Abstract
The β2 integrins are important for transendothelial migration of leukocytes as well as for T-cell activation during antigen presentation. Despite abundant expression of β2 integrins on antigen-presenting cells (APCs), their functional relevance for antigen presentation is completely unclear. We show here that dendritic cells (DCs) from CD18-deficient mice, which lack all functional β2 integrins, have no defect in antigen presentation. Moreover, DCs from normal mice express inactive β2 integrins that do not become activated on contact with T cells, at least in vitro. Pharmacologic activation of β2 integrins on DCs results in a significant reduction of their T cell–activating capacity. This effect is mediated by Mac-1 (CD11b/CD18) on DCs because it could be reversed via blocking antibodies against CD18 and CD11b. Furthermore, the antigen-presenting capacity of macrophages, which express constitutively active β2 integrins, is significantly enhanced on Mac-1 blockade. We therefore conclude that active CD11b/CD18 (Mac-1) on APCs directly inhibits T-cell activation.
Introduction
The β2 integrins (CD11/CD18) are heterodimeric leukocyte adhesion molecules exclusively expressed on hematopoietic cells. They play an important role for cell-to-cell contacts between leukocytes as well as for contacts between leukocytes and endothelial cells.1,2 The common β chain (CD18) associates with 4 different α subunits, αL, αM, αX, and αD, forming distinct functional heterodimers termed leukocyte functional antigen-1 (LFA-1, CD11a/CD18), Mac-1 (CD11b/CD18), gp150,95 (CD11c/CD18), and CD11d/CD18.2-4 These interact with more than 20 ligands, many of which belong to the family of ICAMs.5,6
Adhesion between T cells and antigen-presenting cells (APCs) is necessary for the formation of the immunologic synapse (IS).7,8 Interactions between LFA-1 on the surface of T cells and intercellular adhesion molecules ICAM-1, 2, and 3 (in humans) on the APC surface were described to be involved in formation of the IS.9,10 Moreover, ligation of LFA-1 on T cells is required for optimal activation and differentiation of T cells,11-21 although the downstream signaling events are still not fully understood. On T cells, experiments with blocking antibodies against LFA-1 and with LFA-1–deficient T cells have shown that those T cells are impaired in numerous effector functions.22-24
Dendritic cells (DCs), on the other hand, are known to be the most relevant APCs in the immune system.25,26 DCs express at least 3 β2 integrins, LFA-1 (CD11a/CD18), Mac-1 (CD11b/CD18), and gp150,95 (CD11c/CD18).27,28 Compared to LFA-1 on T cells, very little information is available about the role and function of β2 integrins on DCs, especially regarding the process of antigen presentation and T-cell activation. Studies with splenocytes of mice deficient in CD11a, CD11b, CD11c, and CD11d showed impaired T-cell activation in CD11a-, CD11b-, and CD11d-deficient splenocytes on stimulation with superantigen, but this was due to dysfunctional T cells rather than a defect in APCs. Using CD11b- and CD11d-deficient irradiated splenocytes as APCs, no defect was detectable in activation of wild-type (WT) T cells.29 Until now, there has been no report on the use of CD11a- or CD11c-deficient DCs for T-cell stimulation in vitro.
Here, we examined the role of β2 integrins for antigen presentation by DCs. We show that active β2 integrins are not necessary for the antigen-presenting capacity of DCs because DCs that lack β2 integrins (CD18−/− DCs) are fully capable of T-cell activation. Although β2 integrins are expressed on bone marrow (bm)–derived DCs (bmDCs), they are unable to bind to plate-bound ICAM-1–Fc, suggesting that they are functionally inactive. In contrast, when artificially activated by Mg2+ treatment, β2 integrins on DCs inhibit T-cell activation. This effect is mostly due to activation of CD11b/CD18 (Mac-1) because it can be blocked by anti-CD11b antibodies. In turn, constitutively active Mac-1 that is present on other APC types such as macrophages, but not on DCs, inhibits antigen presentation by these “nonprofessional” APCs. By this mechanism, antigen presentation in vivo could be restricted to DCs, despite the presence of Ag-MHC on other leukocyte subsets. These data reveal a new role for the β2 integrin Mac-1 (CD11b/CD18) in the regulation of antigen presentation.
Materials and methods
Mice
CD18−/− 129X1/SvJ × C57BL/6 (H-2b) mice were generated as described.30 CD18+/+ litters from heterozygote crosses served as WT control mice. BALB/c or C57BL/6 mice were obtained from Harlan (Horst, The Netherlands). Mice used were 8 to 12 weeks of age and were housed according to federal and institutional guidelines.
Antibodies and reagents
All antibodies were purchased from BD PharMingen (Heidelberg, Germany), including anti-CD3 (145-2C11), anti-CD8a (53-6.7), anti-CD11a (2D7), anti-CD11b (M1/70), anti-CD11c (HL-3), anti-CD18 (GAME 46, M18.2), anti-CD16/32 (2.4G2,“Fc-block”), anti-CD24 (M1/69), anti-CD25 (PC61), anti-CD28 (37.51), anti-CD40 (1C10), anti-CD45/B220 (RA3-6B2), anti–GR-1/Ly-6G (RB6-8C5), anti-γδ-T cells (GL3), anti-NKT (U5A2-13), anti-CD44 (Ly-24), anti-CD62L (Ly-22), anti-CD80 (16-10A1), anti-CD86 (GL1), anti–MHC II (I-A/I-E, 2G9), anti–ICAM-1 (αCD54), and anti–ICAM-2 (αCD102). Cytokines were quantitated using the cytometric bead array (CBA) for Th1/Th2 cytokine detection (BD PharMingen). For cell separation, anti–rat immunoglobulin beads, streptavidin beads, anti-PE beads, and anti-Thy 1.2 (CD90) beads were from Miltenyi Biotech (Bergisch-Gladbach, Germany). PMA/ionomycin and LPS were purchased from Sigma-Aldrich (Taufkirchen, Germany). Bovine type I collagen (Vitrogen 100) was purchased from Cohesion Technologies (Palo Alto, CA). MgCl2 was from Merck (Darmstadt, Germany).
ICAM-1 adhesion assay
P-selectin Fc and ICAM-1–Fc human IgG chimeric proteins were prepared and adhesion assays were performed as described,31 with slight modifications. Briefly, 10 μg/mL protein was coated onto 96-well flat-bottom plates (Maxisorp, Nunc, Wiesbaden, Germany). Antibodies (10 μg/mL) or Mg2+ (5 mM) were added to the cells prior to the assay in cold Hanks balanced salt solution (HBSS) without Ca2+ and Mg2+. Then, 1 μg Fc-block (BD PharMingen) was used to minimize nonspecific binding. To allow adhesion, 5 × 105 cells/well were added and incubated for 45 minutes at 4°C. Plates were washed 4 times, and adherent cells were fixed using 1% paraformaldehyde. Numbers were evaluated by computer-aided image analysis with National Institutes of Health (NIH; Bethesda, MD) Image 1.55 software as described.32
Mg2+ treatment
In preliminary experiments, we tested Mn2+ and Mg2+ (1-20 mM) for toxic effects on DCs or T cells. Mg2+ (5 mM) treatment for up to 6 days did not significantly affect cell viability (data not shown). Thus, 5 mM Mg2+ was used for subsequent experiments. In contrast, Mn2+, which enhanced β2-integrin affinity to ICAM-1 even more than Mg2+33-36 (data not shown), was toxic for DCs, even at low concentrations (data not shown).
Generation of DCs and macrophages and isolation of T cells
DCs were generated essentially as described.37,38 Briefly, single-cell suspensions from murine bone marrow were seeded into 90-mm tissue culture dishes (Becton Dickinson Biosciences, Heidelberg, Germany) in complete medium (RPMI 1640 containing 5% heat-inactivated FCS, 50 μM β-mercaptoethanol, 2 mM l-glutamine, 0.1 mM nonessential amino acids [NAAs], and 20 μg/mL gentamicin (all from PAA Laboratories, Linz, Austria) for 2 hours at 37°C and 10% CO2. Nonadherent cells were cultured in the presence of 150 U/mL GM-CSF and IL-4 (both from conditioned cell culture supernatants kindly provided by Thomas Blankenstein, MDC Berlin, Germany) for 7 days, with a medium change every second day. The yield of CD11c+ cells was routinely greater 80%. To activate DCs, LPS (100 ng/mL) was added for 48 hours on day 7 of culture, as described.38 Macrophages were generated from bone marrow cells as described.39 Briefly, cells were grown in DMEM (PAA Laboratories) containing L cell-conditioned media (15% vol/vol), 2 mM l-glutamine, 0.1 mM NAAs, 20 μg/mL gentamicin, and 10% FCS. Cells were cultured for 6 days in Teflon bags (Heraeus, Hanau, Germany), and subsequently used in experiments.
T cells from spleens and lymph nodes of mice were prepared as described.40 Since peripheral lymphatic organs of CD18−/− mice contain a large proportion of preactivated T cells and lineage-negative cells, a nonstandard T-cell purification protocol had to be adapted. Thus, CD69+ cells were removed using anti–CD69-PE antibodies plus ferromagnetic anti-PE microbeads by AutoMACS technology (Miltenyi Biotech). The resulting CD69− cells were further enriched with anti-CD90 microbeads by AutoMACS, yielding more than 95% CD3+CD69− CD90+ T cells, which were used in subsequent experiments.
Flow cytometry
For flow cytometry, cells (5 × 105) were incubated in 50 μL PBS/1% FCS with the indicated antibodies (1 μg/mL) for 30 min at 4°C. Cells were washed twice in 1 mL PBS/1% FCS. For intracellular staining, cells were fixed in 100 μL 1% (wt/vol) paraformaldehyde for 15 minutes at 37°C, washed twice, and permeabilized using 50 μL 1% (wt/vol) saponin for 10 minutes at 37°C. Then 1 μg of the indicated antibodies was added to the permeabilized cells and incubated for 30 minutes on ice. Finally, cells were washed twice, resuspended in 500 μL PBS/1% FCS, and analyzed using a FACSCalibur flow cytometer, equipped with CellQuestPro software (BD PharMingen).
T-cell proliferation and MLRs
T cells (1 × 105–2 × 105) were plated in 96-well microtiter plates in triplicate. Cells were stimulated with plate-bound anti-CD3 antibody (5 μg/mL) plus soluble anti-CD28 antibody (2.5 μg/mL), and were cultured for 5 days at 37°C and 5% CO2. For mixed lymphocyte reactions (MLRs), APCs were washed twice in PBS to remove cytokines from the culture medium. Then, T cells and APCs were resuspended in complete medium. T cells (1 × 105) were plated into 96-well round-bottom microtiter plates and APCs were added at the indicated ratios. Cells were incubated for 4 days at 37°C and 5% CO2. Then supernatants were taken for analysis of cytokine content. Subsequently, cells were incubated for another 18 hours with 1 μCi (0.037 MBq) 3H-thymidine (Hartman Analytics, Braunschweig, Germany) per well. Cells were harvested and measured for thymidine incorporation using a β counter (Wallac, Turku, Finland).
Cytokine measurement
Cytokine production was measured from cell culture supernatants using CBA array technology (BD PharMingen) according to the manufacturer's instructions and subsequently analyzed by FACS.
CFDA labeling of T cells
T cells (1 × 107) T cells were incubated for 5 minutes in PBS containing 0.5 μM CFDA or 1.0 μM SNARF (Molecular Probes, Leiden, The Netherlands) at 37°C in the dark, washed in PBS/1% FCS, and used in proliferation assays.
Three-dimensional collagen gel
Time-lapse video microscopy analysis was performed as described earlier.41 Briefly, 1 × 105 DCs and 1 × 106 T cells were inserted into 3-dimensional collagen gels at 37°C and monitored by time-lapse video microscopy. DC–T-cell interactions were analyzed using an Olympus BX61 microscope equipped with a UAPO 340 air objective lens (20 ×/1.6 magnification changer/0.75 numerical aperture) and an F-View camera and analySIS software (Olympus, Hamburg, Germany). Images were processed using Cell P software (Olympus).
Results
Phenotype of DCs from CD18−/− mice
Recently, we demonstrated that lack of β2 integrins on DCs does not influence their migratory capacities in vivo, whereas migration of T cells was greatly impaired.42 This suggested that β2 integrins might be inactive on DCs and led us to examine their role for DC function. First, we compared bmDCs from CD18−/− and CD18+/+ (WT) mice phenotypically. FACS analysis confirmed that bmDCs of CD18−/− mice do not express the α chains (CD11a, CD11b, CD11c) or the β chain (CD18) of β2 integrins on their surface (Figure S1, available on the Blood website; see the Supplemental Figures link at the top of the online article). Lack of β2 integrins did not alter expression of MHC class II, CD40, CD80, CD86, ICAM-1, and ICAM-2 on either immature DCs (iDCs, data not shown) or mature DCs (mDCs; Figure S1). On maturation of bmDCs, MHC class II and costimulatory molecules were up-regulated on WT and CD18−/− bmDCs (data not shown). Likewise, splenic DCs or epidermal Langerhans cells were phenotypically unaltered in CD18−/− mice42 (data not shown). Thus, except for β2-integrin expression, the phenotype of CD18−/− and CD18+/+ DCs was not significantly different.
Binding activity of β2 integrins on DCs
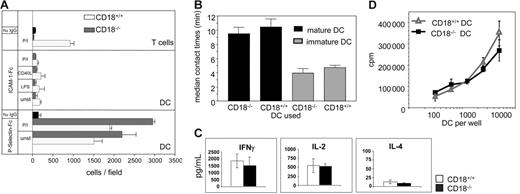
We then asked whether β2 integrins on WT bmDCs can be activated to confer ligand-binding capacity. Using plate-bound recombinant ICAM-1–Fc chimeric protein we determined the amount of bound cells as a measure of β2 integrin activity (Figure 1A). Because LFA-1 is the only β2 integrin on T cells and can be activated to bind to its ligand ICAM-1, we used T cells as a reference. On PMA/ionomycin stimulation, CD18+/+ T cells readily bound to ICAM-1 (Figure 1A upper panel, □), whereas CD18−/− T cells did not bind (upper panel, ⊡). Plate-bound human IgG (upper panel, ▪) served as specificity control for WT T cells. Interestingly, DCs were unable to bind to ICAM-1 significantly, not even on stimulation with LPS, CD40 ligand (CD40L), and PMA/ionomycin, respectively (Figure 1A middle panel, CD18+/+ DCs, □; CD18−/−, ⊡). To determine whether DCs were generally able to adhere to plate-bound proteins we examined binding to recombinant P-selectin Fc via PSGL, which was reported to be active on DCs.31 As a control, CD18+/+ DCs and CD18−/− DCs, unstimulated or stimulated, readily bound to P-selectin, suggesting that DCs were functional and viable, but did not express active β2 integrins. Coated human IgG served as a specificity control (Figure 1A lower panel). To explore the capacity of β2 integrins on DCs to bind cells, WT T cells and DCs were coincubated in 3-dimensional collagen gels and the duration of DC–T-cell interaction was analyzed as reported earlier.41 Overall contact times between iDCs and T cells were significantly shorter than those between mDCs and T cells (Figure 1B). However, the average duration of DC–T-cell contact did not depend on β2-integrin expression on DCs because CD18+/+ DCs and CD18−/− DCs showed similar contact times to T cells (Figure 1B). These data indicate that β2 integrins on DCs are unable to mediate binding to either plate-bound ICAM or to cellular β2-integrin ligands expressed on T cells.
Binding activity and antigen-presenting capacity of DCs from CD18−/− mice. (A) Wells of a 96-well plate were coated with 10 μg/mL of either recombinant ICAM-1–Fc (top panel), recombinant P-selectin–Fc (bottom panel), or human IgG (▪). Then 1 × 106 T cells/well or 5 × 105 bmDCs (CD18+/+, □; CD18−/−, ⊡) were left unstimulated or treated with PMA/ionomycin (3 ng/300 ng/mL), LPS (100 ng/mL), or CD40L (1 μg/mL), and were incubated for 45 minutes at 37°C. Wells were washed 4 times and assessed for bound cells by digital microscopy. (B) bmDCs (5 × 104) of either CD18+/+ or CD18−/− mice were incubated with 5 × 105 CD18+/+ T cells in a collagen gel. Contacts of T cells and bmDCs were recorded by video microscopy and individual DC–T-cell pairs were analyzed for their contact times. Data are representative of 3 separate experiments ± SEM. (C) BALB/C T cells (2 × 105) were incubated with allogeneic bone marrow DCs of either CD18−/− or CD18+/+ mice for 3 days and pulsed for 18 hours with 1 μCi (0.037 MBq) 3H-thymidine before 3H-thymidine incorporation was assessed. Data are representative of 3 independent experiments. (D) Supernatants of panel C were measured for cytokine content using CBA and analyzed by flow cytometry. Data are expressed as means of 3 independent experiments.
Binding activity and antigen-presenting capacity of DCs from CD18−/− mice. (A) Wells of a 96-well plate were coated with 10 μg/mL of either recombinant ICAM-1–Fc (top panel), recombinant P-selectin–Fc (bottom panel), or human IgG (▪). Then 1 × 106 T cells/well or 5 × 105 bmDCs (CD18+/+, □; CD18−/−, ⊡) were left unstimulated or treated with PMA/ionomycin (3 ng/300 ng/mL), LPS (100 ng/mL), or CD40L (1 μg/mL), and were incubated for 45 minutes at 37°C. Wells were washed 4 times and assessed for bound cells by digital microscopy. (B) bmDCs (5 × 104) of either CD18+/+ or CD18−/− mice were incubated with 5 × 105 CD18+/+ T cells in a collagen gel. Contacts of T cells and bmDCs were recorded by video microscopy and individual DC–T-cell pairs were analyzed for their contact times. Data are representative of 3 separate experiments ± SEM. (C) BALB/C T cells (2 × 105) were incubated with allogeneic bone marrow DCs of either CD18−/− or CD18+/+ mice for 3 days and pulsed for 18 hours with 1 μCi (0.037 MBq) 3H-thymidine before 3H-thymidine incorporation was assessed. Data are representative of 3 independent experiments. (D) Supernatants of panel C were measured for cytokine content using CBA and analyzed by flow cytometry. Data are expressed as means of 3 independent experiments.
Antigen-presenting capacity of CD18−/− and CD18+/+ DCs
To further assess the activity of β2 integrins on DCs, we performed functional assays using either CD18−/− or CD18+/+ DCs in MLR experiments with allogeneic WT T cells. Proliferation of T cells was identical when either CD18+/+ or CD18−/− DCs were used as stimulators (Figure 1C). Also, overall T-cell proliferation was lower when we used iDCs, but there was no difference between bmDCs from WT or CD18−/− mice (data not shown). CD18−/− DCs not only were fully capable of stimulating T-cell proliferation, they also induced secretion of the same pattern and amounts of cytokines by T cells compared to their WT counterparts (Figure 1D).
Taken together, active β2 integrins on DCs do not seem to be required for stimulation of T-cell proliferation and cytokine production in this experimental setting.
Activation of β2 integrins on DCs
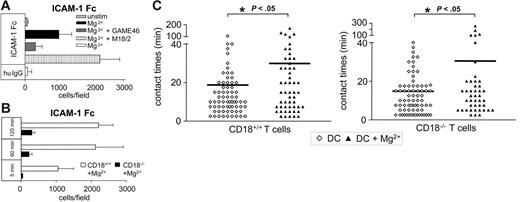
Ligand-binding activity of β2 integrins, especially LFA-1, can be regulated by divalent cations such as Mg2+, Mn2+, and Ca2+.33-36,43 Indeed, preincubation of bmDCs with 5 mM Mg2+ significantly activated the ICAM-1–binding capacity of DCs (Figure 2A, ▪). This activity was blocked by the anti-CD18 antibody GAME 46 (Figure 2A, dark gray bar) that has been described to inhibit binding of LFA-1 to ICAM-1.44 In contrast, the nonblocking anti-CD18 antibody M18.2 did not influence ICAM-1 binding of DCs when used alone (data not shown), but even enhanced Mg2+ induced binding activity (Figure 2A, ▥). Coated human IgG served as a control for nonspecific effects (Figure 2A, □). In time-course experiments, ICAM-1–binding activity of DCs was already measurable after 5 minutes of Mg2+ treatment and was sustained up to at least 120 minutes (Figure 2B, □). CD18−/− DCs served as a specificity control and did not bind substantially to ICAM-1–Fc (Figure 2B, ▪). Various stages of DC maturity, reflected by culture of DCs for 7, 8, or 9 days, were all able to bind ICAM-1–Fc with the same capacity when activated by Mg2+ (Figure S2). We also tested earlier stages of bmDCs (days 4-6), with similar results (data not shown). The viability of DCs was not affected by Mg2+ treatment at concentrations of up to 20 mM for up to 72 hours (Figure S3).
Binding activity of β2 integrins on DCs. (A) bmDCs (day 7) were pretreated with 5 mM Mg2+ either alone or together with the anti-CD18 antibodies 18.2 or GAME46 (10 μg/mL). Cells (5 × 105) were placed in 96-well plates in triplicate for 45 minutes at 37°C. Wells were either coated with 10 μg/mL recombinant ICAM-1–Fc or human IgG as a control. After washing, plates were analyzed for bound cells by microscopy (original magnification × 10) using the NIH Image 1.55 software. One representative experiment of 4 is shown. (B) bmDCs from CD18+/+ and CD18−/− mice were prepared and used to assay ICAM-1 binding as in panel A. One of 3 experiments is shown. (C) T cells were cocultured with either untreated or Mg2+ pretreated CD18+/+ or CD18−/− bmDCS (day 7) in collagen gels. Contact times of individual T-DC pairs were analyzed as in Figure 1B. One representative experiment of 2 is shown. Horizontal bars represent mean values.
Binding activity of β2 integrins on DCs. (A) bmDCs (day 7) were pretreated with 5 mM Mg2+ either alone or together with the anti-CD18 antibodies 18.2 or GAME46 (10 μg/mL). Cells (5 × 105) were placed in 96-well plates in triplicate for 45 minutes at 37°C. Wells were either coated with 10 μg/mL recombinant ICAM-1–Fc or human IgG as a control. After washing, plates were analyzed for bound cells by microscopy (original magnification × 10) using the NIH Image 1.55 software. One representative experiment of 4 is shown. (B) bmDCs from CD18+/+ and CD18−/− mice were prepared and used to assay ICAM-1 binding as in panel A. One of 3 experiments is shown. (C) T cells were cocultured with either untreated or Mg2+ pretreated CD18+/+ or CD18−/− bmDCS (day 7) in collagen gels. Contact times of individual T-DC pairs were analyzed as in Figure 1B. One representative experiment of 2 is shown. Horizontal bars represent mean values.
Thus, Mg2+ ions induced β2-integrin binding activity to ICAM-1, independently of the differentiation status of DCs. Of note, we did not detect β2-integrin activation using 1 mM Mg2+, which is the physiologic Mg2+ concentration in routine culture media (data not shown). Mg2+ also enhanced the capacity of CD18+/+ DCs to bind to cellular β2-integrin ligands, as shown by prolongation of median DC–T-cell contact duration in collagen gels (Figure 2C). This was not due to integrin activation on T cells, because contact times between WT DCs and CD18−/− T cells also increased after addition of Mg2+. In contrast, DCs from CD18−/− mice showed similar contact times to T cells in the presence or absence of Mg2+ (Figure S4).
Active β2 integrins on DCs modulate T-cell activation
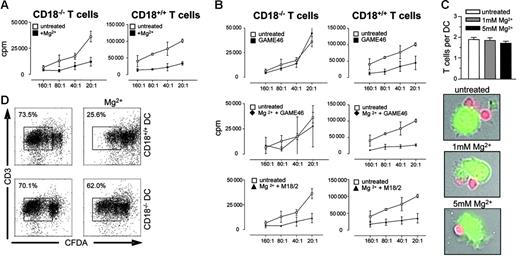
Do active β2 integrins on DCs modify antigen presentation? To address this question we used purified CD18+/+ or CD18−/− T cells and performed MLR experiments with allogeneic DCs. DC-induced T-cell proliferation was significantly impaired in the presence of Mg2+ (Figure 3A left and right graphs). The impaired antigen-presenting capacity of Mg2+-treated DCs could be reversed by incubation with the anti-CD18 antibody GAME46, suggesting that Mg2+-induced integrin activation indeed inhibits antigen presentation by DCs (Figure 3B middle panel, left graph). In these experiments, CD18−/− T cells were used in MLR cultures to ensure that the antibody blockade could only act on DCs (Figure 3A-B). When using CD18+/+ T cells, the same anti-CD18 antibody inhibited T-cell proliferation (Figure 3B upper right graph), indicating that active β2 integrins are required for optimal T-cell activation but inhibit antigen presentation by DCs. Control experiments showed that GAME46 treatment did not affect the antigen-presenting capacity of non–Mg2+-treated DCs (Figure 3B, upper left graph) and also did not reverse the inhibitory effect of Mg2+-treated DCs on CD18+/+ T cells (Figure 3B, middle panel). Moreover, the nonblocking anti-CD18 antibody M18.2 did not reverse the decreased antigen-presenting capacity of Mg2+-activated DCs (Figure 3B lower panel, left and right graphs). As further controls, Mg2+ did not inhibit the anti-CD3/anti-CD28–induced proliferation of T cells alone (Figure S5), and also did not result in enhanced aggregation of DC (Figure 3C), thus excluding the possibility that the decrease in antigen-presenting capacity was simply due to decreased access of T cells to an APC surface due to clumping of the Mg2+-activated DCs. We also verified the data using CFDA as a second read-out for T-cell proliferation. Again, Mg2+ treatment of CD18+/+, but not of CD18−/−, bmDCs substantially reduced the proliferation of CFDA-labeled allogeneic T cells (Figure 3D). Thus, Mg2+-induced activation of β2 integrins on DCs inhibits their capacity to stimulate proliferation of allogeneic T cells.
Active β2 integrins on DCs modulate T-cell activation. (A) T cells (1 × 105) from CD18+/+ or CD18−/− mice were cocultured with allogeneic BALB/c DCs at the indicated ratios. DCs were left untreated or were preincubated with 5 mM Mg2+ or with (B) 5 mM Mg2+ plus 10 μg/mL anti-CD18 antibody either of the GAME46 or M18.2, as indicated. After 3 days, 1 μCi (0.037 MBq) 3H-thymidine was added to the cultures for another 18 hours and thymidine incorporation was determined. Data are mean values (± SEM) of triplicates. One representative experiment of 3 is shown. (C) CFDA-labeled DCs and SNARF-labeled T cells were coincubated for 1 hour and sedimented on poly-l-lysine–coated slides. DC–T-cell contacts were analyzed by fluorescence microscopy. Bar graphs show the average number of DC–T-cell contacts in various concentrations of Mg2+, photomicrographs show representative pictures. (D) CFDA-labeled allogeneic BALB/c T cells (1 × 105) were cocultured with either bmDCs from CD18+/+ or CD18−/− mice at a T/DC ratio of 10:1. Cells were left untreated or treated with 5 mM Mg2+. After 5 days of culture, cells were labeled with anti-CD3 and proliferation of T cells was measured by flow cytometry.
Active β2 integrins on DCs modulate T-cell activation. (A) T cells (1 × 105) from CD18+/+ or CD18−/− mice were cocultured with allogeneic BALB/c DCs at the indicated ratios. DCs were left untreated or were preincubated with 5 mM Mg2+ or with (B) 5 mM Mg2+ plus 10 μg/mL anti-CD18 antibody either of the GAME46 or M18.2, as indicated. After 3 days, 1 μCi (0.037 MBq) 3H-thymidine was added to the cultures for another 18 hours and thymidine incorporation was determined. Data are mean values (± SEM) of triplicates. One representative experiment of 3 is shown. (C) CFDA-labeled DCs and SNARF-labeled T cells were coincubated for 1 hour and sedimented on poly-l-lysine–coated slides. DC–T-cell contacts were analyzed by fluorescence microscopy. Bar graphs show the average number of DC–T-cell contacts in various concentrations of Mg2+, photomicrographs show representative pictures. (D) CFDA-labeled allogeneic BALB/c T cells (1 × 105) were cocultured with either bmDCs from CD18+/+ or CD18−/− mice at a T/DC ratio of 10:1. Cells were left untreated or treated with 5 mM Mg2+. After 5 days of culture, cells were labeled with anti-CD3 and proliferation of T cells was measured by flow cytometry.
Regulatory T cells are not affected by active β2 integrins on DCs
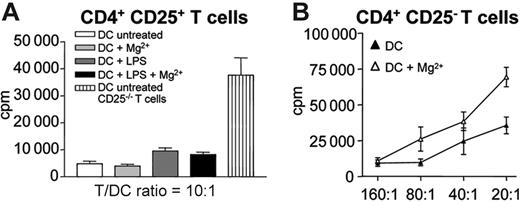
We now asked whether active β2 integrins on DCs might induce or generate regulatory T cells that would act on effector T cells. To test this we isolated CD4+CD25+ regulatory T cells and incubated them with bmDCs, either with or without Mg2+. As shown in Figure 4A, Mg2+ treatment did not enable DCs to stimulate the proliferation of CD4+CD25+ T cells. In turn, active β2 integrins on DCs inhibited the proliferation of T cells that had been depleted of CD4+CD25+ regulatory T cells, again indicating that the effect was not mediated by regulatory T cells (Figure 4B).
Reduced T-cell proliferation by active β2 integrins on DCs is not due to regulatory T-cell activity. (A) CD4+CD25+ T cells (C57BL/6; 1 × 105/well) were cocultured with bmDCs from BALB/c mice at a T/DC ratio of 10:1. DCs were either pretreated with 100 ng/mL LPS for 24 hours or left untreated. Additionally, cocultures were treated with 5 mM Mg2+ where indicated. CD4+CD25− T cells served as controls. After 3 days of culture, 1 μCi (0.037 MBq) 3H-thymidine was added for another 18 hours and proliferation was measured using a β-counter. (B) CD4+CD25− T cells (1 × 105) from C57BL/6 mice were coincubated with bmDCs from BALB/c mice at the indicated ratios, with or without addition of 5 mM Mg2+. Three days later, 1 μCi/well (0.037 MBq) 3H-thymidine was added for another 18 hours and thymidine incorporation was measured. Experiments were performed in triplicate; 1 representative experiment of 2 is shown. Error bars represent SEM of triplicates.
Reduced T-cell proliferation by active β2 integrins on DCs is not due to regulatory T-cell activity. (A) CD4+CD25+ T cells (C57BL/6; 1 × 105/well) were cocultured with bmDCs from BALB/c mice at a T/DC ratio of 10:1. DCs were either pretreated with 100 ng/mL LPS for 24 hours or left untreated. Additionally, cocultures were treated with 5 mM Mg2+ where indicated. CD4+CD25− T cells served as controls. After 3 days of culture, 1 μCi (0.037 MBq) 3H-thymidine was added for another 18 hours and proliferation was measured using a β-counter. (B) CD4+CD25− T cells (1 × 105) from C57BL/6 mice were coincubated with bmDCs from BALB/c mice at the indicated ratios, with or without addition of 5 mM Mg2+. Three days later, 1 μCi/well (0.037 MBq) 3H-thymidine was added for another 18 hours and thymidine incorporation was measured. Experiments were performed in triplicate; 1 representative experiment of 2 is shown. Error bars represent SEM of triplicates.
Active Mac-1 (CD11b/CD18) on DCs inhibits T-cell activation
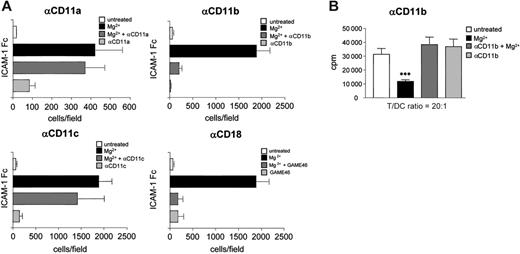
To investigate whether a specific β2 integrin accounts for the inhibitory effect on T-cell activation, we assayed the ICAM-1–binding activity of DCs in the presence of antibodies against the individual α chains of β2 integrins (CD11a, CD11b, and CD11c). Whereas anti-CD11a and anti-CD11c antibodies did not significantly affect ICAM-1 binding of Mg2+-treated DCs, the anti-CD11b antibody prevented Mg2+ induced ICAM-1 binding of DCs to a similar extent as the anti-CD18 antibody GAME46, indicating that CD11b/CD18 (Mac-1) was the Mg2+-activated ICAM-1 ligand on DC (Figure 5A). To address whether active CD11b is also responsible for reduced T-cell activation, we blocked Mac-1–binding activity in MLR experiments. In the absence of Mg2+, DC-induced T-cell proliferation was not affected by anti-CD11b treatment, whereas Mg2+ treatment resulted in the expected decrease in T-cell proliferation. Indeed, addition of anti-CD11b completely restored the T cell-stimulating capacity of Mg2+-activated DCs (Figure 5B).
Active Mac-1 (CD11b/CD18) on DCs modulates T-cell activation. (A) DCs (5 × 105/well) were placed into 96-well plates that had been coated with 10 μg/mL ICAM-1–Fc. DCs were left untreated or were preincubated with 5 mM Mg2+ or 10 μg/mL of the indicated antibodies for 1 hour. Plates were incubated for 45 minutes at 37°C, and wells were washed 4 times and analyzed for bound cells by microscopy as described in “Materials and methods.” Error bars represent SD of 5 independent experiments. (B) CD3+ T cells (BALB/c; 1 × 105/well) were cocultured with allogeneic bmDCs (C57BL/6) at a T/DC ratio of 20:1 for 3 days in 96-well plates. DCs were pretreated with 10 μg/mL anti-CD11b antibody and where indicated 5 mM Mg2+ was added. After 3 days of coculture, 1 μCi (0.037 MBq) 3H-thymidine/well was added to the culture for another 18 hours to determine T-cell proliferation. Error bars represent SD of 3 independent experiments.
Active Mac-1 (CD11b/CD18) on DCs modulates T-cell activation. (A) DCs (5 × 105/well) were placed into 96-well plates that had been coated with 10 μg/mL ICAM-1–Fc. DCs were left untreated or were preincubated with 5 mM Mg2+ or 10 μg/mL of the indicated antibodies for 1 hour. Plates were incubated for 45 minutes at 37°C, and wells were washed 4 times and analyzed for bound cells by microscopy as described in “Materials and methods.” Error bars represent SD of 5 independent experiments. (B) CD3+ T cells (BALB/c; 1 × 105/well) were cocultured with allogeneic bmDCs (C57BL/6) at a T/DC ratio of 20:1 for 3 days in 96-well plates. DCs were pretreated with 10 μg/mL anti-CD11b antibody and where indicated 5 mM Mg2+ was added. After 3 days of coculture, 1 μCi (0.037 MBq) 3H-thymidine/well was added to the culture for another 18 hours to determine T-cell proliferation. Error bars represent SD of 3 independent experiments.
As a control, we determined CD11b/CD18 (Mac-1) surface expression after Mg2+treatment of DCs. Because Mac-1 mediates phagocytosis on binding to many of its ligands,45-47 inhibition of T-cell activation might have been mediated by Mg2+-induced reduction of Mac-1 expression on DCs. However, Mg2+ did not alter the expression of either surface or intracellular CD11b in DCs (Figure S6). We therefore conclude that activated Mac-1 on DCs inhibits their antigen-presenting capacity.
Active Mac-1 (CD11b/CD18) on macrophages inhibits T-cell activation
Because activation of Mac-1 on DCs decreased their T-cell activation capacity, we wondered whether constitutively active Mac-1 on other APC types would naturally impair antigen presentation to T cells. Compared to DCs, macrophages are weak APCs, but are known to express high amounts of active Mac-1. Consequently, blocking Mac-1 on macrophages should improve their capacity to activate T cells. First, we verified the ICAM-1–binding activity of CD11b on macrophages using bone marrow-derived CD18+/+ and CD18−/− macrophages. As shown in Figure 6A, WT macrophages constitutively bound to ICAM-1–Fc (▪). CD18−/− macrophages bound significantly less ICAM-1–Fc, although they showed substantial background binding, presumably due to plastic adherence. Anti-CD11b antibody blockade confirmed that the specific ICAM-1–Fc–binding activity was largely mediated by Mac-1 (dark gray bar). Anti-CD11a or anti-CD11c antibodies did not prevent ICAM-1 binding of macrophages (Figure 6B). Mg2+ did not further increase ICAM-1 binding, suggesting that Mac-1 was indeed constitutively active on macrophages (data not shown). Interestingly, macrophage-induced proliferation of allogeneic T cells was significantly enhanced when CD11b on macrophages was blocked (Figure 6C). Likewise, CD18−/− macrophages were significantly more potent stimulators of allogeneic T cells than their WT counterparts (Figure 6D). Thus, active Mac-1 on APC exerts a direct inhibitory effect on antigen presentation to naive T cells.
Inactivation of CD11b/CD18 (Mac-1) on macrophages enhances T-cell proliferation. (A-B) Macrophages from bone marrow of CD18+/+ and CD18−/− mice were generated as described in “Materials in methods.” Then, 5 × 105 macrophages/well were preincubated with 10 μg/mL anti-CD11a, -b, or -c antibodies and placed into 96-well plates that had been coated with 10 μg/mL recombinant ICAM-1–Fc. Cells were incubated for 45 min at 37°C, washed 4 times, and analyzed for bound cells by microscopy as described in “Materials and methods.” One representative of 3 experiments is shown. Error bars represent SEM of triplicates. (C) CD3+ T cells (1 × 105/well) were cocultured with allogeneic macrophages at the indicated T cell–macrophage ratios. Macrophages were left untreated or preincubated with 20 μg/mL anti-CD11b antibody for 2 hours. After 5 days of coculture, 1 μCi (0.037 MBq) of 3H-thymidine/well was added for another 18 hours and T-cell proliferation was assessed. One representative experiment of 3 is shown. Error bars represent SD. (D) CD4+ T cells (1 × 105/well) were cocultured with allogeneic CD18+/+ or CD18−/− macrophages in the presence of 10 ng/well soluble αCD3 for 4 days, and proliferation was determined by 3H-thymidine incorporation. Proliferation of αCD3-stimulated T cells alone was always less than 1000 cpm. One experiment of 3 is shown. Error bars represent SEM of triplicates.
Inactivation of CD11b/CD18 (Mac-1) on macrophages enhances T-cell proliferation. (A-B) Macrophages from bone marrow of CD18+/+ and CD18−/− mice were generated as described in “Materials in methods.” Then, 5 × 105 macrophages/well were preincubated with 10 μg/mL anti-CD11a, -b, or -c antibodies and placed into 96-well plates that had been coated with 10 μg/mL recombinant ICAM-1–Fc. Cells were incubated for 45 min at 37°C, washed 4 times, and analyzed for bound cells by microscopy as described in “Materials and methods.” One representative of 3 experiments is shown. Error bars represent SEM of triplicates. (C) CD3+ T cells (1 × 105/well) were cocultured with allogeneic macrophages at the indicated T cell–macrophage ratios. Macrophages were left untreated or preincubated with 20 μg/mL anti-CD11b antibody for 2 hours. After 5 days of coculture, 1 μCi (0.037 MBq) of 3H-thymidine/well was added for another 18 hours and T-cell proliferation was assessed. One representative experiment of 3 is shown. Error bars represent SD. (D) CD4+ T cells (1 × 105/well) were cocultured with allogeneic CD18+/+ or CD18−/− macrophages in the presence of 10 ng/well soluble αCD3 for 4 days, and proliferation was determined by 3H-thymidine incorporation. Proliferation of αCD3-stimulated T cells alone was always less than 1000 cpm. One experiment of 3 is shown. Error bars represent SEM of triplicates.
Discussion
We reported previously that β2 integrins had no detectable relevance for DC migration in vivo.42 In accordance with this, we demonstrate here that β2 integrins on DCs are functionally inactive, because they exhibit no ligand-binding activity to either recombinant ICAM-1–Fc or to T cells, even when DCs were stimulated with PMA/ionomycin, LPS, or CD40L (Figure 1). Although abundantly expressed on WT DCs, antigen presentation to T cells is not dependent on β2 integrins (LFA-1, Mac-1, CD11c/CD18), as shown by unaltered T-cell proliferation and cytokine production induced by CD18−/− DCs compared to WT DC (Figure 1). However, divalent cations such as Mg2+ in supraphysiologic concentrations (5 mM) can activate β2 integrins on DCs to bind to ICAM-1–Fc as well as physiologic ligands on T cells. The viability of DCs was not affected by Mg2+ treatment at these concentrations (Figure S3). Thus, β2 integrins on bone marrow DCs are not active per se, but they can be activated by divalent cations. Because each segment of the β2 integrins (α and β chain) contains metal ion-dependent adhesion sites (MIDASs) that are reported to be critical for their ligand binding function,36,48-51 these data were not unexpected.
Surprisingly, however, although contact times of DCs and T cells were prolonged (Figure 2C), the functional consequence of active β2 integrins on DCs was inhibition of T-cell proliferation (Figure 3A). In experiments with CD18−/− T cells and WT DCs we confirmed that active β2 integrins on DCs, and not on T cells, were responsible for inhibited T-cell activation (Figure 3). Additionally, Mg2+ activated CD18+/+ DC, but not Mg2+-treated CD18−/− DCs, inhibited T-cell proliferation substantially in MLR experiments. The same results were obtained using primary spleen DCs as T-cell stimulators (data not shown). We excluded that Mg2+ inhibited T-cell activation directly (Figure S5), because anti-CD3/anti-CD28–induced T-cell proliferation was unaffected (CD18−/−), or even enhanced (CD18+/+), by addition of Mg2+. Furthermore, we excluded nonspecific effects of Mg2+ on the antigen-presenting capacity of DCs, because (1) the expression of the costimulatory molecules CD80 and CD86 on CD11c+ DCs was not altered by the use of Mg2+ (5-20 mM for up to 72 hours), (2) expression of the activation marker CD44 or the β2-integrin CD18 were not altered on Mg2+ treatment of DCs, whereas expression of MHC class II was increased (data not shown), and (3) the effect of Mg2+ on T-cell activation was fully reversed by anti-CD18 antibody treatment. Hence, Mg2+ specifically activates β2 integrins on DCs, which results in impaired T-cell activating capacity. Moreover, regulatory T cells were not expanded by Mg2+-treated DCs (Figure 4A), and CD4+CD25+ regulatory T cells that had been cocultured with Mg2+-treated DCs for 1 week did not exert inhibitory function toward effector T-cell proliferation induced by αCD3/αCD28 (data not shown), although in this allogeneic setting, antigen-specific inhibition by regulatory T cells could not be assayed sufficiently. However, when we removed regulatory T cells from the MLR culture, the inhibitory effect of Mg2+-treated DCs on T-cell proliferation was still present (Figure 4B). Thus, Mg2+-treated DCs neither expand nor activate regulatory T cells.
Antibody-blocking experiments revealed that active Mac-1 was responsible for almost all of the ICAM-1–binding activity of DCs and also for inhibition of T-cell activation (Figure 5). Mac-1 (αMβ2, CD11b/CD18, CR3) is expressed on monocytes, macrophages, DCs, granulocytes, and natural killer cells and has been implicated in diverse responses of these cells, including phagocytosis, cell-mediated killing, chemotaxis, and cellular activation, and consequently, plays a central role in inflammation. The characterization of Mac-1–deficient mice has confirmed these findings by showing that a variety of leukocyte-dependent responses are compromised in these animals.52,53 Mac-1 is the most promiscuous member of the β2-integrin family with more than 30 reported ligands, such as fibrinogen,54,55 complement fragment iC3B,56,57 hookworm neutrophil inhibitory factor,58,59 blood coagulation factor X,60 denatured proteins,61 bacterial and fungal products,62,63 and ICAM-1.64,65 It is unknown how this single integrin recognizes such a vast repertoire of structurally diverse ligands and how such broad ligand-binding specificity of Mac-1 translates into biologic significance. Additionally, there has been no report so far examining the role of Mac-1 for DC function.
Macrophages, on the other hand, express high amounts of Mac-1 on their surface but are weak APCs compared to DCs. When we tested their capacity to bind to ICAM-1, we confirmed that Mac-1 was constitutively active and responsible for most of the ICAM-binding activity of these cells (Figure 6). We thus hypothesized that active Mac-1 could be partially responsible for the relatively low potency of macrophages to induce T-cell activation. Indeed, blocking Mac-1 on macrophages significantly increased their T-cell stimulatory capacity, thus supporting this hypothesis. Recently, it has been reported that T cells from mice that express constitutively active LFA-1 (LFA-1d/d) were impaired in activation in vivo. APCs from LFA-1d/d mice were not examined in this study, but it is the first report to show that constitutively active β2 integrins can exert inhibitory effects in the immune system.66 This study implicates that regulation of β2-integrin activity has to be very strict and tight to not interfere with important mechanisms of immune activation. In support of this hypothesis we clearly demonstrate that β2 integrins on DCs cannot be activated by a variety of stimuli (Figure 1A) and are strictly regulated presumably by an active mechanism. For LFA-1 on human DCs one such mechanism has been described. CYTIP, a cytosolic protein that is expressed in high amounts on maturation of DCs binds the LFA-1–activating protein cytohesin-1 and captures it in the cytosol, thereby keeping LFA-1 on DCs inactive.67 Whether CYTIP additionally controls Mac-1 activity on DCs still needs to be shown. However, a recent study reports that silencing of CYTIP by siRNA induces binding of mDCs to fibronectin,68 which is a ligand for Mac-1.69 Thus, CYTIP may well play a role in regulation of β2-integrin activation in DCs, and experiments in our laboratory are under way to address this issue. Likewise, Kumartsev et al70 recently showed that so called immature myeloid suppressor cells (iMSCs) exert immunosuppressive effects on T cells via induction of reactive oxygen species (ROS) that was dependent on Mac-1 on these cells. Although it still needs to be determined whether iMSCs are related to DCs and whether the inhibitory effect on T cells is based on the same mechanisms as described here, this report clearly supports our observation that active Mac-1 may down-regulate T-cell activation.
An alternative explanation for the observed effects might be that antigen presentation requires the formation of an organized APC–T-cell contact plane, the so-called immunologic synapse (IS). In previous studies, we demonstrated that the physicodynamics of the APC–T-cell contact differ significantly when either DCs, macrophages, or B cells serve as APCs.41,71 At least for DCs, our data suggest that optimal T-cell activation is associated with short, repetitive, and multifocal APC–T-cell contacts, rather than with a stable IS.41,71,72 Thus, insufficient DC–T-cell adhesion, for example, in the case of CD18−/− T cells 14,20,30,41,71 as well as too strong or dysregulated adhesion (as shown here) may both interfere with optimal formation of the IS.
Taken together, this is the first report on the role of β2 integrins on DCs for antigen presentation to T cells. We show that β2 integrins, although abundantly expressed on DCs, are held in an inactive state. On DCs, Mac-1 can be activated by divalent cations and is then the predominant ICAM-1–binding integrin on DCs. Active Mac-1 on DCs or on macrophages exerts inhibitory effects on T-cell activation. Here, we describe a new function of the β2 integrin Mac-1, that is, potentially regulating T-cell activation.
The online version of this manuscript contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
G.V. and S.B. contributed equally to this study.
The authors wish to thank E. Nathkaemper for excellent technical assistance. We also thank B. Roters for performing some of the preliminary experiments.
This work was supported by grants SFB 293/B1 and SFB629/B3 (S.G.) and grant SFB 293/A1 (M.K.W. and D.V.) from the Deutsche Forschungsgemeinschaft (DFG).


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal