Abstract
Dendritic cells (DCs) play a key role in immune homeostasis and maintenance of self-tolerance. Tolerogenic DCs can be established by an encounter with apoptotic cells (ACs) and subsequent inhibition of maturation and effector functions. The receptor(s) and signaling pathway(s) involved in AC-induced inhibition of DCs have yet to be defined. We demonstrate that pretreatment with apoptotic but not necrotic cells inhibits activation of IκB kinase (IKK) and downstream NF-κB. Notably, receptor tyrosine kinase Mer (MerTK) binding of ACs is required for mediating this effect. Monocyte-derived DCs lacking MerTK expression (MerTKKD) or treated with blocking MerTK-specific antibodies (Abs) are resistant to AC-induced inhibition and continue to activate NF-κB and secrete proinflammatory cytokines. Blocking MerTK activation of the phosphatidylinositol 3-kinase (PI3K)/AKT pathway prevents AC-induced inhibition. These results demonstrate an essential role for MerTK-mediated regulation of the PI3K/AKT and NF-κB pathways in AC-induced inhibition of monocyte-derived DCs.
Introduction
Dendritic cells (DCs) are potent mediators of T-cell activation and proinflammatory immune responses to foreign antigens and pathogens.1,2 However, DCs also have an important role in maintaining immune homeostasis and tolerance to self-proteins.3-7 These 2 opposing functions are believed in part to reflect differences in DC activation, maturation, and/or subset. Tolerogenic DCs typically exhibit an immature phenotype characterized by low cell-surface expression of MHC and costimulatory molecules and do not secrete proinflammatory cytokines. Furthermore, soluble and cellular mediators that inhibit DC activation and maturation can establish a tolerogenic phenotype. For example, binding to and phagocytosis of apoptotic cells (ACs) by immature DCs inhibits activation and maturation induced by various stimuli.8,9 This inhibitory effect serves an important role because ACs are present in tissues under both homeostatic and inflamed conditions and provide a potential source of self-proteins to mediate autoimmunity. Defective clearance of ACs has been linked to different types of autoimmunity.10,11 A number of receptors expressed by immature DCs such as the phosphatidylserine (PS) receptor, CD36, αvβ5 integrin, and complement receptor C1qR are involved in AC binding and/or ingestion.12-15 However, the relative contribution of these receptors in mediating the immunoregulatory effect(s) of ACs on immature DCs is unclear, and the molecular basis for this inhibition has not been defined in DCs.
Recently, the Axl/Mer/Tyro3 receptor tyrosine kinase (RTK) family has been implicated in homeostatic regulation of antigen-presenting cell (APC) activation.16,17 This family, consisting of Axl, Tyro3, and MerTK, is expressed by a variety of cell types, including macrophages (Mφs) and DCs. Mice lacking expression of all 3 RTKs exhibit hyperactivated Mφs and DCs, which in turn drive lymphoproliferation and systemic autoimmunity.16 Similarly, our group has shown that mice lacking MerTK expression (MerTKKD) develop lupuslike autoimmunity and are more prone to lipopolysaccharide (LPS)–induced endotoxic shock.18-20 Autoimmunity in MerTKKD mice correlates with a reduced rate of in vivo clearance of ACs, which is consistent with findings that MerTK mediates AC phagocytosis by Mφs.19,20 A ligand for MerTK is growth arrest–specific gene 6 (GAS6), which binds to PS expressed on the inverted plasma membrane of ACs.21 Recognition of a GAS6-PS complex facilitates binding of ACs and subsequent phagocytosis by Mφs. Accordingly, MerTK has been proposed to facilitate phagocytosis of ACs and down-regulate activation in Mφs.17-20 Whether MerTK functions similarly in DCs has yet to be determined.
We and others22-27 have demonstrated a key role for the transcription factor NF-κB in regulating gene expression associated with the development, activation, maturation, and APC function of DCs. The NF-κB complex consists of homodimers and heterodimers of the structurally related proteins p50, p52, p65 (RelA), c-Rel, and RelB. NF-κB is typically sequestered in the cytoplasm bound by the inhibitory molecules IκBα, IκBβ, and IκBϵ.28-30 In response to a broad range of stimuli, including LPS and CD40 engagement, the multisubunit complex IκB kinase (IKK) consisting of IKK1/IKKα, IKK2/IKKβ, and IKKγ/NEMO is activated upon phosphorylation.31-34 Activated IKK phosphorylates the IκB proteins, which in turn undergo polyubiquitination and subsequent degradation via the 26S proteosome.29,30 The latter permits nuclear translocation of NF-κB that binds to consensus sequences and induces gene transcription. We recently demonstrated that the immunosuppressive effect of IL-10 on DC maturation and APC function is mediated by inhibition of IKK activity and downstream NF-κB activation,35 further arguing that the NF-κB pathway is a key target for immunoregulation of DCs. In addition, IL-10–induced inhibition of DCs was dependent on suppression of the phosphatidylinositol 3-kinase (PI3K)/AKT signaling pathway. Studies have shown that NF-κB activation can be regulated by the PI3K/AKT pathway via different mechanisms.36-39
The current study was initiated to define the molecular basis of AC-induced inhibition of DC activation and effector function. In view of observations indicating that MerTK is involved in AC engulfment by Mφs and may also negatively regulate DC activation, we investigated a role for MerTK in AC-mediated inhibition of DCs. Evidence is provided that ACs inhibit activation of the NF-κB signaling pathway in DCs and that MerTK via PI3K/AKT signaling serves a major role in mediating this immunoregulatory effect.
Materials and methods
Mice
Nonobese diabetic (NOD)/LtJ, BALB/c, and C57BL/6 (B6) mice were maintained and bred under specific-pathogen free conditions. Establishment of MerTKKD mice has been described.18 Briefly, the tyrosine kinase domain of Mertk was replaced with a neomycin resistance gene, and B6.MerTKKD mice were established. NOD MerTKKD mice were generated by breeding B6.MerTKKD and NOD mice and then backcrossing the MertkKD gene onto the NOD genome for an additional 11 generations. At N11, Mouse MapPairs (Invitrogen, Carlsbad, CA) distinguishing B6, 129/Ola, and NOD/LtJ chromosome 2 (D2Mit378, D2Mit94, D2Mit14, D2Mit393, D2Mit395, D2Mit190, D2Mit164, D2Mit256, D2Mit304, D2Mit224, D2Mit338, D2Mit307, D2Mit260, D2Mit309, D2Mit493, D2Mit451, D2Mit496, D2Mit287, D2Mit456, D2Mit265) were used in polymerase chain reaction (PCR) according to the supplier's directions to define a 17-cM segment derived from 129/Ola and containing MertkKD. Use of mice was approved by the Institutional Animal Care and Use Committee of the University of North Carolina at Chapel Hill.
Preparation of DCs
Bone marrow–derived DCs (BMDCs) and splenic DCs (sDCs) were prepared from male or female mice between 8 to 12 weeks of age as described.35
Flow cytometry
The following monoclonal antibodies (Abs) used for fluorescence staining were purchased from BD PharMingen (San Diego, CA): FITC-αCD40, FITC-αCD86, FITC-αCD80, PE-αCD11c, PE-αH2Kd, and PE-αCD11b. PE-αmouse IgG, FITC-αmouse IgG, and streptavidin-PE were also purchased from BD PharMingen. Polyclonal goat-αMerTK and normal goat IgG were purchased from R&D Systems (Minneapolis, MN), and biotin-αgoat IgG was purchased from Vector (Burlingame, CA). Stained cells were analyzed on a FACSCalibur (BD Biosciences, San Jose, CA) using Summit Software (Cytomation, Ft Collins, CO).
DC pretreatment with ACs, necrotic cells, PI3K, or protein synthesis inhibitors
For the respective experiments, DCs were cultured in RPMI 1640 supplemented with 10% heat-inactivated fetal bovine serum, 50 μM 2-ME, 1× nonessential amino acids, 1 mM sodium pyruvate, 2 mM l-glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin. To prepare ACs, thymocytes isolated from 4- to 6-week-old mice were adhered to plastic for 2 hours to remove APCs, irradiated at 600 Gy, and then cultured in complete RPMI medium for 12 hours. Flow cytometry demonstrated more than 95% apoptotic and less than 1% necrotic thymocytes based on annexin V and propidium iodide staining. Apoptosis was confirmed via DNA fragmentation analysis. DCs were cocultured with ACs at a ratio of 1:5 (DC/AC) for indicated times. For necrotic cell preparations, thymocytes were frozen at −80°C, thawed for 4 cycles, and then cocultured with DCs at a 5:1 ratio (necrotic cell–DC equivalence) for 3 hours. Following pretreatment with ACs or necrotic cells, DCs were resuspended accordingly and stimulated with LPS.
In some experiments, DCs were treated with αMerTK Ab prior to AC incubation. Briefly, DCs (5 × 106 per well) were incubated with αmouse FcγIII/II (BD PharMingen) in 6-well ultralow cluster plates for 0.5 hours at 37°C to block Fc receptor binding. DCs were then treated for 1 hour at 37°C with 20 μg/mL of either goat αMerTK Ab (AF591; R&D Systems) or goat IgG (R&D Systems), an isotype control.
Alternatively, DCs (5 × 106 cells per well) were treated with wortmannin (Wort) (200 nM) or Ly294002 (LY) (50 μM) (Cell Signaling Technology, Beverly, MA) for 1 hour prior to AC treatment or LPS stimulation as described.35 Finally, DCs (5 × 106 cells per well) were pretreated for 0.5 hours with either cyclohexamide (10 μg/mL) or Ebulin 1 (50 ng/mL) prior to AC treatment.
EMSA and Western blotting
Nuclear and cytoplasmic extracts were prepared from DCs as described.40 Electrophoretic mobility shift assay (EMSA) was performed using 32P-labeled DNA probes containing NF-κB binding sites derived from MHC class I H2K promoter: 5′-CAGGGCTGGGGATTCCCCATCTCCACAGTTTCACTTC-3′.25 A double-stranded OCT-1 DNA probe, 5′-TGTCGAATGCAAATCACTAGAA-3′, was used as control. Bands were visualized using a phosphoimager (Molecular Dynamics, Sunnyvale, CA). For each treatment at least 3 EMSAs were run.
Western blotting was carried out as described.35 Membranes were probed with Abs specific for IκBα, IκBβ, IκBϵ, IKK1, ERK1, and ERK2 (Santa Cruz Biotechnology, Santa Cruz, CA); IKK2, pIκBα, pIKK1 (Ser180)/pIKK2 (Ser181), pAKT (Thr308), AKT, pSAPK/JNK (Thr183/Tyr185), SAPK/JNK, phospho-p38MAPK (Thr180/Tyr182), p38MAPK, pERK1/pERK2 (Thr202/Tyr204), and phosphotyrosine (Cell Signaling Technology); MerTK (R&D Systems); and β-actin (Sigma, St Louis, MO). Binding of secondary HRP-labeled goat αrabbit, donkey αgoat, or goat αmouse Abs (Santa Cruz Biotechnology) was analyzed using SuperSignal West Pico or West Dura Chemiluminescent Substrate (Pierce, Rockland, IL).
IKK assay
IKK signalosome was immunoprecipitated from 700 μg of a whole DC lysate using protein G–agarose beads (Upstate Biotechnology, Lake Placid, NY) and rabbit polyclonal αIKK1 per the manufacturer's instruction. In vitro kinase reaction was performed by incubating the IKK signalosome-bead complex with IκBα-GST and 20 μL of a magnesium/ATP cocktail (Upstate Biotechnology) for 60 minutes at 30°C in kinase buffer. Supernatants were collected and kinase activity determined by measuring phosphorylation of the IκBα-GST substrate via immunoblot probed with αpIκBα Ab.
AKT kinase assay
DCs (5 × 106) were stimulated as described in “EMSA and Western blotting,” and whole cell lysates prepared. AKT kinase activity was determined using an AKT kinase assay kit (Cell Signaling Technology). Briefly, AKT was immunoprecipitated according to the manufacturer's instructions, and kinase activity was assessed by measuring phosphorylation of a GSK3 substrate via immunoblot using αpGSK3 Ab.
Immunoprecipitation of MerTK
Whole cell lysates prepared from DCs (5 × 106) were precleared with protein G–Sepharose beads, and MerTK was immunopreciptated using protein G–Sepharose beads precoated with αMerTK Ab. For “pull-down” experiments, DCs (107) were treated with ACs (1:5) for varying times, chilled on ice, resuspended in 1 mL of the cell-permeable protein crosslinker dimethyl 3,3′-dithiopropionimidate dihydrochloride (Sigma) in PBS (2 mg/mL), and incubated at room tempertature for 20 minutes. A whole cell lysate was prepared and MerTK immunoprecipitated with αMerTK Ab bound protein G–Sepharose beads. In some experiments, Western blots were probed with Ab specific for the PI3K subunits p85α and p110α (Cell Signaling Technology) and p110β and p110δ (Santa Cruz Biotechnology), or Axl- (R&D Systems) and Tyro3- (BD PharMingen) specific Ab.
Measurement of TNFα production from DCs
DCs (9 × 105 per well) were pretreated with or without ACs for 3 hours, washed, and then stimulated with LPS for 48 hours. Supernatants were collected and assayed for TNFα in triplicate using an ELISA kit (BD PharMingen) following the manufacturer's instructions.
Results
NF-κB activation is inhibited in DCs by AC pretreatment
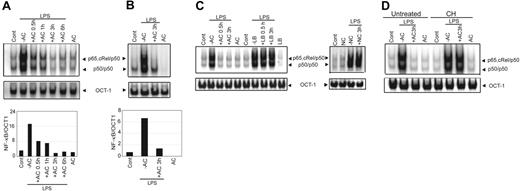
Binding of ACs by immature DCs impairs subsequent activation, maturation, and APC function.8,9 Because these events are largely regulated by NF-κB, the effect of ACs on the NF-κB signaling pathway was investigated. For this purpose, NOD BMDCs, which are CD11b+CD8α− and exhibit an immature phenotype (CD40lo, CD80lo, CD86lo),25 were examined. BMDCs were pretreated at a ratio of 1:5 with ACs for varying times, stimulated with LPS (50 ng/mL) for 0.5 hours, and nuclear NF-κB DNA binding activity was assessed via EMSA. BMDCs treated only with LPS exhibited a 5.7-fold increase in NF-κB DNA binding relative to untreated BMDCs (Figure 1A). In contrast, AC pretreatment resulted in a temporal loss of LPS-stimulated NF-κB DNA binding, which was completely inhibited after a 3-hour incubation with ACs (Figure 1A). No effect on DNA binding of OCT-1 was detected, indicating that the inhibitory effect of ACs was NF-κB specific (Figure 1A). Titrating ACs showed that 1:5 BMDC/AC was the lowest ratio at which maximum suppression of NF-κB activity induced by LPS (50 and 500 ng/mL) was observed (data not shown). This ratio was used for all subsequent experiments. Analogous to BMDCs, a reduction (about 5-fold) in LPS-stimulated nuclear NF-κB activity was detected in NOD DCs prepared from the spleen (sDCs) and pretreated with ACs (Figure 1B). Inhibition of NF-κB activity was specific for ACs. For example, LPS-stimulated NF-κB DNA binding was unaffected by pretreatment of BMDCs with either necrotic cells or polystyrene latex beads (Figure 1C). Furthermore, de novo protein synthesis was required for AC-induced inhibition. Despite AC pretreatment, LPS-stimulated NF-κB activation was readily detected in BMDCs incubated with 10 μg/mL cyclohexamide (Figure 1D). Similar results were obtained with the protein synthesis inhibitor Eublin 1 (Figure S1, available on the Blood website; see the Supplemental Figures link at the top of the online article).
Pretreatment of DCs with ACs inhibits DNA binding activity of NF-κB. NOD BMDCs (A) or sDCs (B) were pretreated with ACs at a ratio of 1:5 (DC/AC) for the indicated times or left untreated and then were stimulated with LPS (50 ng/mL) for 0.5 hours. Nuclear NF-κB DNA binding was measured via EMSA. Composition of NF-κB complexes was previously determined via supershift analysis. A double-stranded OCT-1 DNA probe was used as an internal control. Densitometric analyses represent the ratio of intensity of NF-κB to OCT-1 binding per unit area and are represented as arbitrary units for the respective EMSAs. (C) Nuclear NF-κB binding activity was measured in NOD BMDCs pretreated with ACs, polystyrene latex beads (LB), or necrotic cells (NC) for specified times or left untreated and then were stimulated with LPS for 0.5 hours. (D) NOD BMDCs were preincubated with cyclohexamide (10 μg/mL) (CH) for 15 minutes, treated with ACs for 3 hours, stimulated with LPS (50 ng/mL) for 0.5 hours, and nuclear NF-κB DNA binding activity was determined. For this and all other figures, the control lane AC represents data obtained with DCs plus ACs without stimulation (eg, LPS). Data are representative of 3 independent experiments.
Pretreatment of DCs with ACs inhibits DNA binding activity of NF-κB. NOD BMDCs (A) or sDCs (B) were pretreated with ACs at a ratio of 1:5 (DC/AC) for the indicated times or left untreated and then were stimulated with LPS (50 ng/mL) for 0.5 hours. Nuclear NF-κB DNA binding was measured via EMSA. Composition of NF-κB complexes was previously determined via supershift analysis. A double-stranded OCT-1 DNA probe was used as an internal control. Densitometric analyses represent the ratio of intensity of NF-κB to OCT-1 binding per unit area and are represented as arbitrary units for the respective EMSAs. (C) Nuclear NF-κB binding activity was measured in NOD BMDCs pretreated with ACs, polystyrene latex beads (LB), or necrotic cells (NC) for specified times or left untreated and then were stimulated with LPS for 0.5 hours. (D) NOD BMDCs were preincubated with cyclohexamide (10 μg/mL) (CH) for 15 minutes, treated with ACs for 3 hours, stimulated with LPS (50 ng/mL) for 0.5 hours, and nuclear NF-κB DNA binding activity was determined. For this and all other figures, the control lane AC represents data obtained with DCs plus ACs without stimulation (eg, LPS). Data are representative of 3 independent experiments.
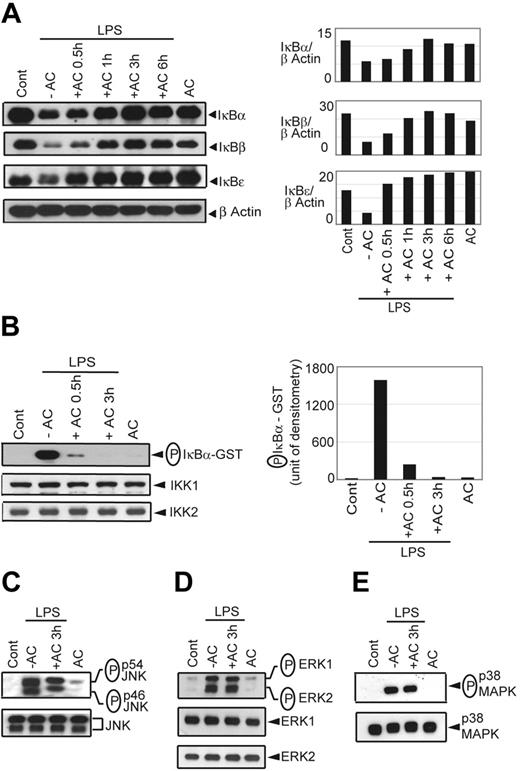
In agreement with the EMSA data, LPS-stimulated degradation of IκBα, IκBβ, and IκBϵ in BMDCs was progressively inhibited with increasing times of AC pretreatment (Figure 2A). Furthermore, inhibition of LPS-induced IκB degradation by AC pretreatment coincided with a reduction in IκBα phosphorylation (Figure S2A). IκBα degradation stimulated by LPS was also inhibited in sDCs pretreated with ACs for 3 hours (Figure S2B).
AC pretreatment inhibits IκB protein degradation and IKK activity. NOD BMDCs were pretreated with ACs and stimulated with LPS as in Figure 1. (A) Cytoplasmic IκBα, IκBβ, IκBϵ, and β-actin protein were detected by Western blot using the same blot. Densitometric readings represent the ratio of intensity of IκB protein to β-actin expression per unit area and are represented as an arbitrary unit. (B) In vitro IKK activity was determined by measuring phosphorylation of an IκBα-GST substrate. Densitometric analysis represents the intensity of phosphorylated (P) IκBα-GST in arbitrary units. The amount of IKK1 and IKK2 immunoprecipitated in the samples was analyzed by Western blot. Western blot was used to detect (C) phospho-JNK versus JNK, (D) phospho-ERK versus ERK, and (E) phospho-p38MAPK versus p38MAPK in whole cell lysates. Data are representative of 3 independent experiments.
AC pretreatment inhibits IκB protein degradation and IKK activity. NOD BMDCs were pretreated with ACs and stimulated with LPS as in Figure 1. (A) Cytoplasmic IκBα, IκBβ, IκBϵ, and β-actin protein were detected by Western blot using the same blot. Densitometric readings represent the ratio of intensity of IκB protein to β-actin expression per unit area and are represented as an arbitrary unit. (B) In vitro IKK activity was determined by measuring phosphorylation of an IκBα-GST substrate. Densitometric analysis represents the intensity of phosphorylated (P) IκBα-GST in arbitrary units. The amount of IKK1 and IKK2 immunoprecipitated in the samples was analyzed by Western blot. Western blot was used to detect (C) phospho-JNK versus JNK, (D) phospho-ERK versus ERK, and (E) phospho-p38MAPK versus p38MAPK in whole cell lysates. Data are representative of 3 independent experiments.
The lack of LPS-induced IκB degradation suggested that upstream IKK activity was inhibited by AC pretreatment. To test this possibility, BMDCs were pretreated with ACs for 0.5 or 3 hours and stimulated with LPS for 15 minutes. Temporal analyses demonstrated that maximum IKK activity in BMDCs was detected after a 15-minute incubation with LPS (data not shown). Cytoplasmic extracts were prepared, the IKK signalosome immunoprecipitated, and kinase activity of the complex determined by measuring phosphorylation of an IκBα-GST substrate in vitro. Phosphorylation of the IκBα-GST substrate was readily detected in LPS-stimulated versus untreated BMDCs (Figure 2B). In comparison, when LPS-stimulated BMDCs where pretreated with ACs for 0.5 and 3 hours, phosphorylation of the IκBα-GST substrate was reduced 6.8-fold and 54-fold, respectively (Figure 2B). No significant difference was detected in the amount of IKK1 and IKK2 protein in the respective BMDC lysates (Figure 2B), indicating that the lack of in vitro phosphorylation of the IκBα-GST substrate was due to reduced IKK activity and not inefficient immunoprecipitation of the IKK complex.
AC pretreatment of DCs had no significant effect on LPS-stimulated activation of the mitogen-activated protein kinase (MAPK) pathway. LPS-stimulated phosphorylation of JNK, ERK1/2, and p38 MAPK was unaffected by AC pretreatment (Figure 2C-E). These data demonstrate that AC pretreatment of either BMDCs or sDCs results in inhibition of IKK signalosome activation, downstream phosphorylation and degradation of the IκB proteins, and NF-κB DNA binding activity induced by LPS stimulation. Furthermore, AC pretreatment selectively inhibits LPS-induced NF-κB signaling.
MerTK is required to mediate AC-induced inhibition of NF-κB activation in DCs
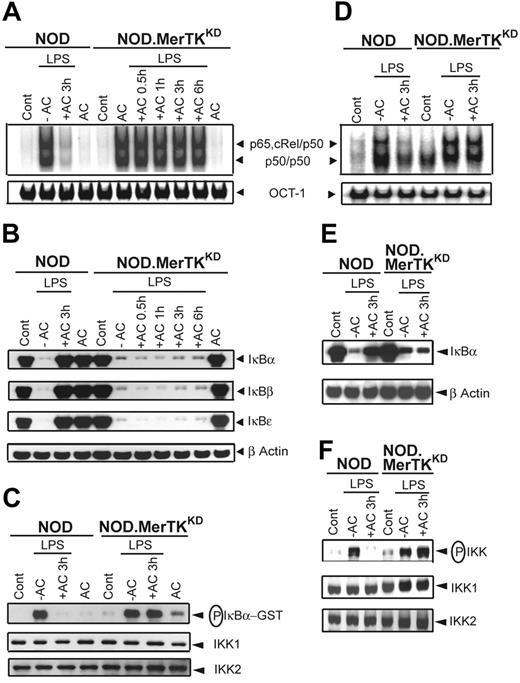
MerTK is necessary for efficient phagocytosis of ACs by Mφs and is associated with down-regulation of Mφ activation.16,18,19,41,42 Whether MerTK mediates AC-induced inhibition of NF-κB activity in DCs was therefore investigated. DCs that lack MerTK expression were prepared from NOD mice homozygous for the MertkKD null mutation (NOD.MerTKKD) and the effect of AC pretreatment on LPS-stimulated NF-κB activation was determined. Whereas NF-κB DNA binding was inhibited in NOD BMDCs, pretreatment with ACs had no significant effect on the induction of NF-κB activity in NOD.MerTKKD BMDCs stimulated with LPS (Figure 3A). LPS-induced NF-κB DNA binding was also unaffected by AC pretreatment in NOD.MerTKKD sDCs (Figure 3D).
Pretreatment with ACs inhibits NF-κB and IKK activation in NOD but not NOD.MerTKKD DCs. NOD and NOD.MerTKKD BMDCs (A-C) or sDCs (D-F) were pretreated with ACs and stimulated with LPS as in Figure 1. (A,D) DNA binding activity of nuclear NF-κB or OCT-1 was determined via EMSA. (B,E) Cytoplasmic IκBα, IκBβ, IκBϵ, and β-actin protein were detected via Western blot using the same blot. (C) In vitro IKK activity and IKK1 and IKK2 protein expression were determined as in Figure 2. (F) IKK phosphorylation was detected via Western blot and the same blot reprobed for IKK1 and IKK2 protein. Data are representative of 3 independent experiments.
Pretreatment with ACs inhibits NF-κB and IKK activation in NOD but not NOD.MerTKKD DCs. NOD and NOD.MerTKKD BMDCs (A-C) or sDCs (D-F) were pretreated with ACs and stimulated with LPS as in Figure 1. (A,D) DNA binding activity of nuclear NF-κB or OCT-1 was determined via EMSA. (B,E) Cytoplasmic IκBα, IκBβ, IκBϵ, and β-actin protein were detected via Western blot using the same blot. (C) In vitro IKK activity and IKK1 and IKK2 protein expression were determined as in Figure 2. (F) IKK phosphorylation was detected via Western blot and the same blot reprobed for IKK1 and IKK2 protein. Data are representative of 3 independent experiments.
Analyses of IκB protein degradation and IKK activity confirmed that ACs failed to inhibit NF-κB activity in NOD.MerTKKD DCs. Degradation of the IκB proteins was observed in NOD.MerTKKD but not NOD BMDCs or sDCs pretreated with ACs and stimulated with LPS (Figure 3B,E). Furthermore, pretreatment with ACs failed to inhibit in vitro IKK activity in lysates prepared from NOD.MerTKKD but not NOD BMDCs (Figure 3C). Similarly, LPS-stimulated IKK activation as measured by phosphorylation of IKK1 and IKK2 was unaffected by AC pretreatment in NOD.MerTKKD sDCs but was significantly reduced in NOD sDCs (Figure 3F). Moreover, ACs failed to inhibit NF-κB DNA binding and phosphorylation of IKK in LPS-stimulated BMDCs prepared from B6 mice lacking MerTK (B6.MerTKKD) (Figure S3), demonstrating that the inability of ACs to block NF-κB activation in NOD.MerTKKD DCs was not intrinsic to the NOD genotype.
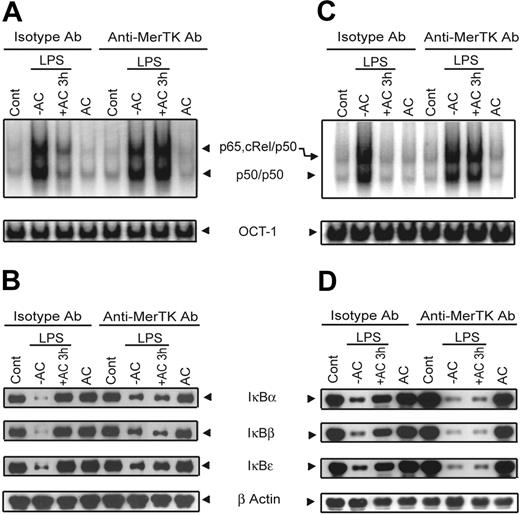
To confirm the role of MerTK in AC-induced inhibition of NF-κB activity, the effect of treating wild-type DCs with a blocking αMerTK Ab was investigated. BMDCs were treated with either αMerTK or isotype control Ab, incubated with ACs for 3 hours, and then stimulated with LPS. LPS-induced NF-κB DNA binding was efficiently inhibited in BMDCs treated with the isotype control Ab and ACs (Figure 4A). In contrast, LPS-induced NF-κB DNA binding was readily detected in BMDCs treated with the αMerTK Ab despite AC pretreatment (Figure 4A). Furthermore, ACs failed to block LPS-induced degradation of the IκB proteins in DCs treated with αMerTK but not isotype control Ab (Figure 4B). The effect of MerTK Ab blocking on AC-induced inhibition of NF-κB signaling was also detected in BMDCs cultured from BALB/c. Whereas ACs inhibited LPS-induced NF-κB DNA binding and IκB protein degradation in BALB/c BMDCs treated with the isotype control Ab, αMerTK Ab blocked this effect (Figure 4C-D). Collectively, these results demonstrate that MerTK signaling is necessary to mediate AC-induced inhibition of the NF-κB pathway independently of the genotype of the DCs.
αMerTK Ab blocks AC-mediated inhibition of NF-κB activation in NOD and BALB/c DCs. NOD (A-B) or BALB/c (C-D) BMDCs were pretreated with 20 μg/mL of αMerTK or isotype control Ab. BMDCs were then treated with ACs and stimulated with LPS as in Figure 1. (A,C) EMSA was used to measure DNA binding activity of nuclear NF-κB or OCT-1. (B,D) Cytoplasmic IκBα, IκBβ, IκBϵ, and β-actin were detected via Western blot with the same blot. Data are representative of 3 independent experiments.
αMerTK Ab blocks AC-mediated inhibition of NF-κB activation in NOD and BALB/c DCs. NOD (A-B) or BALB/c (C-D) BMDCs were pretreated with 20 μg/mL of αMerTK or isotype control Ab. BMDCs were then treated with ACs and stimulated with LPS as in Figure 1. (A,C) EMSA was used to measure DNA binding activity of nuclear NF-κB or OCT-1. (B,D) Cytoplasmic IκBα, IκBβ, IκBϵ, and β-actin were detected via Western blot with the same blot. Data are representative of 3 independent experiments.
AC-induced inhibition of NF-κB activation is mediated through the PI3K/AKT pathway in DCs
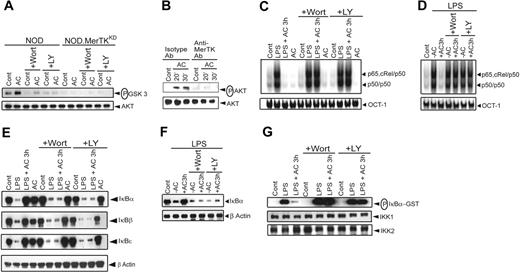
Signaling via a MerTK chimeric molecule or MerTK activates the PI3K/AKT pathway in 32D transfectant cells and retinal pigment epithelial cells, respectively.43,44 In addition, the PI3K/AKT pathway negatively regulates NF-κB activation in human monocytes.37 Accordingly, a role for the PI3K/AKT pathway in mediating AC-induced inhibition of NF-κB activation was assessed. Initially, AKT kinase activity was measured in lysates prepared from NOD BMDCs treated with ACs. AC treatment induced a 3-fold increase in AKT kinase activity relative to untreated NOD BMDCs (Figure 5A). Furthermore, pretreatment of DCs with the PI3K inhibitors Wort or LY for 1 hour effectively blocked AC-induced AKT kinase activity (Figure 5A). Importantly, AC treatment failed to induce AKT kinase activity in NOD.MerTKKD BMDCs (Figure 5A). In addition, phosphorylation of AKT induced by ACs (Figure 5B) was blocked by αMerTK but not isotype Ab treatment.
The PI3K/AKT pathway mediates AC-induced inhibition of NF-κB activation in NOD DCs. NOD or NOD.MerTKKD BMDCs (A-C,E,G) and sDCs (D,F) were incubated with 200 nM Wort or 50 μM LY for 1 hour and then treated with ACs for 3 hours or left untreated. In some experiments (C-G), DCs were subsequently stimulated with LPS (50 ng/mL) for 0.5 hours. (A) In vitro AKT activity was determined by measuring phosphorylation of a GSK3 substrate by Western blot. The same blot was reprobed for AKT protein. (B) NOD BMDCs were pretreated either with isotype control or αMerTK Ab for 1 hour and then incubated with ACs at specified times. Phosphorylation of AKT in cytoplasmic extracts was determined via Western blot using an αphospho AKT Ab. The same blot was reprobed for AKT protein. (C-D) Nuclear NF-κB or OCT-1 DNA binding activity was determined via EMSA. (E-F) Cytoplasmic IκBs and β-actin protein were detected by Western blot using the same blot. (G) In vitro IKK activity was measured as in Figure 2. The same blot was reprobed for IKK1 and IKK2 protein. Data are representative of 3 independent experiments.
The PI3K/AKT pathway mediates AC-induced inhibition of NF-κB activation in NOD DCs. NOD or NOD.MerTKKD BMDCs (A-C,E,G) and sDCs (D,F) were incubated with 200 nM Wort or 50 μM LY for 1 hour and then treated with ACs for 3 hours or left untreated. In some experiments (C-G), DCs were subsequently stimulated with LPS (50 ng/mL) for 0.5 hours. (A) In vitro AKT activity was determined by measuring phosphorylation of a GSK3 substrate by Western blot. The same blot was reprobed for AKT protein. (B) NOD BMDCs were pretreated either with isotype control or αMerTK Ab for 1 hour and then incubated with ACs at specified times. Phosphorylation of AKT in cytoplasmic extracts was determined via Western blot using an αphospho AKT Ab. The same blot was reprobed for AKT protein. (C-D) Nuclear NF-κB or OCT-1 DNA binding activity was determined via EMSA. (E-F) Cytoplasmic IκBs and β-actin protein were detected by Western blot using the same blot. (G) In vitro IKK activity was measured as in Figure 2. The same blot was reprobed for IKK1 and IKK2 protein. Data are representative of 3 independent experiments.
Next, the effect of Wort or LY pretreatment on AC-induced inhibition of NF-κB activation was determined. Whereas ACs blocked LPS-stimulated nuclear NF-κB DNA binding, NF-κB activation was readily detected in NOD BMDCs pretreated with Wort or LY and then incubated with ACs (Figure 5C). In addition, ACs failed to inhibit LPS-stimulated IκB protein degradation (Figure 5E) and IKK activity (Figure 5G) in NOD BMDCs treated with Wort or LY. The 2 PI3K inhibitors similarly prevented AC-induced inhibition of nuclear NF-κB DNA binding activity and degradation of the IκBs in NOD sDCs (Figure 5D,F) or BALB/c BMDCs (Figure S4) stimulated with LPS.
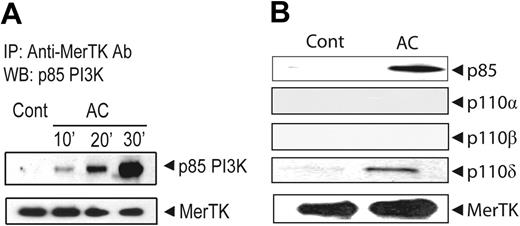
The intracytoplasmic domain of MerTK contains a PI3K binding motif (YDIM). To determine whether PI3K is directly associated with MerTK, NOD BMDCs were treated with ACs and proteins chemically crosslinked. MerTK was then immunoprecipitated and the resulting complexes analyzed via Western blot. MerTK but not p85α/PI3K was detected in untreated NOD BMDCs (Figure 6A). In contrast, a temporal increase in p85α/PI3K was seen in AC-treated NOD BMDCs, with no significant change in the level of MerTK (Figure 6A). Furthermore, the PI3K catalytic subunit p110δ, but not p110α or p110β, was found complexed with immunoprecipitated MerTK (Figure 6B). These results demonstrate that AC-induced inhibition of NF-κB signaling is dependent on MerTK activation of the PI3K/AKT pathway.
A PI3K p85α/p110δ complex is associated with MerTK upon AC pretreatment of NOD BMDCs. MerTK was immunoprecipitated with αMerTK Ab from total cell lysates prepared from NOD BMDCs pretreated with ACs at specified times (A) or for 30 minutes (B) or not (Cont). Western blots were probed for p85α/PI3K, MerTK (A-B), and p110α, p110β, and p110δ (B) using the same membranes.
A PI3K p85α/p110δ complex is associated with MerTK upon AC pretreatment of NOD BMDCs. MerTK was immunoprecipitated with αMerTK Ab from total cell lysates prepared from NOD BMDCs pretreated with ACs at specified times (A) or for 30 minutes (B) or not (Cont). Western blots were probed for p85α/PI3K, MerTK (A-B), and p110α, p110β, and p110δ (B) using the same membranes.
AC-induced inhibition of DC maturation is mediated by MerTK and PI3K/AKT signaling
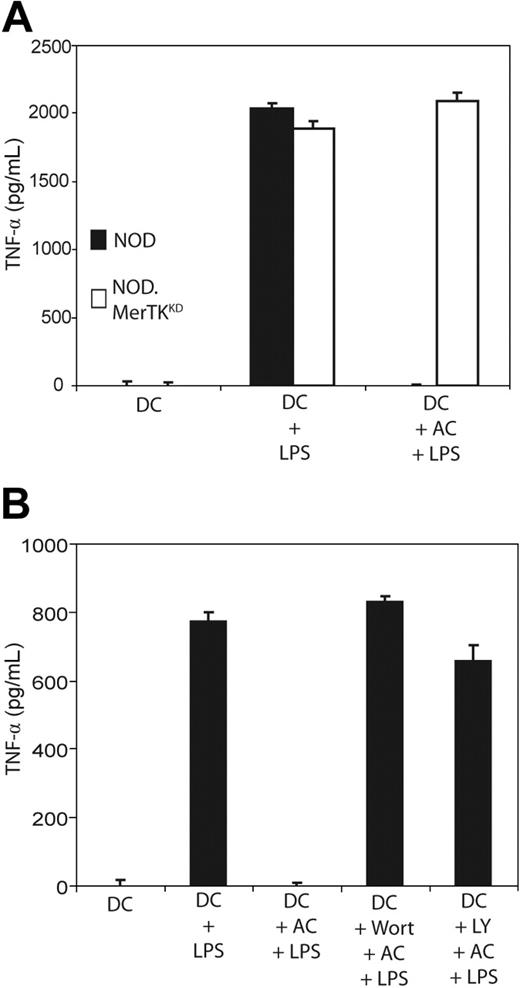
The role of MerTK and PI3K/AKT signaling in AC-induced inhibition of DC maturation, as determined by TNFα secretion, was investigated. Initially, NOD and NOD.MerTKKD BMDCs were compared. As demonstrated in Figure 7A, NOD and NOD.MerTKKD BMDCs secreted similar levels of TNFα upon LPS stimulation. However, AC pretreatment significantly inhibited LPS-stimulated TNFα secretion by NOD BMDCs (P < 10−3), whereas NOD.MerTKKD BMDCs continued to produce TNFα (Figure 7A). Secretion of TGFβ and IL-10 was not detected in either NOD or NOD.MerTKKD BMDCs following pretreatment with ACs (data not shown). Furthermore, LPS-stimulated TNFα secretion was significantly increased in cultures of NOD BMDCs pretreated with Wort or LY plus ACs relative to cultures treated with ACs alone (P < 10−3) (Figure 7B). LPS-stimulated IL-12p70 secretion was similarly affected under these conditions (M.A.W. and R.M.T., unpublished results, February 2006). These data demonstrate that AC inhibition of DC maturation is mediated by MerTK and PI3K/AKT signaling.
AC-induced inhibition of TNFα secretion by NOD DCs is mediated by MerTK-dependent PI3K signaling. (A) NOD and NOD.MerTKKD BMDCs were pretreated with ACs for 3 hours, stimulated with LPS as in Figure 1, and TNFα secretion was measured via ELISA. (B) NOD BMDCs were incubated with 200 nM Wort or 50 μM LY for 1 hour, treated with ACs and LPS, and TNFα secretion was measured as above. Error bars indicate SE.
AC-induced inhibition of TNFα secretion by NOD DCs is mediated by MerTK-dependent PI3K signaling. (A) NOD and NOD.MerTKKD BMDCs were pretreated with ACs for 3 hours, stimulated with LPS as in Figure 1, and TNFα secretion was measured via ELISA. (B) NOD BMDCs were incubated with 200 nM Wort or 50 μM LY for 1 hour, treated with ACs and LPS, and TNFα secretion was measured as above. Error bars indicate SE.
Discussion
DCs are key contributors to the establishment and maintenance of peripheral tolerance to self.3-7 Furthermore, ACs are believed to have an important role in establishing “tolerogenic” DCs in vivo.4,5 The latter is supported by reports demonstrating the potent inhibitory properties of ACs on DCs in vitro.8,9,45 However, the molecular basis for AC-induced inhibition of DCs is ill defined. Here we have identified critical signaling pathways targeted in DCs upon binding of ACs, and the key receptor mediating this inhibitory effect is MerTK.
Pretreatment with ACs blocked activation of the NF-κB signaling pathway in both BMDCs and sDCs. This effect was marked by inhibition of LPS-induced IKK activation as measured by in vitro kinase activity or phosphorylation of IKK1 and IKK2 (Figures 2–3). Consistent with inhibition of the IKK complex was the lack of downstream IκB protein phosphorylation and degradation, and NF-κB nuclear translocation and DNA binding following LPS stimulation of DCs pretreated with ACs (Figures 1,Figure 2–3). In addition, NF-κB activation induced by CD40 crosslinking was inhibited (Figure S5), indicating that ACs block multiple pathways that engage NF-κB. Blockade of NF-κB signaling was seen only by AC pretreatment. Incubation with necrotic cells or phagocytosis of latex beads had no significant effect on LPS-induced NF-κB activation (Figure 1C). Furthermore, the inhibitory effect of ACs showed selectivity among LPS-induced pathways—in this case the activation of NF-κB. For example, LPS-stimulated phosphorylation of the MAPK molecules JNK, ERK1/2, and p38 MAPK was unaltered by AC pretreatment (Figure 2C-E). This observation further supports the hypothesis that blockade of NF-κB activation is essential for promoting AC-mediated DC suppression. Indeed, the effects of ACs can be mimicked by gene transfer of a modified IκBα recombinant that specifically inhibits NF-κB activation and alone is sufficient to prevent up-regulation of costimulatory molecule expression, proinflammatory cytokine secretion, and T-cell stimulation by immature DCs.25-27,46 Interestingly, inhibition of NF-κB signaling by ACs appears to be DC specific. In Mφs, proinflammatory responses are down-regulated by AC pretreatment, but NF-κB activation is still inducible.47,48 This disparity may reflect the relative importance of the NF-κB pathway in regulating activation and the effector functions of DCs versus Mφs.
The second important observation made in this study is that MerTK is required for AC-induced inhibition of DCs. This was shown in both genetic and specific Ab-blocking experiments. First, AC pretreatment of NOD.MerTKKD BMDCs or sDCs failed to establish a tolerogenic phenotype seen in MerTK-expressing DCs. Despite AC pretreatment, NF-κB signaling was readily induced in NOD.MerTKKD DCs, which correlated with continued APC function (Figures 3 and 7). Second, the inability of ACs to inhibit NF-κB activation was also observed when wild-type NOD or BALB/c DCs were treated with αMerTK Ab to block MerTK binding of ACs (Figure 4). The αMerTK Ab inhibits signaling by preventing AC-induced phosphorylation of MerTK tyrosine residues (Y.H. and R.M.T., unpublished results, January 2006). The failure of ACs to inhibit NOD.MerTKKD DCs therefore was due to the lack of MerTK expression and not a secondary defect. One possibility is that family members Axl and Tyro3 are also involved in AC-induced inhibition. For example, ACs may bind a heterodimeric complex consisting of MerTK and Axl and/or Tyro3, and in turn the absence of MerTK expression or blocking MerTK activation with Ab may disrupt such a complex. However, this scenario is unlikely because neither Axl nor Tyro3 was coimmunoprecipitated with MerTK prepared from chemically crosslinked lysates of AC-pretreated NOD BMDCs (Figure S6). Of particular importance is whether MerTK functions similarly in human monocyte-derived DCs. Unfortunately, initial Ab-blocking experiments to test this possibility have been hindered by the lack of an αhuman MerTK Ab that binds native MerTK well (M.A.W. and R.M.T., unpublished results, July 2006).
Another key finding is that AC-induced inhibition of DCs is dependent on MerTK activation of the PI3K/AKT pathway. AC treatment elicited AKT kinase activity in NOD but not NOD.MerTKKD BMDCs (Figure 5A). A role for PI3K/AKT signaling was demonstrated by the use of Wort and LY, which effectively blocked the capacity of ACs to inhibit NF-κB signaling and DC maturation (Figures 5 and 7B). The latter was observed by measuring TNFα secretion, although the capacity of ACs to inhibit up-regulation of CD40, CD80, and CD86 expression following LPS stimulation was also blocked by the PI3K inhibitors (Figure S7). These results are consistent with a consensus PI3K docking site located in the MerTK intracytoplasmic domain and with detection of a p85α/p110δ complex associated with MerTK following AC incubation (Figure 6). How PI3K/AKT signaling blocks downstream activation of the IKK signalsome is currently under investigation. The fact that cyclohexamide and Eublin 1 pretreatment of BMDCs blocked AC-induced inhibition of NF-κB activation (Figures 1D and S1) suggests that PI3K/AKT signaling mediates downstream de novo protein synthesis of regulatory molecules. Although other molecules (and pathways) such as phosphatidylinositol-specific phospholipase Cγ242 and the guanine exchange factor Vav149 may be involved in MerTK signaling, inhibition of the PI3K/AKT pathway in DCs is nevertheless sufficient to block the effects of ACs. A chimeric molecule containing the intracellular domain of MerTK has been reported to activate both PI3K/AKT and NF-κB pathways in pro–B-cell transfectants.50 This finding coupled with our own suggests that the nature of MerTK signaling is cell dependent. It is also noteworthy that induction of the PI3K/AKT pathway in DCs can either inhibit (Figure 5) or promote activation of the NF-κB pathway.35,51 Distinct effects of PI3K/AKT signaling may reflect the subunit composition of the PI3K heterodimer and/or the isoform of AKT. Fukao et al reported that the inhibitory effect of PI3K on LPS-stimulated activation of p38MAPK in DCs is mediated by a p85α/p110β complex.52 In contrast, AC induction of a p85α/p110δ complex appears to have no effect on LPS-stimulated p38MAPK activation (Figure 2E). Finally, activation of other signaling events engaged by a given receptor may alter the “context” and in turn the downstream effect(s) of the PI3K/AKT pathway.
An essential role for MerTK in efficient phagocytosis of ACs by Mφs has been previously demonstrated.19,20,41,42 In contrast, Behrens et al demonstrated unaltered AC phagocytosis by B6.MerTKKD BMDCs,53 suggesting that the primary function of MerTK is to transduce inhibitory signals upon binding of ACs. Importantly, the role of MerTK in regulating DC activation and function is AC dependent. No significant differences were observed between NOD and NOD.MerTKKD DCs in NF-κB signaling or TNFα secretion when AC pretreatment was not included and DCs were stimulated with LPS only (Figures 3 and 7A). A number of DC subsets have been defined, and whether MerTK serves the same function among different DCs is of interest. For instance, in addition to BMDCs, splenic CD11c+CD11b+ and CD11c+CD8α+ but not plasmacytoid DCs express surface MerTK (M.A.W. and R.M.T., unpublished results, April 2004). The relative contribution of different receptors for ACs may vary depending on the subset of DCs and the nature and concentration of the corresponding ligand. In this regard, it is notable that our assay conditions only partially mimic the complex interactions ongoing in vivo between ACs and DCs. For example, opsonins such as complement can also contribute to AC-mediated effects on DCs in vivo.54 Nevertheless, the fact that the lack of expression and/or blocking of MerTK efficiently inhibited the effects of ACs on NOD, BALB/c, and B6 BMDCs and/or sDCs argues that this RTK plays a key role in regulating DC activation and maturation. Potential defects in MerTK function may in turn contribute to autoimmunity.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Contribution: P.S., M.A.W., Z.Y., Y.H., M.H., and C.E.M. performed experiments and contributed to the writing of the manuscript; H.S.E. and G.M. provided MerTKKD mice and contributed to the writing of the manuscript; A.S.B. provided key reagents and expertise in the design of experiments; and R.M.T. designed and interpreted experiments and contributed to the writing of the manuscript.
This work was supported by grants from the National Institute of Dental and Craniofacial Research (NIDCR 1-P60-DE 13079), the Juvenile Diabetes Research Foundation (JDRF 1-2005-984), and the National Institute of Allergy and Infectious Diseases (NIAID AI066075) (R.M.T.); JDRF 3-2001-860 and NIAID AI056374 (C.E.M.); NIAID AI35098 (A.S.B.); and NIAID AI50736 (G.M.).

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal