Abstract
Primary systemic amyloidosis (AL) is an incurable plasma cell disorder. Lenalidomide, especially in conjunction with dexamethasone, is highly active in patients with multiple myeloma. We studied the toxicity and efficacy of lenalidomide in patients with AL. Patients with symptomatic AL, a measurable plasma cell disorder, and adequate hematologic and renal reserve were eligible. Patients received single-agent lenalidomide. If there was no evidence of progression after 3 months or of hematologic response after 3 cycles, dexamethasone was added. Twenty-three patients were enrolled. Thirteen were previously treated. Organ involvement was cardiac (64%), renal (73%), hepatic (23%), and nerve (14%). Within the first 3 cycles of therapy, 10 patients discontinued treatment: 4 early deaths, 3 adverse events, and 3 other causes. With a median follow-up of 17 months, 10 patients responded to treatment. In these patients, responses included 9 hematologic, 4 renal, 2 cardiac, and 2 hepatic. All but one of the responders had dexamethasone added to their treatment program. The most common grade 3 or 4 adverse events at least possibly attributable to lenalidomide were neutropenia (45%), thrombocytopenia (27%), rash (18%), and fatigue (18%). In AL patients, we saw limited activity of single-agent lenalidomide, but significant activity of the combination with dexamethasone, which warrants further investigation.
Introduction
Primary systemic amyloidosis (AL) occurs with an incidence of 8 patients per million per year. Virtually all patients die of this disease, with a median survival of 12 to 18 months, and less than approximately 5% survive 10 years with standard chemotherapy.1,2 Low-dose oral therapies produce response in 15% to 40% of patients.3-10 Although peripheral blood stem cell transplantation produces organ responses in nearly half of patients,11-13 this aggressive chemotherapy is not feasible for the majority of AL patients due to poor performance status.14 Thalidomide has limited activity in patients with AL, and its use is restricted due to a high degree of associated toxicity.15-17 Most patients with AL never demonstrate objective evidence of disease regression and ultimately succumb to their disease.
Lenalidomide, an analog of the immunomodulatory drug (IMiD) thalidomide, has been shown to be approximately 50 to 2000 times more potent than thalidomide in vitro.18 In multiple myeloma, activity was seen in phase 1 studies.19,20 Subsequent studies have demonstrated activity of lenalidomide both as a single agent and in combination with dexamethasone in patients with relapsed refractory myeloma.21,22 The combination of lenalidomide and dexamethasone has also been shown to be a potent induction regimen in patients with newly diagnosed myeloma.23
Based on these encouraging results in myeloma patients, we designed a phase 2 trial whose primary objective was hematologic response rate of single-agent lenalidomide in patients with primary systemic amyloidosis. Secondary objectives were the hematologic response rate of the lenalidomide-dexamethasone combination as well as toxicity and rate of organ response for single-agent lenalidomide or the 2-drug combination. Herein, we report our experience with 23 patients so treated.
Patients, materials, and methods
This phase 2 trial (MC0484) was approved by the institutional review board of Mayo Clinic Rochester, and all data were prospectively collected.
Patient eligibility for lenalidomide trial
Patients were eligible for MC0484 if they were consenting adults with symptomatic primary systemic amyloidosis based on both a tissue diagnosis of amyloidosis based on polarizing microscopy of green birefringent material in Congo red–stained tissue specimens and immunohistochemical stains of that tissue demonstrating light chain amyloidosis. Both previously untreated and treated patients were eligible for participation. They were required to have an Eastern Cooperative Oncology Group (ECOG) performance status of less than 4, a serum creatinine level less than or equal to 265.2 μM (3 mg/dL), an absolute neutrophil count of at least 1 × 109/L, a platelet count of at least 75 × 109/L, and a hemoglobin level of at least 80 g/L (8 g/dL). Sexually active individuals of child-bearing capability were required to use contraception.
Patients were excluded from the study if they had non-AL amyloidosis or merely vascular amyloid in bone marrow biopsy or in a plasmacytoma if their only symptoms comprised carpal tunnel syndrome or skin purpura as only evidence of disease. Patients were excluded if they had received other chemotherapy within 4 weeks or if they were receiving concomitant high-dose corticosteroids, although prednisone up to 20 mg per day was allowed if patients were taking this for another cause (eg, adrenal insufficiency or rheumatoid arthritis). Pregnant or nursing women were ineligible. Other exclusionary criteria included uncontrolled infection; clinically overt multiple myeloma (bone marrow plasma cells > 30% and either bone lesions or hypercalcemia); grade 2 or higher peripheral neuropathy; active malignancy; and prior hypersensitivity reaction to thalidomide.
Because decision making after 3 months of therapy was dependent on hematologic status, all patients were required to have hematologically measurable disease as defined by any of the following: serum monoclonal protein greater than or equal to 10 g/L (1.0 g/dL) by protein electrophoresis; more than 200 mg monoclonal protein in the urine on 24-hour electrophoresis; or serum immunoglobulin free light chain greater than or equal to 100 mg/L (10 mg/dL) along with an abnormal serum immunoglobulin kappa to lambda free light chain ratio.
Protocol treatment
Patients were treated with lenalidomide 25 mg by mouth for 21 days followed by a 7-day rest (1 cycle). For the first 12 weeks of therapy, patients were followed for adverse events every 2 weeks and hematologic response every 4 weeks. If there was no hematologic (or organ) response after 3 cycles of single-agent lenalidomide, dexamethasone 40 mg days 1 through 4 and 15 through 18 was added to the treatment program. Patients were required to return to the Mayo Clinic every 4 weeks for the first 3 cycles and then every 12 weeks thereafter. Patients were required to have blood work, urine studies, and a doctors visit before each cycle. If the patient opted not to return to the Mayo Clinic for intervening cycles, telephone interviews were conducted by a Mayo Clinic primary investigator on the cycles that the patient did not return to the Mayo Clinic. Patients were evaluated for organ response no less frequently than every 12 weeks. Patients were to be treated for 12 cycles of therapy with the option to continue beyond as long as there was evidence of response.
Dose modifications
Treatment modifications were based on adverse events (AEs), which were graded according to the CTEP Common Terminology Criteria for Adverse Events (CTCAE Version 3.0; National Cancer Institute, Bethesda, MD). Patients were assessed for toxicity every 2 weeks for the first 12 weeks and every 4 weeks thereafter. For grade 3 or 4 AEs attributable to lenalidomide occurring prior to day 15, but that resolve to less than or equal to grade 2 prior to day 21, drug could be restarted at one dose level lower and continued through day 21. This reduced dose level was used for the subsequent cycle. For those grade 3 or 4 AEs occurring on or after day 15 of a cycle, the patient's lenalidomide was held for the remainder of the cycle and reduced by one dose level beginning with the next cycle. Although the option existed to add filgrastim for grade 3 or 4 neutropenia as the first dose-level reduction, this activity was discouraged given these patients' predilection to retain fluid with filgrastim administration. The stepwise dose level reductions of lenalidomide were to 15 mg, 10 mg, and 5 mg. Grade 3 AEs attributable to dexamethasone or greater than equal to grade 2 muscle weakness, confusion, or mood alteration were cause for dose reduction. The stepwise reductions were as follows: 20 mg on days 1 to 4 and 15 to 18; 10 mg on days 1 to 4 and 15 to 18; 10 mg on days 1 to 4; and discontinuation of dexamethasone.
Treatment evaluation
Organ response was evaluated on the basis of improvement of one or more affected organs as previously described.24 Only one parameter was required to satisfy the organ response criteria, and the response needed to be maintained for a minimum of 3 months to be considered valid. In brief, organ response was defined as follows. Renal response included a 50% reduction in 24-hour urine protein excretion (at least 0.5 g/d) with stable creatinine. Cardiac response was defined as either greater than a 2-mm reduction in the interventricular septal (IVS) thickness by echocardiogram, improvement of ejection fraction by greater than or equal to 20%, or improvement by 2 New York Heart Association classes without an increase in diuretic use. Hepatic response was defined as either greater than or equal to a 50% decrease in (or normalization of) an initially elevated alkaline phosphatase level or reduction in the size of the liver by at least 2 cm by radiographic determination. A neurologic response was defined as either a reduction in the Neuropathy Impairment Score by 10 points or improvement in the summated compound muscle action potential (CMAP) amplitude by 2 mV. This value is derived from summated value of compound muscle action potential amplitudes of the tibial, peroneal, and ulnar nerves from the nerve conduction studies. Gastrointestinal tract improvement required normalization of a low serum carotene level or reduction of diarrhea to less than 50% of previous movements/d or decrease in fecal fat excretion by 50%.
Organ progression was defined by fulfillment of at least one of the following criteria. Renal progression was defined as at least a 50% increase in urinary protein loss (at least 1 g/24 hours), or 25% worsening of creatinine or creatinine clearance (minimum change of 44.2 μM [0.5 mg/dL ] and 0.250 mL/s [15 mL/min ], respectively). A cardiac progression included an increase in cardiac wall thickness by 2 or more millimeters or an increase in New York Heart Association class by 1 grade with a decreasing ejection fraction of greater than or equal to 10%. A hepatic progression included either of the following: a greater than or equal to 50% increase of alkaline phosphatase level above lowest confirmed level or an increase in liver size by at least 2 cm (radiographic determination). A neurologic progression included an increase in the Neuropathy Impairment Score (NIS) by 10 points or worsening in the summated CMAP amplitude by 2 mV. A gastrointestinal progression was defined as a reduction of serum carotene level below normal limit or worsening of diarrhea with increase more than 50% of previous movements per day or fecal fat by 50%.
The primary objective was the hematologic response rate of single-agent lenalidomide in patients with AL. Patients provided informed consent in accordance with the Declaration of Helsinki. All patients meeting the eligibility criteria who signed a consent form and began treatment were considered evaluable for estimation of the response probability. The trial was powered at 90% to detect at hematologic response of 25% according to a modified Fleming design using 20 evaluable patients to test the null hypothesis that the true success proportion in this patient population is at most 5%. According to this design, a total of 23 patients were planned to be enrolled to account for any patients who may later be found to be ineligible, cancel prior to receiving treatment, or have a major treatment violation. Secondary end points of the study included hematologic response rate of the lenalidomide-dexamethasone combination as well as toxicity, organ response for single-agent lenalidomide and the 2-drug combination, progression-free survival, and overall survival. The proportion of organ responses was estimated by the number of organ responses divided by the total number of evaluable patients. Differences between groups were determined by the nonparametric methods of Kruskal-Wallis and Fisher exact test. Kaplan-Meier survival curves were used to calculate both progression-free survival and overall survival. Patients who died while in the study were considered to have had disease progression, unless documented evidence clearly indicated otherwise. As a posthoc analysis, factors predicting for early death or withdrawal were analyzed, including number of prior regimens, serum creatinine level, serum alkaline phosphatase level, serum albumin, NT-proBNP, left ventricle intraventricular septal thickness, and New York Heart Association class.
Results
Between November 5, 2004, and March 17, 2005, 23 patients enrolled in the study. One patient cancelled study participation prior to receiving any study drug and is thus excluded from all of the following analyses. Details of baseline characteristics for the 22 evaluable patients are presented in Table 1 Thirteen patients had received prior therapies, 6 of whom had undergone high-dose chemotherapy with peripheral blood stem cell transplantation. The median age was 62.5 years (range, 44-88 years), and 59% were male. Based only on cardiac, liver, renal, and nerve involvement, the median number of organs involved was 2 (range, 0-3). In addition to these organs, other types involved were stomach, small bowel, colon, and soft tissue. A majority (64%) of patients had cardiac involvement, where 2 patients were New York Heart Association (NYHA) class III and 8 patients were NYHA class II. Only 8 patients had “measurable” serum or urine M-spikes according the EBMTR criteria25 such that 14 patients had their serum immunoglobulin free light chains used for their hematologic measurement. The majority of patients had significant pre-existing symptoms related to their amyloidosis at study entry: peripheral edema (74%), fatigue (74%), elevated creatinine level (43%), and dyspnea (55%). Two patients had a history of cardiogenic syncope at study entry.
Baseline characteristics
| Baseline characteristic . | Value . |
|---|---|
| Median age, y (range) | 62.5 (44-88) |
| Median time from diagnosis to on-study, mo (range) | 8.8 (0.4-216) |
| No. of patients previously treated (%) | 13 (59) |
| Median no. of prior regimens (range) | 1 (0-3) |
| Prior hematopoietic stem cell transplantation, no. (%) | 6 (27) |
| Median NYHA class (range) | 1 (1-3) |
| ECOG performance status of 2 or more, no. (%) | 6 (27) |
| Median no. of organs involved (range) | 2 (0-3) |
| Organ involvement, no. of patients (%) | |
| Heart | 14 (64) |
| Kidney | 16 (73) |
| Liver | 5 (23) |
| Nerve | 1 (5) |
| Clonality, no. λ (%)/ no. κ (%) | 12 (55)/10 (45) |
| Median creatinine level, mg/dL (range) | 1.3 (0.7-2.6) |
| Median alkaline phosphatase level, IU/L (range) | 102.5 (57-1729) |
| Median bilirubin level, mg/dL (range) | 0.5 (0.3-2.8) |
| Median albumin level, g/dL (range) | 2.80 (1.20-3.70) |
| Median beta2 microglobulin level, mg/L (range) | 3.5 (1.4-8.4) |
| Median serum troponin level, μg/L (range) | 0.03 (0.1-0.55) |
| Median NT-proBNP level, ng/L (range) | 1 324.5 (109-42 844) |
| Troponin T/NT-proBNP stage,* no. of patients (%) | |
| Stage I | 4 (18) |
| Stage II | 13 (59) |
| Stage III | 5 (23) |
| Median serum M-protein level, g/dL (range) | 0.1 (0-1.7) |
| Median urine M-protein level, g/24 h (range) | 0.1 (0-0.889) |
| Median involved immunoglobulin free light chain level, mg/dL (range) | 19.6 (2.26-705) |
| Median no. of inv/uninvolved free light chains (range) | 12.1 (0.95-3711) |
| Median total urine protein level, g/24 h (range) | 4 328 (25-14 276) |
| Median interventricular septal thickness, mm (range) | 13 (9-24) |
| Median left ventricular ejection fraction, % (range) | 63.5 (22-72) |
| Baseline characteristic . | Value . |
|---|---|
| Median age, y (range) | 62.5 (44-88) |
| Median time from diagnosis to on-study, mo (range) | 8.8 (0.4-216) |
| No. of patients previously treated (%) | 13 (59) |
| Median no. of prior regimens (range) | 1 (0-3) |
| Prior hematopoietic stem cell transplantation, no. (%) | 6 (27) |
| Median NYHA class (range) | 1 (1-3) |
| ECOG performance status of 2 or more, no. (%) | 6 (27) |
| Median no. of organs involved (range) | 2 (0-3) |
| Organ involvement, no. of patients (%) | |
| Heart | 14 (64) |
| Kidney | 16 (73) |
| Liver | 5 (23) |
| Nerve | 1 (5) |
| Clonality, no. λ (%)/ no. κ (%) | 12 (55)/10 (45) |
| Median creatinine level, mg/dL (range) | 1.3 (0.7-2.6) |
| Median alkaline phosphatase level, IU/L (range) | 102.5 (57-1729) |
| Median bilirubin level, mg/dL (range) | 0.5 (0.3-2.8) |
| Median albumin level, g/dL (range) | 2.80 (1.20-3.70) |
| Median beta2 microglobulin level, mg/L (range) | 3.5 (1.4-8.4) |
| Median serum troponin level, μg/L (range) | 0.03 (0.1-0.55) |
| Median NT-proBNP level, ng/L (range) | 1 324.5 (109-42 844) |
| Troponin T/NT-proBNP stage,* no. of patients (%) | |
| Stage I | 4 (18) |
| Stage II | 13 (59) |
| Stage III | 5 (23) |
| Median serum M-protein level, g/dL (range) | 0.1 (0-1.7) |
| Median urine M-protein level, g/24 h (range) | 0.1 (0-0.889) |
| Median involved immunoglobulin free light chain level, mg/dL (range) | 19.6 (2.26-705) |
| Median no. of inv/uninvolved free light chains (range) | 12.1 (0.95-3711) |
| Median total urine protein level, g/24 h (range) | 4 328 (25-14 276) |
| Median interventricular septal thickness, mm (range) | 13 (9-24) |
| Median left ventricular ejection fraction, % (range) | 63.5 (22-72) |
N = 22.
To convert creatinine level from milligrams per deciliter to micromoles per liter, multiply milligrams per deciliter by 88.4. To convert bilirubin level from milligrams per deciliter to micromoles per liter, multiply milligrams per deciliter by 17.1. To convert albumin level from grams per deciliter to grams per liter, multiply grams per deciliter by 10. To convert beta2 microglobulin from milligrams per liter to nonomoles per liter, multiply milligrams per liter by 85.
Troponin T/NT-proBNP staging uses the cutoffs of < 0.035 μg/L for troponin T and < 334 ng/L for NT-proBNP. Stage I indicates that both levels are below threshold; stage II, one level is above threshold; and stage III, both levels are above threshold.
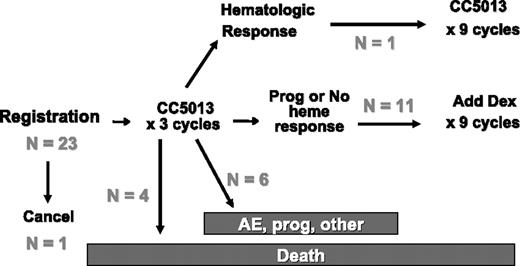
At the time of analysis, the median follow-up of patients remaining on active therapy is 17.0 months (range, 12.6 to 17.6 months) with only 7 of the original 23 patients remaining on the treatment. Within the first 3 cycles of treatment, 10 patients ended active treatment due to early deaths (n = 4), AEs (n = 3), other medical problems (n = 1), alternate therapy (n = 1), and disease progression (n = 1) (Figure 1). The baseline characteristics of these patients with early death are shown in Table 2. All 4 died due to complications of their restrictive cardiomyopathy, 3 in the first cycle and 1 in the third cycle; none of these events was thought to be related to the study medication. Predictive factors for early withdrawal, including early death, included poorer cardiac function, higher NT-proBNP level (5925 versus 537 ng/L, P = .02), and worse NYHA class (greater than II versus I; P = .08).
Schema with outcomes. Prog indicates disease progression; CC5013, lenalidomide.
Characteristics of deceased patients
| Characteristic . | Patient no. . | |||
|---|---|---|---|---|
| 3 . | 12 . | 14 . | 15 . | |
| Time from diagnosis to entry, mo | 0.5 | 8.1 | 55 | 9.5 |
| Age, y | 59 | 67 | 70 | 44 |
| Time from study entry to death, mo | 0.4 | 3.2 | 1.2 | 0.7 |
| Previous treatment | None | MP | MP, alemtuzemab | Tandem SCT |
| No. of organs involved | 2 | 1 | 2 | 3 |
| History of syncope | No | No | No | Yes |
| NYHA class | 3 | 2 | 1 | 2 |
| Seated blood pressure | 90/60 | 85/40 | 130/78 | 70/50 |
| Creatinine level, mg/dL | 1.2 | 2.5 | 1.0 | 1.4 |
| NT-proBNP level, ng/L | 6178 | 5672 | 345 | 42 844 |
| Troponin T level, μg/L | < 0.01 | 0.1 | < 0.01 | 0.6 |
| Cardiac biomarker stage | 2 | 3 | 2 | 3 |
| Interventricular septum, mm | 12 | 17 | 24 | 14 |
| Left ventricular ejection fraction, % | 55 | 61 | 53 | 22 |
| Characteristic . | Patient no. . | |||
|---|---|---|---|---|
| 3 . | 12 . | 14 . | 15 . | |
| Time from diagnosis to entry, mo | 0.5 | 8.1 | 55 | 9.5 |
| Age, y | 59 | 67 | 70 | 44 |
| Time from study entry to death, mo | 0.4 | 3.2 | 1.2 | 0.7 |
| Previous treatment | None | MP | MP, alemtuzemab | Tandem SCT |
| No. of organs involved | 2 | 1 | 2 | 3 |
| History of syncope | No | No | No | Yes |
| NYHA class | 3 | 2 | 1 | 2 |
| Seated blood pressure | 90/60 | 85/40 | 130/78 | 70/50 |
| Creatinine level, mg/dL | 1.2 | 2.5 | 1.0 | 1.4 |
| NT-proBNP level, ng/L | 6178 | 5672 | 345 | 42 844 |
| Troponin T level, μg/L | < 0.01 | 0.1 | < 0.01 | 0.6 |
| Cardiac biomarker stage | 2 | 3 | 2 | 3 |
| Interventricular septum, mm | 12 | 17 | 24 | 14 |
| Left ventricular ejection fraction, % | 55 | 61 | 53 | 22 |
Data are from time of study entry, unless otherwise indicated. All patients had cardiac involvement.
To convert creatinine level from milligrams per deciliter to micromoles per liter, multiply milligrams per deciliter by 88.4.
NT-proBNP indicates N-terminal pro-brain natriuretic peptide; MP, melphalan and prednisone; and SCT, stem cell transplantation.
After 3 cycles of treatment, only 1 patient had achieved a hematologic response to single-agent lenalidomide therapy. The remaining 11 had dexamethasone added to their program as per protocol design. Despite the high drop-out rate, 45% (10/22) of patients achieved either a hematologic or organ response with study treatment (Figure 2A). Hematologic response was seen in 41% of patients (9 of 22), and organ response was seen in 23% of patients (5 of 22). Four patients had both a hematologic and an organ response, where 1 individual achieved a hematologic response to single-agent lenalidomide within the first 3 cycles. This individual ultimately had both a renal and liver response. On an intention-to-treat basis, of the 9 patients with previously untreated AL, the hematologic response and organ response rates were 44% and 33%, respectively. Of the 13 previously treated patients, the respective response rates were 38% and 15%. Figure 2B also shows the response rates of those patients who remained on the drug for at least 3 months and therefore had the opportunity to have dexamethasone added to their treatment program. Of these, 75% had a hematologic response and 42% had an organ response.
Response to therapy. (A) Intention-to-treat analysis. (B) Patients receiving at least 3 cycles of treatment and fulfilling the design option to have dexamethasone added to the treatment program.
Response to therapy. (A) Intention-to-treat analysis. (B) Patients receiving at least 3 cycles of treatment and fulfilling the design option to have dexamethasone added to the treatment program.
The median time to hematologic response was 6.2 months, whereas the median time to organ response was 9.4 months. Median time to progression was 18.8 months, with the 3-, 6-, and 12-month progression-free survival rates being 75%, 56%, and 56%, respectively. So far, only 1 patient with a hematologic response has progressed at 12 months. This patient never achieved an organ response, and after 12 months of therapy qualified as a renal progression; at that time, he had an unconfirmed hematologic progression.
The toxicity profile for lenalidomide was manageable, although 86% of patients experienced grade 3 or higher adverse events; if one excludes hematologic and skin toxicities, 73% of patients had grade 3 or 4 adverse events. The maximum grade of the most common adverse events is shown in Table 3. Over the course of treatment, the most common grade 3 or 4 AEs considered at least possibly related to therapy were neutropenia, thrombocytopenia, rash, infection, and fatigue. Other grade 3 or 4 events not shown in Table 3 include hyperglycemia, pleurisy, and headache, each of which occurred in 1 patient. A total of 2 patients developed thromboembolic events—1 before dexamethasone was added and 1 after. None of these patients was receiving erythropoietin at the time of the event.
Most common adverse events at least possibly related to treatment (percent of patients having event)
| Adverse event . | Grade . | |||
|---|---|---|---|---|
| 1 . | 2 . | 3 . | 4 . | |
| Thrombocytopenia | 23 | 18 | 18 | 9 |
| Anemia | 9 | 55 | 5 | 0 |
| Neutropenia | 5 | 14 | 27 | 18 |
| Rash | 18 | 23 | 18 | 0 |
| Edema | 18 | 27 | 9 | 0 |
| Infection | 0 | 27 | 18 | 0 |
| Constipation | 27 | 9 | 0 | 0 |
| Diarrhea | 27 | 14 | 0 | 0 |
| Dyspnea | 27 | 5 | 9 | 0 |
| Fatigue | 0 | 14 | 18 | 0 |
| AST | 14 | 9 | 5 | 0 |
| Hypocalcemia | 0 | 14 | 9 | 0 |
| Elevated creatinine level | 9 | 14 | 0 | 0 |
| Low consciousness/cognitive disorder | 0 | 14 | 5 | 0 |
| Neurosensory | 23 | 0 | 0 | 0 |
| Elevated alkaline phosphatase/bilirubin level | 0 | 0 | 5 | 0 |
| Dyspepsia | 0 | 5 | 9 | 0 |
| Hyperkalemia | 0 | 9 | 0 | 0 |
| Hypothyroidism | 5 | 5 | 0 | 0 |
| Muscle weakness | 0 | 14 | 0 | 0 |
| Thrombosis | 0 | 0 | 9 | 0 |
| Adverse event . | Grade . | |||
|---|---|---|---|---|
| 1 . | 2 . | 3 . | 4 . | |
| Thrombocytopenia | 23 | 18 | 18 | 9 |
| Anemia | 9 | 55 | 5 | 0 |
| Neutropenia | 5 | 14 | 27 | 18 |
| Rash | 18 | 23 | 18 | 0 |
| Edema | 18 | 27 | 9 | 0 |
| Infection | 0 | 27 | 18 | 0 |
| Constipation | 27 | 9 | 0 | 0 |
| Diarrhea | 27 | 14 | 0 | 0 |
| Dyspnea | 27 | 5 | 9 | 0 |
| Fatigue | 0 | 14 | 18 | 0 |
| AST | 14 | 9 | 5 | 0 |
| Hypocalcemia | 0 | 14 | 9 | 0 |
| Elevated creatinine level | 9 | 14 | 0 | 0 |
| Low consciousness/cognitive disorder | 0 | 14 | 5 | 0 |
| Neurosensory | 23 | 0 | 0 | 0 |
| Elevated alkaline phosphatase/bilirubin level | 0 | 0 | 5 | 0 |
| Dyspepsia | 0 | 5 | 9 | 0 |
| Hyperkalemia | 0 | 9 | 0 | 0 |
| Hypothyroidism | 5 | 5 | 0 | 0 |
| Muscle weakness | 0 | 14 | 0 | 0 |
| Thrombosis | 0 | 0 | 9 | 0 |
Of the 4 patients who withdrew from the trial due to adverse events, 1 patient withdrew during the first cycle of treatment due to headache (grade 4), dyspepsia (grade 3), diarrhea (grade 1), and rash (grade 2). The other 3 patients discontinued therapy due to persistent cytopenias. As seen in Figure 3, only a minority could tolerate the full dose of lenalidomide 25 mg for 21 of 28 days. By cycle 6, only 4 patients were taking the 25-mg dose, while the remaining 6 patients' doses were distributed between 5 to 15 mg/d. The combination of dexamethasone and lenalidomide was reasonably tolerated. Of the 3 patients who went off the therapy due to adverse events after the dexamethasone was added, the most common cause for discontinuation was cytopenias rather than dexamethasone-specific adverse events. By cycle 6 (ie, 3 months of dexamethasone), 7 of 9 patients taking dexamethasone were still taking the full 40 mg days 1 to 4 and 15 to 18.
Discussion
What conclusions can we draw from this study? First, as a single agent, lenalidomide has modest activity in patients with primary systemic amyloidosis that is significantly enhanced when used in conjunction with dexamethasone. Second, as a single agent the standard myeloma dose of 25 mg per day for 21 of 28 days is too high for this population of patients. Third, levels of NT-proBNP appear to be a useful predictor for early attrition.
Although only 1 of 22 patients treated had a response to single-agent lenalidomide in the first 3 cycles, when combined with dexamethasone the overall hematologic response rate was 41%. If one examines the 12 patients who had dexamethasone added at 3 months, 9 had a hematologic response—a hematologic response rate of 75%, which is similar to the response rates seen in myeloma patients.21,26 With a median of 17 months of follow-up in patients still receiving treatment, organ responses were seen in 23% of patients on an intention-to-treat basis and in 42% of patients who received at least 3 cycles of therapy.
As shown in Table 4, the results of this study are comparable to more recent trials that have evaluated either single-agent thalidomide or combinations of thalidomide and dexamethasone.27-29 Palladini et al27 used a combination of thalidomide and dexamethasone and achieved a hematologic response rate of 59% and an organ response rate of 24%. Our early experience would suggest that the combination of lenalidomide and dexamethasone may be more active than the thalidomide and dexamethasone combination. The response rates of the combination of lenalidomide and dexamethasone should be interpreted with care since ours are not intention to treat in that patients had to remain on the treatment for 3 months in order to receive dexamethasone. Although lenalidomide was associated with as many adverse events as the thalidomide experience, the type of AEs was different, with the thalidomide having more side effects relating to fluid retention and cardiac function, constipation, rash, and cognitive disturbances, while for lenalidomide, cytopenias, rash,31 fatigue, and elevations in liver function tests were most common. These data suggest that lower doses of lenalidomide will be better tolerated than thalidomide, and in combination with dexamethasone, more effective
Comparison of immune modulatory derivatives in patients with AL
| Regimen, source . | No. . | No prior Rx, % . | 2 or more organs, % . | Cardiac, % . | Heme response, % . | Organ response, % . | Median f/u, mo . | Grade 3-4 AEs, % . |
|---|---|---|---|---|---|---|---|---|
| Dex | ||||||||
| Palladini et al7 | 23 | 43 | 48 | 39 | 13 | 30 | 39 | NR |
| Gertz et al8 | 25 | 100 | NR | 68 | 40 | 12 | 18 | NR |
| Gertz et al9 | 19 | 0 | NR | 63 | 53 | 16 | 27 | NR |
| Dex-IFN | ||||||||
| Dhodapkar et al10 | 87 | 84 | 71 | 50 | 27 | 17 | 41 | 46/53‡ |
| Thal/Dex | ||||||||
| Palladini et al27 | 31 | 42 | 61 | 38 | 48 | 26 | 32 | 65 |
| Thal | ||||||||
| Seldin et al28 * | 16 | 6 | 31 | 25 | 25 | 0 | NR | 50 |
| Dispenzieri et al29 * | 12 | 58 | 67 | 42 | 0 | 11 | 2.3§ | 58 |
| Dispenzieri et al30 † | 18 | 28 | 50 | 67 | 0 | 11 | 5.6§ | 75 |
| Len with or without Dex | ||||||||
| Current study | 22 | 43 | 57 | 65 | 43 | 26 | 17 | 83 |
| Regimen, source . | No. . | No prior Rx, % . | 2 or more organs, % . | Cardiac, % . | Heme response, % . | Organ response, % . | Median f/u, mo . | Grade 3-4 AEs, % . |
|---|---|---|---|---|---|---|---|---|
| Dex | ||||||||
| Palladini et al7 | 23 | 43 | 48 | 39 | 13 | 30 | 39 | NR |
| Gertz et al8 | 25 | 100 | NR | 68 | 40 | 12 | 18 | NR |
| Gertz et al9 | 19 | 0 | NR | 63 | 53 | 16 | 27 | NR |
| Dex-IFN | ||||||||
| Dhodapkar et al10 | 87 | 84 | 71 | 50 | 27 | 17 | 41 | 46/53‡ |
| Thal/Dex | ||||||||
| Palladini et al27 | 31 | 42 | 61 | 38 | 48 | 26 | 32 | 65 |
| Thal | ||||||||
| Seldin et al28 * | 16 | 6 | 31 | 25 | 25 | 0 | NR | 50 |
| Dispenzieri et al29 * | 12 | 58 | 67 | 42 | 0 | 11 | 2.3§ | 58 |
| Dispenzieri et al30 † | 18 | 28 | 50 | 67 | 0 | 11 | 5.6§ | 75 |
| Len with or without Dex | ||||||||
| Current study | 22 | 43 | 57 | 65 | 43 | 26 | 17 | 83 |
Rx indicates treatment; Cardiac, cardiac involvement; f/u, follow-up; mo, months; AEs, adverse events; Dex, dexamethasone; IFN, interferon; Thal, thalidomide; and Len, lenalidomide.
200-800 mg.
50-200 mg.
Induction phase/maintenance phase.
Median time on the treatment.
Challenges to interpreting the results of lenalidomide trial include the high attrition rate and the high toxicity rate. The lenient entry criteria and the starting dose of drug account for these issues. Patients with ECOG performance scores of 3, NYHA class III, and high cardiobiomarker stage were deliberately included to test the performance of lenalidomide in a general AL population. Why myelosuppression and rash were so common is not clear, but for future studies using single-agent lenalidomide in patients with AL, the starting dose of lenalidomide should be no higher than 15 mg per day. In clinical practice, one would have the option of immediately starting the combination of lenalidomide along with dexamethasone or waiting 1 cycle and then adding the dexamethasone if there were no hematologic response. Although this study tested a schedule of dexamethasone 40 mg days 1 to 4 and 15 to 18 every 28 days, other schedules may prove to be as effective and even better tolerated. The results of the ongoing ECOG study, which randomizes newly diagnosed myeloma patients to lenalidomide with either standard high-dose dexamethasone (days 1-4, 9-12, and 17-20) or low-dose dexamethasone (days 1, 8, 15, and 22), may be informative in this regard.
So where will this regimen belong in the armamentarium against AL amyloidosis? In newly diagnosed patients who are not candidates for high-dose chemotherapy with peripheral blood stem cell transplantation, the combination of lenalidomide and dexamethasone may be an option as either first- or second-line therapy—albeit with lower starting doses of lenalidomide than that used in this study. For patients who have relapsed after other treatments, the lenalidomide and dexamethasone combination is a viable choice.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Contribution: A.D., M.Q.L., M.A.G., and S.M.G. designed and performed the research, collected and analyzed the data, and wrote the manuscript; S.R.Z., S.R.H., S.K.K., J.A.L., T.E.W., S.V.R., P.R.G., and S.J.R. collected the data, performed research, and critically reviewed the manuscript; and J.B.A. and B.K. designed and performed the research, analyzed data, and critically reviewed the manuscript.
Acknowledgments
Gratitude goes to Ann Birgin and Deb Scott for their work with the patients and data collection and management.
This work was supported in part by the Caligiuri Fund for Amyloidosis Research, Celgene, and the Robert A. Kyle Hematologic Malignancies Program. Supported by National Institutes of Health (NIH) grant CA 91561.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal