Abstract
Hodgkin lymphoma (HL) originates from the clonal expansion of malignant Hodgkin and Reed-Sternberg (HRS) cells. These B-cell–derived elements constitute less than 10% of the tumoral mass. The remaining tissue is comprised of an inflammatory infiltrate that includes myeloid cells. Myeloid cells activate B cells by producing BAFF and APRIL, which engage TACI, BCMA, and BAFF-R receptors on the B cells. Here, we studied the role of BAFF and APRIL in HL. Inflammatory and HRS cells from HL tumors expressed BAFF and APRIL. Unlike their putative germinal center B-cell precursors, HRS cells lacked BAFF-R, but expressed TACI and BCMA, a phenotype similar to that of plasmacytoid B cells. BAFF and APRIL enhanced HRS cell survival and proliferation by delivering nonredundant signals via TACI and BCMA receptors through both autocrine and paracrine pathways. These signals caused NF-κB activation; Bcl-2, Bcl-xL, and c-Myc up-regulation; and Bax down-regulation, and were amplified by APRIL-binding proteoglycans on HRS cells. Interruption of BAFF and APRIL signaling by TACI-Ig decoy receptor, which binds to and neutralizes BAFF and APRIL, or by small-interfering RNAs targeting BAFF, APRIL, TACI, and BCMA inhibited HRS cell accumulation in vitro and might attenuate HL expansion in vivo.
Introduction
Classical Hodgkin lymphoma (HL) is a lymphoid neoplasm that stems from the clonal expansion of mononuclear Hodgkin cells and multinuclear Reed-Sternberg cells expressing the CD30 antigen.1 Malignant Hodgkin and Reed-Sternberg (HRS) cells usually constitute less than 10% of the neoplastic mass.2 The remaining tissue is composed of a reactive cellular infiltrate. Although rare cases with T-cell genotype have been described,3,4 the vast majority of classical HL tumors is thought to originate from transformed germinal center (GC) B cells, because their HRS component harbors a monoclonal immunoglobulin (Ig) gene rearrangement and somatically mutated Ig V region genes.1,5-7 Despite their GC B-cell origin, HRS cells lack many molecules usually expressed by B cells and are incapable of producing functional Igs.7-12 While nonmalignant B cells that have lost their capacity to express Igs rapidly undergo apoptosis,13 malignant HRS cells survive. This abnormal survival is thought to be due to dysregulated activation of nuclear factor κB (NF-κB),14-18 a transcription factor essential for the development of both normal and neoplastic B cells.19,20
In classical HL, the reactive infiltrate is composed of nonmalignant T cells, B cells, plasma cells, and myeloid cells, including macrophages and granulocytes.2,21 These cells are thought to enhance HRS cell growth through cytokines and tumor necrosis factor (TNF) family members, such as CD30 ligand (CD30L), receptor activator of NF-κB ligand (RANKL), and CD40 ligand (CD40L).15,22-29 Recent studies show that myeloid cells express B-cell–activating factor of the TNF family (BAFF, also known as BLyS) and its homolog APRIL, a proliferation-inducing ligand,30-33 2 molecules essential for the survival, proliferation, and differentiation of B cells and plasma cells.34,35 BAFF activates B cells and plasma cells by binding to transmembrane activator and calcium modulator and cyclophylin ligand interactor (TACI), B-cell maturation antigen (BCMA), and BAFF receptor (BAFF-R) receptors. APRIL activates B cells and plasma cells by binding to TACI and BCMA, but not BAFF-R.36 By recruiting TNF receptor–associated factor (TRAF) adaptor molecules, TACI, BCMA, and BAFF-R activate an IκB kinase (IKK) complex that in turn elicits phosphorylation-dependent degradation of inhibitor of NF-κB (IκB), which retains p50, c-Rel, and p65 NF-κB proteins in a cytoplasmic inactive form.37-39 IκB degradation causes NF-κB nuclear translocation and transcriptional activation of NF-κB–responsive genes involved in B-cell survival, proliferation, and maturation.31,39-41 Of note, recent studies show that APRIL signaling via TACI and BCMA receptors is reinforced by heparan sulfate proteoglycans (HSPGs) anchored on the cell membrane or associated with the extracellular matrix.35,42,43 The role of TACI, BCMA, BAFF-R, and HSPGs in HL remains unknown.
BAFF and APRIL are implicated in B-cell neoplasias,44-52 including non-Hodgkin lymphoma (NHL).39,53-57 Consistent with this, BAFF and APRIL expression is up-regulated by lymphoma-associated viruses, such as Epstein-Barr virus (EBV) and human immunodeficiency virus (HIV).58,59 The recent observation that HL patients have increased serum levels of BAFF and APRIL60 prompted us to hypothesize BAFF and APRIL involvement in HRS cell accumulation. We found that HRS cells lacked BAFF-R, but expressed TACI, BCMA, and HSPGs. These receptors conveyed powerful survival and growth signals to HRS cells upon engagement by paracrine and autocrine BAFF and APRIL. Interruption of BAFF and APRIL signaling by soluble TACI-Ig decoy receptor, small-interfering RNAs (siRNAs), and HSPG-modifying agents inhibited HRS cell survival and proliferation in vitro and might attenuate expansion of HL tumors in vivo.
Materials and methods
Tissue samples and cells
Frozen tissue samples from 15 cases of classical HL were stored at −80°C. The diagnosis of classical HL was rendered according to the criteria established by the World Health Organization.61 Frozen tissue samples from healthy individuals undergoing tonsillectomy due to tonsillitis were stored at −80°C. All tissue specimens were obtained according to a protocol approved by the institutional review board of Weill Medical College of Cornell University. HD-MyZ, HD-LM2, L428, KMH-2, and L1236 cell lines were derived from primary HRS cells of classical HL tumors. HD-MyZ, HD-LM2, and L428 originate from HLs of the nodular sclerosis subtype, while KMH-2 and L1236 originate from HLs of the mixed cellularity subtype (Table 1)All these cell lines are of B-cell origin with the exception of HD-LM2, which is of T-cell origin.62-64 Although there may be differences between HRS cell lines and primary HRS cells, the HRS cell lines mirror the gene expression and key functional features of the neoplastic HRS cells.11,12 Nonmalignant B cells were purified from tonsils.39,58,59
Histologic subtype of parental HL tumor, origin, and TACI, BCMA, BAFF-R, HSPG, BAFF, and APRIL expression profiles of HRS cell lines
| HRS cell line . | Subtype . | Origin* . | TACI . | BCMA . | APRIL . |
|---|---|---|---|---|---|
| HD-MyZ | NS | B | + | + | + |
| HD-LM2 | NS | T | − | − | −† |
| L428 | NS | B | + | +/−‡ | + |
| KM-H2 | MC | B | + | + | + |
| L1236 | MC | B | + | + | + |
| HRS cell line . | Subtype . | Origin* . | TACI . | BCMA . | APRIL . |
|---|---|---|---|---|---|
| HD-MyZ | NS | B | + | + | + |
| HD-LM2 | NS | T | − | − | −† |
| L428 | NS | B | + | +/−‡ | + |
| KM-H2 | MC | B | + | + | + |
| L1236 | MC | B | + | + | + |
All HRS cell lines were negative for the EBV protein latent-membrane protein-1 and for BAFF-R and were positive for HSPGs expressing the heparinitase-sensitive 3G10 epitope and BAFF.
NS indicates nodular sclerosis; MC, mixed cellularity (Drexler62 ; Kanzler et al63 ; and Wolf et al64 ).
*B- or T-cell derivation was established by detecting rearrangements of Ig and TCR loci, respectively (Drexler62 ; Kanzler et al63 ; and Wolf et al64 ).
†Although containing APRIL transcripts, HD-LM2 cells lacked APRIL protein.
‡Weak expression.
Cultures and reagents
Cells were cultured in complete RPMI 1640 medium supplemented with 10% fetal bovine serum, unless otherwise indicated. Recombinant BAFF, APRIL MegaLigand (Alexis Biochemicals, San Diego, CA), CD40L, and M44 antibody to CD30 (Immunex, Seattle, WA), which mimics engagement of CD30 by CD30L, were used at 100 ng/mL, 100 ng/mL, 500 ng/mL, and 5 μg/mL, respectively. Doxorubicin, heparinitase, heparinase (Sigma-Aldrich, St Louis, MO), and heparin (Calbiochem, San Diego, CA) were used at 100 ng/mL, 10 mU/mL, 10 mU/mL, and 50 μg/mL, respectively, unless otherwise stated. MOPC-21 control Ig (Sigma-Aldrich) as well as soluble TACI-Ig and BAFF-R–Ig decoy receptors (ZymoGenetics, Seattle, WA) were used at 5 μg/mL. TACI and BCMA receptors were selectively cross-linked by immobilizing 5 μg/mL agonistic mouse IgG1 antibodies (ZymoGenetics) on irradiated L-fibroblasts expressing the mouse Fcγ receptor CD32.
Flow cytometry
Cells (0.5 × 106) were stained with fluorescein-conjugated, phycoerythrin-conjugated, and/or cytochrome-3–conjugated antibodies to IgD (Southern Biotechnologies, Birmingham, AL), CD19, CD30, CD38, CD40 (BD Pharmingen, San Diego, CA), TACI, BAFF-R, and membrane-bound BAFF (eBiosciences, San Diego, CA). Biotinylated antibodies to TACI, BCMA (ZymoGenetics), and syndecan-1 (BD Pharmingen) as well as biotinylated BAFF and APRIL proteins were stained with phycoerythrin-conjugated streptavidin. A mouse antibody to the heparinitase-sensitive 3G10 epitope of HSPGs (from Dr Guido David, Catholic University of Leuven, Leuven, Belgium), a goat antibody to syndecan-4 (R&D Systems, Minneapolis, MN), and a rat antibody to membrane-bound APRIL (Alexis Biochemicals) were stained with appropriate phycoerythrin-conjugated secondary antibodies (BD Pharmingen). Cells were acquired and analyzed using a FACScalibur analyzer and CellQuest software (BD Pharmingen).
Fluorescence microscopy
Frozen tissue sections (4 μm each) from 15 cases of classical HL were used for immunofluorescence staining. Goat primary antibodies to TACI, BCMA (Santa Cruz Biotechnologies, Santa Cruz, CA), and APRIL (Alexis Biochemicals); mouse primary antibodies to CD30 (Dako, Carpinteria, CA), IgD (BD Pharmingen), and BAFF-R (eBiosciences); and a rabbit primary antibody to BAFF (Upstate Biotechnology, Lake Placid, NY) were labeled with an appropriate Alexa 488–conjugated or Cy3-conjugated secondary antibody (Jackson Immunoresearch Laboratories, West Grove, PA). Cell nuclei were visualized with DAPI, 4′,6-diamidine-2′-phenylindole dihydrochloride (Boehringer Mannheim, Indianapolis, IN). HRS cell lines were adhered to polylysine-coated glass slides, fixed, and washed as described.31,39,59 Different combinations of goat, mouse, and rabbit primary antibodies to BAFF, APRIL, TACI, BCMA, and TRAF2 were labeled with appropriate Alexa 488–conjugated, Cy3-conjugated, or Cy5-conjugated secondary antibodies. Nuclei were visualized with DAPI and coverslips were applied with Slow Fade reagent (Molecular Probes, Eugene, OR). Images were acquired with an Axiovert 200M microscope (Carl Zeiss, Baujahr, Germany) supplied with an RTE/CCD-1300-Y/HS camera (Princeton Instruments, Trenton, NJ) and 5×/0.15 NA dry, 10×/0.50 NA dry Ph1, 40×/1.3 NA oil-immersion DIC III, and 63×/1.4 NA oil-immersion objective lenses (Carl Zeiss). Acquired images were processed with Adobe Photoshop CS2 for Macintosh, version 9 (Adobe Systems, San Jose, CA).
Cell viability, cell proliferation, and apoptosis assays
Cell viability was evaluated through a trypan blue exclusion test. To measure cell death, cells were fixed in 70% ethanol at 4°C overnight and incubated in 1 mL PBS containing 50 μg/mL propidium iodide and 10 U/mL ribonuclease A (Sigma-Aldrich) for 30 minutes at room temperature in the dark. Hypodyploid dead cells were enumerated through flow cytometry. To measure DNA synthesis, 2 × 104 cells/200 μL were seeded in 96-well plates and pulsed with 1 μCi (0.037 MBq) tritiated thymidine at day 4 of culture. After 18 hours, cells were harvested to measure thymidine uptake. Apoptosis was assayed by using an annexin V–FITC apoptosis detection kit (Oncogene Research Products, San Diego, CA).
Reverse transcriptase–polymerase chain reactions (RT-PCRs) and immunoblots
TACI (323 bp), BCMA (326 bp), BAFF-R (260 bp), BAFF (398 bp), APRIL (417 bp), and β-actin (593 bp) were RT-PCR amplified as previously described.31,39,58,65 Equal amounts of cytoplasmic or nuclear proteins were fractionated onto a 10% sodium dodecyl sulfate–polyacrylamide gel and transferred to nylon membranes (BioRad, Hercules, CA). After blocking, membranes were probed with primary antibodies to BAFF (Upstate Biotechnology), APRIL (Alexis Biochemicals), TACI, BCMA, B-cell–specific activator protein (BSAP, also known as Pax5), actin, Bcl-2, Bcl-xL, Bax, c-Myc, Octamer 1 (Oct1), IκBα, p50, p65, c-Rel (Santa Cruz Biotechnology), IKKγ, phospho (Ser376)-IKKγ, and phospho (Ser32/Ser36)-IκBα (Cell Signaling Technology, Beverly, MA) as reported.31,39,58,65 Membranes were then washed and incubated with an appropriate secondary antibody (Santa Cruz Biotechnology). Proteins were detected with an enhanced chemiluminescence detection system (Amersham, Little Chalfont, United Kingdom), and signal intensity was quantified by using a Quant1 software (BioRad).
RNA interference
The following siRNA duplexes (Qiagen, Valencia, CA) were used: Hs_ATGGCTCTGCTGACCCAACAA_1_HP siRNA to APRIL, Hs_CAGACAGTGAAACACCA ACTA_4_HP siRNA to BAFF, Hs_CAGCGGAGTGGAGAAGTTGAA_1_HP siRNA to TACI, and Hs_ACCATTAAAGGACGAGTTTAA_1_HP siRNA to BCMA. Transfection of experimental and control siRNAs was performed as recommended by the manufacturer. Briefly, 0.4 μL of 20-μM siRNA was incubated with 6 μL HiPerFect Transfection Reagent (Qiagen) in 200 μL serum-free RPMI medium for 5 to 10 minutes at room temperature. This mixture was added dropwise onto 2.5 × 105 HRS cells, which were subsequently incubated at 37°C. Expression of BAFF, APRIL, TACI, and BCMA proteins by transfected HRS cells was evaluated after 24 hours through immunoblotting.
Electrophoretic mobility gel shift assays (EMSAs)
In all assays, except assays involving RNA interference, cells were preincubated for 24 hours in medium with 2% fetal bovine serum to attenuate the constitutively high activation of NF-κB. Nuclear proteins were extracted from 5 × 106 cells as reported.31,39,58,65 A double-strand 5′-AGTTGAGGGGACTTTCCC-3′ oligonucleotide probe encompassing a consensus κB site was end-labeled with [γ-P32] ATP by T4 kinase and used at approximately 30 000 cpm in each EMSA reaction. Reaction samples were prepared as described,31,39,58 incubated at room temperature for 15 minutes, and electrophoresed through a 6% nondenaturing polyacrylamide gel. The composition of DNA-bound protein complexes was determined by incubating the reaction mixture with an antibody to p65, c-Rel, or p50 (Santa Cruz Biotechnology) or with saturating amounts (100 ×) of cold probe before adding the radiolabeled probe.31,39,58
Results
Nonmalignant B cells exhibit distinct TACI, BCMA, and BAFF-R expression profiles
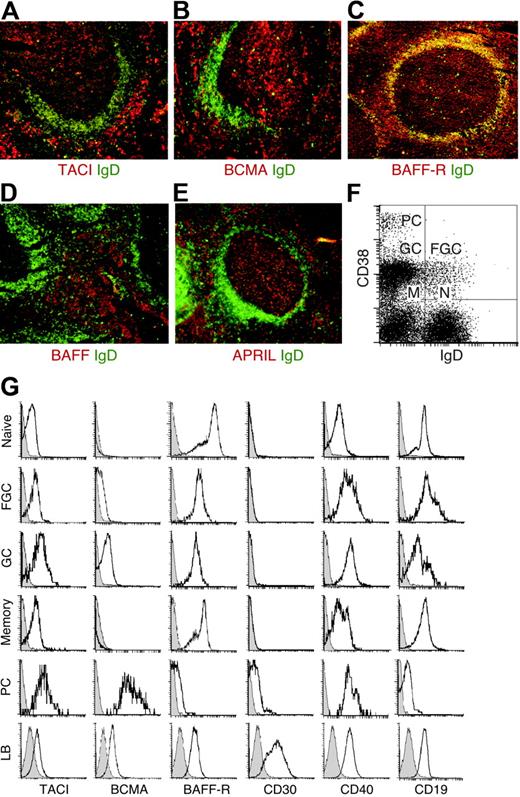
The expression of TACI, BCMA, BAFF-R, BAFF, and APRIL in nonmalignant lymphoid tissue remains unclear. In tonsillar lymphoid follicles, TACI was detected within the GC (Figure 1A), which contains mostly IgD− B cells, and the interfollicular area, which comprises memory IgD−, activated IgDlow/−, and plasmacytoid IgD− B cells. Some TACI was also detected within the follicular mantle, which comprises naive IgDhigh B cells. In contrast, BCMA was detected in the GC and interfollicular area, but not in the follicular mantle (Figure 1B). Although more prominent in the follicular mantle, BAFF-R was also present in the GC and interfollicular area (Figure 1C). Finally, BAFF expression was prominent in the interfollicular area (Figure 1D), whereas APRIL was identified within both GC and interfollicular areas (Figure 1E). Consistent with previous studies,35,36 BAFF and APRIL mostly colocalized with CD21+ follicular dendritic cells, CD83+ dendritic cells, and CD68+ macrophages, but not CD20+ B cells (not shown). These results show that TACI, BCMA, BAFF-R, BAFF, and APRIL exhibit discrete expression profiles in nonmalignant lymphoid tissue.
Nonmalignant GC B-cell precursors of HRS cells express TACI, BCMA, and BAFF-R. (A-E) IgD (green), TACI, BCMA, BAFF-R, BAFF, and APRIL (red) in tonsillar tissue sections. In panel C, yellow indicates follicular mantle B cells coexpressing IgD and BAFF-R. Original magnification ×10. (F) IgD and CD38 on purified tonsillar B cells. N indicates naive B cells; FGC, founder GC B cells; GC, GC B cells; M, memory B cells; and PC, plasmacytoid B cells. (G) TACI, BCMA, BAFF-R, CD30, CD40, and CD19 on purified tonsillar B cells gated into naive, FGC, GC, memory, and plasmacytoid subsets, and on EBV-transformed lymphoblastoid (LB) B cells. Open and shaded profiles correspond to antibody under study and control antibody, respectively. Panels A-G depict 1 of 4 experiments yielding similar results.
Nonmalignant GC B-cell precursors of HRS cells express TACI, BCMA, and BAFF-R. (A-E) IgD (green), TACI, BCMA, BAFF-R, BAFF, and APRIL (red) in tonsillar tissue sections. In panel C, yellow indicates follicular mantle B cells coexpressing IgD and BAFF-R. Original magnification ×10. (F) IgD and CD38 on purified tonsillar B cells. N indicates naive B cells; FGC, founder GC B cells; GC, GC B cells; M, memory B cells; and PC, plasmacytoid B cells. (G) TACI, BCMA, BAFF-R, CD30, CD40, and CD19 on purified tonsillar B cells gated into naive, FGC, GC, memory, and plasmacytoid subsets, and on EBV-transformed lymphoblastoid (LB) B cells. Open and shaded profiles correspond to antibody under study and control antibody, respectively. Panels A-G depict 1 of 4 experiments yielding similar results.
Nonmalignant GC B-cell precursors of HRS cells express TACI, BCMA, and BAFF-R
To further elucidate TACI, BCMA, and BAFF-R expression by nonmalignant HRS cell precursors, purified tonsillar B cells were fractionated into IgD+CD38− naive, IgD+CD38+ founder GC, IgD−CD38+ GC, IgD−CD38− memory, and IgD−CD38++ plasmacytoid B cells (Figure 1F). Although present on all B-cell fractions, TACI was more intensely expressed by GC and plasmacytoid B cells (Figure 1G). In contrast, BCMA was negative on naive and memory B cells, weak on founder GC B cells, positive on GC B cells, and highly positive on plasmacytoid B cells. Like the pan-B-cell antigens CD19 and CD40, BAFF-R was highly expressed by all B-cell fractions, except plasmacytoid B cells. Apart from plasma cells, all B-cell subsets lacked the HL-associated antigen CD30,1 which was instead detected on EBV-transformed lymphoblastoid B cells together with variable levels of TACI, BCMA, and BAFF-R. Thus, CD30− and CD30+ B-cell subsets exhibit distinct TACI, BCMA, and BAFF-R expression profiles, with putative CD30− GG B-cell precursors of HRS cells being positive for all 3 receptors.
Malignant HRS cells express TACI and BCMA but not BAFF-R
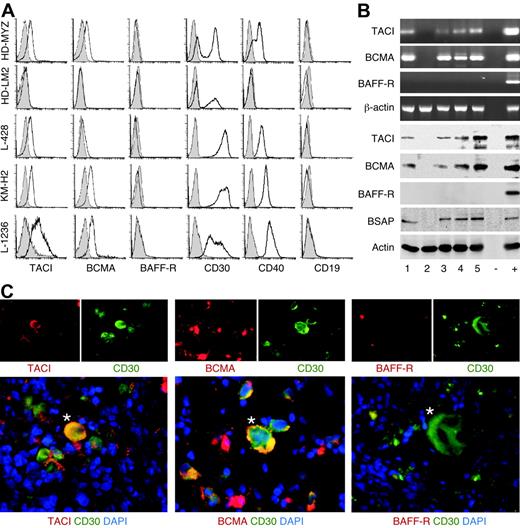
Due to their putative derivation from GC B cells,31 HRS cells were hypothesized to express TACI, BCMA, and BAFF-R. Like HRS cells from primary HL tumors,8-10,12 HRS cell lines, including HD-MyZ, HD-LMZ, L428, KMH-2, and L1236 (Table 1), expressed surface CD30 but not CD19 (Figure 2A). All HRS cell lines, except HD-LM2, also expressed surface CD40 and variable levels of TACI and BCMA, but little or no BAFF-R. Furthermore, all HRS cell lines, except HD-LM2, contained TACI and BCMA, but not BAFF-R transcripts and proteins (Figure 2B). In keeping with its derivation from a transformed T cell,62 HD-LM2 lacked the B-cell–specific protein BSAP as well as CD19, TACI, BCMA, BAFF-R, and CD40. Finally, primary CD30+ HRS cells from classical HL tumors expressed TACI and BCMA, but lacked BAFF-R (Figure 2C). CD30+ HRS cells expressed TACI and BCMA in 93% and 67%, respectively, of classical HL cases examined (Table 2). These results confirm the B-cell–like phenotype of HRS cells and show that, unlike their putative GC B-cell progenitors, HRS cells express TACI and BCMA, but not BAFF-R.
Malignant HRS cells from primary HL tumors express TACI and BCMA but not BAFF-R. (A) TACI, BCMA, BAFF-R, CD30, CD40, and CD19 on HRS cell lines. Open and shaded profiles correspond to antibody under study and control antibody with irrelevant binding activity, respectively. (B) The top 4 panels show TACI, BCMA, BAFF-R, and β-actin transcripts, while the bottom 5 panels show TACI, BCMA, BAFF-R, BSAP, and actin proteins in HRS cell lines. Numbers indicate HD-MyZ (1), HD-LM2 (2), L428 (3), KMH-2 (4), and L1236 (5) HRS cell lines. Minus (−) indicates negative control (no cDNA); plus (+), positive control (cDNA from IARC100 lymphoblastoid cell line). (C) TACI, BCMA, and BAFF-R in CD30+ HRS cells from a classical HL tumor. DAPI (blue) stains nuclei; asterisks indicate representative CD30+ HRS cells. Original magnification ×40. Panel C shows 1 of 15 cases yielding similar results.
Malignant HRS cells from primary HL tumors express TACI and BCMA but not BAFF-R. (A) TACI, BCMA, BAFF-R, CD30, CD40, and CD19 on HRS cell lines. Open and shaded profiles correspond to antibody under study and control antibody with irrelevant binding activity, respectively. (B) The top 4 panels show TACI, BCMA, BAFF-R, and β-actin transcripts, while the bottom 5 panels show TACI, BCMA, BAFF-R, BSAP, and actin proteins in HRS cell lines. Numbers indicate HD-MyZ (1), HD-LM2 (2), L428 (3), KMH-2 (4), and L1236 (5) HRS cell lines. Minus (−) indicates negative control (no cDNA); plus (+), positive control (cDNA from IARC100 lymphoblastoid cell line). (C) TACI, BCMA, and BAFF-R in CD30+ HRS cells from a classical HL tumor. DAPI (blue) stains nuclei; asterisks indicate representative CD30+ HRS cells. Original magnification ×40. Panel C shows 1 of 15 cases yielding similar results.
Clinical characteristics, histologic subtype, and TACI, BCMA, BAFF-R, BAFF and APRIL expression profiles of classical HL cases
| Case no. . | Age, y* . | Sex . | Subtype† . | EBV‡ . | TACI§ . | BCMA§ . | BAFF∥ . | APRIL∥ . |
|---|---|---|---|---|---|---|---|---|
| 1 | 10 | F | NS | + | + | + | + | + |
| 2 | 47 | M | NS | − | + | + | + | + |
| 3 | 32 | M | NS | − | + | + | + | + |
| 4 | 24 | M | NS | + | +/− | − | + | + |
| 5 | 52 | F | MC | + | +/− | +/− | + | +/− |
| 6 | 24 | M | MC | − | + | + | +/− | +/− |
| 7 | 50 | F | MC | + | + | − | +/− | + |
| 8 | 30 | M | NS | − | + | + | + | + |
| 9 | 76 | M | NS | − | + | − | + | + |
| 10 | 37 | F | NS | − | + | + | + | + |
| 11 | 38 | M | LD | − | + | + | + | + |
| 12 | 23 | F | NS | − | − | − | + | +/− |
| 13 | 33 | F | NS | − | + | + | + | + |
| 14 | 35 | F | NS | − | +/− | +/− | + | +/− |
| 15 | 66 | M | NS | − | +/− | − | +/− | +/− |
| Case no. . | Age, y* . | Sex . | Subtype† . | EBV‡ . | TACI§ . | BCMA§ . | BAFF∥ . | APRIL∥ . |
|---|---|---|---|---|---|---|---|---|
| 1 | 10 | F | NS | + | + | + | + | + |
| 2 | 47 | M | NS | − | + | + | + | + |
| 3 | 32 | M | NS | − | + | + | + | + |
| 4 | 24 | M | NS | + | +/− | − | + | + |
| 5 | 52 | F | MC | + | +/− | +/− | + | +/− |
| 6 | 24 | M | MC | − | + | + | +/− | +/− |
| 7 | 50 | F | MC | + | + | − | +/− | + |
| 8 | 30 | M | NS | − | + | + | + | + |
| 9 | 76 | M | NS | − | + | − | + | + |
| 10 | 37 | F | NS | − | + | + | + | + |
| 11 | 38 | M | LD | − | + | + | + | + |
| 12 | 23 | F | NS | − | − | − | + | +/− |
| 13 | 33 | F | NS | − | + | + | + | + |
| 14 | 35 | F | NS | − | +/− | +/− | + | +/− |
| 15 | 66 | M | NS | − | +/− | − | +/− | +/− |
All HL cases were negative for BAFF-R.
*Mean age, 38.5 years (range, 10-76 years).
†NS indicates nodular sclerosis; MC, mixed cellularity; and LD, lymphocyte depletion.
‡EBV was detected by staining tissue sections for the EBV protein latent-membrane protein-1.
§TACI, BCMA, and BAFF-R expression by CD30+ cells with canonical HRS morphology.
∥BAFF and APRIL expression by CD30+ cells with canonical HRS morphology. All HL cases comprised abundant reactive cells expressing BAFF and/or APRIL.
Malignant HRS cells and inflammatory cells infiltrating HL tumors express BAFF and APRIL
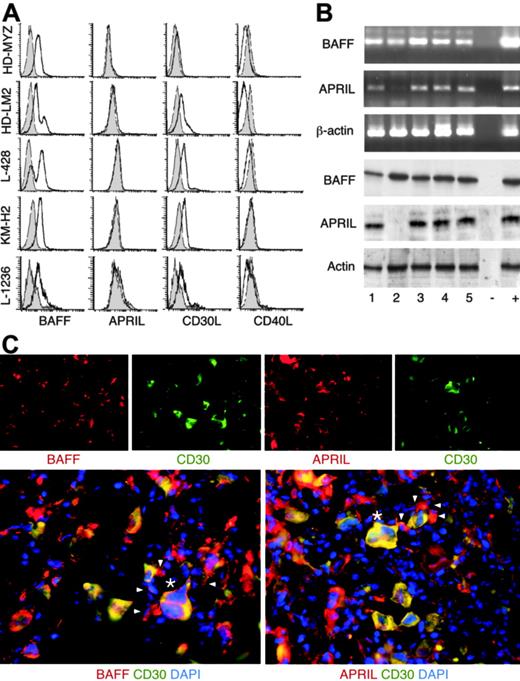
HRS cells express several myelocytic and dendritic cell antigens, including CD15, the chemokine TARC, and fascin,12 and, therefore, were hypothesized to produce BAFF and APRIL. All HRS cell lines expressed surface CD30L, but little or no surface CD40L (Figure 3A), confirming their similarity to primary HRS cells.22,24 CD30L expression was confirmed at the mRNA level (not shown). All HRS cell lines expressed surface BAFF and contained BAFF transcripts and protein (Figure 3B). Although lacking surface APRIL, all HRS cell lines, except HD-LM2, contained APRIL transcripts and protein. HD-LM2 expressed low amounts of APRIL transcripts, but no APRIL protein. CD30+ HRS cells expressed variable levels of BAFF and APRIL in virtually 100% of classical HL tumors examined (Table 2; Figure 3C). BAFF and APRIL were also detected in the reactive infiltrate in 100% of cases of classic HL. As previously shown by others,35,36 BAFF- and APRIL-producing cells included CD68+ macrophages, myeloperoxidase+ neutrophils, eosinophil cationic protein+ eosinophils, tryptase+ mast cells, and CD138+ plasma cells (not shown). Finally, HL cases lacking EBV contained as much BAFF and APRIL as HL cases harboring EBV (Table 2), a BAFF- and APRIL-inducing virus.58 Thus, malignant HRS cells and reactive cells from classical HL tumors synthesize BAFF and APRIL independently of their EBV status.
Malignant HRS cells and reactive cells from primary HL tumors express BAFF and APRIL. (A) BAFF, APRIL, CD30L, and CD40L on HRS cell lines. Open and shaded profiles correspond to antibody under study and control antibody with irrelevant binding activity, respectively. (B) The top 3 panels show BAFF, APRIL, and β-actin transcripts, while the bottom 3 panels show BAFF, APRIL, and actin proteins in HRS cell lines. Numbers indicate HD-MyZ (1), HD-LM2 (2), L428 (3), KMH-2 (4), and L1236 (5) HRS cell lines. Minus (−) indicates negative control (no cDNA); plus (+), positive control (cDNA from IARC100 lymphoblastoid cell line). (C) BAFF and APRIL in CD30+ HRS cells from a classical HL tumor. DAPI (blue) stains nuclei, arrowheads point to reactive CD30− cells surrounding CD30+ HRS cells, and asterisks indicate representative CD30+ HRS cells. Original magnification ×40. Panel C shows 1 of 15 cases yielding similar results.
Malignant HRS cells and reactive cells from primary HL tumors express BAFF and APRIL. (A) BAFF, APRIL, CD30L, and CD40L on HRS cell lines. Open and shaded profiles correspond to antibody under study and control antibody with irrelevant binding activity, respectively. (B) The top 3 panels show BAFF, APRIL, and β-actin transcripts, while the bottom 3 panels show BAFF, APRIL, and actin proteins in HRS cell lines. Numbers indicate HD-MyZ (1), HD-LM2 (2), L428 (3), KMH-2 (4), and L1236 (5) HRS cell lines. Minus (−) indicates negative control (no cDNA); plus (+), positive control (cDNA from IARC100 lymphoblastoid cell line). (C) BAFF and APRIL in CD30+ HRS cells from a classical HL tumor. DAPI (blue) stains nuclei, arrowheads point to reactive CD30− cells surrounding CD30+ HRS cells, and asterisks indicate representative CD30+ HRS cells. Original magnification ×40. Panel C shows 1 of 15 cases yielding similar results.
BAFF and APRIL enhance HRS cell survival and proliferation
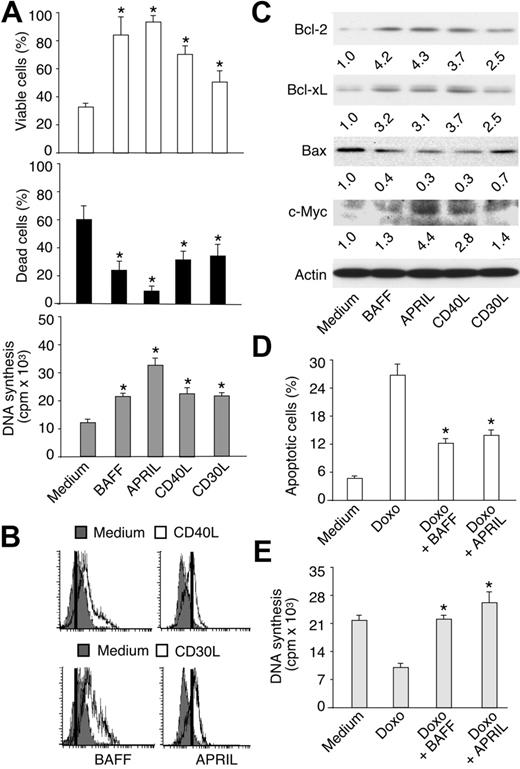
TACI and BCMA play major roles in normal and malignant B cells.35,66 Therefore, BAFF and APRIL could deliver key signals to HRS cells. Due to the scarcity of HRS cells in primary HL tumors, HRS cell lines were used to elucidate the function of BAFF and APRIL in HL. Although there may be differences between HRS cell lines and their primary counterparts, cell lines mirror key phenotypic and functional properties of primary HRS cells.11,12 In B-cell–derived HRS lines (Tables 1 and 3), including HD-MyZ (Figure 4A), BAFF and APRIL increased cell viability and decreased cell death under serum starvation conditions. In addition, BAFF and, to a larger extent, APRIL increased HRS cell proliferation. All B-cell–derived HRS cell lines exhibited similar responses to BAFF and APRIL (Table 3). Only HD-LM2, which derives from a T-cell progenitor, did not respond to BAFF and APRIL, possibly due to the lack of TACI and BCMA receptors (Tables 1 and 3).
Summary of functional responses of HRS cell lines to BAFF and APRIL
| Functional responses to BAFF or APRIL . | HRS cell lines . | |||
|---|---|---|---|---|
| HD-MyZ . | HD-LM2 . | L428 . | L1236 . | |
| BAFF-mediated increase of viable cells | +* | −† | + | + |
| BAFF-mediated decrease of apoptotic cells | +‡ | − | + | − |
| BAFF-mediated induction of DNA synthesis | + | − | + | − |
| APRIL-mediated increase of viable cells | + | − | + | + |
| APRIL-mediated decrease of apoptotic cells | + | − | + | + |
| APRIL-mediated induction of DNA synthesis | +++ | ND | ++ | + |
| Functional responses to BAFF or APRIL . | HRS cell lines . | |||
|---|---|---|---|---|
| HD-MyZ . | HD-LM2 . | L428 . | L1236 . | |
| BAFF-mediated increase of viable cells | +* | −† | + | + |
| BAFF-mediated decrease of apoptotic cells | +‡ | − | + | − |
| BAFF-mediated induction of DNA synthesis | + | − | + | − |
| APRIL-mediated increase of viable cells | + | − | + | + |
| APRIL-mediated decrease of apoptotic cells | + | − | + | + |
| APRIL-mediated induction of DNA synthesis | +++ | ND | ++ | + |
The functional responses were all positive for the KM-H2 cell line.
ND indicates not determined.
Increased viability under serum starvation conditions.
†No detectable response.
‡Decreased apoptosis under serum starvation conditions.
BAFF and APRIL increase malignant HRS cell survival and proliferation. (A) Viability, death, and proliferation of serum-starved HD-MyZ HRS cells incubated for 2 days in the presence or absence of BAFF, APRIL, CD40L, or CD30L as described in “Materials and methods.” Bars indicate SD of 4 experiments; *P < .05 versus cells incubated with medium, respectively. (B) BAFF and APRIL expression on HD-MyZ HRS cells incubated for 2 days with medium (shaded profiles), or CD40L or CD30L (open profiles). Positive cells are on the right of the vertical bar. On the left of this bar are cells stained with a control mouse (for BAFF) or rat (for APRIL) antibody with irrelevant binding activity. One of 3 experiments yielding similar results. (C) Bcl-2, Bcl-xL, Bax, c-Myc, and actin proteins in HD-MyZ HRS cells incubated as in panel A. Bands were quantified (numbers below lanes) after normalization to the actin loading control. One of 3 experiments yielding similar results. (D-E) Apoptosis and proliferation of HD-MyZ HRS cells exposed to BAFF or APRIL in the presence or absence of the anti-HL agent doxorubicin (Doxo). Bars indicate SD of 3 experiments; *P < .05 versus cells incubated with Doxo.
BAFF and APRIL increase malignant HRS cell survival and proliferation. (A) Viability, death, and proliferation of serum-starved HD-MyZ HRS cells incubated for 2 days in the presence or absence of BAFF, APRIL, CD40L, or CD30L as described in “Materials and methods.” Bars indicate SD of 4 experiments; *P < .05 versus cells incubated with medium, respectively. (B) BAFF and APRIL expression on HD-MyZ HRS cells incubated for 2 days with medium (shaded profiles), or CD40L or CD30L (open profiles). Positive cells are on the right of the vertical bar. On the left of this bar are cells stained with a control mouse (for BAFF) or rat (for APRIL) antibody with irrelevant binding activity. One of 3 experiments yielding similar results. (C) Bcl-2, Bcl-xL, Bax, c-Myc, and actin proteins in HD-MyZ HRS cells incubated as in panel A. Bands were quantified (numbers below lanes) after normalization to the actin loading control. One of 3 experiments yielding similar results. (D-E) Apoptosis and proliferation of HD-MyZ HRS cells exposed to BAFF or APRIL in the presence or absence of the anti-HL agent doxorubicin (Doxo). Bars indicate SD of 3 experiments; *P < .05 versus cells incubated with Doxo.
BAFF and APRIL induced prosurvival and growth-inducing effects like CD40L and CD30L, 2 HRS cell-stimulating ligands structurally related to BAFF and APRIL.15,22-27,29 Of note, CD40L and CD30L up-regulated BAFF expression on the surface of HRS cells (Figure 4B). Consistent with previous studies showing APRIL on chronic lymphocytic leukemia malignant B cells,45 CD40L or CD30L induced HRS cells to express surface APRIL. Currently, the prevailing view is that APRIL is expressed within cytosolic compartments, such as the Golgi apparatus.35,67 Activation of HRS cells by CD40L and CD30L might elicit recycling of Golgi-derived vesicles to the plasma membrane, thereby exposing APRIL on the cell surface. Alternatively, our anti-APRIL antibody may recognize surface TWE-PRIL, a hybrid TNF ligand encoded by TNF-like weak inducer of apoptosis (TWEAK) and APRIL transcripts.68 In any case, blockade of autocrine BAFF and APRIL by TACI-Ig attenuated the survival and proliferation of HRS cells exposed to either CD40L or CD30L (not shown), suggesting that BAFF and APRIL cooperate with other TNF-related ligands to elicit HRS cell growth.
BAFF and APRIL up-regulate Bcl-2, Bcl-xL, and c-Myc in HRS cells
Prosurvival Bcl-2 and Bcl-xL, proapoptotic Bax, and growth-inducing c-Myc proteins play a key role in lymphoma cells.69 Thus, we wondered whether BAFF and APRIL modulate Bcl-2, Bcl-xL, and Bax expression in HRS cells. In B-cell–derived HRS cell lines, including HD-MyZ (Figure 4C), BAFF and APRIL up-regulated the expression of Bcl-2 and Bcl-xL, but down-regulated that of Bax. In addition, APRIL up-regulated the expression of c-Myc, whereas BAFF did not. In some HRS cell lines, such as L1236, BAFF and APRIL down-regulated the expression of p53 (not shown), a proapoptotic and cell cycle arrest–inducing protein. Similar effects were induced by CD40L and CD30L. Thus, BAFF and APRIL may enhance HRS cell accumulation by shifting the intracellular balance between prosurvival/growth-inducing and proapoptotic/growth-inhibiting proteins toward survival and growth.
BAFF and APRIL attenuate the anti-HL activity of doxorubicin
Chemotherapeutic agents, such as doxorubicin, attenuate the expansion of HL tumors by inhibiting the survival and proliferation of HRS cells. Given their ability to deliver powerful prosurvival and growth-inducing signals, BAFF and APRIL could make HRS cells less responsive to chemotherapy. When exposed to HRS cell lines, including HD-MyZ (Figure 4D-E), doxorubicin induced apoptosis and inhibited DNA synthesis. These effects were partially or completely reverted by BAFF and APRIL. Of note, BAFF and APRIL interfered also with the proapoptotic and growth-inhibiting activities of prednisone (not shown), another anti-HL agent. These results indicate that BAFF and APRIL make HRS cells less sensitive to chemotherapeutic agents.
BAFF and APRIL activate NF-κB in HL cells
Constitutive NF-κB activation is a hallmark of HRS cells.14 TNF-related receptors, such as CD40, CD30, RANK, TACI, and BCMA, activate NF-κB by inducing TRAF-dependent phosphorylation of IKK, an enzymatic complex that comprises 2 catalytic subunits, α and β, and a regulatory subunit, γ. Activated IKK phosphorylates IκBα an inhibitor of NF-κB that retains p50, p65, and c-Rel in the cytoplasm of resting cells.36,40 Phosphorylated IκBα undergoes degradation,40 thereby allowing NF-κB to translocate into the nucleus. Once in the nucleus, NF-κB activates genes involved in B-cell survival, proliferation, and differentiation.39,40 Given their ability to express TACI and BCMA, HRS cells were hypothesized to activate NF-κB in response to BAFF or APRIL.
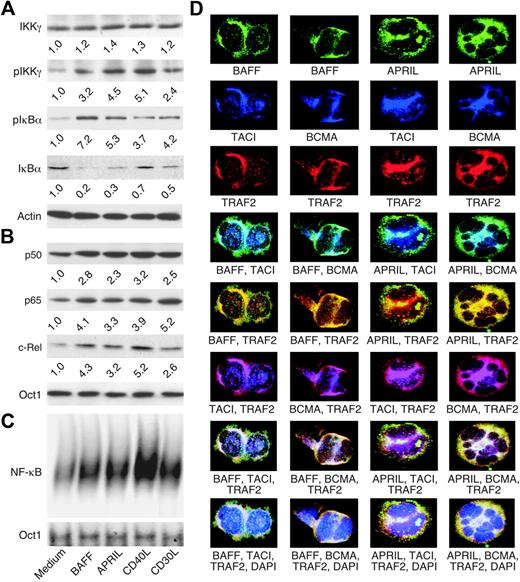
The NF-κB–inducing activity of BAFF and APRIL was evaluated in B-cell–derived HRS cell lines expressing a functional IκBα protein, including HD-MyZ.70 Such HRS cells exhibit less constitutive NF-κB activation than HRS cells expressing nonfunctional IκBα and are more sensitive to NF-κB changes brought about by external stimuli, including BAFF and APRIL. To further attenuate their baseline NF-κB activity, HRS cells were preincubated for 24 hours with serum-depleted medium. In the presence of BAFF or APRIL, HRS cells up-regulated IKKγ and IκBα phosphorylation over baseline levels and induced significant IκBα degradation (Figure 5A). In addition, BAFF or APRIL augmented the translocation of NF-κB proteins, including p50, p65, and c-Rel, to the nucleus (Figure 5B), and increased the binding of NF-κB to DNA (Figure 5C). Similar effects were induced by CD40 and CD30L. Thus, BAFF and APRIL may contribute to the dysregulation of NF-κB in HRS cells.
BAFF and APRIL activate NF-κB in malignant HRS cells and colocalize with TACI and BCMA receptors as well as TRAF2 signal transducer. (A) Cytoplasmic IKKγ, pIKKγ, pIκBα, IκBα, and actin (loading control) proteins in serum-starved HD-MyZ HRS cells incubated for 10 minutes (IKKγ and pIKKγ) or 2 hours (pIκBα, IκBα and actin) in the presence or absence of BAFF, APRIL, CD40L, or CD30L. pIKKγ bands were quantified (numbers below lanes) after normalization to IKKγ, while pIκBα and IκBα bands were quantified after normalization to actin. One of 3 experiments yielding similar results. (B) Nuclear p50, p65, and c-Rel proteins in HD-MyZ HRS cells incubated for 6 hours as in panel A. Bands were quantified (numbers below lanes) after normalization to Octamer-1 (Oct1), a ubiquitous nuclear protein (loading control). One of 3 experiments yielding similar results. (C) NF-κB and Oct1 binding to DNA in HD-MyZ HRS cells incubated for 6 hours as in panel A. Shifts correspond to p50-c-Rel and p50-p65 complexes as shown elsewhere.31,39,58,65 One of 3 experiments yielding similar results. (D) Colocalization of BAFF, TACI, and TRAF2 (first column); BAFF, BCMA, and TRAF2 (second column); APRIL, TACI, and TRAF2 (third column); and APRIL, BCMA, and TRAF2 (fourth column) in L428 cells. DAPI (blue) stains nuclei in the last row. Turquoise (fourth row)–, yellow (fifth row)–, pink (sixth row)–, and white (seventh and eighth rows)–appearing areas indicate colocalization. Original magnification ×64. One of 4 experiments yielding similar results.
BAFF and APRIL activate NF-κB in malignant HRS cells and colocalize with TACI and BCMA receptors as well as TRAF2 signal transducer. (A) Cytoplasmic IKKγ, pIKKγ, pIκBα, IκBα, and actin (loading control) proteins in serum-starved HD-MyZ HRS cells incubated for 10 minutes (IKKγ and pIKKγ) or 2 hours (pIκBα, IκBα and actin) in the presence or absence of BAFF, APRIL, CD40L, or CD30L. pIKKγ bands were quantified (numbers below lanes) after normalization to IKKγ, while pIκBα and IκBα bands were quantified after normalization to actin. One of 3 experiments yielding similar results. (B) Nuclear p50, p65, and c-Rel proteins in HD-MyZ HRS cells incubated for 6 hours as in panel A. Bands were quantified (numbers below lanes) after normalization to Octamer-1 (Oct1), a ubiquitous nuclear protein (loading control). One of 3 experiments yielding similar results. (C) NF-κB and Oct1 binding to DNA in HD-MyZ HRS cells incubated for 6 hours as in panel A. Shifts correspond to p50-c-Rel and p50-p65 complexes as shown elsewhere.31,39,58,65 One of 3 experiments yielding similar results. (D) Colocalization of BAFF, TACI, and TRAF2 (first column); BAFF, BCMA, and TRAF2 (second column); APRIL, TACI, and TRAF2 (third column); and APRIL, BCMA, and TRAF2 (fourth column) in L428 cells. DAPI (blue) stains nuclei in the last row. Turquoise (fourth row)–, yellow (fifth row)–, pink (sixth row)–, and white (seventh and eighth rows)–appearing areas indicate colocalization. Original magnification ×64. One of 4 experiments yielding similar results.
BAFF and APRIL colocalize with TACI, BCMA, and TRAF2 in HRS cells
CD30 and CD40 receptors are thought to be implicated in the dysregulation of NF-κB in HRS cells.15,22,23,26,29 Given their structural and functional homology with CD30 and CD40,35,66 TACI and BCMA may further enhance the dysregulation of NF-κB in HRS cells. Consistent with this possibility, BAFF and APRIL colocalized with TACI and BCMA receptors and with TRAF2, an NF-κB–activating adaptor protein, within discrete areas of B-cell–derived HRS cell lines, including L428 (Figure 5D). In agreement with previous findings,39,67,71 BAFF colocalized with TACI, BCMA, and TRAF2 on the membrane of HRS cells, although some colocalization was also noted in the cytoplasm of these cells. By contrast, APRIL predominantly colocalized with TACI, BCMA, and TRAF2 in the cytoplasm of HRS cells. These data suggest that TACI and BCMA constitutively signal from both membrane and cytosolic compartments as a result of their engagement by autocrine BAFF and APRIL.
BAFF and APRIL stimulate HRS cells through TACI and BCMA
Soluble TACI-Ig and BAFF-R–Ig decoy receptors were used to further elucidate the contribution of BAFF and APRIL to the survival and proliferation of HRS cell lines, including HD-MyZ. While TACI-Ig binds to and neutralizes both BAFF and APRIL, BAFF-R–Ig binds to and neutralizes BAFF only.35,66 TACI-Ig induced more early HRS cell apoptosis than BAFF-R–Ig (Figure 6A). In addition, TACI-Ig attenuated spontaneous HRS cell proliferation, whereas BAFF-R–Ig did not (Figure 6B). These data indicate that autonomous accumulation of HRS cells involves both BAFF and APRIL. Consistent with this interpretation, TACI-Ig decreased the proliferation of HRS cells exposed to either BAFF or APRIL, whereas BAFF-R–Ig interfered with the proliferation of HRS cells exposed to BAFF but had no effect on the proliferation of HRS cells exposed to APRIL.
TACI-Ig attenuates spontaneous as well as BAFF- and APRIL-induced survival and proliferation of malignant HRS cells. (A) Apoptosis and necrosis in HD-MyZ HRS cells incubated for 2 days in the presence or absence of control Ig, TACI-Ig, and BAFF-R–Ig. Early apoptotic, late apoptotic, and necrotic cells were annexin-V+propidium iodide-, annexin-V+propidium iodide+, and annexin-V−propidium iodide+, respectively. Bars indicate SD of 4 experiments; *P < .05 and **P < .005 versus cells incubated with medium, respectively (leftmost bar of each cluster). (B) Proliferation of HD-MyZ HRS cells incubated for 2 days with or without BAFF and APRIL and in the presence or absence of control Ig, TACI-Ig, and BAFF-R–Ig. Bars indicate SD of 4 experiments; *P < .05 and **P < .005 versus cells incubated with medium, respectively (leftmost bar of each cluster). (C-D) Viability and proliferation of HD-MyZ HRS cells incubated for 2 days with or without a control, or anti-TACI or anti-BCMA antibody immobilized on CD32-L cells. Bars indicate SD of 4 experiments; *P < .05 and **P < .005 versus cells incubated with medium, respectively (leftmost bar of each panel). (E-F) Apoptosis, proliferation, and NF-κB–DNA binding activity of HD-MyZ HRS cells incubated for 2 days with control, BAFF, APRIL, TACI, and/or BCMA siRNAs. Bars indicate SD of 4 experiments; *P < .05, *P < .005, and **P < .001 versus cells incubated with control siRNA, respectively (leftmost bar of each panel). Antibodies to p65, p50, and c-Rel as well as a cold NF-κB–binding probe were used in supershift assays (bottom-right gel) to identify NF-κB–DNA complexes.
TACI-Ig attenuates spontaneous as well as BAFF- and APRIL-induced survival and proliferation of malignant HRS cells. (A) Apoptosis and necrosis in HD-MyZ HRS cells incubated for 2 days in the presence or absence of control Ig, TACI-Ig, and BAFF-R–Ig. Early apoptotic, late apoptotic, and necrotic cells were annexin-V+propidium iodide-, annexin-V+propidium iodide+, and annexin-V−propidium iodide+, respectively. Bars indicate SD of 4 experiments; *P < .05 and **P < .005 versus cells incubated with medium, respectively (leftmost bar of each cluster). (B) Proliferation of HD-MyZ HRS cells incubated for 2 days with or without BAFF and APRIL and in the presence or absence of control Ig, TACI-Ig, and BAFF-R–Ig. Bars indicate SD of 4 experiments; *P < .05 and **P < .005 versus cells incubated with medium, respectively (leftmost bar of each cluster). (C-D) Viability and proliferation of HD-MyZ HRS cells incubated for 2 days with or without a control, or anti-TACI or anti-BCMA antibody immobilized on CD32-L cells. Bars indicate SD of 4 experiments; *P < .05 and **P < .005 versus cells incubated with medium, respectively (leftmost bar of each panel). (E-F) Apoptosis, proliferation, and NF-κB–DNA binding activity of HD-MyZ HRS cells incubated for 2 days with control, BAFF, APRIL, TACI, and/or BCMA siRNAs. Bars indicate SD of 4 experiments; *P < .05, *P < .005, and **P < .001 versus cells incubated with control siRNA, respectively (leftmost bar of each panel). Antibodies to p65, p50, and c-Rel as well as a cold NF-κB–binding probe were used in supershift assays (bottom-right gel) to identify NF-κB–DNA complexes.
TACI-Ig and BAFF-R–Ig had no effect on the survival and proliferation of T-cell–derived HRS cell lines lacking TACI and BCMA (not shown), suggesting a key role of TACI and BCMA. Consistent with this interpretation, selective TACI or BCMA cross-linking enhanced HRS cell viability and proliferation (Figure 6C-D). Of note, BCMA cross-linking enhanced HRS cell survival more than TACI cross-linking. The relative contribution of TACI and BCMA to HRS cell growth was further dissected by selectively targeting TACI or BCMA through RNA interference. BCMA siRNA inhibited HRS cell survival more than TACI siRNA, and a combination of BCMA and TACI siRNAs had an additive inhibitory effect (Figure 7E). In contrast, HRS cell proliferation was comparably inhibited by BCMA and TACI siRNAs (Figure 7F). In addition to confirming the key role of autocrine BAFF and APRIL in HL, these data indicate that TACI predominantly delivers proliferation signals, whereas BCMA delivers both survival and proliferation signals to HRS cells.
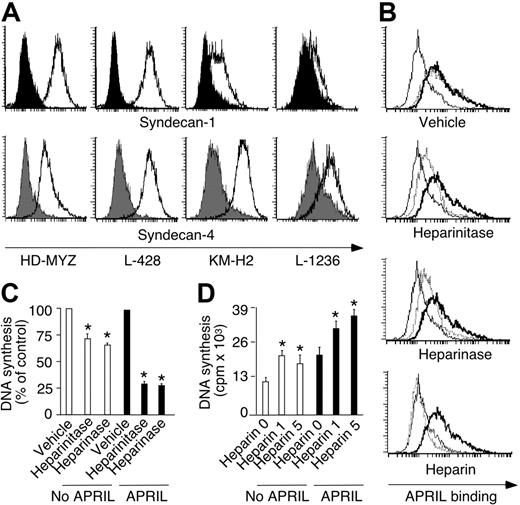
HSPGs enhance spontaneous and APRIL-induced HRS cell survival and proliferation. (A) Expression of syndecan-1 and syndecan-4 by HD-MyZ, L-428, KM-H2, and L-1236 HRS cells. Open and solid profiles correspond to experimental antibody and control antibody with irrelevant binding activity, respectively. (B) Binding of APRIL to HD-MyZ HRS cells pretreated with vehicle, heparinitase, heparinase, or heparin. Thin line corresponds to fluorescence of control streptavidin. Thick line corresponds to APRIL-binding activity of untreated HRS cells. Dashed line corresponds to APRIL-binding activity of HRS cells pretreated for 2 hours with control vehicle, heparinitase, heparinase, or heparin. Background fluorescence generated by control streptavidin was equivalent in HRS cells after various treatments. (C) Proliferation of HD-MyZ HRS cells exposed or not to APRIL after pretreatment with control vehicle, heparinitase, or heparinase. Cpm were converted into percentage of control as follows: cpm with pretreatment/cpm without pretreatment ×100. Bars indicate SD of 4 experiments; *P < .05 versus cells incubated with control vehicle (leftmost bar of each cluster). (D) Proliferation of HD-MyZ HRS cells exposed or not to APRIL in the presence of 0, 1, or 5 μg/mL heparin. Bars indicate SD of 3 experiments; *P < .05 versus cells incubated in the absence of heparin (leftmost bar of each cluster).
HSPGs enhance spontaneous and APRIL-induced HRS cell survival and proliferation. (A) Expression of syndecan-1 and syndecan-4 by HD-MyZ, L-428, KM-H2, and L-1236 HRS cells. Open and solid profiles correspond to experimental antibody and control antibody with irrelevant binding activity, respectively. (B) Binding of APRIL to HD-MyZ HRS cells pretreated with vehicle, heparinitase, heparinase, or heparin. Thin line corresponds to fluorescence of control streptavidin. Thick line corresponds to APRIL-binding activity of untreated HRS cells. Dashed line corresponds to APRIL-binding activity of HRS cells pretreated for 2 hours with control vehicle, heparinitase, heparinase, or heparin. Background fluorescence generated by control streptavidin was equivalent in HRS cells after various treatments. (C) Proliferation of HD-MyZ HRS cells exposed or not to APRIL after pretreatment with control vehicle, heparinitase, or heparinase. Cpm were converted into percentage of control as follows: cpm with pretreatment/cpm without pretreatment ×100. Bars indicate SD of 4 experiments; *P < .05 versus cells incubated with control vehicle (leftmost bar of each cluster). (D) Proliferation of HD-MyZ HRS cells exposed or not to APRIL in the presence of 0, 1, or 5 μg/mL heparin. Bars indicate SD of 3 experiments; *P < .05 versus cells incubated in the absence of heparin (leftmost bar of each cluster).
Like TACI and BCMA, BAFF and APRIL had nonredundant effects on HRS cells, as BAFF siRNA inhibited HRS cell survival more than APRIL siRNA, whereas APRIL siRNA inhibited HRS cell proliferation more than BAFF siRNA. A combination of BAFF and APRIL siRNAs had an additive inhibitory effect on HRS cell survival. In addition to confirming the key role of autocrine BAFF and APRIL in HL, these data indicate that BAFF delivers mainly survival signals, whereas APRIL delivers predominantly proliferation signals to HRS cells. Finally, BAFF, APRIL, TACI, or BCMA siRNA, or a combination thereof, attenuated the constitutive activation of NF-κB and in particular of p65 in HRS cells (not shown), indicating that TACI and BCMA engagement by autocrine BAFF and APRIL contributes to the dysregulation of NF-κB in HL.
HRS cell–anchored and extracellular HSPGs enhance APRIL-induced HRS cell growth
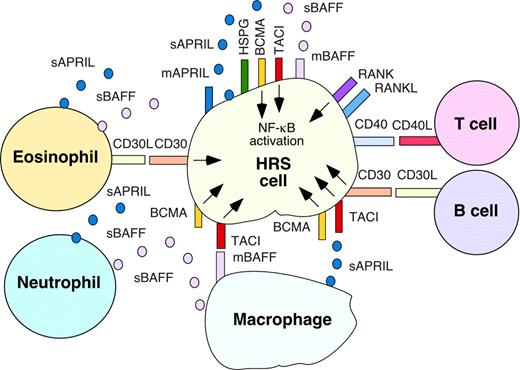
Cationic sulfate glycosaminoglycan side chains of cell-anchored and extracellular HSPGs bind a basic QKQKKQ amino acid sequence proximal to the amino terminus of APRIL.42,43 The ensuing oligomerization of APRIL would enhance TACI and BCMA signaling by promoting the formation of a highly efficient signaling platform. Thus, we verified the role of HSPGs in HRS cell growth. HRS cell lines expressed HSPGs, including syndecan-1 and syndecan-4 (Table 1; Figure 7A). Preincubation of HRS cells with heparinitase and heparinase, which disrupt the cationic side chains of HSPGs, reduced APRIL binding to HRS cells (Figure 7B), but had no effect on BAFF binding (not shown). APRIL binding to HRS cells was abrogated by heparin, a compound that mimics extracellular HSPGs. Finally, heparinitase and heparinase attenuated, whereas heparin increased, spontaneous as well as APRIL-induced HRS cell proliferation (Figure 7C). These data suggest that membrane-anchored and matrix-associated HSPGs enhance HRS cell accumulation by amplifying APRIL binding and signaling to HRS cells (Figure 8).
BAFF and APRIL stimulate the accumulation of malignant HRS cells through autocrine and paracrine signals conveyed by TACI, BCMA, and HSPGs. Malignant HRS cells express TACI and BCMA, which deliver survival and growth signals upon engagement by paracrine BAFF and APRIL released by reactive myeloid cells, including macrophages, neutrophils, and eosinophils. TACI and BCMA are also engaged by autocrine BAFF and APRIL expressed on the membrane of and released by HRS cells. Binding of APRIL to TACI and BCMA is enhanced by HSPGs anchored on the membrane of HRS cells and (not shown) associated with the extracellular matrix. Engagement of CD30 and CD40 on HRS cells by CD30L and CD40L on eosinophils, CD4+ T cells, and/or B cells up-regulates expression of autocrine BAFF and APRIL by HRS cells, thereby further enhancing their survival and proliferation. The TNF family member RANKL and its receptor RANK are also indicated on HRS cells. Together with CD30L, CD40L, and RANKL, BAFF and APRIL trigger abnormal activation of NF-κB in HRS cells, thereby enhancing HL expansion.
BAFF and APRIL stimulate the accumulation of malignant HRS cells through autocrine and paracrine signals conveyed by TACI, BCMA, and HSPGs. Malignant HRS cells express TACI and BCMA, which deliver survival and growth signals upon engagement by paracrine BAFF and APRIL released by reactive myeloid cells, including macrophages, neutrophils, and eosinophils. TACI and BCMA are also engaged by autocrine BAFF and APRIL expressed on the membrane of and released by HRS cells. Binding of APRIL to TACI and BCMA is enhanced by HSPGs anchored on the membrane of HRS cells and (not shown) associated with the extracellular matrix. Engagement of CD30 and CD40 on HRS cells by CD30L and CD40L on eosinophils, CD4+ T cells, and/or B cells up-regulates expression of autocrine BAFF and APRIL by HRS cells, thereby further enhancing their survival and proliferation. The TNF family member RANKL and its receptor RANK are also indicated on HRS cells. Together with CD30L, CD40L, and RANKL, BAFF and APRIL trigger abnormal activation of NF-κB in HRS cells, thereby enhancing HL expansion.
Discussion
We have reported here that reactive and malignant HRS cells from classical HL tumors produce BAFF and APRIL. Unlike their putative germinal center B-cell precursors, HRS cells expressed TACI and BCMA, but lacked BAFF-R. In the presence of BAFF or APRIL, TACI and BCMA conveyed powerful survival and growth-inducing signals to HRS cells. These signals were amplified by APRIL-binding HSPGs on HRS cells. Interruption of BAFF and APRIL signaling by TACI-Ig, siRNAs, or HSPG-modifying agents dampened HRS cell accumulation in vitro and might attenuate HL expansion in vivo.
BAFF and APRIL are innate NF-κB–activating factors essential for the survival, proliferation, and differentiation of nonmalignant B cells.34,36 BAFF and APRIL are also implicated in the expansion of malignant B cells.35,66 These cells have devised at least 2 strategies to dysregulate BAFF and APRIL expression. First, they release cytokines, such as IL-10, capable of up-regulating paracrine BAFF and APRIL production by tumor-associated myeloid cells.39,56,57 Second, they produce autocrine BAFF and APRIL as a result of aberrant NF-κB activation,39,58,72 a hallmark of lymphoma.69 In this fashion, malignant B cells establish a vicious circle in which NF-κB–dependent up-regulation of autocrine BAFF and APRIL causes further activation of NF-κB.
More than 90% of classical HL tumors are composed of a reactive infiltrate that includes macrophages, neutrophils, eosinophils, and mast cells.2,21 These myeloid cells are capable of producing BAFF and APRIL upon activation by cytokines and TNF family members, including CD40L.30-33 Considering that HRS cells synthesize a large spectrum of cytokines and TNF-related molecules,12,22,23,26,29 our observation that the reactive infiltrate of classical HL contains abundant BAFF and APRIL is not surprising and provides a biologic explanation for recent studies showing the presence of high levels of BAFF and APRIL in the serum of HL patients.60
By showing that HRS cells produce BAFF and APRIL, our findings extend previous reports documenting aberrant expression of myelocytic and dendritic cell molecules by HRS cells, despite their B-cell origin.1,12 Like malignant B cells from NHL tumors,39,58,72 HRS cells may undergo autocrine BAFF and APRIL production as a result of dysregulated activation of NF-κB. This transcription factor stimulates BAFF synthesis by binding multiple cis-regulatory κB sites on the BAFF gene promoter.58 In this regard, up to 40% of HL cases harbor latent membrane protein-1 (LMP-1), an EBV-encoded protein that induces NF-κB–dependent BAFF production.58 Four of our 15 cases of classical HL harbored LMP-1. However, these EBV+ cases contained as much BAFF and APRIL as their EBV− counterparts, suggesting a complex genesis of BAFF and APRIL dysregulation in HL tumors.
Intriguingly, primary HRS cells and HRS cell lines lacked BAFF-R, the main driver of BAFF signaling in normal B cells.36 Lack of BAFF-R on HRS cells has been reported by others73,74 and may result from the overall transcriptional down-regulation of B-cell–associated genes in HL tumors.7-10 Of nonmalignant B cells, plasma cells expressed little or no BAFF-R, whereas GC B cells, the putative HRS precursor, expressed abundant BAFF-R. Thus, down-regulation of BAFF-R by HRS cells may reflect the advanced differentiation stage of their nonmalignant B-cell progenitor.
Despite expressing little or no BAFF-R, HRS cell lines generated survival and, to a lesser extent, proliferation signals in response to BAFF. Multiple lines of evidence support the involvement of TACI and BCMA signaling in BAFF-induced HRS cell accumulation. First, TACI and BCMA colocalized with autocrine BAFF and the NF-κB–activating adaptor protein TRAF2 in discrete membrane and cytosolic areas of B-cell–derived HRS cells, suggesting the presence of constitutive BAFF-mediated NF-κB–dependent signaling through TACI and BCMA. Second, T-cell–derived HRS cell lines lacking TACI and BCMA did not respond to exogenous BAFF. Third, TACI or BCMA cross-linking triggered survival and proliferation signals in HRS cells. Conversely, TACI or BCMA deficiency attenuated autonomous HRS cell accumulation. The positive role of TACI and BCMA in HL is in agreement with previous studies showing that TACI and BCMA activate NF-κB34,37,38 and deliver powerful survival, proliferation, and differentiation signals to B cells, including plasma cells expressing little or no BAFF-R.45,47,51,75-77 Of note, TACI and BCMA appeared to have nonredundant functions in HRS cells, as BCMA deficiency attenuated HRS cell survival more than TACI deficiency did.
TACI and BCMA would also enable HRS cells to respond to APRIL. Compared with BAFF, APRIL binds to TACI with a somewhat lower affinity than to BCMA.35,36 Yet, APRIL elicited more robust HRS cell proliferation than BAFF. This observation may be explained by the ability of HRS cell–anchored HSPGs to increase APRIL binding to TACI and BCMA. By showing that HRS cell lines express HSPGs, including syndecan-1 and syndecan-4, our findings extend previous studies documenting syndecan-1 expression by primary HRS cells.78 As shown by recent reports,42,43 the glycosaminoglycan side chains of cell-anchored HSPGs bind a basic QKQKKQ amino acid sequence proximal to the amino terminus of APRIL, thereby generating APRIL oligomers. These oligomers would enhance TACI and BCMA signaling by promoting the formation of a highly efficient signaling platform in HRS cells. Extracellular HSPGs may have the same effect, as heparin enhanced spontaneous as well as APRIL-induced accumulation of HRS cells.
BAFF and APRIL activated the canonical NF-κB pathway in HRS cells, similar to CD40L and CD30L.22,23,26,27,29 By showing that CD30L and CD40L up-regulated BAFF and APRIL on HRS cells, our findings suggest that HL tumor expansion may involve integrated signaling from multiple TNF family members. Together with CD30L and CD40L, BAFF and APRIL would contribute to the dysregulation of NF-κB activation in HRS cells. Such dysregulation would not only enhance aberrant HRS cell accumulation, but also render HRS cells more resistant to anti-HL agents. Consistent with this, HRS cells exposed to BAFF or APRIL responded less to doxorubicin, at least in vitro.
By delivering nonredundant signals, BAFF and APRIL would contribute to the overall dysregulation of prosurvival and proliferation checkpoints in HL tumors.12,17 Accordingly, HRS cells up-regulated antiapoptotic Bcl-2 and Bcl-xL, down-regulated proapoptotic Bax, and up-regulated growth-inducing c-Myc proteins upon exposure to BAFF or APRIL. Like malignant plasma cells,45,47,51 HRS cells transduced survival, growth, and NF-κB signals as induced by autocrine and paracrine BAFF and APRIL through a BAFF-R–independent mechanism involving TACI and BCMA. These signals were attenuated by TACI-Ig, a soluble decoy receptor that binds to and neutralizes both BAFF and APRIL. Although capable of blocking binding of extracellular BAFF and APRIL to membrane-anchored TACI and BCMA receptors, TACI-Ig would have no access to intracellular BAFF and APRIL, which appeared to constitutively interact with cytosolic TACI and BCMA receptors in HRS cells. Intracellular BAFF and APRIL can instead be targeted by siRNAs, which in fact were more efficient than TACI-Ig in inhibiting HRS cell survival. Thus, TACI-Ig could be combined with TACI- and BCMA-targeting siRNAs to attenuate HL tumor expansion in vivo.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Conflict-of-interest disclosure: Several of the authors (S.R.D., J.A.G., and E.S.) are employed by a company (ZymoGenetics, Inc) whose (potential) product, TACI-Ig, was studied in the present work.
Contribution: A.C. and W.X. contributed equally to this work by designing and performing research, analyzing data, and writing the paper; B.H., X.Q., P.S., E.H., and J.L. performed research; S.R.D., J.A.G., and E.S. provided vital new reagents and discussed data; E.C., A.C., and D.M.K. provided tissue samples and discussed data; A.C. designed research, analyzed data, and wrote the paper.
Acknowledgments
This work was supported by the National Institutes of Health (NIH) grants AI057530 and AI057653.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal