Abstract
High-dose melphalan with stem-cell transplantation is believed to be the most effective treatment for systemic light-chain (AL) amyloidosis, but many patients are ineligible because of the extent of their disease, and treatment-related mortality (TRM) remains substantial. We report the use of a risk-adapted oral regimen of cyclophosphamide, thalidomide, and dexamethasone (CTD) or attenuated CTD (CTDa) in 75 patients with advanced AL amyloidosis, including 44 patients with clonal relapse after prior therapy. Fifty-one (68%) patients received CTD and 24 (32%) received CTDa. A hematologic response occurred in 48 (74%) of 65 evaluable patients, including complete responses in 14 (21%) and partial responses in 34 (53%) cases. Median estimated overall survival (OS) from commencement of treatment was 41 months, and from diagnosis median was not reached with a median follow-up of 22 months. Three-year estimated OS was 100% and 82% among complete and partial hematologic responders, respectively. Toxicity necessitating cessation of therapy occurred in 8% and was at least grade 2 in 52% of patients. TRM was 4%. The clonal response rates to CTD reported here are higher than any previously reported nontransplantation regimen in AL amyloidosis, and risk adaptation allows its use in poorer risk patients. CTD merits prospective randomized study.
Introduction
Systemic light-chain amyloidosis (AL) is the most common systemic type with an age-adjusted incidence of 5.1 to 12.8 per million patient years.1 Treatment comprises chemotherapy regimens derived from use in myeloma, and the prognosis remains poor for patients with AL amyloidosis whose underlying plasma-cell dyscrasia (PCD) cannot be suppressed or relapses after therapy. Outcomes following treatment with conventional alkylator-based oral regimens have been poor with fewer than 1 in 5 patients responding to treatment, and the median overall survival being fewer than 2 years.2 Although response rates are much greater following high-dose melphalan and autologous stem cell transplantation (SCT),3 such therapy is limited by high treatment-related mortality (TRM),4 even among the approximately 50% of patients who are considered well enough to be eligible for this intensive approach. Furthermore, the role of SCT in relapsed disease is not well studied. Recent data from our center and other centers suggest that equivalent outcomes can be obtained with intermediate-dose chemotherapy regimens, such as vincristine, adriamycin, and dexamethasone (VAD)5 or intermediate-dose infusional melphalan,6 and modified conventional regimens, such as oral melphalan and dexamethasone,7 although there have been very few direct comparisons. There is certainly no consensus at present on the best treatment for patients with AL amyloidosis.
The discovery of thalidomide as an effective agent in myeloma has ushered in a new era of combination therapies for PCDs.8 In amyloidosis, single-agent thalidomide in “standard” higher doses is tolerated poorly, with most patients discontinuing treatment within 3 months.9,10 Lower doses are better tolerated, but adequate hematologic responses occur rarely when thalidomide is administered alone.11 Addition of dexamethasone to thalidomide improves the hematologic response rate to about 50% but at the cost of increased toxicity, affecting some 60% of patients.12 In myeloma the oral combination of cyclophosphamide, thalidomide, and dexamethasone (CTD) has been evaluated in a number of small studies in relapsed and newly diagnosed cases, in which it has produced hematologic response rates of 61% to 71%, and it has been relatively well tolerated.13,14 Different versions of this regimen have been reported using cyclophosphamide either daily or weekly with similar response rates. Growing optimism has led to CTD being studied as induction therapy for newly diagnosed myeloma in the current UK MRC Myeloma IX trial, including a dose-attenuated version (CTDa) for elderly or poor-risk patients.
We report here the outcome of 75 patients with systemic AL amyloidosis who were treated with CTD. Despite advanced amyloid in many cases, TRM was very low, whereas clonal response rates were higher than any non-transplantation regimen described to date in this disease.
Patients, materials, and methods
Patients, diagnosis, and protocol
The analysis was carried out at the National Amyloidosis Centre (NAC), United Kingdom. In accordance with guidelines in the United Kingdom on the treatment of AL amyloidosis,15 the vast majority of patients attending the NAC since 2000 received intermediate-dose non-transplantation chemotherapy regimens. Since such time, the CTD regimen adapted from the current UK MRC Myeloma IX trial has been included among the offered treatment options. The patients reported here include all patients with AL amyloidosis who were treated with CTD or attenuated CTD (CTDa) between 2000 and 2005 and who underwent systematic prospective evaluation at the NAC. CTD was given as first-line therapy in 31 patients and for refractory or relapsed underlying clonal disease in 44 cases. Only patients with predominant and severe autonomic or peripheral neuropathy were not deemed eligible to receive this regimen because of the potential for exacerbation by thalidomide.
The presence of amyloid was confirmed by characteristic birefringence after Congo red staining of a tissue biopsy, a diagnostic serum amyloid P component (SAP) scan, or both. AL-type amyloidosis was confirmed by immunohistochemical staining when possible and otherwise by characteristic clinical and scintigraphic appearances, supported by demonstration of a plasma-cell dyscrasia and, when necessary, by exclusion of hereditary amyloidosis by demonstration of wild-type sequence for the genes encoding known hereditary amyloidogenic proteins.16 Patients attended the NAC for their initial diagnostic evaluation and were followed up at 6-month intervals for evaluation of clonal disease and organ responses and for whole-body amyloid load by SAP scintigraphy. Blood samples were requested at monthly intervals during CTD treatment and at 2-month intervals thereafter for monoclonal immunoglobulin measurements, which included the serum free light chain (FLC) assay (Freelite; The Binding Site, Birmingham, United Kingdom). The novel medical care described here was performed with informed consent from each patient in accordance with the Declaration of Helsinki, and the study was performed with institutional review board approval by the Royal Free Hospital ethics committee.
Treatment
The CTD regimen was adapted from the current UK MRC Myeloma IX trial and consisted of a 21-day cycle of oral cyclophosphamide 500 mg once weekly, thalidomide 200 mg/day (starting dose, 100 mg/day, increased after 4 weeks if tolerated) continuously, and dexamethasone 40 mg days 1 to 4 and 9 to 12. This was risk attenuated in elderly patients (older than 70 years), in those with heart failure exceeding New York Heart Association (NYHA) class II, and those with significant fluid overload. The attenuated regimen (CTDa) consisted of a 28-day cycle of cyclophosphamide 500 mg days 1, 8, and 15; thalidomide 200 mg/day (starting dose, 50 mg/day, increased by 50 mg at 4-week intervals as tolerated); and dexamethasone 20 mg days 1 to 4 and days 15 to 18. Treatment was given at the referring hospital, and antimicrobial and thromboprophylaxis were given according to local protocol. Patients did not receive routine thromboprophylaxis. Treatment was given until a stable clonal response was achieved on consecutive samples at least 4 weeks apart, or the patient was confirmed as unresponsive to treatment. Thalidomide maintenance therapy was only considered for responders and was decided on by a combination of patient preference and tolerance to treatment.
Outcome measures
Primary outcome measures were hematologic responses and toxicity. Additional outcome variables were overall survival (OS), event-free survival (EFS), organ response rates, and the course of whole-body amyloid burden by serial SAP scintigraphy. Hematologic response was assessed by serum and urine electrophoresis and immunofixation and also by FLC. FLC response is a strong predictor of survival in AL amyloidosis17 and a reliable early marker in myeloma which correlates with clonal plasma-cell burden.18 Conventional response and relapse was defined according to the Blade criteria.19 FLCs were considered interpretable for assessing response if the pretreatment FLC κ/λ ratio was outside the 95% reference range (0.3-1.2)20 and the concentration of the light chain class (ie,κ or λ]) containing the monoclonal component (also called monoclonal class) was at least twice the upper limit of 95% of the reference range for that class (except in renal failure in which only the κ/λ ratio was used). A FLC partial response (PR) was defined as at least a 50% fall in the monoclonal class; a FLC complete response (CR) was defined as normalization of the FLC ratio and both light chain classes, unless there was renal failure causing polyclonal retention of FLC, in which case the ratio alone was used. Minor response has not been defined for FLC; hence, any change which could not be classed as FLC-PR or FLC-CR and patients with clonal disease progression were together labeled as nonresponders. Clonal relapse or progression was classified as an event (EFS) and defined as per the Blade criteria for conventional serum and urine monoclonal protein measurements and for FLC as follows: from FLC-CR as a newly abnormal ratio with a doubling of the monoclonal class, and from FLC-PR as at least a 50% rise in the monoclonal class. The response was assessed as the best-achieved response at least 3 months after completion of CTD therapy. Among patients who subsequently received maintenance therapy with single-agent thalidomide, clonal response was assessed after the last cycle of CTD. Progression-free survival was defined as the time to clonal relapse or death as a result of progressive amyloidosis.
Toxicity was recorded according to the National Cancer Institute Cancer Therapy Evaluation Program, Common Terminology Criteria for Adverse Events (Version 3.0).21
Amyloidotic organ involvement and responses were defined according to the international consensus criteria.22 Responses were assessed 6 months following CTD therapy and before further treatment was delivered. Performance status was assessed as described by the Eastern Cooperative Oncology Group (ECOG) criteria.23
All patients underwent 123I-labeled SAP scintigraphy, and serial studies were used to quantitatively measure the whole-body amyloid load, as previously described.24 Prior to commencement of CTD chemotherapy additional organ involvement that is not encompassed by the amyloidosis consensus criteria was identified by SAP scintigraphy in 22 (29%) cases. Labeled SAP studies were interpreted by a single physician (P.N.H.) with experience of more than 4000 SAP scans.
Statistics
Statistical analysis was undertaken using the SPSS 14 software package (SPSS, Chicago, IL). Survival was assessed by the method of Kaplan and Meier and compared by log-rank test. Categorical variables were compared with chi-square or Fisher tests as appropriate. All P values were 2 sided with a significance level of .05. Multivariate analysis was by Cox or binary logistic regression as appropriate.
Results
Patients
Seventy-five patients received CTD or CTDa between January 2000 and August 2005, among a total of 1577 patients with confirmed AL amyloidosis evaluated at the NAC during this period. Features at the time of commencement of CTD or CTDa therapy are summarized in Table 1. Median age was 60 years (range, 30-81 years). Twelve patients (16%) were dialysis dependent. Twenty-five (33%) patients had interventricular septal thickness of at least 15 mm and typical amyloid echocardiograms, among whom 8 (10%) had heart failure of at least NYHA class III. More than 2 organs were involved in 25 (33%) patients. Forty-six (61%) patients had ECOG performance status of at least 2. Among those receiving CTD as first-line therapy, the median performance score was 2 (range, 1-3) and the median number of organs involved by amyloid at commencement of therapy was 2 (range, 1-3). Thirty-nine (52%) of the 75 patients would not have been eligible for SCT according to the criteria published by the Mayo Group.25
Patient characteristics at commencement of CTD chemotherapy
| . | Value . |
|---|---|
| Age, y, median (range) | 60 (29-81) |
| Sex, male-to-female ratio | 0.9:1 |
| Monoclonal light chain, no. patients (%) | |
| κ | 23 (31) |
| λ | 52 (69) |
| Hemoglobin level, g/L, median (range) | 12.0 (8.2-18.9) |
| Platelet count, × 109/L, median (range) | 248 (89-875) |
| Creatinine level, μM, median (range) | 96 (56-ESRD) |
| Albumin level, g/L, median (range) | 38 (14-55) |
| Bilirubin level, mM, median (range) | 8 (4-64) |
| Alkaline phosphatase level, IU/L, median (range) | 110 (43-1357) |
| 24-h proteinuria, g/24 h, median (range) | 1.8 (<0.1-11) |
| Creatinine clearance, mL/min, median (range) | 58 (ESRD-140) |
| Organ involvement, no. patients (%) | |
| Liver, consensus criteria | 18 (24) |
| Liver, SAP scintigraphy | 32 (43) |
| Renal, consensus criteria | 48 (64) |
| Renal, SAP scintigraphy | 56 (75) |
| Cardiac, any involvement | 44 (59) |
| Cardiac, IVS ≥ 15 mm | 25 (33) |
| Peripheral neuropathy | 0 |
| Total number of organs, international consensus criteria, no. patients (%) | |
| 1 | 16 (22) |
| 2 | 34 (45) |
| 3 or more | 25 (33) |
| ECOG performance status, no. patients (%) | |
| No more than 1 | 29 (39) |
| 2 | 36 (48) |
| At least 3 | 10 (13) |
| . | Value . |
|---|---|
| Age, y, median (range) | 60 (29-81) |
| Sex, male-to-female ratio | 0.9:1 |
| Monoclonal light chain, no. patients (%) | |
| κ | 23 (31) |
| λ | 52 (69) |
| Hemoglobin level, g/L, median (range) | 12.0 (8.2-18.9) |
| Platelet count, × 109/L, median (range) | 248 (89-875) |
| Creatinine level, μM, median (range) | 96 (56-ESRD) |
| Albumin level, g/L, median (range) | 38 (14-55) |
| Bilirubin level, mM, median (range) | 8 (4-64) |
| Alkaline phosphatase level, IU/L, median (range) | 110 (43-1357) |
| 24-h proteinuria, g/24 h, median (range) | 1.8 (<0.1-11) |
| Creatinine clearance, mL/min, median (range) | 58 (ESRD-140) |
| Organ involvement, no. patients (%) | |
| Liver, consensus criteria | 18 (24) |
| Liver, SAP scintigraphy | 32 (43) |
| Renal, consensus criteria | 48 (64) |
| Renal, SAP scintigraphy | 56 (75) |
| Cardiac, any involvement | 44 (59) |
| Cardiac, IVS ≥ 15 mm | 25 (33) |
| Peripheral neuropathy | 0 |
| Total number of organs, international consensus criteria, no. patients (%) | |
| 1 | 16 (22) |
| 2 | 34 (45) |
| 3 or more | 25 (33) |
| ECOG performance status, no. patients (%) | |
| No more than 1 | 29 (39) |
| 2 | 36 (48) |
| At least 3 | 10 (13) |
ESRD indicates end-stage renal disease; IVS, interventricular septal thickness.
Treatment and response
Fifty-one (68%) patients received CTD and 24 (32%) received CTDa. The median time from diagnosis to treatment with full-dose or attenuated CTD for all patients was 4.3 months (range, 1.7-104 months); it was 1.6 months in patients with newly diagnosed AL amyloidosis, and 13.6 months in relapsed patients. The number of prior regimens was none in 31 (41%) patients, 1 in 31 (41%) cases, 2 in 10 (13%) cases, 3 in 3 (4%) cases. Ten (13%) patients had undergone a prior autologous stem cell transplantation. The median dose of thalidomide was 100 mg (range, 50-200 mg), dexamethasone was 20 mg (range, 2-40 mg), and cyclophosphamide was 500 mg (range, 300-500 mg). Median follow-up from the start of treatment was 18 months (range, 1.4-48 months) and from diagnosis 22 months (range, 4-112 months). Patients received a median of 4 cycles (range, 1-12 cycles) of CTD or CTDa. Nineteen (27%) patients continued with thalidomide maintenance.
The underlying PCD was evaluable for response in 65 (87%) patients. Sixty (80%) patients were evaluable by FLC measurements, and 30 by conventional monoclonal protein measurements. In 10 cases the pretreatment FLC concentration, although abnormal, was less than twice the upper limit of normal, although 5 of these patients were evaluable by conventional criteria. Five further patients were not evaluable either because data were incomplete or because insufficient time had elapsed after completing CTD therapy. Responses are shown in Table 2. Forty-eight (74%) evaluable patients responded, with CR in 14 (21%) and PR in 34 (53%) cases. An FLC response was seen in 43 (72%) of 60 patients, with a CR by FLC criteria in 19 (32%) and PR in 24 (40%). A conventional paraprotein response was seen in 22 (73%) of 30 evaluable patients, with a CR in 1 (3%) and a PR in 21 (70%) cases. Among 5 patients with CR by FLC assessment who had a response assessable by conventional criteria, 4 had a PR by conventional criteria. There was no discrepancy between nonresponders by either criteria. Of the patients given CTD, 76% responded as compared with 61% of those given CTDa (P = .331). Among the 34 responders in whom samples were provided for monthly FLC assays, a response (at least PR) occurred by the end of the first, second, and third months of treatment in 50%, 76%, and 100% of cases, respectively. On univariate analysis, receiving treatment for longer than 2 months was a significant positive factor for hematologic response (P = .022; OR, 2.6). On multivariate analysis, the only independent factor (negatively) affecting hematologic response was performance status (P = .02; odds ratio [OR], 0.005), whereas other factors, including organ involvement, light chain isotype, CTD dose attenuation, or development of toxicity, were not significant. There was no significant difference in hematologic response rate between newly diagnosed and relapsed patients.
Clonal response to chemotherapy by serum free light chain (FLC) assay, conventional immunofixation electrophoresis (IFE), and combined FLC plus IFE
| . | FLC response . | Conventional response . | Combined response . |
|---|---|---|---|
| Total n | 60 | 30 | 65 |
| CR, n (%) | 19 (32) | 1 (3) | 14 (21) |
| PR, n (%) | 24 (40) | 21 (70) | 34 (53) |
| Total response, n (%) | 43 (72) | 22 (73) | 48 (74) |
| . | FLC response . | Conventional response . | Combined response . |
|---|---|---|---|
| Total n | 60 | 30 | 65 |
| CR, n (%) | 19 (32) | 1 (3) | 14 (21) |
| PR, n (%) | 24 (40) | 21 (70) | 34 (53) |
| Total response, n (%) | 43 (72) | 22 (73) | 48 (74) |
Numbers in rows do not add up as patients are overlapping or in different response categories for FLC and conventional responses.
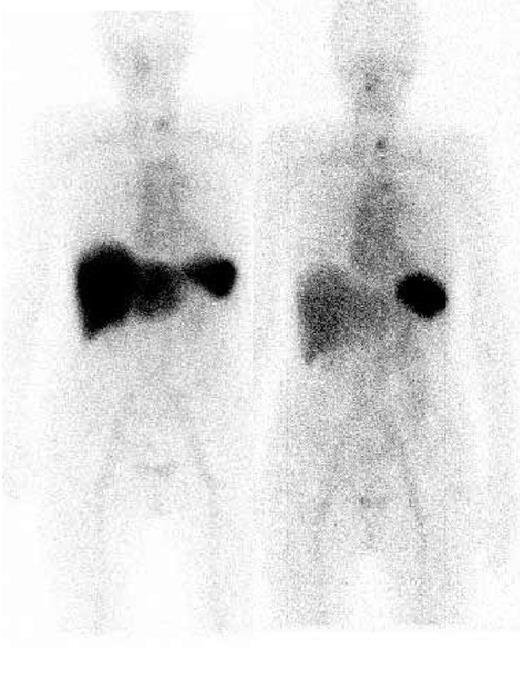
Sixty patients were evaluable for organ responses. Organ responses occurred in 15 (31%) of 48 hematologic responders compared with 1 (5%) hematologic nonresponder (P < .001), a patient who had improvement of serum FLC but less than 50%. Among 11 cases in whom renal function improved, the median decrease in proteinuria from the onset of treatment was 76% (range, 50%-95%). Hepatic function improved in 5 cases, including complete normalization of the liver function tests which included alkaline phosphatase in 2 patients, and the function of other organ systems improved in 6 cases, with some patients showing improvement in more than 1 organ. No patient with end-stage renal failure became dialysis independent following CTD chemotherapy, and no objective cardiac responses were observed. Regression of amyloid deposits by serial SAP scintigraphy was recorded in 16% (7 of 43) of hematologic responders but was not in any nonresponder. Regression of amyloid from the liver (Figure 1) was observed in every patient in whom there was improvement in liver function, whereas regression from the kidneys was only observed in 2 of 11 patients with improvement in renal function, the remainder showing stable deposits.
Serial 123I-labeled anterior whole-body SAP scintigraphy. Visceral amyloid deposits in the spleen and liver pretreatment (left) are shown. Six months after CTD treatment (right), which resulted in a complete clonal response, marked regression of amyloid from the liver was evident.
Serial 123I-labeled anterior whole-body SAP scintigraphy. Visceral amyloid deposits in the spleen and liver pretreatment (left) are shown. Six months after CTD treatment (right), which resulted in a complete clonal response, marked regression of amyloid from the liver was evident.
Survival
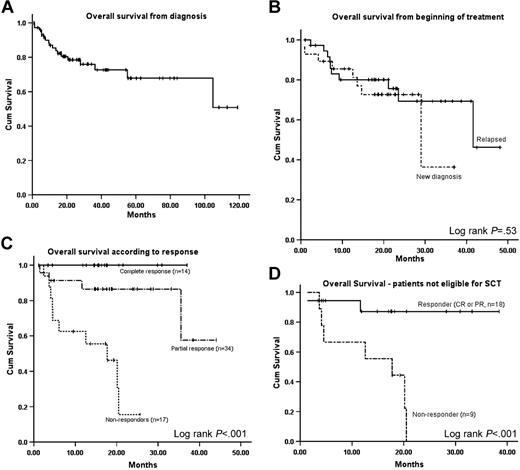
At censor, the Kaplan-Meier estimated median OS for the whole cohort from diagnosis was not reached (Figure 2A). The median OS from commencement of CTD chemotherapy was 41 months (Figure 2B). Patients who had a FLC response had markedly better OS than patients who had less than 50% FLC response (median not reached versus 17 months; P < .001). The estimated 3-year survival (Figure 2C) was 100% for patients achieving a CR (by both conventional and FLC criteria), 82% for those achieving a PR, and nil (median, 17 months) for hematologic nonresponders (P < .001, log-rank test). The median OS in patients not eligible for SCT (Figure 2D) by the Mayo Group criteria25 was 17 months for nonresponders and not reached among responders (P < .001). Factors affecting OS from the end of treatment are shown in Table 3. The only independent factors affecting OS from the end of treatment were ECOG performance status and hematologic response to treatment. Previous treatment(s) was not a significant factor affecting OS. Median EFS from the end of CTD therapy was 21 months. There was no significant difference in EFS between patients with a hematologic CR or PR. The only significant factors affecting EFS on multivariate analysis was receiving thalidomide maintenance (P = .032; OR 3.6) (Table 3). There was no significant effect of thalidomide maintenance on OS.
Overall survival and effect of pretreatment status and hematologic response on survival. (A) Overall survival for all patients from diagnosis of amyloidosis. (B) Overall survival from beginning of CTD chemotherapy stratified by newly diagnosed and relapsed/refractory patients. (C) Overall survival from end of CTD chemotherapy according to degree of hematologic response. (D) Effect of pretreatment eligibility for stem cell transplantation on overall survival from the end of CTD chemotherapy, stratified by hematologic response.
Overall survival and effect of pretreatment status and hematologic response on survival. (A) Overall survival for all patients from diagnosis of amyloidosis. (B) Overall survival from beginning of CTD chemotherapy stratified by newly diagnosed and relapsed/refractory patients. (C) Overall survival from end of CTD chemotherapy according to degree of hematologic response. (D) Effect of pretreatment eligibility for stem cell transplantation on overall survival from the end of CTD chemotherapy, stratified by hematologic response.
Factors affecting overall survival from the end of CTD chemotherapy
| Factor . | Univariate significance (OR; 95% CI) . | Multivariate significance (OR; 95% CI) . |
|---|---|---|
| Overall survival from the end of treatment | ||
| Number of organs | 0.69 (1.1; 0.5-2.1) | — |
| Performance status | < 0.0001 (5.2; 2.2-12.4)* | 0.01 (9.6; 1.9-16.2)* |
| Cardiac involvement | 0.30 (0.5; 0.1-1.8) | — |
| Liver involvement | 0.008 (4.1; 1.4-11.6) | NS |
| Renal involvement | 0.42 (1.5; 0.5-4.5) | — |
| Amyloid load on SAP scan | 0.082 (0.79; 0.61-1) | — |
| Any previous treatment | 0.30 (1.7; 0.58-5.4) | — |
| At least 2 mo on treatment | 0.002 (6.22; 1.9-19.4)* | NS |
| CTD or CTDa | 0.071 (1.4; 0.97-2.1) | — |
| Organ response | 0.14 (1.8; 0.81-4.22) | — |
| Any toxicity | 0.33 (1.7; 0.5-5.1) | — |
| Hematologic response | 0.002 (12.6; 2.6-60.3)* | 0.048 (6.3; 1.1-39.2)* |
| Event-free survival | ||
| Any previous treatment | 0.99 (0.99; 0.42-2.3) | — |
| No. of prior treatments | 0.87 (0.95; 0.56-1.5) | — |
| CTD or CTDa | 0.15 (1.2; 0.92-1.6) | — |
| Thalidomide maintenance | 0.033 (3.2; 1-9.7)* | 0.032 (3.6; 1.1-11.5)* |
| Toxicity | 0.98 (1; 0.44-2.2) | — |
| Factor . | Univariate significance (OR; 95% CI) . | Multivariate significance (OR; 95% CI) . |
|---|---|---|
| Overall survival from the end of treatment | ||
| Number of organs | 0.69 (1.1; 0.5-2.1) | — |
| Performance status | < 0.0001 (5.2; 2.2-12.4)* | 0.01 (9.6; 1.9-16.2)* |
| Cardiac involvement | 0.30 (0.5; 0.1-1.8) | — |
| Liver involvement | 0.008 (4.1; 1.4-11.6) | NS |
| Renal involvement | 0.42 (1.5; 0.5-4.5) | — |
| Amyloid load on SAP scan | 0.082 (0.79; 0.61-1) | — |
| Any previous treatment | 0.30 (1.7; 0.58-5.4) | — |
| At least 2 mo on treatment | 0.002 (6.22; 1.9-19.4)* | NS |
| CTD or CTDa | 0.071 (1.4; 0.97-2.1) | — |
| Organ response | 0.14 (1.8; 0.81-4.22) | — |
| Any toxicity | 0.33 (1.7; 0.5-5.1) | — |
| Hematologic response | 0.002 (12.6; 2.6-60.3)* | 0.048 (6.3; 1.1-39.2)* |
| Event-free survival | ||
| Any previous treatment | 0.99 (0.99; 0.42-2.3) | — |
| No. of prior treatments | 0.87 (0.95; 0.56-1.5) | — |
| CTD or CTDa | 0.15 (1.2; 0.92-1.6) | — |
| Thalidomide maintenance | 0.033 (3.2; 1-9.7)* | 0.032 (3.6; 1.1-11.5)* |
| Toxicity | 0.98 (1; 0.44-2.2) | — |
NS indicates not significant; —, not tested.
Statistically significant.
Toxicity
Toxicities of at least grade 2 were observed in 39 (52%) patients, with all patients reporting grade 1 toxicities. Toxicities are summarized in Table 4. Fatigue and constipation (grade 1) were reported by all patients. Most patients needed an increase in the diuretic dosage during treatment, but this was most often needed during the dexamethasone component of the regimen. Symptomatic bradycardia or other treatment-related cardiac arrhythmias were not seen in this cohort. Toxicity occurred more frequently in patients receiving CTD (60%) than CTDa (50%) (P = .02). On multivariate analysis, receiving full-dose CTD was the only significant independent factor in development of at least grade 2 toxicity (P = .029). Interestingly, there was no correlation between involvement of a particular organ by amyloid and associated toxicity. Toxicity of grade 3 or greater, needing dose reduction or regimen discontinuation, was seen in 24 (32%) patients and led to omission of dexamethasone in 11 (15%), cyclophosphamide in 4 (6%), and thalidomide in 3 (4%) cases. The chemotherapy regimen was discontinued in 6 (8%) patients within 8 weeks. Two (3%) patients had nonfatal but serious thrombotic complications during treatment (both pulmonary emboli). One such patient discontinued thalidomide, and the other patient continued treatment with adequate anticoagulation and remains on thalidomide maintenance. There were 3 (4%) possible treatment-related deaths: one with multiorgan failure after an infection and 2 with massive gastrointestinal bleeding (1 patient with proven and another with unproven but almost certain gastrointestinal amyloid).
Toxicity of CTD chemotherapy
| Side effect ≥ grade 2 . | No. of patients (%) . |
|---|---|
| Fluid retention or worsening congestive heart failure | 16 (21) |
| Tiredness or sleepiness | 30 (40) |
| Peripheral neuropathy | 4 (5) |
| Tremor | 2 (3) |
| Cytopenia | 4 (5) |
| Infections | 5 (7) |
| Constipation | 6 (8) |
| Dizziness | 2 (3) |
| Thrombosis | 2 (3) |
| Gastrointestinal bleeding | 3 (4) |
| Side effect ≥ grade 2 . | No. of patients (%) . |
|---|---|
| Fluid retention or worsening congestive heart failure | 16 (21) |
| Tiredness or sleepiness | 30 (40) |
| Peripheral neuropathy | 4 (5) |
| Tremor | 2 (3) |
| Cytopenia | 4 (5) |
| Infections | 5 (7) |
| Constipation | 6 (8) |
| Dizziness | 2 (3) |
| Thrombosis | 2 (3) |
| Gastrointestinal bleeding | 3 (4) |
Discussion
This is the first report of the safety and efficacy of CTD or CTDa combination chemotherapy in a series of patients with systemic AL amyloidosis, and the resulting hematologic response rates of 74% are higher than for any previously reported non–stem-cell transplantation regimen in this disease. Also note that hematologic responses were rapid, with all responses having occurred within 3 months of commencing chemotherapy. Table 5 summarizes the previously reported clonal response rates and toxicity with standard chemotherapy regimens and following stem-cell transplantation in AL amyloidosis.
Hematologic response rates and toxicity of CTD, thalidomide, and intermediate-dose chemotherapy regimens in AL amyloidosis
| Study . | Regimen . | No. of patients . | Hematologic summated partial and complete response rates, % . | Grade 3 or greater toxicity, % . | Treatment-related mortality, % . |
|---|---|---|---|---|---|
| Dispenzieri et al * (2004)11 | Low-dose thalidomide | 18 | Nil | 17 | NR |
| Dispenzieri et al* (2003)9 | Thalidomide (full dose) | 12 | Nil | 50 | NR |
| Seldin et al* (2003)10 | Thalidomide (full dose) | 16 | 5 | 25 | NR |
| Palladini et al (2005)12 | Thalidomide/dexamethasone | 31 | 48 | 65 | NR |
| Goodman et al (2005)5 | VAD† | 229 | 61 | — | 5 |
| Goodman et al (2004)6 | IDM‡ | 144 | 54 | — | 12 |
| Sanchorawala et al (2005)28 | SCT§ | 66 | 88 | — | 14 |
| Present study | CTD or CTDa | 75 | 74 | 32 | 4 |
| Study . | Regimen . | No. of patients . | Hematologic summated partial and complete response rates, % . | Grade 3 or greater toxicity, % . | Treatment-related mortality, % . |
|---|---|---|---|---|---|
| Dispenzieri et al * (2004)11 | Low-dose thalidomide | 18 | Nil | 17 | NR |
| Dispenzieri et al* (2003)9 | Thalidomide (full dose) | 12 | Nil | 50 | NR |
| Seldin et al* (2003)10 | Thalidomide (full dose) | 16 | 5 | 25 | NR |
| Palladini et al (2005)12 | Thalidomide/dexamethasone | 31 | 48 | 65 | NR |
| Goodman et al (2005)5 | VAD† | 229 | 61 | — | 5 |
| Goodman et al (2004)6 | IDM‡ | 144 | 54 | — | 12 |
| Sanchorawala et al (2005)28 | SCT§ | 66 | 88 | — | 14 |
| Present study | CTD or CTDa | 75 | 74 | 32 | 4 |
NR indicates not reported; —, not tested.
FLC responses not assessed.
Vincristine, Adriamycin, dexamethasone.
Intravenous intermediate-dose melphalan (25 mg/m2).
Autologous stem cell transplantation.
Hematologic response to treatment is a strong predictor of survival in AL amyloidosis. Early series demonstrated prolonged OS among patients with AL amyloidosis who achieved hematologic responses by immunofixation electrophoresis (IFE) and bone marrow biopsy.2,26 Improved hematologic response rates and OS compared with historical controls were demonstrated among patients receiving high-dose melphalan and stem-cell rescue (SCT).27 A greater than 50% FLC response after chemotherapy was shown by our group to be associated with significantly prolonged OS in patients with AL amyloidosis, irrespective of the treatment regimen used.17 More recently, studies have suggested that deeper clonal responses are associated with improved OS.28,29 The Boston investigators reported that decreases in FLC are more readily detected early after chemotherapy in patients with AL amyloidosis than changes in IFE and that a greater than 90% reduction in FLC concentration predicts favorable long-term outcome after SCT regardless of PR or CR by IFE.28 More recently, the Mayo Clinic investigators reported that achieving low absolute FLC concentrations after SCT predicts improved OS among patients with AL amyloidosis.29
Despite the high clonal response rates with SCT in AL amyloidosis, TRM has consistently remained around 13% in all reported US studies and has been considerably higher in all European series.30,31 The increased risks of SCT in AL amyloidosis compared with multiple myeloma are underpinned by the organ dysfunction and reduced functional reserve induced by the presence of amyloid deposits. In the large Boston series32 (in which the TRM with SCT was 13%) 394 (56%) of 701 consecutive patients with AL amyloidosis were considered eligible for SCT, but because of disease progression or patient choice only 312 (45%) initiated treatment. Despite an impressive median OS of 4.6 years among the 45% of patients who initiated treatment, the median OS was only 4 months among the 44% ineligible patients. The median OS of all 701 patients was not specified in that study. It is likely that careful patient selection could reduce TRM,4 but this would also limit SCT to a small proportion of patients with AL amyloidosis. The challenge in AL amyloidosis is therefore to achieve, rapidly after commencement of chemotherapy, a high rate and depth of hematologic response while minimizing TRM.
Conventional chemotherapy regimens such as oral melphalan and prednisolone have poor response rates, take a long time to achieve a response, and are associated with a poor long-term outcome in the majority of treated patients with AL amyloidosis.2,26 Adding more alkylating agent does not improve survival or responses.33 A SWOG study of high-dose single-agent dexamethasone had better responses than the earlier regimens and appeared to show an improvement in outcome.34 We recently reported in abstract form good responses to VAD5 and intermediate-dose intravenous melphalan,6 and similar response rates were reported with oral melphalan and dexamethasone.7 Thalidomide is an oral agent with a mechanism of action that differs from standard chemotherapy agents.35 Hematologic responses occur in 30% to 50% of patients with relapsed multiple myeloma using single-agent thalidomide36 and are increased by the addition of dexamethasone37 or when used in combination with a variety of other agents.13,14,38 In AL amyloidosis, single-agent thalidomide is not effective in low doses11 and poorly tolerated in higher doses9 with 50% experiencing grade 3 or 4 toxicities. Palladini et al12 reported improved rates of hematologic response (48%) with combination thalidomide and dexamethasone (thal-dex) but at least grade 3 toxicity in 64% of patients. A risk-adapted strategy has been successful in reducing TRM with SCT in AL amyloidosis and in allowing delivery of high-dose treatment to patients who would not otherwise be eligible.4 The risk-adapted CTD regimen reported here permitted treatment of patients who would not have tolerated full-dose thalidomide, dexamethasone, or both.
The overall hematologic response rate of 74% seen in the current study with CTD or CTDa chemotherapy is higher than any previously reported in AL amyloidosis in a non-transplantation setting. A relatively low median dose of thalidomide (100 mg), ineffective as monotherapy,11 was used in the current study. Importantly, the hematologic responses to CTD in this series were rapid, with 50% of responses occurring within 1 month, 76% by 2 months, and the remainder by 3 months from commencement of CTD chemotherapy. Although continuing treatment beyond 3 months in patients not achieving a response is probably futile, the merit of continuing treatment for longer in responders is not known and needs to be studied because there is some evidence that total thalidomide dose has an effect on survival in myeloma36 and also possibly in amyloidosis.12 Performance status was the only independent factor (negatively) affecting the response rate, probably accounted for by the positive correlation between better performance status and continuing treatment for longer than 2 months. Note that there was no significant difference in response rates, EFS, or OS from the end of treatment between newly treated and relapsed patients. The reasons for this remain unknown but are likely to reflect the biologic characteristics of the underlying plasma-cell clone and the unique mechanism of actions of thalidomide.
The median OS of the current cohort from diagnosis was not reached and was estimated at 41 months from commencement of CTD chemotherapy; median follow-up was 22 months from diagnosis and 18 months from start of chemotherapy. The specific effect of CTD on survival is difficult to assess and needs to be interpreted with caution because of the possible contribution of prior or subsequent treatments. The only independent factors affecting OS were performance status and hematologic response to treatment. The median OS was significantly better in patients achieving a greater than 50% reduction in FLC (P < .001) and better still for patients in CR with a 100% estimated 3-year survival. This provides further support to our previously published observations that a greater than 50% FLC response remains a strong predictor of improvement in survival.17 Although there were too few patients in the current series to make a meaningful comparison between patients having 50% to 90% FLC response versus greater than 90% FLC response, the excellent survival of patients achieving CR appears to lend support to the observation from SCT data of better outcomes in patients with a greater than 90% FLC response.28,29 In contrast with myeloma whereby patients who do not attain a CR after a first autologous stem cell transplantation appear to benefit from a second stem-cell transplantation and improvement of response,39 there are no data to answer the question of merit in continuing with therapy until attainment of a CR in patients with amyloidosis, an area that needs addressing as part of a prospective randomized trial.
The estimated median event-free survival in this series was 21 months and, unlike OS, was significantly prolonged by thalidomide maintenance, an observation which needs confirmation in a larger series. The durability of hematologic responses in AL amyloidosis with intermediate-dose chemotherapy regimens such as VAD and CTD may well be reduced when compared with (higher dose) melphalan-based chemotherapy regimens, another area for investigation by a prospective randomized study. Organ responses were observed in 27% evaluable patients in this series, with renal improvement being most frequent. Objective cardiac improvement was not seen in this study. The relatively poor rate of organ responses is likely to reflect, in part, the short duration of follow-up, because improvements are often substantially delayed after chemotherapy in patients with AL amyloidosis.
Despite the poor-risk cohort, it was encouraging that 68% of patients were able to continue with the regimen without dose modification. All patients reported grade 1 side effects. Side effects (≥ grade 2 toxicity) were seen in 52% of patients with 32% developing at least grade 3 toxicity despite the risk-adapted strategy. This is favorable compared with the reported toxicity profile of single-agent dexamethasone (52% ≥ grade 3 toxicity)34 or thal-dex (64% ≥ grade 3 toxicity)12 and is probably explained by the use of higher doses of dexamethasone (median, 40 mg) and thalidomide (median, 300 mg) in those respective studies compared with the current study (20 mg and 100 mg, respectively). Fatigue and lethargy were most commonly reported, with fluid retention or worsening of congestive cardiac failure being the next most common problems. Most patients needed an increase in diuretic dosage during treatment. Dexamethasone was discontinued more frequently than was thalidomide. Peripheral neuropathy was not a major problem in this cohort in keeping with other thalidomide series, although no patients with a preexisting amyloid peripheral neuropathy were included. Routine thromboprophylaxis was not administered because amyloid patients are predisposed to bleeding. Four percent of patients in the current study had a serious thrombotic event while on chemotherapy treatment, none of whom had received thromboprophylaxis. This number is less than comparative data for thalidomide when used as part of combination chemotherapy in myeloma,36 including CTD.40 Because of the serious nature of the adverse events, prophylaxis with aspirin, low molecular weight heparin, or warfarin is now routinely recommended for high-risk patients receiving thalidomide, such as those with nephrotic syndrome. However, thromboprophylaxis has to be individually tailored according to bleeding risk, taking into consideration presence of gastrointestinal or liver amyloid or clotting factor deficiencies. The TRM of 4% with CTD was comparable to that with VAD5 and significantly lower than that with intermediate-dose melphalan (12%).6 Two patients, neither of whom were taking anticoagulants or had an overt bleeding diathesis, died as a result of massive gastrointestinal bleeding. Patients with amyloidosis have previously been reported to have a high risk of gastrointestinal toxicity (9% patients ≥ grade 3 toxicity) with single-agent dexamethasone.34 Dexamethasone is a critical component of the CTD regimen, but neither the minimum effective dose nor the role of early-dose reduction has been studied. Use of stool occult blood screening prior to each chemotherapy cycle may be useful and needs further study in this context. Routine prophylaxis with a proton pump inhibitor is recommended for all amyloid patients receiving high-dose dexamethasone. A single patient developed multiorgan failure and died following an infection in the absence of significant cytopenia.
In conclusion, CTD appears to be a highly effective initial chemotherapy regimen for the treatment of systemic AL amyloidosis. A risk-adapted strategy permitted the use of this regimen in patients with advanced disease, in which few studies have shown benefit, and, although toxicity was common, thalidomide-related side effects were less apparent than in single-agent studies. More stringent use of risk adaptation may improve the tolerability of the regimen. At present, the long-term durability of the responses is unknown and remains to be determined. Only SCT achieves higher FLC response rates of 83% to 88% in patients with amyloidosis, but whether this advantage is counterbalanced by the requirement for careful patient selection and the increased risk of TRM with SCT compared with risk-adapted CTD merits further prospective randomized study.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Conflict-of-interest statement: the authors declare no competing financial interests.
Contribution: A.D.W. performed the research, analyzed the data, and wrote the paper; H.J.B.G., P.N.H., and J.D.G. performed the research and wrote the paper; H.J.L. and M.O. performed the research.
Acknowledgments
We thank the hematologists who administered chemotherapy and were primarily responsible for the care of these patients during such time. We thank Ms Dorothea Gopaul for undertaking SAP scintigraphy, Ms Dorota Rowczenio for performing all relevant genotyping, and Ms Jayshree Joshi for providing specialist echocardiography. We also thank Beth Jones for expert preparation of the manuscript.
This work was supported by the MRC Programme Grant (G97900510) (P.N.H.), Leukaemia Research Fund (A.D.W.), UCL Amyloidosis Research Fund, and NHS Research and Development Funds.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal