). Selective activation of the alternative NF-κB pathway by BAFF indicates that signaling through BR3 may be distinct from that induced by the ligation of BCMA or TACI. This is similar to the interaction of BAFF with BR3 on normal B cells, which also promotes the processing of NF-κB2/p100.35,39 Morrison et al40 reported that this specific function of BR3 is mediated by a sequence motif, PVPAT, homologous to the TRAF-binding site (PVQET) in CD40. They also showed that BR3 preferentially induced the alternative NF-κB pathway. In our studies, we found that anti-BR3 could not block BAFF-induced activation of the canonical NF-κB pathway (Figures 2C, 3A-B). This is in contrast to a recent report suggesting that signaling through BR3 activates the canonical and alternative NF-κB pathways.35 One possible explanation is that the canonical NF-κB pathway might be sufficiently activated through the other BAFF receptors, BCMA and TACI, in CLL cells. This explanation is supported by the observation that BR3-Fc, which can bind to BAFF and block BAFF binding to its receptors, could inhibit the canonical and alternative NF-κB pathways (Figure 3B).
Investigators have shown that some tumor cells that did not bind BAFF responded to APRIL.4,41 These findings suggest that APRIL may have a specific receptor (APRIL-R) expressed on these tumor cells that cannot bind BAFF. It is unclear whether such a hypothetical APRIL-R also is expressed on CLL cells. If so, then the studies presented here suggest that such a specific APRIL-R does not activate the alternative NF-κB pathway in these leukemia cells (Figure 2A).
Studies have shown that IKKα, which is involved in the canonical and alternative NF-κB pathways, is essential for B-cell maturation and formation of secondary lymphoid tissues in mice.25,42 IKKβ, which is involved in the canonical NF-κB pathway, also is reported to be required for the survival and proliferation of normal blood B cells in mice.43 BR3-knockout mice have reduced numbers of late transitional and follicular B cells and essentially are devoid of marginal zone B cells. Overexpression of the antiapoptotic protein Bcl-2 rescued mature B-cell development in these mice.44 In addition, NF-κB2–deficient mice also were reported to have a marked reduction in B-cell numbers.45 These findings indicate that BR3 mediates a survival signal in B cells and that NF-κB2, which is involved in the alternative NF-κB pathway, has an important role in the maintenance of the normal B cell population in mice.
On the other hand, the level of NF-κB activation may be high in CLL cells compared with that of nonneoplastic, normal B cells.28 Moreover, sustained activation of NF-κB may be critical for the survival of CLL cells.27 The results presented here indicated that activation of the alternative NF-κB pathway did not play a dominant role in promoting BAFF-induced survival of CLL cells (Figure 3C). Similarly, B1 cell development may be unaffected by the disruption of BAFF or BR3, which again contrasts with findings made with conventional B2 cells.46 On the other hand, the canonical NF-κB pathway appears to play the dominant role in promoting survival induced by BAFF and APRIL. The viability of CLL cells was markedly reduced when we blocked the canonical NF-κB pathway by an IKKβ inhibitor (UTC) or through ectopic expression of IκBα-SR (Figures 4E, 5B). Activation of the canonical NF-κB pathway in CLL may obviate the requirement for the alternative NF-κB pathway to promote leukemia cell survival, at least under the in vitro culture conditions used in this study. In this regard, Sasaki et al47 found that activation of the canonical NF-κB pathway could obviate BAFF-receptor signaling and promote B-cell proliferation in IKKβ-transgenic mice.
A number of selective IKKβ inhibitors have been developed.31 Several groups report that IKKβ inhibitors could induce the apoptosis of neoplastic cells, such as myeloma, lymphoma, and myeloid leukemia cells.48-50 We examined the effect of one IKKβ inhibitor, UTC, on CLL cells or on isolated normal B cells from healthy donors. This compound (Figure 4A) is identical to TPCA-1, which was reported by Podolin et al51 to be a specific inhibitor of IKKβ. They examined the activity of TPCA-1 against 13 kinases: IKKα, IKKβ, p38α, p38β, p38γ, p38δ, MAPKAPK2, MKK1, MAPK2, COX-1, COX-2, JNK1, and JNK3. The activity of TPCA-1 was 22- and 200-fold selective for IKKβ compared with IKKα and JNK3, respectively, and more than 550-fold selective for IKKβ compared with the other 10 kinases. Consequently, the compound seemed to have high specificity for IKKβ.
We found that UTC, which can selectively block the canonical NF-κB pathway, can entirely abrogate the protective effects of rhBAFF on CLL cell survival. Furthermore, the viability of CLL cells cultured with UTC was less than that of cells cultured in medium alone (Figure 4E). However, UTC did not have any effect on the survival of normal B cells cultured in medium alone (Figure 4F). These results suggest that the canonical NF-κB pathway is constitutively activated in CLL cells, even in CLL cells cultured in medium alone, and are in agreement with those reported by Furman et al.28 The constitutive activation of the canonical NF-κB pathway in CLL cells may arise from an autocrine mechanism of BAFF and APRIL, as reported previously.14 In addition, the survival benefit provided by exogenous rhBAFF to the leukemic cells was blocked by incubation with UTC (Figure 4D-E). These data imply that the antiapoptotic effect of BAFF or APRIL on CLL cells is highly dependent on activation of the canonical NF-κB pathway. As such, we speculate that inhibitors of IKKβ that inhibit the canonical NF-κB pathway may have a therapeutic role in this disease.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Contribution: T. Endo designed and performed the research, analyzed the data, and wrote the paper. M.N., T. Enzler, T.F., and D.F.J. performed the research and analyzed the data. H.B.C. synthesized the IKKβ inhibitor. M.K. designed the research and analyzed the data. T.J.K. designed the research, analyzed the data, and wrote the paper.
Acknowledgments
We thank Dr G. Zhang (National Jewish Medical and Research Center, Denver, CO) for the kind gift of rhBAFF. We also thank Dr Laura Z. Rassenti (Chronic Lymphocytic Leukemia Research Consortium) for collaboration and Drs Susan Kalled (Biogen-Idec) and Melissa A. Starovasnik (Genentech) for helpful discussions.
This work was supported in part by a Specialized Center of Research Award from the Leukemia and Lymphoma Society (T.J.K.).

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal