Abstract
Graft versus host disease (GVHD) typically results in impaired T-cell reconstitution characterized by lymphopenia and repertoire skewing. One of the major causes of inadequate T-cell reconstitution is that T-cell survival and expansion in the periphery are impaired. In this report, we have performed adoptive transfer studies to determine whether the quantitative reduction in T-cell numbers is due to an intrinsic T-cell defect or whether the environmental milieu deleteriously affects T-cell expansion. These studies demonstrate that T cells obtained from animals with graft-versus-host disease (GVHD) are capable of significant expansion and renormalization of an inverted CD4/CD8 ratio when they are removed from this environment. Moreover, these cells can generate complex T-cell repertoires early after transplantation and are functionally competent to respond to third-party alloantigens. Our data indicate that T cells from mice undergoing GVHD can respond to homeostatic signals in the periphery and are not intrinsically compromised once they are removed from the GVHD environment. We thereby conclude that the host environment and not an intrinsic T-cell defect is primarily responsible for the lack of effective T-cell expansion and diversification of complex T-cell repertoires that occurs during GVHD.
Introduction
Graft-versus-host disease (GVHD) is initiated by donor T-cell recognition of host alloantigens presented in the context of host antigen presenting cells (APCs).1,2 This results in the activation and expansion of donor T cells leading to cytokine production and acquisition of effector function which both play a role in mediating target organ damage.2–7 The ensuing expansion of alloactivated donor T cells typically results in impaired T-cell immunity characterized by lymphopenia and repertoire skewing as donor T cells respond to host antigens.3–5 Early after transplantation, T-cell immunity and repertoire complexity is primarily dependent on mature T cells that are transferred in the marrow graft.8,9 The generation of new bone marrow (BM)–derived donor T cells, in contrast, occurs over time to enhance T-cell reconstitution,10,11 although in adults this process is retarded and constrained by direct thymic damage from the conditioning regimen and/or age-related involution.12–14 In these patients, mature T cells assume the primary role in T-cell immunity because of limited thymic production of new T cells. In patients with GVHD, thymic production of new T cells is further compromised as a result of direct epithelial damage along with reduced production of cytokines necessary for thymopoiesis.15–18 This places a larger burden on the mature T-cell compartment for the maintenance of T-cell immunity.
GVHD also deleteriously affects expansion of mature T cells transferred in the donor marrow graft.19 This has been attributed, in part, to the propensity of these cells to have shortened survival in the periphery20–22 and to undergo activation-induced cell death,23 suggesting that an intrinsic T-cell defect may be responsible for T-cell lymphopenia. Alternatively, GVHD may effect alterations in the microenvironment that constrain the ability of resident mature T cells to expand. Delineating the etiology for the impairment in peripheral T-cell reconstitution has clinical implications, given that strategies designed to directly augment T-cell expansion may be of limited value if the host environment is not able to support T-cell regeneration. To discriminate between these 2 possible explanations, we used a murine adoptive transfer model to simulate what occurs in older aged adults, where thymic production of de novo–generated T cells is limited, to assess the capability of GVHD T cells for homeostatic expansion and repertoire regeneration once removed from this environment.
Materials and methods
Mice
C57BL/6 (B6) (H-2b, Thy1.2+), B6.PL (H-2b, Thy 1.1+), B6.129S7-Rag 1 (H-2b), Balb/cJ (H-2d), and AKR/J (H-2k) mice were obtained from The Jackson Laboratories (Bar Harbor, ME). All animals were housed in the American Association for Laboratory Animal Care (AALAC)–accredited Animal Resource Center of the Medical College of Wisconsin. Mice received regular mouse chow and acidified tap water ad libitum. Experiments were carried out under protocols approved by the Medical College of Wisconsin Institutional Animal Care and Use Committee.
Bone marrow transplantation and adoptive transfer studies
Bone marrow (BM) was flushed from donor femurs and tibias with Dulbecco modified media and passed through sterile mesh filters to obtain single-cell suspensions. BM was T-cell depleted in vitro with anti-Thy 1.2 monoclonal antibody (clone 30-H12, rat IgG2b; American Tissue Culture Collection, Rockville MD) plus low-toxicity rabbit complement (C-6 Diagnostics, Mequon, WI). Host mice were conditioned with total body irradiation administered as a single exposure at a dose rate of 62 cGy using a Shepherd Mark I Cesium Irradiator (J.L. Shepherd and Associates, San Fernando, CA). Sublethally irradiated (750 cGy) AKR mice were transplanted with TCD B6 BM (107 cells) plus B6 spleen cells adjusted to yield a T-cell dose of 5 × 105 cells. This model was used to simulate the situation in man where the competing risks of graft rejection and GVHD are both operative. Spleen cells from these chimeras 19 to 21 days after BM transplantation (BMT) were analyzed by flow cytometry to confirm complete donor T-cell engraftment prior to transplantation, along with B6 Rag-1 BM cells (5 × 106), into lethally irradiated (1100 cGy) secondary AKR mice. A congenic B6 murine model was used to determine that approximately 98% of donor T cells were splenic (B6.PL) as opposed to BM derived (B6) at the time of transfer into secondary recipients (data not shown). Spleen cells from individual B6→AKR chimeras were transplanted into 2 to 3 secondary AKR animals so that the reproducibility of T-cell repertoire regeneration could be assessed in replicate mice.
Mixed lymphocyte culture (MLC)
Spleen cells (adjusted to a T-cell dose of 1 × 105 cells/well) from normal B6 or chimeric B6→AKR mice were cocultured with 5 × 104 AKR or Balb/c dendritic cell–enriched stimulator cells in U-bottomed microwell plates (Becton Dickinson, Lincoln Park, NJ) at 37°C. Stimulator cells were obtained by digestion of spleens with collagenase D (1 mg/mL; Roche, Indianapolis, IN) followed by positive selection of CD11c+ “dendritic cells” using the magnetic cell separation system (Miltenyi Biotech, Auburn, CA). Nonirradiated stimulator cells were then seeded into microwell plates. One microcurie (0.037 MBq) of 3H-thymidine was added to triplicate wells for the final 12 to 18 hours prior to harvest. Thymidine incorporation was assessed using a liquid scintillation counter (Micromedics Systems, Huntsville, AL). Control wells consisted of responders only without stimulators.
Flow cytometric analysis and assessment of chimerism
Monoclonal antibodies conjugated to either fluorescein isothiocyanate (FITC) or phycoerythrin (PE) were used to assess chimerism in marrow transplant recipients. PE–anti-CD8 (clone CT-CD8a, rat IgG2a) was obtained from Caltag (San Francisco, CA). PE–anti-T-cell receptor (TCR) αβ (clone H57-597, hamster IgG), PE–anti-CD4 (clone GK1.5, rat IgG2b), FITC–anti-CD3 (clone 145-2C11, hamster IgG), PE–anti-Thy1.1 (clone OX-7, mouse IgG1), FITC–anti-Thy1.2 (clone 30-H12, rat IgG2b), and FITC–anti-H-2Kb (clone AF6-88.5, mouse IgG2a) were all purchased from Pharmingen (San Diego, CA). Spleen cells were analyzed on a fluorescence-activated cell scanner flow cytometer (Becton Dickinson, Mountain View, CA). At least 10 000 cells were analyzed for each determination whenever possible.
T-cell spectratype analysis
TCR spectratyping was performed as previously described.24–27 Briefly, Vβ total RNA was prepared from splenocytes using Trizol (Gibco-BRL, Gaithersburg, MD) and converted to cDNA as previously described.24 Rearrangement analysis was performed by polymerase chain reaction (PCR) amplification of the CDR3 for 19 Vβ (BV) families using Vβ and Cβ region-specific primers.25,26 The C region primer was labeled with fluorescein, and the PCR products were analyzed on denaturing polyacrylamide gels. The fluorescent PCR products for 19 Vβ families were quantitated using a FluorImager (Molecular Dynamics, Sunnyvale, CA) or an ABI 3100 Genetic Analyzer (Foster City, CA). Intensities were analyzed using ImageQuant (Molecular Dynamics) or Genescan (ABI) software. Intensity data were converted to relative band frequency, and pairs of samples were compared using P-P plots as described.26,27
Histologic analysis
Representative samples of lung, liver, and colon were obtained from recipients of transplants and fixed in 10% neutral-buffered formalin. Samples were then embedded in paraffin, cut into 5-μm thick sections, and stained with hematoxylin and eosin. Semiquantitative scoring systems were used to account for histologic changes consistent with GVHD. Changes in representative organs deemed to be compatible with GVHD were as follows: lung (peribronchial or perivascular mononuclear infiltration), colon (crypt cell apoptosis, crypt destruction, mucosal ulceration, goblet cell depletion, and lamina propria lymphocytic infiltration), and liver (periportal infiltration, lobular infiltration, hepatocyte apoptosis, endothelialitis, and bile duct necrosis). The scoring system that was used categorized 0 as normal, 1 for mild, 2 for moderate, and 3 for severe for each of these organs. All slides were read in a blinded fashion.
Isolation of intrahepatic lymphocytes
Livers were perfused with phosphate-buffered saline to remove peripheral blood cells and then passed through wire mesh screens. The liver cell suspension was then incubated with collagenase D (1 mg/mL; Roche) for 90 minutes, and cells were then passed over a nylon wool column to remove cellular debris. The resulting cell suspension was layered on a 44%/67% Percoll gradient and the interface was removed after centrifugation. The absolute number of T cells in the interface was determined by flow cytometry.
Statistics
Survival curves were constructed using the Kaplan-Meier product limit estimator and compared using the log-rank rest. Group comparisons of donor T-cell engraftment, histology scores, absolute numbers of CD4+ and CD8+ T cells, and CD4/CD8 ratios were performed using the Mann-Whitney U test. The magnitude of thymidine incorporation between groups was compared using the Student t test. Weight curves were comparatively analyzed using a mixed model for repeated measures. In this model, days after transplantation was included as a covariate so that the mean weight was compared between groups separately at each time point. The mixed model was used to account for the random mouse effect. A P value less than or equal to .05 was deemed to be significant in all experiments.
Results
Splenic-derived donor T cells from mice undergoing a GVH reaction have a markedly reduced capacity to transfer GVHD into secondary recipients
We have previously shown that splenic T cells obtained from GVHD mice early after BMT have a significantly reduced ability to transfer the disease when reexposed to host APCs and host alloantigens in nonirradiated immunodeficient animals.28 We therefore sought to determine the capability of these cells to expand and regenerate complex T-cell repertoires after transfer into secondary hosts. Initial studies were performed to confirm that splenic T cells from mice undergoing GVHD had a reduced capacity to respond to host alloantigens presented by host APCs in this murine model. When these cells were placed into culture in the absence of stimulator cells, T cells from GVHD mice had a modest degree of spontaneous proliferation when compared with normal T cells (P = .007) (Figure 1A). These same cells, however, had a significantly decreased proliferative response in MLCs compared with naive T cells (P < .001), indicating that the ability of the former cells to respond to host alloantigens was significantly attenuated.
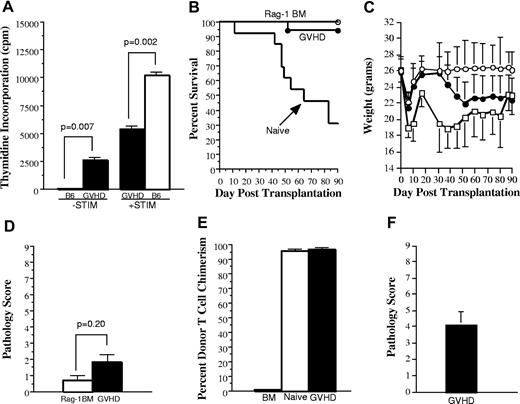
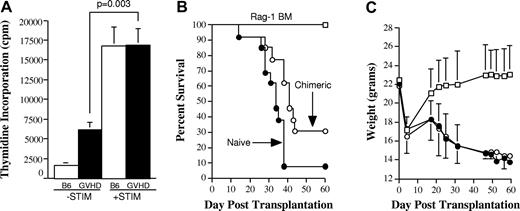
Splenic T cells from GVHD mice have a reduced capacity to mediate alloreactivity in vitro and to transfer GVHD in secondary recipients. (A). Spleen cells (adjusted to yield 1 × 105 T cells/well) from either normal B6 (□) or from B6→AKR chimeras undergoing GVHD (■) 19 days after BMT were cultured in triplicate wells in the absence (−STIM) or the presence of AKR CD11c+ dendritic “stimulator” cells (+STIM) (5 × 104) in a standard MLC for 5 days. Data are presented as the mean ± SEM. One of 2 representative experiments is shown. (B-C). Lethally irradiated (1100 cGy) AKR mice were transplanted with Rag-1 BM (5 × 106) alone (n = 19; ○) or together with B6 spleen cells adjusted to yield a dose of 4 to 5 × 105 naive (n = 13; □) or GVHD (n = 17; ●) T cells. Naive T cells were obtained from the spleens of normal B6 mice. GVHD T cells were obtained from sublethally irradiated (750 cGy) AKR animals that received a transplant with TCD B6 BM plus B6 spleen cells adjusted to a dose of 5 × 105 T cells 19 to 20 days after BMT. Spleen cells from individual GVHD mice were each transplanted into 2 to 3 secondary AKR hosts. Actual survival (B) and serial weight curves ± 1 SD (C) are depicted. Data are cumulative results from 6 independent experiments. (D). Colon, lung, and liver tissues from mice transplanted with Rag-1 BM alone (n = 6; □) or Rag-1 BM plus GVHD T cells (n = 17; ■) that were killed 90 days after BMT and examined histologically for evidence of GVHD using a grading system as described in “Material and methods.” Pathology score data are presented as the mean ± SEM. (E). Percentage of donor T-cell chimerism in the spleens of recipients of Rag-1 BM alone (n = 19; hatched bar) or Rag-1 BM plus either naive T cells (n = 3; □) or GVHD T cells (n = 17; ■) 3 to 4 months after BMT. Data are shown as the mean percentage of donor T cells ± SEM and are pooled from 6 independent experiments. Statistics: Rag versus naive, P < .001; Rag versus GVHD, P < .001; naive versus GVHD, P = .07. (F). Lethally irradiated AKR mice were transplanted with B6 Rag-1 BM plus 5 × 105 B6 T cells obtained from the livers of lethally irradiated B6→AKR chimeras with GVHD 24 days after transplantation. Pathology score data are presented as the mean ± SEM and are pooled from 2 experiments.
Splenic T cells from GVHD mice have a reduced capacity to mediate alloreactivity in vitro and to transfer GVHD in secondary recipients. (A). Spleen cells (adjusted to yield 1 × 105 T cells/well) from either normal B6 (□) or from B6→AKR chimeras undergoing GVHD (■) 19 days after BMT were cultured in triplicate wells in the absence (−STIM) or the presence of AKR CD11c+ dendritic “stimulator” cells (+STIM) (5 × 104) in a standard MLC for 5 days. Data are presented as the mean ± SEM. One of 2 representative experiments is shown. (B-C). Lethally irradiated (1100 cGy) AKR mice were transplanted with Rag-1 BM (5 × 106) alone (n = 19; ○) or together with B6 spleen cells adjusted to yield a dose of 4 to 5 × 105 naive (n = 13; □) or GVHD (n = 17; ●) T cells. Naive T cells were obtained from the spleens of normal B6 mice. GVHD T cells were obtained from sublethally irradiated (750 cGy) AKR animals that received a transplant with TCD B6 BM plus B6 spleen cells adjusted to a dose of 5 × 105 T cells 19 to 20 days after BMT. Spleen cells from individual GVHD mice were each transplanted into 2 to 3 secondary AKR hosts. Actual survival (B) and serial weight curves ± 1 SD (C) are depicted. Data are cumulative results from 6 independent experiments. (D). Colon, lung, and liver tissues from mice transplanted with Rag-1 BM alone (n = 6; □) or Rag-1 BM plus GVHD T cells (n = 17; ■) that were killed 90 days after BMT and examined histologically for evidence of GVHD using a grading system as described in “Material and methods.” Pathology score data are presented as the mean ± SEM. (E). Percentage of donor T-cell chimerism in the spleens of recipients of Rag-1 BM alone (n = 19; hatched bar) or Rag-1 BM plus either naive T cells (n = 3; □) or GVHD T cells (n = 17; ■) 3 to 4 months after BMT. Data are shown as the mean percentage of donor T cells ± SEM and are pooled from 6 independent experiments. Statistics: Rag versus naive, P < .001; Rag versus GVHD, P < .001; naive versus GVHD, P = .07. (F). Lethally irradiated AKR mice were transplanted with B6 Rag-1 BM plus 5 × 105 B6 T cells obtained from the livers of lethally irradiated B6→AKR chimeras with GVHD 24 days after transplantation. Pathology score data are presented as the mean ± SEM and are pooled from 2 experiments.
Experiments were then conducted to confirm that splenic-derived donor T cells did not cause significant GVHD after transferinto irradiated, as opposed to immunodeficient, hosts. Lethally irradiated (1100 cGy) AKR animals were transplanted with Rag-1 BM alone or together with 4 to 5 × 105 T cells from either normal B6 or GVHD (B6→AKR) mice. Rag-1 BM was used so that T-cell expansion or lack thereof was due only to transferred T cells without any potential contribution from the bone marrow. Mice that received transplants with Rag-1 BM (n = 16) all survived until day 90 (Figure 1B). In contrast, animals transplanted with naive T cells (n = 13) had significantly worse survival because of mortality attributable to GVHD (31% survival by day 90; P < .001). Notably, mice transplanted with GVHD T cells (n = 18) had no statistically significant difference in survival when compared with animals transplanted with Rag-1 BM (P = .35) alone, but their survival was superior to that of mice transplanted with naive T cells (P < .001). When weight curves were assessed, mice transplanted with GVHD T cells had similar weights for the first 30 days after BMT, but thereafter weights were significantly less than control animals (P < .01; Figure 1C). In contrast, animals transplanted with naive T cells had significantly greater weight loss than both control and GVHD animals at all time points after transplantation (P < .02). To determine whether late weight loss in transplant recipients of chimeric T cells was associated with pathologic damage, we examined representative tissues from the liver, lung, and colon of these mice and those that received a transplant with Rag-1 BM alone. The latter mice had no appreciable evidence of GVHD in any of the organs examined (Figure 1D). Similarly, the histologic score in animals transplanted with GVHD T cells (1.8 ± 0.4) was not significantly different from that observed in control mice (0.7 ± 0.2; P = .20), indicating that the transfer of T cells from GVHD animals did not cause histologic damage. To confirm that lack of observed GVHD in secondary recipients was not attributable to a failure of donor T-cell engraftment, surviving mice in all 3 groups were analyzed at the completion of these studies. Mice transplanted with either naive T cells or GVHD T cells had complete donor T-cell engraftment (Figure 1E), demonstrating that the absence of GVHD mortality in the latter animals was not attributable to failure of these cells to engraft. These results were in contrast to those observed when T cells were obtained from the livers of GVHD animals and subsequently transferred into lethally irradiated secondary AKR animals (n = 9). In these studies, transferred T cells did cause pathologic damage in the liver, lung, and colon (mean pathologic score 4.1 ± 0.8) (Figure 1F). Collectively, these studies indicate that splenic T cells have a significantly reduced ability to cause GVHD when reexposed to host APCs and host antigens compared with either naive T cells or T cells obtained directly from a GVHD target organ.
Donor splenic T cells from mice undergoing GVHD are capable of significant homeostatic expansion after adoptive transfer into secondary recipients
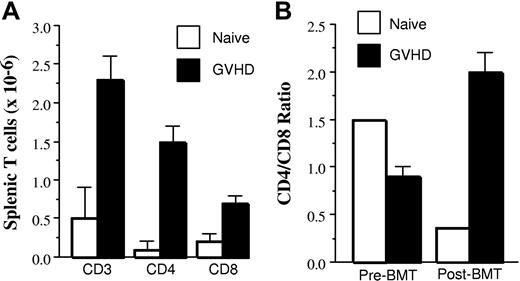
We then examined whether splenic T cells from GVHD mice were capable of homeostatic expansion upon transfer into secondary recipients. To account for the possibility that stem cells present in the splenic inoculum had emigrated into the Rag thymus and generated new T cells, mice were analyzed for T-cell reconstitution only if there was no evidence of de novo T-cell generation. Thirteen (76%) of 17 animals met this criterion because these mice had a mean thymus size of only 2.4 ± 0.6 × 106 cells with a mean percentage of double-positive thymocytes (CD4+CD8+) of 11% ± 2.5%. Animals transplanted with naive T cells (n = 3) that had survived at least 90 days had severe T-cell hypoplasia as total splenic T cells averaged 0.5 × 106 cells (Figure 2A). In contrast, the absolute number of splenic T cells in secondary recipients transplanted with GVHD T cells (n = 13) was significantly higher (2.3 × 106; P = .014). These data indicated that GVHD T cells were capable of approximately 5-fold expansion in the spleen compared with input T-cell numbers, whereas T cells from primary animals with ongoing GVHD failed to expand and these mice remained severely T-cell lymphopenic.
Homeostatic expansion of CD4+ and CD8+ GVHD T cells after transfer into secondary recipients. Lethally irradiated (1100 cGy) AKR mice from Figure 1 that were transplanted with Rag-1 BM (5 × 106) and a spleen cell-adjusted dose of either 4 to 5 × 105 naive or GVHD T cells were killed 90 to 119 days after transplantation and examined for the extent of T-cell expansion in the spleen. (A) Absolute numbers of CD3+, CD4+, and CD8+ splenic T cells in mice transplanted with naive (n = 3; □) or GVHD (n = 13; ■) T cells. (B) CD4/CD8 ratio before and after transfer of naive or GVHD splenic T cells into secondary AKR hosts is shown. Analysis in panels A and B was performed on 13 (76%) of 17 animals transplanted in Figure 1 that had received Rag-1 BM plus chimeric (B6→AKR) spleen cells and had a mean thymus size of 2.4 ± 0.6 × 106 cells with a mean percentage of double-positive thymocytes (CD4+CD8+) of 11% ± 2.5%, indicative of limited de novo T-cell generation. Data are presented as mean ± SEM and are cumulative results from 6 experiments.
Homeostatic expansion of CD4+ and CD8+ GVHD T cells after transfer into secondary recipients. Lethally irradiated (1100 cGy) AKR mice from Figure 1 that were transplanted with Rag-1 BM (5 × 106) and a spleen cell-adjusted dose of either 4 to 5 × 105 naive or GVHD T cells were killed 90 to 119 days after transplantation and examined for the extent of T-cell expansion in the spleen. (A) Absolute numbers of CD3+, CD4+, and CD8+ splenic T cells in mice transplanted with naive (n = 3; □) or GVHD (n = 13; ■) T cells. (B) CD4/CD8 ratio before and after transfer of naive or GVHD splenic T cells into secondary AKR hosts is shown. Analysis in panels A and B was performed on 13 (76%) of 17 animals transplanted in Figure 1 that had received Rag-1 BM plus chimeric (B6→AKR) spleen cells and had a mean thymus size of 2.4 ± 0.6 × 106 cells with a mean percentage of double-positive thymocytes (CD4+CD8+) of 11% ± 2.5%, indicative of limited de novo T-cell generation. Data are presented as mean ± SEM and are cumulative results from 6 experiments.
Prior to transplantation, T cells obtained from primary GVHD animals had a marked inversion in the CD4/CD8 ratio when compared with normal animals (Figure 2B). When these T cells were subsequently transplanted into secondary AKR mice, however, there was a significant increase in the absolute number of both CD4+ and CD8+ T cells (ie, 1.5 × 106 CD4+ and 0.7 × 106 CD8+ T cells, respectively) (Figure 2A) when analyzed 90 to 119 days after BMT. This represented a 7-fold expansion of CD4+ T cells and 3.5-fold expansion of CD8+ T cells from input cell numbers and resulted in normalization of the CD4/CD8 ratio (2.1 ± 0.2) (Figure 2B) because of the preferential expansion of the CD4+ T-cell population. This was in marked contrast to what was observed when animals were transplanted with T cells from normal B6 mice where there was an overall decline in the absolute number of T cells (Figure 2A) accompanied by an inversion in the ratio of CD4+ to CD8+ T cells (Figure 2B) due to the development of GVHD (Figure 1). The net effect was that the absolute number of CD4+ (P = .007) and CD8+ (P = .014) T cells was significantly higher in mice transplanted with T cells from GVHD as opposed to normal animals.
GVHD T cells are able to regenerate a complex T-cell repertoire in secondary hosts
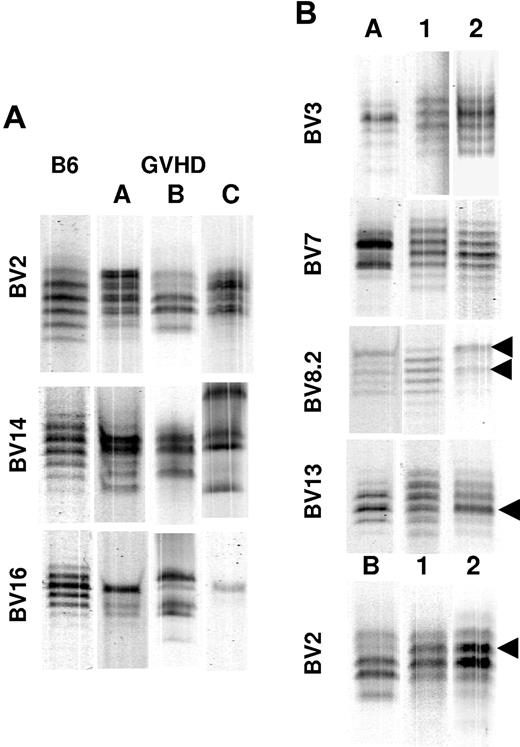
Early after transplantation, the T-cell repertoire is attributable primarily to expansion of mature T cells present in the donor graft. In recipients with GVHD, the repertoire is often skewed and/or contracted,27,29 and this has been purported to contribute to the propensity of patients with GVHD to develop opportunistic infections.30 We sought to determine whether skewing of the T-cell repertoire was fixed or whether there was plasticity that allowed for regeneration in the absence of significant thymic production. Animals were transplanted as in Figure 1, and spleen cells from day 19 GVHD mice were transplanted along with Rag-1 BM into lethally irradiated secondary AKR recipients. Prior to transplantation into secondary recipients, spleen cells were analyzed from GVHD animals (n = 6) to determine the complexity of the T-cell repertoire prior to secondary transfer. Repertoire complexity was measured by using CDR3 spectratyping, an approach that divides the repertoire on the basis of which Vβ family is expressed and further subdivides each family on the basis of CDR3 length. A normal repertoire will be comprised of examples of rearrangement for each Vβ family that is specific for the particular mouse strain, and the CDR3 length will follow a Gaussian distribution. Our analysis divided the repertoire into 19 families or subfamilies where significant skewing was observed in all Vβ families, including families that are not typically deleted in AKR recipients (ie, non–superantigen-related) (Table 1). Although spleen cells from normal B6 mice showed the expected Gaussian distribution, the predominant pattern observed in representative GVHD mice was a highly skewed one with many families showing only few intense bands (Figure 3A). Although the skewing was not always identical, there tended to be identical skewed bands in more than one mouse, indicating some similarity in responses between animals. These 6 animals represented a total of 114 data points of which only 10 families showed little or no skewing, and in 8 cases the signal for the family was too faint to determine whether there was skewing. Thus, approximately 90% (96 of 106) of the Vβ families in the spleen had undergone large changes in T-cell population frequencies as a result of GVHD (Table 1).
Summary of spectratype analysis in the spleen
| . | Experiment 1 . | Experiment 2 . | ||||
|---|---|---|---|---|---|---|
| Number of recipients | 2 | 2 | 2 | 2 | 3 | 2 |
| Input (BV families) | ||||||
| Skewed* | 15 | 17 | 16 | 14 | 14 | 19 |
| Missing† | 1 | 0 | 0 | 4 | 4 | 0 |
| Complex‡ | 3 | 2 | 3 | 1 | 1 | 0 |
| Percent Complex | 17 | 11 | 16 | 7 | 7 | 0 |
| Degree of Reconstitution§ | ||||||
| Partial | 5 | 3 (5) | 3 (4) | 2 (4) | 7 (1) | 7 (1) |
| Complete | 8 (3) | 5 (5) | 11 (2) | 2 (1) | 2 (1) | 1 (1) |
| None | 1 (3) | 1 (7) | 0 (2) | 3 (5) | 3 (2) | 5 |
| Loss | 2 | 1 (1) | 1 | 5 (4) | 5 | 4 (2) |
| Percent Reconstitution‖ | 76 | 68 | 90 | 34 | 51 | 47 |
| Concordance¶ | 0.84 | 0.58 | 0.88 | 0.63 | 0.79 | 0.89 |
| . | Experiment 1 . | Experiment 2 . | ||||
|---|---|---|---|---|---|---|
| Number of recipients | 2 | 2 | 2 | 2 | 3 | 2 |
| Input (BV families) | ||||||
| Skewed* | 15 | 17 | 16 | 14 | 14 | 19 |
| Missing† | 1 | 0 | 0 | 4 | 4 | 0 |
| Complex‡ | 3 | 2 | 3 | 1 | 1 | 0 |
| Percent Complex | 17 | 11 | 16 | 7 | 7 | 0 |
| Degree of Reconstitution§ | ||||||
| Partial | 5 | 3 (5) | 3 (4) | 2 (4) | 7 (1) | 7 (1) |
| Complete | 8 (3) | 5 (5) | 11 (2) | 2 (1) | 2 (1) | 1 (1) |
| None | 1 (3) | 1 (7) | 0 (2) | 3 (5) | 3 (2) | 5 |
| Loss | 2 | 1 (1) | 1 | 5 (4) | 5 | 4 (2) |
| Percent Reconstitution‖ | 76 | 68 | 90 | 34 | 51 | 47 |
| Concordance¶ | 0.84 | 0.58 | 0.88 | 0.63 | 0.79 | 0.89 |
Lethally irradiated (1100 cGy) AKR mice were transplanted with Rag-1 BM (5 × 106) and a spleen cell-adjusted dose of 5 × 105 T cells obtained 19 to 29 days after BMT from B6→AKR chimeras. Spleen cells from individual chimeras (input) were each then transplanted into 2 to 3 secondary AKR hosts. Transplanted secondary AKR animals were killed 90 days after BMT, and spleen cells were analyzed by CDR3 spectratyping.
Skewed input refers to at least one band showing more than 25% intensity deviation from a normal pattern.
Missing refers to no appreciable signal.
Complex input refers to the pattern observed in normal B6 spleen cells.
Reconstitution in secondary recipients 90 days after BMT was graded as complete if the pattern was similar to that observed in a normal spleen and partial if it was more complex than observed in transferred T cells but not entirely normal. No reconstitution indicated that the original skewed pattern was maintained, whereas loss referred to loss of signal from the Vβ family analyzed, either because there was no input signal or the cells corresponding to the input did not expand. Included in the partial category were those cases in which the original skew disappeared but reconstitution was limited to 2 or 3 new bands, but not a fully complex repertoire. In calculating the percent reconstitution, only those families in which there was an observable signal were compared.
Percent reconstitution = partial + complete/19 total Vβ families. The increase in repertoire complexity is indicated by comparison of the percent reconstitution versus the percent complex repertoire in the input spleen cell population.
Concordance refers to the extent to which both recipients, or 2 of 3 recipient mice showed the same pattern of T-cell repertoire reconstitution. A Vβ family was scored as concordant if both of the mice showed the same pattern or if 2 of 3 mice showed the same pattern. Data in parentheses shows the outcomes from nonconcordant pairs or outliers in the triplicate.
Splenic T cells from mice undergoing GVHD are able to regenerate complex T-cell repertoires in secondary hosts. Lethally irradiated (1100 cGy) AKR mice were transplanted with Rag-1 BM (5 × 106) and a spleen cell-adjusted dose of 5 × 105 T cells obtained 19 to 29 days after BMT from B6→AKR chimeras as in Figure 1. Spleen cells from individual chimeras were each then transplanted into 2 to 3 secondary AKR hosts. AKR animals that received a secondary transplant were killed 90 days after BMT, and spleen cells were analyzed by CDR3 spectratyping. (A) Examples of skewed spectratypes of BV2, BV14, and BV16 families derived from day 19 splenocytes obtained from 3 primary GVHD (B6→AKR) mice (labeled A-C) that served as donor cells for secondary transfers (input) are shown. For comparison, unskewed spectratypes from normal B6 spleen cells are shown in the lane labeled B6. (B) Spectratypes of splenocytes from secondary AKR recipients recovered 90 days after adoptive transfer. Selected BV families from 2 secondary recipient mice (lanes 1-2) receiving GVHD T cells from either mouse A (lane A) or B (lane B) are depicted. Arrows denote skewed bands that were also present in the input splenocyte population except for BV2 where a new skew develops. The particular BV gene being analyzed is identified to the left of each set of spectratypes.
Splenic T cells from mice undergoing GVHD are able to regenerate complex T-cell repertoires in secondary hosts. Lethally irradiated (1100 cGy) AKR mice were transplanted with Rag-1 BM (5 × 106) and a spleen cell-adjusted dose of 5 × 105 T cells obtained 19 to 29 days after BMT from B6→AKR chimeras as in Figure 1. Spleen cells from individual chimeras were each then transplanted into 2 to 3 secondary AKR hosts. AKR animals that received a secondary transplant were killed 90 days after BMT, and spleen cells were analyzed by CDR3 spectratyping. (A) Examples of skewed spectratypes of BV2, BV14, and BV16 families derived from day 19 splenocytes obtained from 3 primary GVHD (B6→AKR) mice (labeled A-C) that served as donor cells for secondary transfers (input) are shown. For comparison, unskewed spectratypes from normal B6 spleen cells are shown in the lane labeled B6. (B) Spectratypes of splenocytes from secondary AKR recipients recovered 90 days after adoptive transfer. Selected BV families from 2 secondary recipient mice (lanes 1-2) receiving GVHD T cells from either mouse A (lane A) or B (lane B) are depicted. Arrows denote skewed bands that were also present in the input splenocyte population except for BV2 where a new skew develops. The particular BV gene being analyzed is identified to the left of each set of spectratypes.
The extent of repertoire regeneration was then assessed in secondary AKR recipients 90 to 100 days after transplantation. After transfer of GVHD T cells into these animals, mice (n = 13) were killed, and the spleens were harvested to assess the T-cell repertoire in long-term chimeras. The different patterns of reconstitution that we observed in these animals are illustrated in Figure 3B. The panels on the left depict skewed T-cell repertoires obtained from splenocytes obtained from primary GVHD animals 19 to 29 days after BMT (lanes A-B). For the BV3 and BV7 families, the repertoire recovers in both mice that received skewed splenocytes, whereas for BV8.2, one animal recovered a normal CDR3 length distribution whereas the other maintained a skewed pattern similar to the input (arrows, mouse 2). The BV13 data represents an example of relatively unskewed input. In one animal, the day 90 sample shows a normal repertoire distribution for BV13, whereas in a second there is a slight skew that corresponds to the most intense band in input T cells (arrow, mouse 2). For BV2, the repertoires become more complex, but there is a new skew that is not observed in the transferred T cells in one of the replicate mice. Overall, whereas a complex repertoire was observed in only approximately 10% of Vβ families in primary GVHD animals, approximately 61% of Vβ families analyzed in splenic T cells from secondary recipients had evidence of reconstitution that was either partial or complete (Table 1). The concordance between mice that received the same GVHD splenocytes was also good, indicating that the extent to which repertoire reconstitution occurred between replicate animals was generally reproducible. In general, even for the BV families in which the repertoire at day 90 did not show reconstitution of a normal pattern, the repertoires observed at day 90 were generally more complex than those observed in the input T cells (Table 1).
Regeneration of complex repertoires can be demonstrated early after transplantation
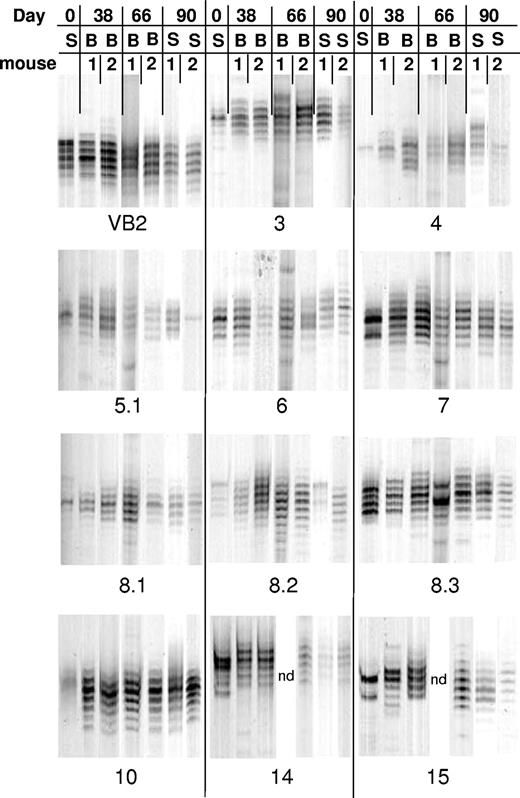
Because the risk of opportunistic infection is highest early after transplantation, we sought to determine the rapidity with which animals were able to generate complex repertoires after BMT. To address this question, peripheral blood samples (from mice in Table 1, Experiment 1) that had been serially obtained 38 and 66 days after BMT were analyzed by TCR spectratyping. In this experiment, 68% to 90% of Vβ families had evidence of reconstitution in the spleen when assessed 90 days after BMT (Table 1). A representative example of the data from 1 of the 3 cohorts of mice that are part of this experiment is shown in Figure 4. The most revealing data that address the tempo of reconstitution are depicted in Vβ families such as BV7, BV14, and BV15 where pronounced skewing was observed in the input splenocyte population. The BV7 family data are an example of complete normalization of the repertoire by day 38. The data depicted for BV14 and BV15, however, show the emergence of an almost normal repertoire by day 38, although there is some residual skewing that resolves completely by day 66. Of note, new skews that were not present in T cells from GVHD mice developed in a number of animals at both 38 and 66 days after transplantation. This finding occurred in 10% of the families that were analyzed at either of the 2 time points (Table 2). The significance of these new skews is not clear, although the fact that the majority of mice had near complete reconstitution by day 66 suggests that this phenomenon may have been a manifestation of a dynamic reconstitution process, possibly as a result of an asynchronous pattern of T-cell reconstitution or a response by a subpopulation of T cells to an undefined stimulus. An example of this is shown in Figure 4 where the BV 8.3 family shows a new skew in mouse 1 at day 66 that subsequently disappears from both the blood and spleen by day 90. Overall, the results of this analysis demonstrated that the majority of Vβ families had evidence of either partial or complete reconstitution when compared with input splenocytes (Table 2). In fact, 40% of Vβ families had normal complex distributions by day 38, whereas 65% were complex by day 66 after BMT.
GVHD T cells can generate complex T-cell repertoires early after transplantation. Lethally irradiated (1100 cGy) AKR mice were transplanted with Rag-1 BM (5 × 106) and 5 × 105 T cells that were obtained 19 to 20 days after BMT from B6→AKR chimeras as in Figure 1. Spleen cells from individual chimeras were then each transplanted into 2 secondary AKR hosts, and animals were serially bled at days 38 and 66 days after BMT. Spectratypes from 2 mice that received splenocytes from mouse A (see Figure 3) are depicted. Repertoire skewing of input splenocytes when present is shown in lane S. Spectratypes of selected BV families from serial peripheral blood (B) samples are shown at 38 and 66 days after BMT, whereas the corresponding T-cell repertoire in the spleen (S) is depicted 90 days after transplantation. Data in these studies were obtained from mice whose day 90 splenic T-cell repertoires are also depicted in Table 1, Experiment 1. ND indicates not done because of technical failure.
GVHD T cells can generate complex T-cell repertoires early after transplantation. Lethally irradiated (1100 cGy) AKR mice were transplanted with Rag-1 BM (5 × 106) and 5 × 105 T cells that were obtained 19 to 20 days after BMT from B6→AKR chimeras as in Figure 1. Spleen cells from individual chimeras were then each transplanted into 2 secondary AKR hosts, and animals were serially bled at days 38 and 66 days after BMT. Spectratypes from 2 mice that received splenocytes from mouse A (see Figure 3) are depicted. Repertoire skewing of input splenocytes when present is shown in lane S. Spectratypes of selected BV families from serial peripheral blood (B) samples are shown at 38 and 66 days after BMT, whereas the corresponding T-cell repertoire in the spleen (S) is depicted 90 days after transplantation. Data in these studies were obtained from mice whose day 90 splenic T-cell repertoires are also depicted in Table 1, Experiment 1. ND indicates not done because of technical failure.
Generation of complex repertoires in the peripheral blood early after transplantation
| Input (mouse) . | A . | B . | C . | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BV families skewed* | 15 | 17 | 16 | |||||||||
| Day after BMT | 38 | 66 | 38 | 66 | 38 | 66 | ||||||
| Recipient mouse | 1 | 2 | 1 | 2 | 1 | 2 | 1 | 2 | 1 | 2 | 1 | 2 |
| Degree of reconstitution† | ||||||||||||
| None | 1 | 1 | 0 | 0 | 4 | 4 | 0 | 0 | 3 | 3 | 0 | 0 |
| Partial | 10 | 5 | 2 | 2 | 6 | 6 | 6 | 7 | 3 | 4 | 3 | 2 |
| Complete | 7 | 12 | 10 | 13 | 9 | 8 | 12 | 11 | 3 | 5 | 13 | 13 |
| Loss | 1 | 1 | 1 | 2 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 2 |
| New skew | 0 | 0 | 3 | 2 | 0 | 1 | 0 | 0 | 8 | 5 | 2 | 1 |
| Input (mouse) . | A . | B . | C . | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BV families skewed* | 15 | 17 | 16 | |||||||||
| Day after BMT | 38 | 66 | 38 | 66 | 38 | 66 | ||||||
| Recipient mouse | 1 | 2 | 1 | 2 | 1 | 2 | 1 | 2 | 1 | 2 | 1 | 2 |
| Degree of reconstitution† | ||||||||||||
| None | 1 | 1 | 0 | 0 | 4 | 4 | 0 | 0 | 3 | 3 | 0 | 0 |
| Partial | 10 | 5 | 2 | 2 | 6 | 6 | 6 | 7 | 3 | 4 | 3 | 2 |
| Complete | 7 | 12 | 10 | 13 | 9 | 8 | 12 | 11 | 3 | 5 | 13 | 13 |
| Loss | 1 | 1 | 1 | 2 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 2 |
| New skew | 0 | 0 | 3 | 2 | 0 | 1 | 0 | 0 | 8 | 5 | 2 | 1 |
Lethally irradiated (1100 cGy) AKR mice were transplanted with Rag-1 BM (5 × 106) and a spleen cell-adjusted dose of 5 × 105 T cells obtained 19 to 29 days after BMT from B6→AKR chimeras. Spleen cells from individual chimeras were each then transplanted into 2 to 3 secondary AKR hosts. AKR animals that received a secondary transplant were bled 38 and 66 days after BMT, and peripheral blood cells were analyzed by CDR3 spectratyping.
The number of BV families that were skewed from input splenocytes. A total of 19 families were examined in all animals.
Reconstitution of BV families was graded as none, partial, or complete, using the same criteria as noted in the legend to Table 1 Loss refers to absence of a spectratype that was attributed to technical failure. Consequently, the total number of BV families at each time point for each animal does not total 19 families in all cases. New skew refers to the emergence of skewed bands that were not present in the input spleen cell populations from primary GVHD animals.
GVHD T cells are functionally competent to mediate third-party alloreactive responses
To determine whether T cells that were capable of homeostatic expansion and repertoire regeneration were functionally competent, we examined whether these cells could respond to third-party alloantigens. This was first assessed in vitro in an MLC. As previously observed (Figure 1), T cells from chimeric mice had baseline proliferation that was greater than T cells from normal animals (Figure 5A). When these cells were cultured with allogeneic stimulators, however, there was significantly higher thymidine incorporation than observed in control wells (P = .003), indicating that these T cells could proliferate in response to third-party antigens. To determine whether these T cells could respond to alloantigens in vivo, lethally irradiated AKR mice were transplanted with B6 Rag-1 BM and GVHD T cells that were obtained 22 days after BMT from B6→AKR chimeras. Spleen cells were pooled from fully donor T-cell–engrafted B6→AKR animals mice 70 days after BMT and transplanted along with B6 Rag-1 BM into lethally irradiated MHC-incompatible Balb/c (H-2d) animals. Chimeric spleen cells caused lethal GVHD in the majority of Balb recipients (P = .007 versus Rag-1 BM controls; Figure 5B) and induced severe weight loss that was similar to that observed in mice transplanted with spleen cells from normal B6 animals (Figure 5C). Collectively, these data demonstrated that T cells which had expanded in secondary recipients were able to recognize third-party alloantigens and were not anergic.
GVHD T cells that have undergone homeostatic expansion in secondary hosts are capable of responding to third-party alloantigens. (A) Spleen cells (adjusted to yield 1 × 105 T cells/well) from either normal B6 (□) or from secondary B6→AKR chimeras that had been transplanted 70 days earlier with T cells from primary B6→AKR GVHD animals (■) were cultured in triplicate wells in the absence (−STIM) or presence of third-party Balb/c CD11c+ dendritic stimulator cells (+STIM) (5 × 104) in a standard MLC for 5 days. Data are presented as the mean ± SEM. (B-C) Lethally irradiated (1100 cGy) AKR mice were first transplanted with Rag-1 BM (5 × 106) and splenocytes that were obtained 22 days after BMT from donor T-cell–engrafted primary B6→AKR chimeras. Spleen cells (adjusted to yield a T-cell dose of 5 × 105/mouse) were pooled from these mice 70 days after BMT and transplanted along with Rag-1 BM (6 × 106) into lethally irradiated (900 cGy) Balb/c animals (n = 13; ○). Control mice received either Rag-1 BM alone (n = 6; □) or Rag-1 BM plus spleen cells (adjusted to yield a T-cell dose of 5 × 105/mouse) from normal B6 animals (n = 13; ●). Actual survival (B) and serial weight curves ± 1 SD (C) for each cohort are depicted. Data are cumulative results from 2 experiments.
GVHD T cells that have undergone homeostatic expansion in secondary hosts are capable of responding to third-party alloantigens. (A) Spleen cells (adjusted to yield 1 × 105 T cells/well) from either normal B6 (□) or from secondary B6→AKR chimeras that had been transplanted 70 days earlier with T cells from primary B6→AKR GVHD animals (■) were cultured in triplicate wells in the absence (−STIM) or presence of third-party Balb/c CD11c+ dendritic stimulator cells (+STIM) (5 × 104) in a standard MLC for 5 days. Data are presented as the mean ± SEM. (B-C) Lethally irradiated (1100 cGy) AKR mice were first transplanted with Rag-1 BM (5 × 106) and splenocytes that were obtained 22 days after BMT from donor T-cell–engrafted primary B6→AKR chimeras. Spleen cells (adjusted to yield a T-cell dose of 5 × 105/mouse) were pooled from these mice 70 days after BMT and transplanted along with Rag-1 BM (6 × 106) into lethally irradiated (900 cGy) Balb/c animals (n = 13; ○). Control mice received either Rag-1 BM alone (n = 6; □) or Rag-1 BM plus spleen cells (adjusted to yield a T-cell dose of 5 × 105/mouse) from normal B6 animals (n = 13; ●). Actual survival (B) and serial weight curves ± 1 SD (C) for each cohort are depicted. Data are cumulative results from 2 experiments.
Discussion
One of the major complications associated with GVHD is the development of T-cell lymphopenia that predisposes patients to the risk of acquiring opportunistic infections. Lymphopenia is thought to result from reduced thymic production of naive BM-derived donor T cells8,9,31 as well as impaired survival of mature peripheral T cells that are susceptible to apoptosis.20–22 This is particularly problematic in older aged patients when diminished thymic production places the onus on mature donor-derived T cells for effective T-cell immunity. The question that we sought to address in these studies is whether the quantitative reduction in T-cell numbers is due to an intrinsic defect in these cells or whether the environmental milieu that arises from GVHD deleteriously affects T-cell expansion. Our data indicate that 3 weeks after BMT there is a population of splenic T cells in lymphopenic GVHD animals that have a significantly reduced ability to transfer the disease when they reencounter host alloantigens presented in the context of host APCs. Upon transfer into an environment in which GVH reactivity is substantially reduced, however, these T cells retain the capacity for significant expansion as cell numbers increased 5-fold after transfer into secondary recipients (Figure 2). This was in direct contrast to primary GVHD control mice transplanted with naive T cells where GVHD significantly constrained T-cell expansion. These results extend the findings of Dulude et al19 who demonstrated that host-tolerant T cells have a limited capability to expand when transferred into GVHD mice. In contrast, T cells obtained from animals undergoing GVHD were competent to expand in thymectomized secondary hosts, supporting the premise that these T cells were capable of significant expansion once removed from the GVHD milieu. In their report, however, the majority of transferred T cells from GVHD mice were BM-derived which does not simulate what occurs in older transplant recipients where de novo T-cell production is severely constrained. Our study demonstrates that mature splenic-derived T cells in the absence of BM-derived T cells also possess this capability.
Impaired regeneration of CD4+ T cells is a common finding in recipients of allogeneic marrow grafts and in patients receiving intensive chemotherapy.11,32,33 This abnormality contributes to the propensity of these patients to develop opportunistic infections. Mackall et al33 have inversely correlated CD4+ recovery with age and demonstrated that regeneration of CD8+ T cells occurs more rapidly via thymic-independent pathways than CD4+ T cells. The fact that this has been observed in patients undergoing chemotherapy where there is no GVH response to drive preferential expansion of CD8+ T cells is consistent with the interpretation that chemotherapy and GVHD can both result in host defects that affect regeneration of CD4+ T cells. We observed that the CD4/CD8 ratio normalized, and there was a marked increase in the absolute number of CD4+ T cells in secondary AKR recipients, indicative of the preferential expansion of these cells once removed from the GVHD environment. Collectively, these data support the premise that the quantitative reduction in T-cell numbers and the profound CD4 deficiency observed in GVHD animals, and by extrapolation patients, is, to a large extent, a function of the GVHD environmental milieu in secondary lymphoid organs and not a fixed intrinsic T-cell defect.
In subsequent studies, we demonstrated that T-cell populations obtained from mice undergoing GVHD were also able to regenerate molecular diversity as assessed by CDR3 spectratyping. This was evident early after transplantation because the majority of mice had either partial or complete reconstitution within the first 40 days after BMT (Table 2). Skewing of the T-cell repertoire has been demonstrated to be a characteristic of GVHD in both humans and murine models.19,27,32 The presence of skewing is indicative of dominant populations or subpopulations of T cells within any given Vβ family, but does not necessarily exclude the presence of other T-cell subfamilies within that same group. Our data indicate that “holes” in the T-cell repertoire are not necessarily fixed in GVHD animals, but rather subdominant T-cell populations persist in mice and have the capacity for expansion and the ability to contribute to normalization of the T-cell repertoire. The extent to which repertoire diversity occurred was dependent upon the degree of skewing observed in T cells obtained from primary GVHD animals (Table 1). Mice with the most pronounced skewing had somewhat less repertoire normalization, whereas mice with less skewing had significant regeneration and, in some instances, normalization of the repertoire. The presence of holes, as determined by spectratyping, therefore did not denote absence of these clonotypes in all cases but rather underrepresentation of these T-cell populations that could be corrected by homeostatic expansion in a relatively GVHD-free environment. These T cells also retained their functional capability because they were able to mediate a third-party response when exposed to MHC and alloantigens distinct from both donor and host (Figure 5).
Given the significantly reduced capability of T cells from GVHD mice to transfer the disease in secondary recipients along with the ability of these same T cells to expand and reconstitute a complex repertoire when removed from the host environment, a central question is why T-cell hypoplasia and repertoire skewing persist during GVHD. A possible explanation for how this occurs is that GVHD may deleteriously affect signals necessary for T-cell survival. The major survival signals that have been identified for both naive and memory T cells are cytokines and MHC molecules.34 With respect to cytokines, IL-7 and IL-15 both have been shown to have a pivotal role in T-cell homeostasis. IL-7 is necessary for survival of naive T cells35,36 ; however, the inability of IL-7 to enhance immune reconstitution in mice undergoing GVHD is evidence that it does not appear to be responsible for T-cell lymphopenia in these animals.37,38 Moreover, we have shown that lethally irradiated mice undergoing GVHD have significantly increased levels of IL-7 mRNA transcripts,39 providing additional evidence that the relative absence of IL-7 does not appear to be an explanation for this observation. Although survival of memory CD8+ T cells is dependent upon the presence of IL-15,40–42 this cytokine does not appear to be required for CD4+ memory T-cell survival. Therefore, lack of IL-15 is not a likely explanation for the quantitative reduction in CD4+ T cells in GVHD animals. Thus, there is presently no direct evidence that cytokine deprivation is responsible for the impairment in T-cell reconstitution in GVHD recipients.
The extent to which T cells require contact with MHC molecules for maintenance of survival is a controversial area and appears to be influenced by whether survival is assessed under steady state or lymphopenic conditions.43 The latter is perhaps most relevant for comparison to what occurs during GVHD where a myeloablative conditioning regimen induces a state of lymphopenia. Although naive T cells require self peptide/MHC signals for survival,35,44 memory T cells do not share this requirement, although the lack of MHC ligands may affect memory T-cell function.43,45,46 Destruction of lymphoid niches by GVHD or reductions in the numbers of class I– and class II–expressing cells in the periphery may therefore inhibit T:MHC contacts necessary for survival and is a potential explanation for the observed lymphopenic state. The expansion of GVHD T cells in secondary hosts (Figure 2) could therefore be explained by the reencounter of T cells with appropriate MHC ligands that were absent or reduced in numbers in primary GVHD mice.
In summary, these studies demonstrate that GVHD T cells that are transferred into secondary hosts remain capable of significant expansion, normalization of CD4/CD8 ratios, and repertoire regeneration. This leads us to conclude that the homeostatic expansion that occurs in the absence of significant de novo T-cell generation is evidence that the host environment and not an intrinsic T-cell defect per se is the primary cause of GVHD-associated lymphopenia and repertoire skewing. Delineation of the specific defect(s) present in the GVHD environment may open the door to novel approaches designed to correct this abnormal milieu and thereby enhance T-cell immunity within the existing resident T-cell population.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by grants from the National Institutes of Health (grant HL64603) and the Midwest Athletes Against Childhood Cancer Fund (Milwaukee, WI).
National Institutes of Health
Authorship
Contribution: J.G. analyzed data, contributed to scientific design of studies, and wrote the paper; X.C. performed research; M.G. performed research; M.Y. performed research and analyzed data; A.K. performed research; E.T. performed research; B.L. performed statistical analysis; R.K. performed pathological analysis; S.V.-J. performed research; and W.R.D. designed research and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: William R. Drobyski, Bone Marrow Transplant Program, 9200 W Wisconsin Ave, Milwaukee, WI 53226; e-mail: wdrobysk@mcw.edu.