Abstract
Von Willebrand factor (VWF) is a chaperone molecule for procoagulant factor VIII (FVIII). Its role in the reduction of the immunogenicity of therapeutic FVIII in patients with hemophilia A has been evoked but lacks clear cellular and molecular rationale. Here, we demonstrate that VWF protects FVIII from being endocytosed by human dendritic cells (DCs) and subsequently presented to FVIII-specific T cells. The immunoprotective effect of VWF requires a physical interaction with FVIII because the endocytosis of FVIII was significantly restored on hindering the formation of the VWF-FVIII complex. Interestingly, VWF had no direct inhibitory effect either on the ability of DCs to present antigenic peptides or on the activation potency of CD4+ T cells. We thus propose that VWF may reduce the immunogenicity of FVIII by preventing, upstream from the activation of immune effectors, the entry of FVIII in professional antigen-presenting cells.
Introduction
A role for von Willebrand factor (VWF) as a chaperone molecule for procoagulant factor VIII (FVIII) has been extensively documented.1-4 Under physiologic conditions, VWF binds to FVIII after its release in the circulation. VWF protects FVIII from proteolysis by lipid-bound proteases, stabilizes the FVIII heterodimeric structure, modulates its activity by thrombin, and further regulates its elimination by lipoprotein-related receptors.5,6
Patients with severe hemophilia A lack functional endogenous FVIII. In up to 30% of the patients, injection of exogenous FVIII to treat hemorrhages results in the development of anti-FVIII antibodies that inhibit the therapeutically administered FVIII. In vivo experimental evidence and clinical observations suggest that the presence of VWF in FVIII preparations is associated with reduced FVIII immunogenicity.7,8 Cellular and molecular mechanisms underlying a putative protective effect of VWF remain, however, unclear.
The induction of a primary anti-FVIII immune response requires the administered FVIII to be endocytosed by professional antigen-presenting cells (APCs) and to be presented to FVIII-specific naive CD4+ T lymphocytes. In previously untreated patients, dendritic cells (DCs) are presumably the only candidate professional APCs. We hypothesized that VWF protects FVIII from being endocytosed by DCs, thus leading to reduced antigen presentation and stimulation of immune effectors.
Materials and methods
DCs were generated from circulating monocytes of healthy blood donors on 5-day culture in X-VIVO15-1% AB serum or in RPMI-10% FCS, supplemented with rhGM-CSF (1000 UI/106 cells; Immunotools, Friesoythe, Germany) and rhIL-4 (500 UI/106 cells; R&D Systems, Lille, France). Surface phenotypic expression confirmed their immature status (data not shown).
DCs generated in X-VIVO15-1% AB serum were incubated with FVIII-FITC (molar ratio, 1:25) alone or in the presence of VWF (Wilfactin) or human serum albumin (HSA). Conjugation of FVIII with FITC did not alter its specific activity (>4000 IU/mg) and its interaction with a series of monoclonal anti-FVIII IgG (data not shown) and with VWF (Figures S1 and S4, available on the Blood website; see the Supplemental Figures link at the top of the online article).
DCs from donors with the DRB1*1501/DRB5*01 haplotype, generated in RPMI-10% FCS, were used for T-lymphocyte activation studies. The synthetic FVIII-derived I2144-T2161 peptide (NeoMPS, Strasbourg, France) and human recombinant factor IX (Benefix) were used as controls.
For confocal microscopy studies, DCs were fixed with 100% ethanol and mounted on glass slides. Images were acquired using a Leica SP2 confocal microscope (Leica, Mannheim, Germany) coupled to a Leica DMIRE2 inverted microscope (Wetzlar, Germany). The detection wavelength range was 500 to 580 nm for FITC.
Results and discussion
We first analyzed the kinetics of internalization of FITC-labeled FVIII by DCs. Incubation of DCs with FVIII-FITC resulted in a dose-dependent labeling of the cells and internalization of FVIII as shown by confocal microscopy (Figure 1A-B). Preincubation of the DCs with 5 mM EDTA significantly reduced the uptake of FVIII (data not shown), demonstrating the involvement of bivalent ion-dependent endocytic receptors in FVIII internalization. The hallmark of DCs is their ability to trigger the activation and proliferation of T cells in an antigen-specific manner.9,10 We confirmed that internalization of FVIII by DCs led to presentation of FVIII-derived peptides and activation of the anti-FVIII C1 domain-specific human CD4+ T-cell clone, D9E9,11 in a dose-dependent manner (Figure 1C). Activation of D9E9 was detected for FVIII concentrations close to that reached in patients with hemophilia A on administration of therapeutic FVIII (ie, 26±8 and 711±63 pg/mL IFN-γ at 1 and 7 nM, respectively; mean ± SD). D9E9 was not activated when it was incubated with DCs in medium alone or with an irrelevant antigen (Figure 1D). D9E9 was, however, strongly activated by DCs incubated in the presence of its target FVIII-derived synthetic peptide I2144-T2161.11
Endocytosis of FVIII by human DCs and presentation to FVIII-specific T cells. (A) Dose-dependent labeling of DCs following incubation with FVIII-FITC. Five-day-old monocyte-derived human DCs were incubated in X-VIVO15 medium containing 0 to 357 nM FVIII-FITC for 2 hours at 37°C. Following incubation, cells were washed extensively and fluorescent cells were analyzed by flow cytometry. Representative of more than 2 experiments. (B) Intracellular localization of FITC-FVIII. DCs were incubated with FVIII-FITC for 2 hours at 37°C. Cells were then fixed on a glass slide and observed with a confocal microscope equipped with a 63 ×/1.32 numerical aperture oil objective. Gray-level images were obtained by differential interference contrast. The fluorescence image was chosen in the middle of the cell, at the level of the nuclei. The fluorescence image and the corresponding gray-level image were acquired using Leica Confocal System software, and were merged using Adobe Photoshop 7.0 software (Adobe Systems, San Jose, CA). (C) Activation of the FVIII-specific CD4+ T-cell clone D9E9 by FVIII-loaded DCs. DCs were generated from blood donors with matched MHC haplotypes (thanks to J. Treton, Institut National de la Santé et de la Recherche Médicale [INSERM] Unité [U] 662, Paris, France) and were incubated at 10 000 cells/well with D9E9 (5000 cells/well) in DMEM-10% FCS-20 IU/mL rhIL-2, alone or in the presence of 0 to 36 nM FVIII for 20 hours at 37°C. Activation of D9E9 was assessed by measuring released IFN-gamma by enzyme-linked immunosorbent assay (ELISA). Data are representative of 3 independent experiments. (D) DCs (10 000 cells/well) were cultured with D9E9 (5000 cells/well) alone or in the presence of 20 nM FVIII, human recombinant factor IX (FIX, 36 nM) or a synthetic FVIII peptide (I2144-T2161) known to activate D9E9 (2 üg/mL), for 20 hours at 37°C. Data represent the average of 7 independent experiments. Error bars indicate SD.
Endocytosis of FVIII by human DCs and presentation to FVIII-specific T cells. (A) Dose-dependent labeling of DCs following incubation with FVIII-FITC. Five-day-old monocyte-derived human DCs were incubated in X-VIVO15 medium containing 0 to 357 nM FVIII-FITC for 2 hours at 37°C. Following incubation, cells were washed extensively and fluorescent cells were analyzed by flow cytometry. Representative of more than 2 experiments. (B) Intracellular localization of FITC-FVIII. DCs were incubated with FVIII-FITC for 2 hours at 37°C. Cells were then fixed on a glass slide and observed with a confocal microscope equipped with a 63 ×/1.32 numerical aperture oil objective. Gray-level images were obtained by differential interference contrast. The fluorescence image was chosen in the middle of the cell, at the level of the nuclei. The fluorescence image and the corresponding gray-level image were acquired using Leica Confocal System software, and were merged using Adobe Photoshop 7.0 software (Adobe Systems, San Jose, CA). (C) Activation of the FVIII-specific CD4+ T-cell clone D9E9 by FVIII-loaded DCs. DCs were generated from blood donors with matched MHC haplotypes (thanks to J. Treton, Institut National de la Santé et de la Recherche Médicale [INSERM] Unité [U] 662, Paris, France) and were incubated at 10 000 cells/well with D9E9 (5000 cells/well) in DMEM-10% FCS-20 IU/mL rhIL-2, alone or in the presence of 0 to 36 nM FVIII for 20 hours at 37°C. Activation of D9E9 was assessed by measuring released IFN-gamma by enzyme-linked immunosorbent assay (ELISA). Data are representative of 3 independent experiments. (D) DCs (10 000 cells/well) were cultured with D9E9 (5000 cells/well) alone or in the presence of 20 nM FVIII, human recombinant factor IX (FIX, 36 nM) or a synthetic FVIII peptide (I2144-T2161) known to activate D9E9 (2 üg/mL), for 20 hours at 37°C. Data represent the average of 7 independent experiments. Error bars indicate SD.
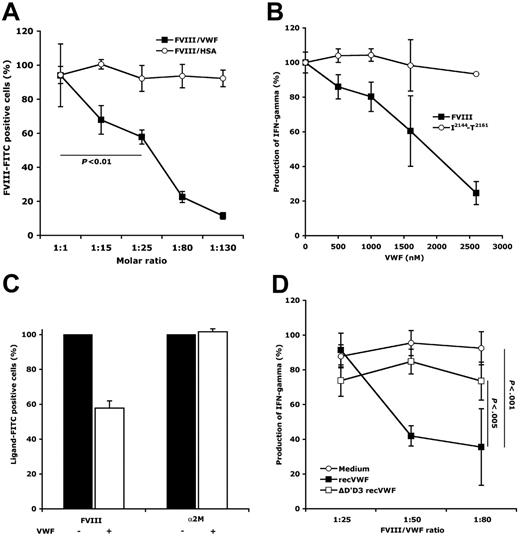
Preincubation of FVIII-FITC (107 nM) with VWF at 1- to 130-fold molar excess, prior to incubation with DCs, resulted in a dose-dependent inhibition of FVIII endocytosis (12%±2% to 94%±18%; Figure 2A). Incubation of FVIII in the presence of a 25-fold molar excess of VWF yielded a significant reduction of FVIII uptake (ie, 58%±4%; P <.01), as compared to incubation of FVIII alone. Blocking DC-mediated FVIII endocytosis by VWF resulted in a dose-dependent reduction of the activation of D9E9 (up to 75%, Figure 2B). Interestingly, D9E9 activation by DCs loaded with the I2144-T2161 peptide was not altered in the presence of concentrations of VWF similar to those used in the presence of FVIII, thus indicating that VWF does not have a direct inhibitory effect on T-cell activation.
VWF reduces FVIII endocytosis by DCs and the consequent presentation to FVIII-specific T cells. (A) Reduction of FVIII endocytosis by DCs in the presence of VWF. FVIII-FITC (107 nM) was incubated with DCs for 2 hours at 37°C, following preincubation in medium alone or in the presence of increasing concentrations of VWF or HSA (ie, molar ratios of 1:1 to 1:130). Cells were then analyzed by flow cytometry. Results depict relative percentage of FVIII-FITC+ cells considering 100% when preincubation was done in medium alone. Data are the average of 6 independent experiments. Significant differences were assessed using the Mann-Whitney test. (B) Reduction of the activation of D9E9 in the presence of VWF. FVIII (20 nM) and the I2144-T2161 peptide (2 üg/mL) were incubated with DCs (10 000 cells/well) and D9E9 (5000 cells/well) in medium alone or in the presence of increasing amounts of VWF, for 20 hours at 37°C. Activation of D9E9 was assessed by measuring the released IFN-γ by ELISA. Data are from 1 representative experiment. (C) Specificity of the protective effect of VWF on FVIII endocytosis by DCs. FVIII-FITC (107 nM) and α2M-FITC (27.8 nM), preincubated alone or with a 25-fold molar excess of VWF, were incubated with DCs for 2 hours at 37°C. Ligand-FITC+ cells (ie, FVIII-FITC or α2M-FITC) were analyzed as described. Average of the results obtained with cells from 2 different donors. (D) Requirement of FVIII-VWF binding for inhibition of FVIII endocytosis. FVIII (5 nM) was incubated with DCs (10 000 cells/well) and D9E9 (5000 cells/well) in medium alone or in the presence of full-length recombinant VWF (recVWF) or D′D3 deleted recombinant VWF (ΔD′D3 recVWF), for 20 hours at 37°C. Activation of D9E9 was assessed by measuring the released IFN-γ by ELISA. Statistical significance was assessed using the ANOVA Fisher PLSD test. Error bars indicate SD.
VWF reduces FVIII endocytosis by DCs and the consequent presentation to FVIII-specific T cells. (A) Reduction of FVIII endocytosis by DCs in the presence of VWF. FVIII-FITC (107 nM) was incubated with DCs for 2 hours at 37°C, following preincubation in medium alone or in the presence of increasing concentrations of VWF or HSA (ie, molar ratios of 1:1 to 1:130). Cells were then analyzed by flow cytometry. Results depict relative percentage of FVIII-FITC+ cells considering 100% when preincubation was done in medium alone. Data are the average of 6 independent experiments. Significant differences were assessed using the Mann-Whitney test. (B) Reduction of the activation of D9E9 in the presence of VWF. FVIII (20 nM) and the I2144-T2161 peptide (2 üg/mL) were incubated with DCs (10 000 cells/well) and D9E9 (5000 cells/well) in medium alone or in the presence of increasing amounts of VWF, for 20 hours at 37°C. Activation of D9E9 was assessed by measuring the released IFN-γ by ELISA. Data are from 1 representative experiment. (C) Specificity of the protective effect of VWF on FVIII endocytosis by DCs. FVIII-FITC (107 nM) and α2M-FITC (27.8 nM), preincubated alone or with a 25-fold molar excess of VWF, were incubated with DCs for 2 hours at 37°C. Ligand-FITC+ cells (ie, FVIII-FITC or α2M-FITC) were analyzed as described. Average of the results obtained with cells from 2 different donors. (D) Requirement of FVIII-VWF binding for inhibition of FVIII endocytosis. FVIII (5 nM) was incubated with DCs (10 000 cells/well) and D9E9 (5000 cells/well) in medium alone or in the presence of full-length recombinant VWF (recVWF) or D′D3 deleted recombinant VWF (ΔD′D3 recVWF), for 20 hours at 37°C. Activation of D9E9 was assessed by measuring the released IFN-γ by ELISA. Statistical significance was assessed using the ANOVA Fisher PLSD test. Error bars indicate SD.
We then explored the molecular mechanisms underlying the reduced endocytosis of FVIII by VWF. We first tested the specificity of the protective effect of VWF on endocytosis of FVIII by DCs: (1) when used at concentrations equimolar to that of VWF, HSA (which is used as a stabilizing agent in the excipient of different therapeutic proteins, including FVIII) did not inhibit FVIII endocytosis (Figure 2A); (2) endocytosis of α2M-FITC (Biomac, Leipzig, Germany) by DCs was not inhibited by a 25-fold molar excess of VWF (Figure 2C). We then investigated whether the integrity of the FVIII/VWF complex is required for blocking the endocytosis of FVIII by DCs. FVIII and VWF were incubated in the presence of F(ab′)2 fragments of a monoclonal anti-FVIII IgG and of a monoclonal anti-VWF IgG, both of which disrupt the interaction between FVIII and VWF. The preincubation of FVIII and VWF with both monoclonal IgGs restored the endocytosis of FVIII by DCs to a significant extent (Figures S2–S3). Furthermore, whereas recombinant human VWF12 inhibited the activation of D9E9 in a manner similar to that of plasma-derived VWF, recombinant VWF lacking the D′D3 domains did not prevent the activation of D9E9 (Figure 2D).
LRP/CD91 and other members of the LDL receptor (LDLR) family have been implicated in FVIII uptake by scavenger cells.5,13-15 LRP is expressed by DCs.16,17 Interestingly, VWF has been shown to block the interaction of FVIII with receptors of the LDLR family.5 However, our recent findings show that LDLR family members are not implicated in FVIII endocytosis by DCs (S. Dasgupta, J.B., S.V.K., and S.L.-D., unpublished data, September 2006) and suggests that FVIII endocytosis by DCs involves yet unidentified alternative bivalent ion-dependent receptors. Whether VWF can prevent the interaction of FVIII with the latter receptors will have to be established.
Our data highlight the role of VWF as an immunoprotective chaperone for FVIII, that is, by preventing, upstream from the activation of immune effectors, the entry of FVIII in professional APCs. Further, we demonstrate in vitro that increasing the VWF/FVIII ratio reduces FVIII endocytosis by DCs in a dose-dependent manner. It remains, however, to be demonstrated whether VWF exerts similar effects in vivo.
Interestingly, coadministration with FVIII of exogenous VWF was suggested to reduce FVIII immunogenicity in vivo.7,8 In normal plasma, the FVIII/VWF molar ratio is 1:50.3,18 It ranges from 1:9 to 1:174 in the different plasma-derived FVIII preparations (manufacturers' communications, June-July 2006), yielding expected molar ratios of 1:64 to 1:229 following intravenous administration to the patients. It would be of interest to investigate whether higher amounts of VWF in therapeutic preparations are associated with lesser incidence of FVIII inhibitors. If confirmed, increasing VWF amounts in FVIII preparations above physiologic levels within limits that are compatible with normal hemostasis may be beneficial to the patients by inhibiting the initiation of immune responses to FVIII. Alternatively, reducing the risk for development of FVIII inhibitors may be achieved using minimal VWF-derived peptidic constructs endowed with similar protective abilities, while being devoid of prothrombotic effects.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Contribution: S. Dasgupta, J.B., P.J.L., A.B.-D., S.V.K., and S.L.-D. designed research; S. Dasgupta, Y.R., A.-M.N., B.W., T.I., S. Delignat, and C.K. performed research; P.J.L., J.-M.S.-R., M.J., and A.B.-D. contributed vital new reagents or analytical tools; S. Dasgupta, Y.R., A.-M.N., B.W., and C.K. collected data; S. Dasgupta, Y.R., P.J.L., and S.L.-D. analyzed data; and S. Dasgupta, Y.R., J.B., S.V.K., and S.L.-D. wrote the paper.
S. Dasgupta and Y.R. have contributed equally to the work.
Acknowledgments
We wish to thank Joelle Treton (INSERM U662, Hospital Saint-Louis, Paris), Renaud Lavend'homme (Center for Molecular and Vascular Biology, Katolische Universtitat Leuven, Belgium), and Dr Jean-Pierre Girma (INSERM U770, BicêAxetre, France) for providing us with blood from MHC-matched donors, with the D9E9 CD4+ T-cell clone, and with the anti-VWF IgG Ac418. We are indebted to Dr Olivier Christophe (INSERM U770) and Dr Yves Laurian (Laboratoire d'Hématologie, HêAxopital Jean Verdier, Bondy, France) for invaluable discussions. VWF (Wilfactin) and HSA were kind gifts from Laboratoire FranêAccais du Fractionnement et des Biotechnologies (Les Ulis, France). FIX (Benefix) and FVIII (Helixate NexGen) were kind gifts from Baxter (Maurepas, France) and ZLB-Behring, respectively.
This work was supported by INSERM, by the Centre National de la Recherche Scientifique, by the Université Pierre et Marie Curie, by grants from Indo-French Center for Promotion of Advanced Research (CEFIPRA), from Agence Nationale de la Recherche (ANR-05-MRAR-030), and from ZLB-Behring (Paris, France). P.J.L. is a recipient of a grant from Biotest (Dreieich, Germany). S. Dasgupta, B.W., J.B., and Y.R. are recipients of fellowships from CEFIPRA, LFB, Les Entreprises du Médicament (LEEM; Paris, France), and ZLB-Behring, respectively.

![Figure 1. Endocytosis of FVIII by human DCs and presentation to FVIII-specific T cells. (A) Dose-dependent labeling of DCs following incubation with FVIII-FITC. Five-day-old monocyte-derived human DCs were incubated in X-VIVO15 medium containing 0 to 357 nM FVIII-FITC for 2 hours at 37°C. Following incubation, cells were washed extensively and fluorescent cells were analyzed by flow cytometry. Representative of more than 2 experiments. (B) Intracellular localization of FITC-FVIII. DCs were incubated with FVIII-FITC for 2 hours at 37°C. Cells were then fixed on a glass slide and observed with a confocal microscope equipped with a 63 ×/1.32 numerical aperture oil objective. Gray-level images were obtained by differential interference contrast. The fluorescence image was chosen in the middle of the cell, at the level of the nuclei. The fluorescence image and the corresponding gray-level image were acquired using Leica Confocal System software, and were merged using Adobe Photoshop 7.0 software (Adobe Systems, San Jose, CA). (C) Activation of the FVIII-specific CD4+ T-cell clone D9E9 by FVIII-loaded DCs. DCs were generated from blood donors with matched MHC haplotypes (thanks to J. Treton, Institut National de la Santé et de la Recherche Médicale [INSERM] Unité [U] 662, Paris, France) and were incubated at 10 000 cells/well with D9E9 (5000 cells/well) in DMEM-10% FCS-20 IU/mL rhIL-2, alone or in the presence of 0 to 36 nM FVIII for 20 hours at 37°C. Activation of D9E9 was assessed by measuring released IFN-gamma by enzyme-linked immunosorbent assay (ELISA). Data are representative of 3 independent experiments. (D) DCs (10 000 cells/well) were cultured with D9E9 (5000 cells/well) alone or in the presence of 20 nM FVIII, human recombinant factor IX (FIX, 36 nM) or a synthetic FVIII peptide (I2144-T2161) known to activate D9E9 (2 üg/mL), for 20 hours at 37°C. Data represent the average of 7 independent experiments. Error bars indicate SD.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/109/2/10.1182_blood-2006-05-022756/4/m_zh80020706330001.jpeg?Expires=1765907073&Signature=nVPtuKMxsmwkhRcRoRP39xW-vs26GgNJyz0Hzl-hhMdqzppWeDg7au1BchY3-~C0BXWebG6n9Kq0eH8LxHcxXT4zK062AxmbBxZ9y3Iq7LpE4GyH8jDAtxASpLnMySC9sjhHrPlK6pB0uxSpqGHRZfeOkVdMvYUm8tSRN5Vg-6GwIljlJpZ3y7Yhvl5A94tnkVDbNxQJ0KdOAFgzNnhRm1GklasVU6LjZwMrUmzoxZUKr8FyM~Jdt3cDQW1~Z0k6TNak0AXRTpOGhJV-ESM3fwWS~5FS4f9Qow1ThCMJABJ41WmK9M8lNX~dXvza0Lt0De98uFQsychORVHF5mVNdA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal