Abstract
The findings that many primitive human hematopoietic cells give rise to daughter cells that adopt different cell fates and/or show different proliferation kinetics suggest that hematopoietic stem cells (HSCs) and hematopoietic progenitor cells (HPCs) can divide asymmetrically. However, definitive experimental demonstration is lacking due to the current absence of asymmetrically segregating marker molecules within the primitive hematopoietic cell compartment. Thus, it remains an open question as to whether HSCs/HPCs have the capability to divide asymmetrically, or whether the differences that have been observed are established by extrinsic mechanisms that act on postmitotic progenitors. Here, we have identified 4 proteins (CD53, CD62L/L-selectin, CD63/lamp-3, and CD71/transferrin receptor) that segregate differentially in about 20% of primitive human hematopoietic cells that divide in stroma-free cultures. Therefore, this indicates for the first time that HSCs/HPCs have the capability to divide asymmetrically. Remarkably, these proteins, in combination with the surrogate stem-cell marker CD133, help to discriminate the more primitive human cultivated HSCs/HPCs. Since 3 of these proteins, the transferrin receptor and the tetraspanins CD53 and CD63, are endosomal-associated proteins, they may provide a link between the endosomal compartment and the process of asymmetric cell division within the HSC/HPC compartment.
Introduction
Somatic stem cells are undifferentiated cells that can self-renew over a long period of time in vivo and give rise to progenitor cells that are committed to differentiate. Since both uncontrolled expansion as well as loss of stem cells would be fatal for multicellular organisms, the decision of self-renewal versus differentiation needs to be tightly controlled. Therefore, key questions in stem cell biology are how and which mechanisms govern these decisions. Although mammalian hematopoietic stem cells (HSCs) are the most intensively investigated somatic stem cells, the nature of the factors controlling self-renewal and differentiation remain largely unknown.
There is good evidence that HSCs can expand in vivo1 and be maintained in vitro in close contact to adequate stroma cells.2–5 These observations point toward the existence of specialized HSC niches, which was already hypothesized as early as 1978.6 Indeed, it was recently shown that osteoblasts are key elements of HSC niches in the endosteum of bone marrow (BM) and sinusoidal endothelial cells of vascular HSC niches found in the spleen and BM.7–9 In addition to the data supporting the HSC niche model that likely provides cell extrinsic cue, evidence suggest that cells of the HSC and hematopoietic progenitor cell (HPC) compartment contain capabilities to divide asymmetrically. Ogawa and colleagues showed that after separation of paired murine and human HPCs that were cultured in stroma-free suspension conditions, siblings gave rise to colonies with significantly different characteristics.10–12 More recently, HSC-enriched cell populations were found to be highly heterogeneous in respect to their function and their proliferation kinetics (ie, the proliferation rate of more primitive cells is slower than that of committed ones).13–15 Furthermore, it was observed that approximately 30% of primitive hematopoietic cells (CD34+CD38− cells) give rise to daughter cells with heterogeneous proliferation kinetics and functions.14–16
Recently, we showed that human myeloid-lymphoid initiating cells (ML-ICs), a subfraction of the CD34+CD38− cells, when cultured under stroma-free conditions give rise to daughters that adopt different cell fates, with 1 cell inheriting the developmental capacity of the mother cell, and 1 cell becoming more specified.17 Similarly, in mice, up to 62% of primitive hematopoietic cells (lin−CD34low/−c-kit+Sca-1+) give rise to daughter cells with different myeloid developmental potentials.18,19 Although all these observations are in accordance with the model of asymmetric cell division in which primitive hematopoietic cells contain the potential to give birth to 2 intrinsically different daughter cells, it cannot be concluded that the observed differences are indeed the result of an asymmetric cell division. In principle, these differences could have been established by postmitotic, extrinsic decision processes.17,20,21
In organisms like Drosophila melanogaster and Caenorhabditis elegans, in which asymmetric cell divisions have been proven to occur, cells that divide asymmetrically are polarized during cell division and localize specific molecules to distinct regions of the cell, which are then transmitted unequally into the daughters.22 Therefore, to demonstrate that primitive hematopoietic cells can indeed divide asymmetrically, molecules that clearly segregate asymmetrically during mitoses of these cells need to be identified. Here, we describe the identification of proteins containing extracellular epitopes, which segregate differentially to daughters during mitosis of approximately 20% of the human CD34+CD133+ hematopoietic cells and confirm the occurrence of asymmetric cell division within the human HSC and HPC compartment.
Materials and methods
Cell source and preparation
Human umbilical cord blood (CB), BM, and peripheral blood (PB) of granulocyte colony-stimulating factor (G-CSF)–treated stem cell donors were obtained from unrelated donors after informed consent was obtained in accordance with the Declaration of Helsinki. The use of human cord blood was approved by the ethics committee of Heinrich-Heine University. Mononuclear cells (MNCs) were isolated from individual sources by Ficoll (Biocoll Separating Solution; Biochrom AG, Berlin, Germany) density gradient centrifugation as described previously.23 CD34+ cells were isolated by magnetic cell separation using the MidiMacs technique according to the manufacturer's instructions (Miltenyi Biotec, Bergisch Gladbach, Germany).
If not stained immediately, freshly purified MNCs were cultured in a humidified atmosphere at 37°C and 5% CO2 at a density of approximately 1 × 106 cells/mL and freshly enriched CD34+ cells at a density of approximately 1 × 105 cells/mL in I20 (Iscove modified Dulbecco medium [IMDM; Invitrogen, Karlsruhe, Germany] supplemented with 20% fetal calf serum [FCS; Biochrom AG], 1000 U/mL penicillin, and 100 U/mL streptomycin [Invitrogen]) in the presence of early-acting cytokines (fetal liver tyrosine kinase 3 ligand [FLT3L], stem cell factor [SCF], and thrombopoietin [TPO], each at 10 ng/mL final concentration [all from PeproTech, Rocky Hill, NJ]).
Flow cytometry
For the proliferation kinetics, freshly isolated CD34+ cells were stained for 4 minutes with 2 μM PKH2 (Sigma-Aldrich Chemie, Taufkirchen, Germany). After 1 washing step, stained cells were cultured in I20 in the presence of early-acting cytokines. For flow cytometric analyses, cells were stained with AC133-phycoerythrin (PE; Miltenyi Biotec) and anti-CD34–PE/cytochrome 5 (PCy5) antibodies (BD PharMingen, Heidelberg, Germany).
For the screening procedure, MNCs were stained with different combinations of 3 different antibodies as described in paragraph 3 of Results. A list of the antibodies used is given in Table S1, available on the Blood website; see the Supplemental Table link at the top of the online article).
Flow cytometric analyses were performed on a Cytomics FC 500 flow cytometer equipped with the RXP software (Beckman Coulter, Krefeld, Germany).
For the functional assays, the cells were highly purified using a Coulter EPICS Elite ESP fluorescence cell sorting system equipped with the Expo32 software (Beckman Coulter).
Immunofluorescence and microscopy
For subcellular localization studies of polarized and dividing primitive hematopoietic cells, CD34+ cells were cultured for 3 or 4 days in the presence of early-acting cytokines in I20 medium before immunostaining. To conserve their morphology, the CD34+ cells were prefixed for 5 minutes at room temperature with paraformaldehyde (Sigma-Aldrich Chemie) at a final concentration of 0.2% in the medium. As the AC133 epitope of CD133 is sensitive to formaldehyde and paraformaldehyde fixation, the cells for the extracellular staining procedure were then incubated with the AC133-PE antibody (1:10; AC133/1; Miltenyi Biotec) diluted in PBS containing 10% donkey serum (Jackson ImmunoResearch Laboratories, West Grove, PA). They were stained for at least 10 minutes at 4°C, and then postfixed with 4% paraformaldehyde in PBS for 20 minutes at 4°C. Because PE is not a suitable fluorochrome for immunofluorescence microscopy, we counterstained the AC133-labeled cells with Cy3-conjugated AffiniPure Fab fragment donkey anti-mouse IgG (1:20; Jackson ImmunoResearch Laboratories) diluted in PBS containing 10% donkey serum for at least 10 minutes at 4°C. Remaining mouse epitopes were saturated with unconjugated AffiniPure Fab fragment rabbit anti-mouse IgG (1:10; Jackson ImmunoResearch Laboratories). Cells were divided in different aliquots and stained with 1 of the following primary mouse antibodies: anti-CD43–FITC (1G10; BD PharMingen), anti-CD44–FITC (J173; Coulter Immunotech, Krefeld, Germany), anti-CD50–FITC (TU41; BD PharMingen), anti-CD54–FITC (84H10; Coulter Immunotech), anti-CD53–FITC (HI29; BD PharMingen), anti-CD62L (FREG56; Coulter Immunotech), anti-CD63–FITC (H5C6; BD PharMingen), or anti-CD71 (YDJ1.2.2; Coulter Immunotech). These antibodies were counterstained using Cy2-conjugated secondary antibodies (1:100; goat anti-mouse IgG; Jackson ImmunoResearch Laboratories).
For the intracellular staining procedure, cultured and prefixed CD34+ cells were fixed with 4% paraformaldehyde in PBS for 20 minutes at 4°C and permeabilized using 0.1% Triton X100 (Sigma-Aldrich Chemie) in PBS. After blocking in 10% donkey serum (Jackson ImmunoResearch Laboratories), cells were incubated with the anti-CD63 antibody (1:50; H5C5; BD PharMingen) for at least 30 minutes at room temperature and counterstained with Cy3-conjugated AffiniPure Fab fragment donkey anti-mouse IgG (1:100; Jackson ImmunoResearch Laboratories) diluted in PBS containing 10% donkey serum for another 30 minutes at room temperature. After saturating remaining mouse epitopes with unconjugated AffiniPure Fab fragment rabbit anti-mouse IgG (1:10; Jackson ImmunoResearch Laboratories), cells were stained with the anti-CD71 antibody (1:50; YDJ1.2.2; Coulter Immunotech) and counterstained with Cy2-conjugated secondary antibodies (1:400 goat anti-mouse IgG; Jackson ImmunoResearch Laboratories).
Labeled cells were mounted in 75% glycerin containing propylgallat (50 mg/mL) and DAPI (200 ng/mL; Roche, Mannheim, Germany) and observed with an Axioplan 2 fluorescence microscope (Carl Zeiss, Goettingen, Germany) using a Zeiss Plan-Neofluar 100× objective lens (1.3 NA O). Pictures were taken with an Axiocam digital camera and processed using Axiovision 4.5 Software (Carl Zeiss).
Functional assays
For the long-term culture-initiating cell (LTC-IC) assays, approximately 6000 sorted cells were cocultured in a limiting dilution with the irradiated murine fetal liver stroma cell line AFT024 in IMDM (Invitrogen) supplemented with 12.5% FCS, 12.5% horse serum (Cell Systems, St. Katharinen, Germany), 2 mM l-glutamine (Invitrogen), 1000 U/mL penicillin, 100 U/mL streptomycin (Invitrogen), and 10−6 M hydrocortisone as extensively described previously.14,24 Briefly, cultures were maintained for 5 weeks in a humidified atmosphere at 37°C and 5% CO2 and fed once a week. At week 5, all wells were overlaid with clonogenic methylcellulose medium (Methylcel MC; Fluka, Sigma-Aldrich Chemie) in a final concentration of 1.2% containing IMDM, supplemented with 30% FCS for colony-forming cells (CFCs), 5 U/mL erythropoietin (Cell Systems), and supernatant of the bladder carcinoma cell line 5637 (10%). Wells were scored for the occurrence of secondary CFCs after an additional 10 days.
Statistics
Experimental results from different experiments were reported as standard deviation of the mean. Significance analyses were performed with the paired Student t test. LTC-IC frequencies were calculated as the reciprocal of the concentration of test cells that gives 37% negative cultures using Poisson statistics and the weighted mean method.
Results
Proliferation kinetics of cultured CD34+ cells
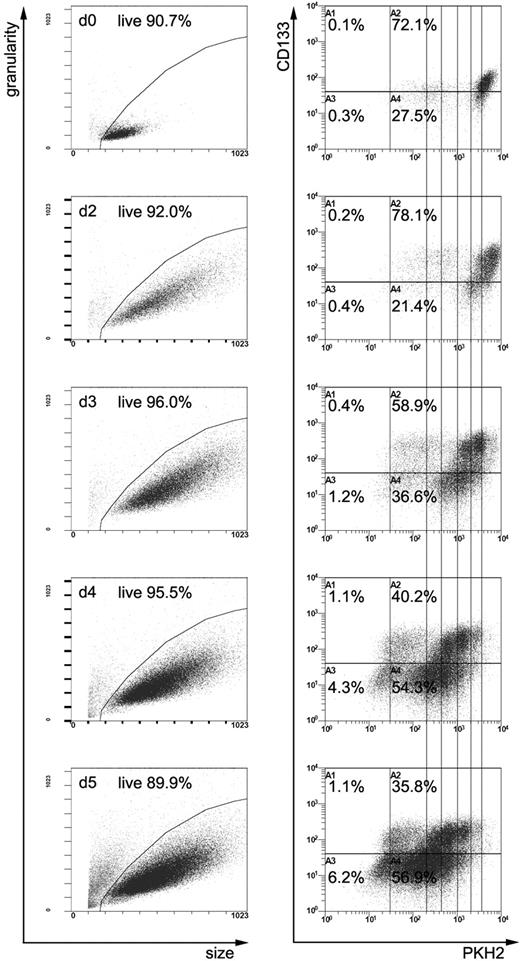
Recently, we have shown that human CD34+ cells grown for 5 days in the presence of early- or late-acting cytokines in suspension cultures segregate into CD34+CD133+ and CD34+CD133low/− subfractions. Furthermore, we realized that early-acting cytokines stimulate proliferation of CD34+ cells more homogeneously than late-acting cytokines.17 Since we aimed to study the subcellular distribution of antigens in dividing human CB–derived primitive hematopoietic cells and had the experimental requirement to obtain sufficient mitotic cells numbers, we first estimated the proliferation kinetics of CD34+ cells labeled with the fluorescence dye PKH2 and subsequently cultured in the presence of early-acting cytokines. Cells were harvested at different time points, counted, and the expression of PKH2, CD133, and CD34 was analyzed by flow cytometry. According to the PKH2 staining (Figure 1) and in agreement with our previous studies,23 only a few CD34+ cells undergo cell division within the first 48 hours of cultivation. The proportions of both CD133+ cells (Figure 1) and CD34+ cells (data not shown) were not altered during this period. Analysis of the PKH2 staining at later time points revealed that most of the CD34+ cells in vitro undergo their first cell division between day 2 and day 3 in culture, and subsequent divisions during the next few days (Figure 1). In agreement with our previous report,17 the content of CD34+ cells does not change during the first 5 days of culture (data not shown). However, with the onset of cell division, the proportion of CD133+ cells decreases between day 2 and 5 in culture (Figure 1). This corresponds to the maximum expansion period of CD34+ cells between days 3 and 4 seen in 3 different CB-derived CD34+ cell samples (expansion rate, 2.3 ± 0.4 times).
Flow cytometric analyses of PKH2-stained CD34+-enriched cells. PKH2-stained CD34+-enriched cells, either noncultivated (d0) or cultivated in the presence of early-acting cytokines for 2, 3, 4, or 5 days (d2, d3, d4, d5), were measured after labeling with anti-CD34 and anti-CD133 antibodies. The size and the granularity of all cells is plotted in the panels of the first column, and the PKH2 and anti-CD133 staining of the cells located in the live gates (shown in the first column) in the panels of the second column. Quadrants are adjusted according to corresponding isotype controls. Upon cultivation, CD34+ cell increase in size and slightly up-regulate CD133 on their cell surface (compare d0 with d2 plots). Starting at day 3, the content of CD133+ cells and the intensity of the PKH2 staining diminishes over time. Since PKH2 is a plasma membrane intercalating dye, its staining gets diluted with each cell division; the PKH2 intensity therefore reflects the number of cell divisions a given cell has performed during cultivation. The perpendicular lines should help to cluster cells regarding the number of cell divisions they had performed. The amount of cells depicted in all plots is normalized to the cell numbers of day 0; therefore, plots can be compared semiquantitatively. Note the small population with the weaker PKH2 staining follows the same kinetics as the large brightly stained population, demonstrating the reliability of the PKH2 experiment.
Flow cytometric analyses of PKH2-stained CD34+-enriched cells. PKH2-stained CD34+-enriched cells, either noncultivated (d0) or cultivated in the presence of early-acting cytokines for 2, 3, 4, or 5 days (d2, d3, d4, d5), were measured after labeling with anti-CD34 and anti-CD133 antibodies. The size and the granularity of all cells is plotted in the panels of the first column, and the PKH2 and anti-CD133 staining of the cells located in the live gates (shown in the first column) in the panels of the second column. Quadrants are adjusted according to corresponding isotype controls. Upon cultivation, CD34+ cell increase in size and slightly up-regulate CD133 on their cell surface (compare d0 with d2 plots). Starting at day 3, the content of CD133+ cells and the intensity of the PKH2 staining diminishes over time. Since PKH2 is a plasma membrane intercalating dye, its staining gets diluted with each cell division; the PKH2 intensity therefore reflects the number of cell divisions a given cell has performed during cultivation. The perpendicular lines should help to cluster cells regarding the number of cell divisions they had performed. The amount of cells depicted in all plots is normalized to the cell numbers of day 0; therefore, plots can be compared semiquantitatively. Note the small population with the weaker PKH2 staining follows the same kinetics as the large brightly stained population, demonstrating the reliability of the PKH2 experiment.
Because upon stimulation with late-acting cytokines CD133 expression was associated with the more primitive, slowly dividing CD34+ cell fraction,17 we assumed and verified (fourth paragraph of Results section) that cultivated CD34+CD133+ cells are more primitive than CD34+CD133low/− cells, and decided to preferentially analyze CD34+CD133+ cells in the course of this project. Since the content of CD34+CD133+ cells was significant higher at culture day 3 compared with days 4 and 5 (day 3, 57.5% ± 1.9%; day 4, 44.7% ± 1.6%; day 5, 33.0% ± 5.2%; Pday3/day4 < .003; Pday3/day5 < .02; n = 3), we mainly used day-3 CB-derived CD34+ cells for our further analyses.
Distribution of uropod markers in dividing CD34+ cells
We have recently shown that several surface molecules, and especially CD133, become distributed in a localized fashion in cultivated primitive hematopoietic cells.23 Now, we have studied their distribution in dividing CD34+ cells. To minimize extrinsic effects on the distribution of these molecules, we generally cultured the cells under stroma-free, nonadherent culture conditions before staining. We analyzed in total 2409 mitotic CD34+ cells from 14 different CB samples that were cultured for 3 or 4 days, respectively. A total of 1379 mitotic cells represented late mitotic stages (telophase), in which the 2 cellular poles can clearly be discriminated (Figure 2). Of the latter, 899 (65.2%) were found to be positive for CD133, a ratio consistent with the content of CD133+ cells within the fraction of CD34+ cells at day 3 of culture. In 892 of these mitotic cells, CD133 was distributed in a symmetrical fashion, displaying its highest concentration at the cleavage furrow or at the midbody, respectively (Figure 2). Only 7 (0.8%) late mitotic cells were found in which the anti-CD133 staining was not symmetrically distributed to the 2 prospective daughter cells; however, according to the appearance of these cells, the nonsymmetric distribution was nonspecific rather than specific.
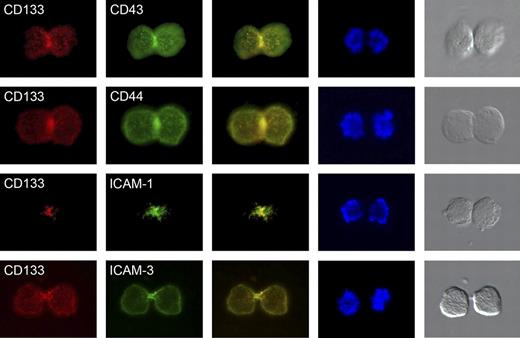
Cell-surface distribution of uropod markers in late mitotic CB-derived CD34+CD133+ cells. Before double labeling with anti-CD133 (red staining) and either anti-CD43, anti-CD44, anti–ICAM-1, or anti–ICAM-3 antibodies (green staining), cells were cultured for 3 days in serum-containing medium supplemented with early-acting cytokines. Note that CD133 as well as the other uropod markers are highly enriched at the cleavage furrow or midbody of dividing CD34+CD133+ cells. Column 1 shows anti-CD133 staining; column 2, anti-CD43, anti-CD44, anti–ICAM-1, and anti–ICAM-3 staining of cultivated CD34+ cells; column 3, merge of columns 1 and 2; column 4, DAPI staining; and column 5, light microscopy images of the stained cells.
Cell-surface distribution of uropod markers in late mitotic CB-derived CD34+CD133+ cells. Before double labeling with anti-CD133 (red staining) and either anti-CD43, anti-CD44, anti–ICAM-1, or anti–ICAM-3 antibodies (green staining), cells were cultured for 3 days in serum-containing medium supplemented with early-acting cytokines. Note that CD133 as well as the other uropod markers are highly enriched at the cleavage furrow or midbody of dividing CD34+CD133+ cells. Column 1 shows anti-CD133 staining; column 2, anti-CD43, anti-CD44, anti–ICAM-1, and anti–ICAM-3 staining of cultivated CD34+ cells; column 3, merge of columns 1 and 2; column 4, DAPI staining; and column 5, light microscopy images of the stained cells.
Furthermore, we analyzed the distribution of other uropod markers in late mitotic CD34+ cells (CD43, CD44, ICAM-1, and ICAM-3). All of these proteins were highly enriched at the cleavage furrow or midbody of the stained cells studied (Table 1; Figure 2). In none of the latter cells we did find any evidence for an asymmetric distribution of these proteins. Together with the results presented in paragraph 6 of the Results section, we assume that under the conditions used here, CD133 and the other uropod markers become highly enriched at the cleavage furrow and at the midbody of all dividing CD34+CD133+ cells, and thus do not identify putative asymmetric cell divisions.
Analyses of the uropod marker distribution in mitotic CD34+ cells
| . | n . | No. stained telophases . | Content of CD133+ telophases, % . |
|---|---|---|---|
| CD43 | 3 | 95 | 74.7 |
| CD44 | 3 | 195 | 87.2 |
| ICAM-1 | 3 | 124 | 95.2 |
| ICAM-3 | 4 | 135 | 80.7 |
| . | n . | No. stained telophases . | Content of CD133+ telophases, % . |
|---|---|---|---|
| CD43 | 3 | 95 | 74.7 |
| CD44 | 3 | 195 | 87.2 |
| ICAM-1 | 3 | 124 | 95.2 |
| ICAM-3 | 4 | 135 | 80.7 |
n indicates number of CB samples analyzed. The distribution of different antigens was studied in late mitotic cells counterstained with CD133. In all of the late mitotic cells analyzed, antigens were distributed in a symmetric fashion.
Phenotypical characterization of the CD34+CD133+ versus the CD34+CD133low/− cell fraction
Although our data argues against the possibility that CD133 segregates asymmetrically in dividing CD34+CD133+ cells, the kinetics of the cultured CD34+ cells are principally compatible with a model in which asymmetric cell divisions give rise to more primitive cells that will maintain the CD34+CD133+ phenotype, and to committed cells that will reduce their CD133 surface expression postmitotically. At this point we hypothesized that if CD34+CD133+ cells indeed can divide asymmetrically to give rise to CD34+CD133+ and CD34+CD133low/− cells, any protein that is expressed differentially among these populations might be a candidate for a protein that segregates differentially in asymmetrically dividing CD34+CD133+ cells.
Thus, to challenge this hypothesis, we next screened for proteins that are expressed differentially on both populations. For the screening procedure, we cultured CB-derived MNCs in the presence of early-acting cytokines for 3 days. Then, cells were stained with anti-CD133–PE and anti-CD34–PCy5 antibodies as well as with 1 of 58 different FITC-conjugated antibodies that recognize different surface antigens. The expression levels of the corresponding antigens were measured by flow cytometry. Using a gating strategy on CD34+ cells, the expression of the different antigens on CD34+CD133+ cells as well as on CD34+CD133low/− cells was analyzed and compared with each other (Figure 3). According to our results, 39 of these antigens were judged to be expressed on cultivated CD34+ cells, with 19 of them uncovering differences between the CD34+CD133+ and CD34+CD133low/− subpopulations (data not shown).
Flow cytometric analyses of CB-derived MNCs that have been cultivated for 3 days in the presence of early-acting cytokines. Plots represent MNCs that are ungated or gated on CD34+ cells. The CD34+ gate used is shown in the first plot of the first row. The size of these cells plotted against their granularity is shown in the second plot of row 1. The remaining plots represent the intensity of CD133 staining against the intensity of the isotype control, an anti-CD38, anti-CD47, anti-CD53, anti-CD62L, anti-CD63, or anti-CD71 staining, respectively. Quadrants are adjusted according to isotype controls of CD34 negative cells. Note that CD34+CD133+ cells contain different levels of CD53, CD63, CD62L, and CD71 than CD34+CD133low/− cells.
Flow cytometric analyses of CB-derived MNCs that have been cultivated for 3 days in the presence of early-acting cytokines. Plots represent MNCs that are ungated or gated on CD34+ cells. The CD34+ gate used is shown in the first plot of the first row. The size of these cells plotted against their granularity is shown in the second plot of row 1. The remaining plots represent the intensity of CD133 staining against the intensity of the isotype control, an anti-CD38, anti-CD47, anti-CD53, anti-CD62L, anti-CD63, or anti-CD71 staining, respectively. Quadrants are adjusted according to isotype controls of CD34 negative cells. Note that CD34+CD133+ cells contain different levels of CD53, CD63, CD62L, and CD71 than CD34+CD133low/− cells.
Following this initial screening procedure, we chose a panel of 11 different antibodies and studied the expression of the corresponding antigens on 2 additional CB-derived MNC fractions. While CD47 and CD59 were expressed homogeneously on CD34+ cells, CD13, CD31, CD53, CD62L, CD63, CD71, CD74, and CD105 were expressed in different amounts on CD34+CD133+ cells compared with CD34+CD133low/− cells in all samples. The expression levels of CD164 on these subpopulations were not consistent among the different samples (data not shown). It should be mentioned that in comparative analyses, we have studied the expression of these proteins on MNCs of 2 BM-derived and 2 PB-derived MNC fractions as well and obtained comparable results (data not shown).
According to our results, the expression levels of CD53, CD62L, CD63, and CD71 displayed the highest contrast between CD34+CD133+ and CD34+CD133low/− cells of the MNC fractions (Figure 3). To exclude any influence of CD34− MNCs on the expression levels of these antigens, we analyzed the expression of CD53, CD62L, CD63, and CD71 on purified CB-derived CD34+ cells that were cultured for 3 days in the presence of early-acting cytokines and obtained comparable results (Figure S1).
Functional characterization of the newly identified CD34+ subpopulations
To functionally characterize the newly identified CD34+ subfractions, we have purified CD133+ and CD133low/− fractions in combination with CD38, CD53, CD62L, CD63, or CD71 by fluorescent cell sorting (Figure S1B) and analyzed their primitive myeloid developmental capacity, measured as LTC-ICs. CD34+ cells of the same samples were purified in parallel and analyzed as controls. According to our results, the LTC-IC frequency within the purified CD34+CD133+ cell fractions was significantly higher than in their CD34+CD133low/− counterparts or in the total CD34+ cell fractions (Table 2). Compared with the subfraction of cultivated CD133+CD38low/− and CD133+CD71low cells, cells of the CD133+CD53+, CD133+CD62L+, and CD133+CD63low subfractions can be recognized as distinct cell populations (Figures 3, S1). Therefore, CD53, CD62L, and CD63 provide markers, which can be used in combination with CD133 to objectively identify potentially more primitive cells within the fraction of cultivated CD34+ cells.
Functional analyses of sorted CD34+ sub-fractions in LTC-IC assays
| LTC-IC frequency . | n . | CD34′, % . | CD133+, % . | CD133low/−, % . | P CD133+ to CD133low/− . | P CD133+ to CD34+ . |
|---|---|---|---|---|---|---|
| CD38 | 3 | 4.2 ± 3.5 | CD38low, 13.4 ± 1.8 | CD38+, 0.3 ± 0.4 | < .01 | < .02 |
| CD53 | 5 | 3.4 ± 2.7 | CD53+, 7.9 ± 4.2 | CD53low/−, 1.1 ± 1.0 | < .03 | < .03 |
| CD62L | 5 | 3.4 ± 2.7 | CD62L+, 8.9 ± 3.9 | CD62Llow/−, 0.3 ± 0.1 | < .01 | < .01 |
| CD63 | 5 | 5.3 ± 2.8 | CD63low/−, 11.4 ± 3.0 | CD63+, 0.3 ± 0.2 | < .01 | < .01 |
| CD71 | 5 | 3.4 ± 2.7 | CD71low, 12.7 ± 10.0 | CD71+, 1.0 ± 0.8 | < .06 | < .05 |
| LTC-IC frequency . | n . | CD34′, % . | CD133+, % . | CD133low/−, % . | P CD133+ to CD133low/− . | P CD133+ to CD34+ . |
|---|---|---|---|---|---|---|
| CD38 | 3 | 4.2 ± 3.5 | CD38low, 13.4 ± 1.8 | CD38+, 0.3 ± 0.4 | < .01 | < .02 |
| CD53 | 5 | 3.4 ± 2.7 | CD53+, 7.9 ± 4.2 | CD53low/−, 1.1 ± 1.0 | < .03 | < .03 |
| CD62L | 5 | 3.4 ± 2.7 | CD62L+, 8.9 ± 3.9 | CD62Llow/−, 0.3 ± 0.1 | < .01 | < .01 |
| CD63 | 5 | 5.3 ± 2.8 | CD63low/−, 11.4 ± 3.0 | CD63+, 0.3 ± 0.2 | < .01 | < .01 |
| CD71 | 5 | 3.4 ± 2.7 | CD71low, 12.7 ± 10.0 | CD71+, 1.0 ± 0.8 | < .06 | < .05 |
n indicates number of experiments; CD34+, LTC-IC frequency of CD34+ cells purified at culture day 3 (note: identical CB- samples for CD53, CD62L, and CD71 analyses were used); CD133+, LTC-IC frequency of purified, cultured CD34+ CD133+ cell fractions which are CD38low, CD53+, CD62L+, CD63low/−, or CD71low, respectively; CD133low, LTC-IC frequency of purified, cultured CD34+ CD133low/− cell fractions which are CD38+, CD53low/−, CD62Llow/−, CD63+, or CD71+, respectively; P CD133+ to CD133low/−, P values of significance analyses of the LTC-IC frequency of CD34+CD133+ cell fractions compared with that of corresponding CD34+CD133low/− cell fractions; P CD133+ to CD34+, P values of significance analyses of the LTC-IC frequency of CD34+CD133+ cell fractions compared with that of corresponding CD34+ cell fractions.
Subcellular distribution of identified antigens in polarized CD34+CD133+ cells
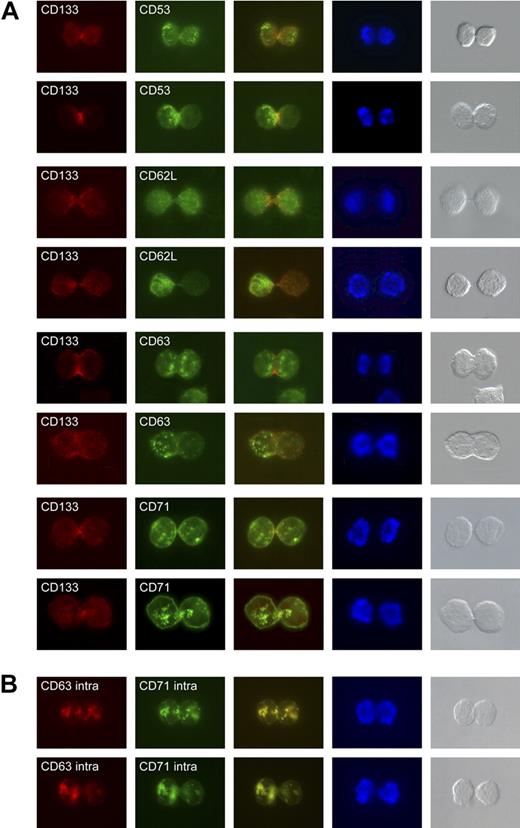
To subcellularly localize the identified antigens on polarized CD34+ cells, we stained CD34+ cells cultured for 3 days with antibodies against these markers and an anti-CD133 antibody. CD53 and CD63 were highly concentrated in vesicular-like structures at the base of the uropod. CD71 was distributed over the cell surface, including the leading edge of CD34+ cells; in addition, it was highly concentrated in vesicular-like structures at the base of the uropod. CD62L was highly concentrated at the tip of the uropod, where it seemed to colocalize with CD133; in addition, faint staining was sometimes found at the base of the uropod (Figure 4). It should be mentioned that compared with the staining with the other antibodies, the anti-CD62L antibody staining is generally very weak on fixed cells.
Localization of CD53, CD62L, CD63, and CD71 in polarized CD34+CD133+ cells. CD53, CD63, and CD71 are localized in vesicular-like structures at the base of the uropod. In addition, CD71 is expressed all over the cell surface, including the leading edge (left side of the cell shown in column 2). CD62L is highly localized at the tip of the uropod and colocalizes with CD133. Column 1 shows anti-CD133 staining; column 2, anti-CD53, anti-CD62L, anti-CD63, and anti-CD71 staining of CD34+ cells; column 3, merge of columns 1 and 2; column 4, DAPI staining; and column 5, light microscopy images of the stained cells.
Localization of CD53, CD62L, CD63, and CD71 in polarized CD34+CD133+ cells. CD53, CD63, and CD71 are localized in vesicular-like structures at the base of the uropod. In addition, CD71 is expressed all over the cell surface, including the leading edge (left side of the cell shown in column 2). CD62L is highly localized at the tip of the uropod and colocalizes with CD133. Column 1 shows anti-CD133 staining; column 2, anti-CD53, anti-CD62L, anti-CD63, and anti-CD71 staining of CD34+ cells; column 3, merge of columns 1 and 2; column 4, DAPI staining; and column 5, light microscopy images of the stained cells.
CD53, CD62L, CD63, and CD71 segregation during mitosis of CD34+CD133+ cells
Hence, the proteins identified in our screen are candidates that might segregate differently during mitosis. Therefore, we have analyzed the distribution of CD53, CD62L, CD63, and CD71 on CD34+CD133+ cells of late mitotic stages (telophase). On approximately 20% of the mitotic cells studied, CD53+ (19.6%), CD63+ (20.5%), and CD71+ (22.1%) vesicular structures were found preferentially in 1 of the prospective daughter cells, suggesting that these structures segregate differentially (Table 3, Figure 5). We also found late telophase CD34+CD133+ cells that displayed an asymmetric distribution of the CD62L antigen (15.2%). However, due to the weak anti-CD62L antibody staining of fixed cells, the number of asymmetrically dividing CD133+CD62L+ cells might be underestimated.
Distribution of CD53, CD62L, CD63, and CD71 in late mitotic CD34+ cells
| Antigen . | n . | Total no. telophases . | Total no. asym telophases . | Content of asym telophases, % . | Average rate of asym telophases per CB, % . |
|---|---|---|---|---|---|
| CD53/CD133 | 3 | 97 | 19 | 19.6 | 18.9 ± 9.4 |
| CD62L/CD133 | 4 | 112 | 17 | 15.2 | 17.8 ± 13.7 |
| CD63/CD133 | 5 | 146 | 30 | 20.5 | 23.6 ± 6.5 |
| CD71/CD133 | 4 | 131 | 29 | 22.1 | 29.9 ± 16.3 |
| CD63/CD71 | 4 | 221 | 40 | 18.1 | 20.3 ± 6.5 |
| Antigen . | n . | Total no. telophases . | Total no. asym telophases . | Content of asym telophases, % . | Average rate of asym telophases per CB, % . |
|---|---|---|---|---|---|
| CD53/CD133 | 3 | 97 | 19 | 19.6 | 18.9 ± 9.4 |
| CD62L/CD133 | 4 | 112 | 17 | 15.2 | 17.8 ± 13.7 |
| CD63/CD133 | 5 | 146 | 30 | 20.5 | 23.6 ± 6.5 |
| CD71/CD133 | 4 | 131 | 29 | 22.1 | 29.9 ± 16.3 |
| CD63/CD71 | 4 | 221 | 40 | 18.1 | 20.3 ± 6.5 |
The distribution of different antigens was studied in late mitotic cells counterstained with CD133 or in cells that were intracellularly stained with CD63 and CD71.
n indicates number of CB samples analyzed; asym, asymmetric.
*Data shows telophases.
Localization of CD53, CD62L, CD63 and CD71 in dividing CD34+CD133+ cells. (A) Cell-surface distribution of CD53, CD62L, CD63, and CD71 on late mitotic CD34+CD133+ cells. For each of these markers, 1 mitotic cell is shown containing a symmetric distribution of the given antigen (top row), and 1 containing an asymmetric distribution (bottom row; green staining). Cells are counterstained with an anti-CD133 antibody (red staining) and DAPI (blue staining). Light microscopy images of the stained cells are presented in the fifth column. (B) Intracellular distribution of CD63 (red staining) and CD71 (green staining). The overlay of the 2 is given in the third column.
Localization of CD53, CD62L, CD63 and CD71 in dividing CD34+CD133+ cells. (A) Cell-surface distribution of CD53, CD62L, CD63, and CD71 on late mitotic CD34+CD133+ cells. For each of these markers, 1 mitotic cell is shown containing a symmetric distribution of the given antigen (top row), and 1 containing an asymmetric distribution (bottom row; green staining). Cells are counterstained with an anti-CD133 antibody (red staining) and DAPI (blue staining). Light microscopy images of the stained cells are presented in the fifth column. (B) Intracellular distribution of CD63 (red staining) and CD71 (green staining). The overlay of the 2 is given in the third column.
Intracellular distribution of CD63 and CD71 in late mitotic CD34+ cells
Since CD53, CD63, and CD71 are expressed on vesicular-like structures and have been reported to be associated with the endosomal traffic,25–28 the vesicular-like structures might correspond to budding endosomes. To exclude the possibility the asymmetric distribution of these structures is connected to a very dynamic process, which might frequently switch among the daughter cells, we decided to study the intracellular distribution of the identified antigens. Due to an incompatibility between the anti-CD53 antibody and the intracellular staining procedure, we double-stained cultured CD34+ cells with anti-CD63 and anti-CD71 antibodies. In all cases studied, the anti-CD63 and the anti-CD71 staining colocalized (Figure 5B). In support of the data presented in the previous paragraph, we found an asymmetric distribution of the anti-CD63– and anti-CD71–stained structures in approximately 20% of the mitotic cells (Table 3, Figure 5B).
Discussion
Challenging the hypothesis of asymmetric cell division within the primitive hematopoietic cell compartment, we demonstrate here for the first time that primitive human hematopoietic cells indeed contain capabilities to divide asymmetrically. Furthermore, we report the identification of cell surface proteins that, in combination with CD133, can be used to define more primitive hematopoietic cells within the CD34+ cell fraction. In addition, these proteins led to the discovery of a potentially new subcellular plasma-membrane domain on migrating CD34+ cells in which endosomes seem to be formed to bud into the interior of the cell.
Asymmetric cell division of primitive hematopoietic cells
It has been suggested that HSCs can divide asymmetrically to give rise to 1 cell maintaining stem cell fate and to a daughter that is committed to differentiate. In agreement with this hypothesis, the findings of several groups, including our own, have demonstrated that primitive hematopoietic cells give rise to daughter cells adopting different cell fates or realizing different proliferation kinetics, respectively.10–15,17 Following a definition in which a cell division is defined as asymmetric or symmetric according to the cell fates of its daughter cells,29 these results would clearly demonstrate that primitive hematopoietic cells divide asymmetrically. However, if a more restrictive definition is used, in which asymmetrically dividing cells are defined as cells that by the different segregation of certain molecules become qualitatively different, these results are not sufficient to demonstrate asymmetric cell divisions within the primitive human hematopoietic cell compartment. The observed differences in the cell fate or the proliferation kinetics could theoretically be a result of extrinsic mechanisms that act postmitotically and alter the developmental capacities of initially identical daughter cells. A well-analyzed process in which cells with identical developmental capacities become different is the process of lateral inhibition, sometimes also referred as mutual inhibition or lateral specification. In this process, cells with identical developmental capacities mutually influence each others cell fate to become different.20,21 Since this process is mediated by the Notch signaling pathway, which plays important roles during early and late hematopoiesis,30 its action is one of several other predictable, postmitotically acting mechanisms which theoretically could account for the observed differences.
However, by the identification of marker proteins that segregate differently in approximately 20% of the mitotic, CB-derived CD34+CD133+ cells, it now becomes evident that primitive hematopoietic cells can indeed give rise to qualitatively different daughter cells and thus divide asymmetrically. Since we raised the cells in stroma-free suspension cultures, and the cells grew nonadherently, we suppose that the asymmetric distribution of the proteins in mitotic cells is assigned intrinsically rather than induced by extrinsic signals that might directly affect the subcellular localization of the identified proteins. This assumption is further enforced by the findings that (1) the ratio of late mitotic cells with asymmetric protein distributions fits into the same range with which primitive human hematopoietic cells deposited as single cells in suspension cultures generate daughter cells realizing different cell fates and different proliferation kinetics, and (2) that the latter rate was not influenced by the different cytokine conditions used to raise these cells in vitro.14,16
Although we cannot exclude that daughter cells inheriting different levels of the identified marker proteins can compensate for these differences and adopt identical developmental potentials, this congruency suggests that there is a high correlation between the marker distribution and the acquired cell fates of the daughter cells, which is supported by the fact that CD133 and the expression of the marker proteins we describe here correlates very well with the primitive state of CD34+ cells. More primitive CD34+CD133+ cells express higher levels of CD53 and CD62L as well as lower levels of CD63 and CD71 than the more mature CD34+CD133low/− cells. In this context, it should be noted that, in combination with CD133 and CD62L, CD53 or CD63 more objectively defined subpopulations of CD34+ cells can be recognized and purified than with the CD38 antigen, which is commonly used to discriminate more primitive human HPCs (CD34+CD38low/−) from more mature ones (CD34+CD38+).31,32 According to our findings, we postulate that asymmetrically dividing CD34+CD133+ cells obtaining more CD53 or CD62L or less CD63 are more primitive than their sister cells. As CD71 is distributed along the whole plasma membrane, we suggest that the daughter cells inheriting more of the CD71-stained vesicular-like structures will express higher levels of CD71 than their sister cells. Since CD34+CD133+CD71low cells are more primitive than CD34+CD133lowCD71+ cells, we assume that the daughter cells inheriting more of the CD71+ vesicular-like structures are more mature than their sister cells. This view is supported by the result of our intracellular staining procedure, in which CD63+ vesicular-like structures, which are supposed to label more mature cells, cosegregate with the CD71-stained structures. In addition, and consistent with our functional data, it was reported several years ago that CD34+CD71low cells are more primitive than CD34+CD71+ cells.33,34 Similarly, previous evidence showed that CD62L expressing CD34+ cells are more primitive than CD62L− CD34+ cells.35,36 To our knowledge, neither CD53 nor CD63 have been associated with the fate of CD34+ cells. Therefore, we have qualified these as new markers to discriminate primitive cultured CD34+ cells from more mature cells.
Tetraspanins
Both CD53 and CD63 encode members of the evolutionary conserved tetraspanin family, a large superfamily of small cell-surface membrane proteins characterized by 4 transmembrane and 2 extracellular domains. At present, 28 members of this family have been documented in humans, many of them expressed on multiple cell types. They seem to organize novel types of cell-surface membrane microdomains, the tetraspanin-enriched microdomains (TEMs), novel signaling platforms which are distinct from lipid rafts.37,38 However, little is known about their function, though tetraspanins seem to take part in regulating the activation, motility, and antigen presentation of different leukocytes.37,38 Interestingly, it has been shown that, in addition to the cell surface, many tetraspanins, including CD53 and CD63, are localized on the internal vesicles of multivesicular endosomes and on exosomes.26 Due to the interaction between CD63 and subunits of AP-2 and AP-3 complexes, it is linked to clathrin-dependent endocytotic pathways and seems to play a role in the recycling of plasma membrane components and their transport to appropriate intracellular compartments.25,27,39–42
Since the transferrin receptor (CD71) also cycles between the plasma membrane and the endosomal compartment and can be secreted on exosomes,28,39 3 of the 4 identified proteins that frequently segregate asymmetrically in dividing CD34+CD133+ cells are linked to the endosomal/exosomal compartment. This, together with the fact that anti-CD53, anti-CD63, and anti-CD71 staining is highly enriched in vesicular-like structures in polarized as well as in mitotic CD34+CD133+ cells, suggest that the vesicular-like structures correspond to budding endosomes. This assumption is further supported by our finding that in living CD34+ cells, antibodies against CD63 and CD71 become internalized within the region that is highly enriched for these vesicular-like structures (data not shown). Since these presumptive budding endosomes are highly enriched in a region at the base of the uropod, they define a membrane domain that to our knowledge has not been previously described in polarized CD34+ cells. Furthermore, these results reveal a link between the endosomal compartment and mechanisms governing the asymmetric cell division of primitive human hematopoietic cells. It will, in the future, be interesting to determine the similarities between this asymmetric cell division in human HPCs and the role of the endosomal compartment and mechanisms governing asymmetric cell divisions that have been described in Drosophila.43
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Michael Punzel, Dr Andreas Wodarz, and Dr Verdon Taylor for general discussion and critiques on the manuscript; and Svenja Jünger, Jan Spanholtz, and Gregor von Levetzow for general experimental support. Umbilical cord blood samples were kindly provided by Gesine Kögler of our institute (Institute for Transplantation Diagnostics and Cellular Therapeutics).
This work was supported by grants from the Deutsche Forschungsgemeinschaft (SPP1109 GI 336/1-4 to B.G. and P.W.) and from the Forschungskommission of the HHU-Duesseldorf (B.G.).
Authorship
Contribution: J.B. helped to design the study, performed experiments and assisted in the writing; S.S. performed experiments; P.W. provided intellectual input by discussing the data; J.C.F. provided essential experimental support; and B.G. designed the study, performed experiments, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Bernd Giebel, Institute for Transplantation Diagnostics and Cell Therapeutics, Heinrich-Heine-University Duesseldorf, D-40225 Duesseldorf, Germany; e-mail: giebel@itz.uni-duesseldorf.de.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal