Abstract
A patient with adenosine deaminase–deficient severe combined immune deficiency (ADA-SCID) was enrolled in a study of retroviral-mediated ADA gene transfer to bone marrow hematopoietic stem cells. After the discontinuation of ADA enzyme replacement, busulfan (75 mg/m2) was administered for bone marrow cytoreduction, followed by infusion of autologous, gene-modified CD34+ cells. The expected myelosuppression developed after busulfan but then persisted, necessitating the administration of untransduced autologous bone marrow back-up at day 40. Because of sustained pancytopenia and negligible gene marking, diagnostic bone marrow biopsy and aspirate were performed at day 88. Analyses revealed hypocellular marrow and, unexpectedly, evidence of trisomy 8 in 21.6% of cells. Trisomy 8 mosaicism (T8M) was subsequently diagnosed by retrospective analysis of a pretreatment marrow sample that might have caused the lack of hematopoietic reconstitution. The confounding effects of this preexisting marrow cytogenetic abnormality on the response to gene transfer highlights another challenge of gene therapy with the use of autologous hematopoietic stem cells.
Introduction
A clinical trial of gene transfer for adenosine deaminase–deficient severe combined immune deficiency (ADA-SCID) was initiated based on retroviral gene delivery to autologous bone marrow (BM) CD34+ cells with bone marrow cytoreduction using busulfan and discontinuation of pegylated bovine ADA (PEG-ADA) enzyme replacement. Approval of this study was obtained from the Committee on Clinical Investigations at Childrens Hospital Los Angeles (institutional review board). Informed consent was obtained according to the Declaration of Helsinki.
Patient, materials, and methods
Patient ADA 302C was born on January 28, 2002, to Hispanic-American parents. At 23 months of age, a workup for severe anemia (hemoglobin level, 21 g/L; reticulocyte count, 0.001) included a BM biopsy that revealed hypocellularity, decreased erythroid lineage, and active parvovirus B19 infection (positive staining of intranuclear viral inclusion bodies within pronormoblasts). Parvovirus titers were negative. Cytogenetic examination of 20 metaphase cells showed normal 46,XX female karyotype. Intravenous immune globulin (IVIg) administration was initiated, and the reticulocyte count increased. Dapsone was administered for Pneumocystis carinii pneumonia prophylaxis because the patient's lymphocyte counts were depressed (range, 0.18-0.3 × 109/L). Anemia improved over time, hemoglobin values remained stable (range, 100-110 g/L), and the patient was maintained on monthly doses of intravenous immunoglobulin (IVIg). ADA-SCID was diagnosed when the patient was 38 months of age, when she had RSV pneumonia and recurrent diarrhea with persistent lymphopenia. Her red blood cells (RBCs) had an undetectable level of ADA enzyme activity and an elevated concentration of deoxyadenosine nucleotides (dAXPs) (0.617 μmol/mL or 22.7% of total adenine nucleotides; normal levels lower than 0.002 μmol/mL or less than 0.2%). The patient was found to be heteroallelic for 2 previously reported ADA missense mutations, R101Q and R211H.
The patient was treated with intramuscular polyethylene glycol–modified adenosine deaminase (PEG-ADA) enzyme replacement therapy twice a week and responded with normalization of RBC dAXPs and increased lymphocyte counts. However, because lymphocyte counts remained below the normal range, the patient was considered for unrelated donor hematopoietic stem cell transplantation or gene therapy. The family opted for participation in the gene transfer research study.
Results and discussion
Physical examination at the time of study entry revealed developmental delay with delayed speech, deafness in the left ear and markedly diminished hearing in the right ear; a tympanostomy tube was in place. Screening ophthalmologic examination demonstrated optic atrophy of uncertain etiology. Laboratory studies showed a hemoglobin level of 109 g/L, a platelet count of 131 × 109/L, and an absolute neutrophil count 1.8 × 109/L. These hematologic values met study inclusion criteria: hemoglobin level greater than 100 g/L, platelet count greater than 100 × 109/L and absolute neutrophil count greater than 1.0 × 109/L. Polymerase chain reaction (PCR) of peripheral blood for parvovirus B19 was performed twice, with negative results.
PEG-ADA was discontinued 1 week before BM harvest, and the patient remained off enzyme replacement therapy throughout the subsequent gene transfer trial. She underwent BM harvest without incidents. CD34+ cells were enriched by the Isolex 300i (Baxter, Glendale, CA); only 35% of the CD34+ cells were isolated. A CD34dim population of BM CD34+ cells was not recovered, even after repeat passage of the negative fraction through the Isolex 300i (Table 1). CD34+ cells were placed in culture with flt3 ligand (300 ng/mL), stem cell factor (50 ng/mL), and MGDF (50 ng/mL; Amgen, Thousand Oaks, CA). After 2 days of prestimulation, half the cells were transduced with the GCsap-M-ADA vector and half were transduced with the MND-ADA vector. Each vector included the identical ADA cDNA but slightly different vector backbones.1,2
Bone marrow cell yields, transduction efficiency, and clonogenicity
| Data . | |
|---|---|
| CD34+ cell count, × 106 | |
| After Ficoll | 46.6 |
| After Isolex | 16.1 |
| Transduction efficiency, % | 9 |
| Back-up MNCs, × 107/kg | 3.7 |
| BM clonogenicity, %; | |
| D 0 | 11.5 |
| D 88 | 0.01 |
| Data . | |
|---|---|
| CD34+ cell count, × 106 | |
| After Ficoll | 46.6 |
| After Isolex | 16.1 |
| Transduction efficiency, % | 9 |
| Back-up MNCs, × 107/kg | 3.7 |
| BM clonogenicity, %; | |
| D 0 | 11.5 |
| D 88 | 0.01 |
Busulfan was administered per protocol (2 doses at 37.5 mg/m2 intravenously 6 hours apart; actual total, 77.6 mg/m2 or 3.1 mg/kg). Pharmacokinetic analysis of the first dose of busulfan showed an area under the curve of 1507 mg∗min/mL per minute, with a steady state concentration of 1031 ng/mL (values as expected). Cells met release criteria (10.5 × 106 cells, 92% viability, negative Gram stain, cultures, and endotoxin assay), and infusion was uneventful (day 0). Colony formation of cells plated in methylcellulose was normal at 10% to 13%. Quantitative PCR analysis of pooled transduced cells revealed 7.5% to 11% gene marking (Table 1).
The development of thrombocytopenia on day 11, followed by granulocytopenia on day 27, was expected after busulfan. However, persistent peripheral blood cytopenia (RBC and platelet transfusion dependence, absolute neutrophil count lower than 0.200 × 109/L) prompted back-up marrow administration (3.7 × 107/kg mononuclear cells [MNCs], viability 100%; Table 1) on day 40, as mandated by the study protocol. Because of the possibility that it could exacerbate neutropenia and optic atrophy,3,4 dapsone administration was discontinued on day 58, and inhaled pentamidine was substituted. Folinic acid and vitamin B12 administration were started, even though blood levels were normal.
Moderate pancytopenia persisted, despite administration of the back-up marrow, and the patient remained dependent on packed RBC and platelet support over subsequent months. Analysis of peripheral blood samples documented the absence of replicationcompetent retrovirus by PCR at baseline and after 3 months. Diagnostic BM biopsy and aspirate were performed on day 88. Wright-Giemsa stain showed hypocellular marrow without megakaryocytes and without myelofibrosis. PCR of the marrow for adenovirus, human herpesvirus 6 (HHV-6), cytomegalovirus (CMV), and parvovirus B19 revealed negative findings.
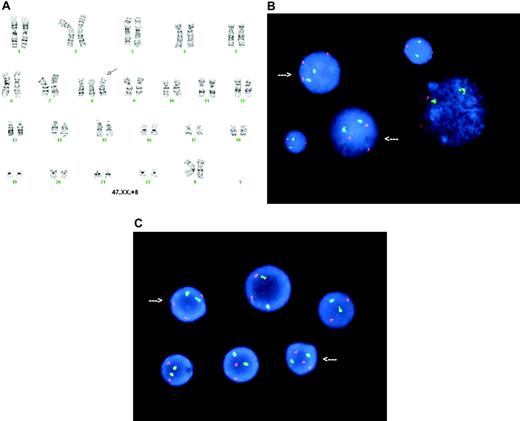
Unexpectedly, cytogenetic analysis of the bone marrow showed one of 9 metaphase cells to have trisomy 8 by karyotype analysis (Figure 1A). Fluorescence in situ hybridization (FISH) revealed trisomy 8 in 108 (21.6%) of 500 of the bone marrow cells (Figure 1B). Retrospective analysis by FISH of a viably frozen bone marrow sample obtained at the time of initial marrow harvest (day −5, before busulfan and transduced cell infusion), revealed trisomy 8 in 16 (3.2%) of 500 cells (Figure 1C). It was unclear whether this represented an acquired trisomy 8 abnormality or constitutional trisomy 8 mosaicism (T8M).
Cytogenetic analysis of bone marrow cells. (A) G-banding karyotype from a posttreatment (day 88) bone marrow cell showing 47,XX,+8 (arrow indicates trisomy 8). (B) FISH analysis in posttreatment (day 88) bone marrow using ETO (8q22, red; Vysis, Downer's Grove, IL) as a test probe for numerical study of chromosome 8, normalized to acute myeloid leukemia 1 (AML1; 21q22, green; Vysis) as an internal ploidy control probe showing 21.6% of the bone marrow interphase cells with 3 copies of chromosome 8 (arrows). FISH images were captured using a MAX-BX51 Olympus fluorescence microscope (Olympus, Tokyo, Japan) equipped with a 100×/1.30 numerical aperture oil objective. These images were captured and processed using MacProbe software (Applied Imaging, Santa Clara, CA). (C) FISH analysis of a pretreatment bone marrow sample showing trisomy 8 mosaicism. Arrows indicate trisomy 8 interphase nuclei.
Cytogenetic analysis of bone marrow cells. (A) G-banding karyotype from a posttreatment (day 88) bone marrow cell showing 47,XX,+8 (arrow indicates trisomy 8). (B) FISH analysis in posttreatment (day 88) bone marrow using ETO (8q22, red; Vysis, Downer's Grove, IL) as a test probe for numerical study of chromosome 8, normalized to acute myeloid leukemia 1 (AML1; 21q22, green; Vysis) as an internal ploidy control probe showing 21.6% of the bone marrow interphase cells with 3 copies of chromosome 8 (arrows). FISH images were captured using a MAX-BX51 Olympus fluorescence microscope (Olympus, Tokyo, Japan) equipped with a 100×/1.30 numerical aperture oil objective. These images were captured and processed using MacProbe software (Applied Imaging, Santa Clara, CA). (C) FISH analysis of a pretreatment bone marrow sample showing trisomy 8 mosaicism. Arrows indicate trisomy 8 interphase nuclei.
In the first 3 months after gene therapy, only low levels of gene marking in peripheral blood cells (less than 1.0 × 10−4 in peripheral blood mononuclear cells [PBMCs] and granulocytes) were demonstrated. Twice-weekly intramuscular injections of PEG-ADA were reinstituted on day 96. The patient was discharged and received regular transfusions of platelets and packed RBCs until she underwent transplantation with matched unrelated donor marrow 8 months after the gene transfer procedure. A marrow sample before transplantation showed trisomy 8 in approximately 50% of cells.
Although RBC, absolute neutrophil, and platelet counts measured in this patient at study entry met the eligibility criteria for the ADA gene transfer clinical trial, they were lower than those we saw in other ADA-SCID patients in this trial (D.B.K. and F.C., unpublished observations, January 2002) and might have indicated marrow dysfunction not associated with ADA-SCID. It is unclear whether the earlier parvovirus B19 infection or the T8M contributed to the decreased blood cell counts, inefficient CD34+ cell recovery, and low transduction frequency.
Patients with constitutional T8M may have persistent peripheral blood cytopenias (particularly thrombocytopenia and anemia) with progression from myelodysplasia to leukemia, ocular manifestations, developmental and speech delays, and hearing deficits.5-8 Neurologic abnormalities observed in our patient have also been described in other ADA-deficient patients.9,10 Although busulfan administration precipitated the onset of cytopenia, the underlying T8M might have played a role in the lack of marrow function recovery from the transduced CD34+ cells or from the untransduced back-up marrow. In the context of T8M, the bone marrow microenvironment has been shown to have alterations in cytokine production11 that may interfere with engraftment. Myelodysplastic syndrome has not been reported in association with ADA deficiency, though a case report has been published of myelodysplasia in a patient with purine nucleoside phosphorylase deficiency.12 To our knowledge, this is the first description of ADA-SCID (the ADA gene is on chromosome 20) occurring with trisomy 8 abnormalities.
The lack of response to gene transfer in this patient with the unfortunate coincidence of ADA deficiency and a marrow cytogenetic abnormality is contrasted by the successful outcome of gene therapy for multiple children with ADA-SCID in other trials.13,14 The current case serves as a cautionary note for gene therapy with autologous hematopoietic stem cells and suggests that cytogenetic studies should be considered for patients with subnormal blood cell counts during enrollment.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Contribution: B.C.E., G.M.P., J.L.I., E.M.S., and D.A.C. performed the clinical/regulatory work and laboratory work for this clinical trial. K.W., A.S., N.K., M.S., M.B., G.M.C., K.I.W., R.P., and H.M.R. administered the clinical care of this patient during the clinical trial. S.-Q.W. performed the cytogenetic studies. M.S.H. performed the ADA genotype and measured the adenine nucleotide and ADA enzyme levels. F.C. and D.B.K. are coprincipal investigators of the study, recruited the patient, and coordinated the research. B.C.E., G.M.P., and D.B.K. wrote the paper.
Acknowledgments
This work was supported by a Distinguished Clinical Scientist Award from the Doris Duke Charitable Foundation (D.B.K.) and by grants from the National Institutes of Health (grants HL54850, MO1 RR-43, DK20902 [M.S.H.]) and NIH intramural funds (F.C.) and was performed at the General Clinical Research Center at Childrens Hospital Los Angeles.
References
Author notes
B.C.E. and G.M.P. contributed equally to this study.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal