Abstract
The present work deals with the mechanisms of signal transduction mediated via CD38 in normal and neoplastic human B lymphocytes. The results indicate that CD38 is a receptor and that CD38-mediated signals are tightly regulated at 3 distinct levels. The first concerns the structural organization of CD38, which is clearly divided into monomeric and dimeric forms. The second level of regulation is based on the dynamic localization of CD38 molecules in lipid microdomains within the plasma membrane. Lateral associations with other proteins, namely with the CD19/CD81 complex, determine the third level of control. Raft localization and association with the CD19 complex are prerequisites for CD38-mediated signals in tonsillar B cells and in continuous lines. Lastly, the results indicate that lipid microdomain disruption and silencing of CD19 directly impacts on CD38's ability to mediate Ca2+ fluxes, while leaving its surface expression unchanged. CD38 is also an enzyme capable of producing several calcium-mobilizing metabolites including cyclic adenosine diphosphate ribose (cADPR). Our inability to identify a correlation between the production of cADPR and the receptorial functions support the hypothesis that CD38 is a pleiotropic molecule whose behavior as a receptor is independent from its enzymatic activity.
Introduction
Human CD38 is a cell surface glycoprotein of approximately 45 kDa expressed in a wide variety of cell types, including thymocytes and activated T lymphocytes, B-cell precursors and plasma cells, natural killer cells (NK), monocytes, and dendritic cells.1,2 Its C-terminal extracellular domain contains a catalytic site capable of metabolizing NAD and NADP.3 Two of the intermediate compounds generated during these reactions, namely cyclic ADP ribose (cADPR) and NAADP, are potent intracellular second messengers.4,5 CD38 also controls a signaling pathway involved in the activation, growth, and survival of lymphoid6–9 and myeloid10,11 cells, which appears to be completely independent from the molecule's enzymatic functions. The dual behavior of CD38 as both enzyme and receptor is likely the long-term outcome of evolutionary pressure on what was once a soluble enzyme, culminating in its metamorphosis into a “social” cell surface protein.12,13 The receptor functions are regulated extracellularly through interactions with CD31, the only nonsubstrate ligand for CD38 identified to date.14 Understanding the mechanisms of signal transduction is less straightforward, for the cytoplasmic domain of CD38 lacks signaling motifs, docking sites, and critical tyrosines, suggesting that lateral associations play important functional roles. The emerging picture is that CD38 associates with cell surface molecules specialized in signal transduction and with membrane adaptors.15–17 Further, these associations are lineage dependent but vary according to the differentiation/maturation steps within each lineage.13 Finally, these associations are a necessary condition for signal transduction, but not for the enzymatic functions, similar to what has been described for murine B cells.18,19 Recent findings have opened a new area for inquiry by suggesting that further regulation occurs through membrane movement of CD38 molecules in and out of specialized membrane microdomains (or rafts).20–22
The present work deals with the mechanisms of signal transduction via CD38 in normal and neoplastic human B lymphocytes. B cells were selected for study because of the growing body of data implicating CD38 in the pathogenetic network underlying chronic lymphocytic leukemia (CLL).23 CD38 is a useful negative prognostic marker that correlates with shorter survival, more frequent therapeutic need, and diminished sensitivity to chemotherapeutic agents (reviewed in Matrai24 ). Our hypothesis is that CD38 is not merely a marker, but directly contributes to the worsening of CLL prognosis. This has already been shown in vitro by mimicking the interactions between CLL and nurselike cells in the blood and in peripheral lymphoid organs.25
Here we show that the prerequisites for CD38-mediated signals are (1) interaction with the CD31 ligand, (2) localization in membrane rafts, and (3) functional association with CD19. Lastly, these requisites are maintained unaltered during distinct differentiation/maturation steps and neoplastic transformation.
Materials and methods
Tonsil samples were collected after informed consent was provided according to the Declaration of Helsinki and after approval from the local ethics committee, Azienda Ospedaliera San Giovanni Battista.
Cells
Tonsillar B lymphocytes were purified as described.26 The cell lines were Nalm-6 (B-cell precursor leukemia), Raji, Daudi and Namalwa (Burkitt lymphoma), U266 (myeloma), and K562 (erythroleukemia). The DL06 cell line was established in the lab from a patient with myeloma; the phenotype is compatible with plasma cells (CD38+/CD138+/CD19−/CD20−/sIg−) and the cells actively secrete IgAλ (F.M., manuscript in preparation). Murine L cells stably transfected with human CD31 (L-CD31+) and with the empty plasmid (L-mock) were previously obtained.14 Cells were cultured as described.25
Antibodies and reagents
The anti-CD38 antibodies used were IB4 (ligation of the molecule),27 OKT10 (immunoprecipitation), SUN-4B7 (Western blot), and AT-1 (immunofluorescence).28 Other in-house antibodies were CB19 (anti-CD19), I.33.22 (anti-CD81), O1.65 (anti–HLA class I), and the irrelevant isotype-matched JAS (anti–HIV-1 gp120). Other reagents were polyclonal anti-sIgM (Southern Biotech, Birmingham, AL), rabbit anti-CD79α and anti-CD79β (Santa Cruz, Santa Cruz, CA), rabbit anti-CD19 (Cell Signaling Technology, Danvers, MA), anti-phospho(p-)ERK1/2, control anti-ERK1/2, and anti-CD45 (BD Biosciences, Milan, Italy), donkey anti–mouse IgG (DαMIgG; Jackson ImmunoResearch, Soham, United Kingdom), goat anti–mouse IgG horseradish-peroxidase (HRP) labeled (Perkin Elmer, Boston, MA), fluorescein isothiocyanate (FITC)– and Texas Red–conjugated goat anti–mouse IgG, and FITC- and HRP-conjugated cholera toxin (CTX; Sigma, Milan, Italy).
CD19 siRNA was from Santa Cruz and gene silencing was achieved according to the manufacturer's instructions.
Membrane fractionation
Detergent-insoluble membrane (DIM, containing the lipid microdomains) and detergent-soluble membrane (DSM) fractions were purified by density gradient centrifugation.29,30 Cells (50 × 106 cells/sample) were washed in PBS and resuspended (30 minutes on ice) in ice-cold buffer A (25 mM Tris, pH 7.5, 150 mM NaCl, 5 mM EDTA) with protease inhibitors (1 μg/mL leupeptin, 1 μg/mL pepstatin, 0.2 mg/mL sodium orthovanadate, and 1 mM phenyl-methyl-sulphonyl-fluoride). The cells were next quickly frozen and thawed before adding Brij98 (Brij 20 oleyl ether, 5 minutes, 37°C, 1% final concentration; Sigma). The cells were then diluted in 2 M sucrose, chilled (55 minutes, on ice), and placed at the bottom of a decreasing sucrose gradient (0.9 M ÷ 0.2 M). The gradient was centrifuged in a SW55Ti rotor (250 000g, 16 hours, 4°C; Beckman Coulter, Fullerton, CA). Of the 8 fractions collected from the top, 2 to 4 contain the DIM; 5 to 7, the intermediate; and 8 to 9, the DSM. Where indicated, fractions were pooled and proteins precipitated with methanol, chloroform, and water.31 In some experiments, DIMs and DSMs were obtained by centrifugation (20 000g, 15 minutes, 4°C) of cells lysed in 1% Brij98.32 DSMs are in the supernatant, while the pellet contains the DIM. Solubilization of raft-associated proteins was obtained by addition of octyl-d-glucopyranoside (ODG; Sigma) to the lysis buffer.
Receptor engagement
Cells were starved in serum-free medium (20 hours, 37°C), washed, and incubated with the agonistic anti-CD38 IB4 antibody (1 μg/106 cells). A DαMIgG (10 μg/106 cells) was used as the cross-linker. Cells were then incubated at 37°C for the indicated times and the reaction was stopped with ice-cold PBS. In selected experiments, CD38 ligation was obtained by using L-CD31+ cells (optimal ratio = 10 B lymphocytes–1 fibroblast).25
Immunoprecipitation and Western blot
Lysates, obtained as described in Membrane fractionation paragraph of Materials and methods, were immunoprecipitated with OKT10 (0.2 μg/100 μg lysate, overnight, 4°C); the immunocomplexes were collected with anti–mouse IgG-agarose (1-2 hours, 4°C; Sigma) and eluted by boiling in 1% Triton-X100 (Sigma) sample buffer. Whole-cell lysates and immunoprecipitates were resolved by 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and electrophoretically transferred to nitrocellulose membranes, using a semidry apparatus (Hoefer SemyPhor; Pharmacia Biotech, San Francisco, CA). Blots were blocked and incubated with primary and secondary antibodies, as indicated by the manufacturer's instructions before development using an enhanced chemiluminescence (ECL) detection system (Perkin Elmer).
Densitometric analyses were performed using the public domain NIH ImageJ program (version 1.36, available at http://rsb.info.nih.gov/nih-image/).
Immunofluorescence and confocal microscopy
B cells (0.5 × 106) were incubated with the specified antibody (30 minutes on ice), washed, and reacted with Texas Red GαMIg (20 minutes on ice). Samples were then moved for 40 minutes to 37°C to induce capping, blocked by ice-cold PBS + 0.5% BSA and 0.1% NaN3. Counterstaining was performed with directly FITC-labeled antibodies or CTX. In selected experiments, B cells were preincubated (20 minutes, 37°C) with methyl-β-cyclodextrin (MβCD, 10 mM; Sigma) to deprive cell membranes of cholesterol.
Cells were then fixed (4% paraformaldehyde) and analyzed with an Olympus IX71 confocal microscope, using a × 60 oil immersion objective. The FluoView software (Olympus, Milan, Italy) was used to acquire data. Images were processed using Adobe Photoshop CS2 software (San Jose, CA).
The CD38/CD31 interactions were evaluated as described.25
Calcium mobilization
Intracellular Ca2+ concentrations were measured by flow cytometry.33 B cells (106/mL) were loaded (1 hour, 30°C) with 5 μM Fluo 3-AM (Invitrogen, Frederick, MD), washed twice, and analyzed using a FACSort (BD Biosciences) by continuously recording 525 nm emissions on a linear scale. The A23187 ionophore (Sigma) was added to check for effective loading of the cells. Data are presented as density plots of FL1-H fluorescence (y-axis) over time (x-axis) or as percentage of fluorescence increase, calculated as [(highest value of FL1-H of the sample under analysis/highest value of FL1-H of cells treated with an irrelevant antibody)-1].
cADPR radioreceptor assays
[32P]cADPR was synthesized from [32P]NAD (Perkin Elmer) using ADP-ribosyl cyclase (Sigma). Briefly, 100 μCi (3.7 MBq) [32P]NAD was incubated with ADP-ribosyl cyclase, 400 ng/mL, for 2 hours at 25°C in 5 mM Tris-HCl, pH 7.5.34 The reaction was then separated by high-performance liquid chromatography (HPLC).35
NAD (1 μM) was incubated with the specified cells (2 × 105, 30 minutes) in PBS (200 μL final volume). Aliquots (10 μL each) were taken and assayed for cADPR concentration by comparing the specific displacement induced by 10 μL of the reaction to binding curves generated using authentic cADPR (Sigma), as described.36
Results
Membrane localization of CD38 in normal and neoplastic B cells
CD38 engagement in human B lymphocytes generates powerful signals that affect activation, survival, and proliferation differently during B-cell ontogenesis (reviewed in Campana et al8 ). The aim of this section is to understand the mechanisms of signal transduction through CD38. The working hypothesis based on experience with other cell lineages is that (1) the localization of CD38 molecules in critical membrane domains, and (2) the formation of supramolecular complexes are necessary conditions for the transduction of CD38-mediated signals.
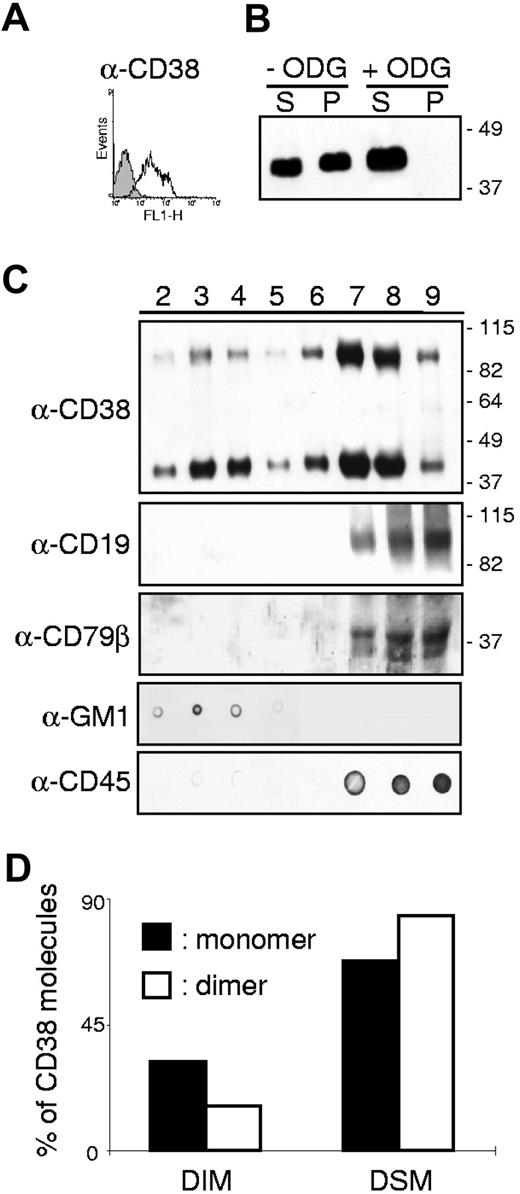
Our strategy was to analyze the distribution of CD38 in membrane fractions of B lymphocytes in resting conditions and after CD38 ligation. First, B lymphocytes obtained from human tonsils (Figure 1A) were used to determine how CD38 molecules are distributed in discrete membrane compartments. Cells were lysed in 1% Brij98 at 37°C and the lysates fractionated into supernatant and pellet after centrifugation. The results indicate that approximately 50% of CD38 molecules are constitutively present in DIM (Figure 1B). Insolubility of a membrane protein in a nonionic detergent could be due to its association with detergent-resistant lipid rafts and/or its anchoring to cytoskeletal elements. These 2 possibilities were distinguished by means of ODG, a gentle nonionic detergent that solubilizes proteins associated with glycolipid-enriched membranes without disrupting the cytoskeleton. CD38 was completely solubilized following treatment and was recovered largely from the supernatant fraction, supporting the premise that its insolubility in Brij98 is mostly due to raft association (Figure 1B). To confirm this, normal resting B lymphocytes were lysed in Brij98, overlaid on sucrose gradient, and ultracentrifuged. The presence of CD38 in the 8 recovered fractions was detected by Western blotting under nonreducing conditions. The first finding was that CD38 molecules are clearly distinct in quantitative terms in the portions inside and outside the rafts, as described at the beginning of the Results section. The second finding was the presence of CD38 dimers also variably distributed in the 8 fractions. Several independent observations support the notion that CD38 is a approximately 90-kDa homodimer,37 as does the crystal structure.38 Only the use of the mild detergent Brij98 followed by a sucrose gradient clearly highlights the presence of the dimers, conventionally seen after immunoprecipitation and SDS-PAGE under nonreducing conditions.37 The third finding was that the monomeric and dimeric CD38 show different distributions in the 8 membrane fractions. Under resting conditions, approximately 30% of the monomer is constitutively located in the DIM (Figure 1C-D). Another approximately 30% of CD38 is associated with the DSM and the remaining 40% found in the intermediate areas (Figure 1C-D). Similar results were obtained using tonsils from 5 different donors, including a young adult, yielding highly reproducible results. The CD38 dimer behaves differently, with only approximately 15% present in the DIM and the remaining approximately 85% evenly distributed among the DSM and intermediate fractions.
Membrane localization of CD38 molecules in human tonsillar B cells. (A) Cell surface expression of CD38 (open profile) in tonsillar B lymphocytes. Background staining is shown in gray. X-axis: intensity of fluorescence; y-axis: number of events. (B) Tonsillar B cells were lysed in 1% Brij98 and the lysates fractionated into a supernatant of soluble proteins (S) and a pellet (P) of insoluble proteins by centrifugation. Proteins were separated on 10% SDS-PAGE under nonreducing conditions, transferred to nitrocellulose membranes, and blotted with the anti-CD38 antibody SUN-4B7. Where indicated, ODG was added to the lysis buffer. The data shown are representative of 3 independent experiments. (C) Tonsillar B cells lysed as indicated in the Membrane fractionation paragraph of Materials and methods were fractionated on a sucrose gradient. Aliquots of the 8 recovered fractions were separated on a 10% SDS-PAGE and immunoblotted with the indicated antibody. The localization of the GM1 ganglioside and of CD45 was checked with a dot blot as a control of the correct separation of the different membrane fractions. The data are representative of 5 independent experiments (D) The intensity of the bands corresponding to the CD38 monomer (black histogram) and dimer (open histogram) in each fraction was measured using the ImageJ software. The bars represent the sum of the bands in fractions 2 to 4 (DIM) or 5 to 9 (DSM) divided by the sum of all fractions.
Membrane localization of CD38 molecules in human tonsillar B cells. (A) Cell surface expression of CD38 (open profile) in tonsillar B lymphocytes. Background staining is shown in gray. X-axis: intensity of fluorescence; y-axis: number of events. (B) Tonsillar B cells were lysed in 1% Brij98 and the lysates fractionated into a supernatant of soluble proteins (S) and a pellet (P) of insoluble proteins by centrifugation. Proteins were separated on 10% SDS-PAGE under nonreducing conditions, transferred to nitrocellulose membranes, and blotted with the anti-CD38 antibody SUN-4B7. Where indicated, ODG was added to the lysis buffer. The data shown are representative of 3 independent experiments. (C) Tonsillar B cells lysed as indicated in the Membrane fractionation paragraph of Materials and methods were fractionated on a sucrose gradient. Aliquots of the 8 recovered fractions were separated on a 10% SDS-PAGE and immunoblotted with the indicated antibody. The localization of the GM1 ganglioside and of CD45 was checked with a dot blot as a control of the correct separation of the different membrane fractions. The data are representative of 5 independent experiments (D) The intensity of the bands corresponding to the CD38 monomer (black histogram) and dimer (open histogram) in each fraction was measured using the ImageJ software. The bars represent the sum of the bands in fractions 2 to 4 (DIM) or 5 to 9 (DSM) divided by the sum of all fractions.
As expected,39,40 CD19 and CD79β were completely excluded from the DIM in resting conditions (Figure 1C). The quality of the membrane fractionation was confirmed by testing the localization of the raft-associated GM1 ganglioside and of non–raft-associated CD45 in dot blots (Figure 1C).
Next, to obtain an overall picture of CD38 molecule distribution in the B-cell compartment, we turned to cell line analysis. The results reported in Table 1 indicate that CD38 is also present in a monomeric and dimeric form in all the cell lines analyzed. The table shows the percentage of CD38 monomers and dimers (ie, sum of CD38 band intensities in the relevant fractions/sum of CD38 band intensities in all fractions) in distinct membrane domains. As seen with tonsillar B lymphocytes, CD38 is not restricted to a discrete membrane compartment. However, the percentage of monomer present in the DIM is generally higher than in nonneoplastic B cells. Lastly, the intermediate fractions contain a significant amount of CD38 monomer and dimer, suggesting that membrane relocalization is an important functional step in CD38 signal transduction.
Distribution (%) of CD38 molecules in distinct membrane domains
| Cells . | DIM . | Intermediate . | DSM . | |||
|---|---|---|---|---|---|---|
| Monomer . | Dimer . | Monomer . | Dimer . | Monomer . | Dimer . | |
| Tonsil B lymphocytes | 32 | 16 | 38 | 44 | 30 | 40 |
| Nalm-6 | 46 | 19 | 46 | 36 | 8 | 45 |
| Raji | 33 | 36 | 50 | 45 | 17 | 19 |
| Daudi | 34 | 46 | 60 | 52 | 6 | 2 |
| DL06 | 27 | 21 | 50 | 59 | 23 | 20 |
| Cells . | DIM . | Intermediate . | DSM . | |||
|---|---|---|---|---|---|---|
| Monomer . | Dimer . | Monomer . | Dimer . | Monomer . | Dimer . | |
| Tonsil B lymphocytes | 32 | 16 | 38 | 44 | 30 | 40 |
| Nalm-6 | 46 | 19 | 46 | 36 | 8 | 45 |
| Raji | 33 | 36 | 50 | 45 | 17 | 19 |
| Daudi | 34 | 46 | 60 | 52 | 6 | 2 |
| DL06 | 27 | 21 | 50 | 59 | 23 | 20 |
CD38 engagement is followed by raft translocation
To establish whether membrane reorganization accompanies the onset of signal transduction via CD38, the distribution of the molecule on the cell surface after cross-linking was monitored using live microscopy and biochemical approaches.
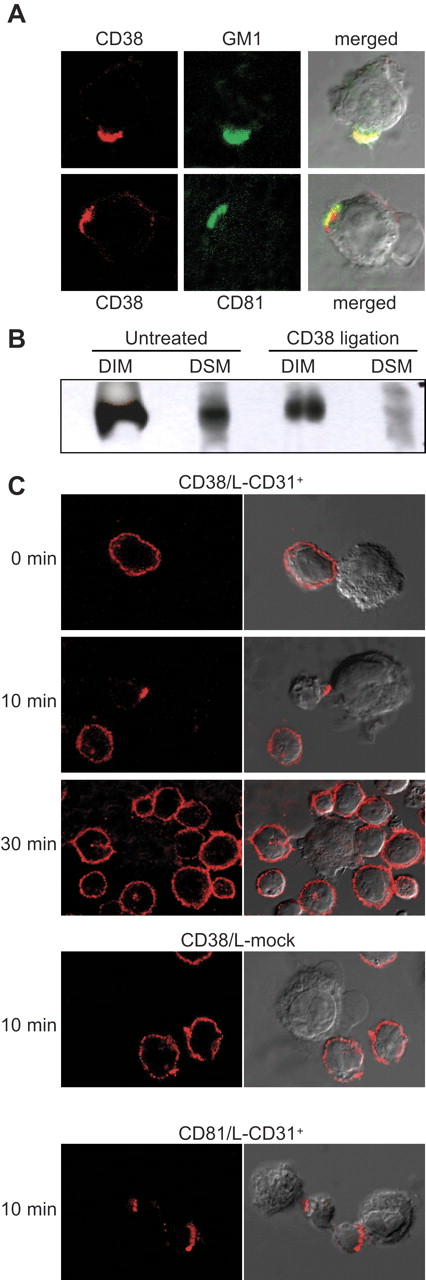
Antibody-mediated capping is an energy-dependent redistribution of cell surface molecules to a single pole of the cell. In general, only molecules bound by the antibody will be redistributed to the area of the cap, unless they have a particular association with other structures that are in turn induced to undergo capping in the same area.41 Upon primary and secondary antibody biding, CD38 molecules expressed by tonsillar B cells polarize to the area of the cell membrane with the highest concentration of GM1 ganglioside, suggesting that receptor engagement triggers recruitment of all CD38 molecules into the rafts (Figure 2A). Cocapping experiments between CD38 and CD81, a raft-resident tetraspanin,42 confirmed this result. CD38 molecules were induced to cap following antibody ligation at 37°C and the membrane fluidity and energy supplies were blocked by transferring the cells on ice in the presence of NaN3. Results indicate that the 2 molecules are laterally associated in normal and neoplastic B cells and that the association is bidirectional: capping of CD38 induces cocapping of CD81 and vice versa (Figure 2A; Table 2). These results were confirmed in all the cell lines tested (not shown).
CD38 engagement is followed by raft translocation. (A) CD38 molecules were stained with the AT-1 antibody followed by a Texas Red–GαMIg. The cells were then moved to 37°C for 40 minutes before stopping the experiment and staining for the GM1 ganglioside and CD81 using FITC-labeled reagents. (B) Tonsillar B cells were treated with the agonistic anti-CD38 antibody followed by a goat anti–mouse IgG used as a cross-linker for 5 minutes. After treatment, cells were lysed with Brij98 and the different membrane fractions separated by ultracentrifugation. Fractions 2 to 4 and 8 to 9 were pooled together and considered DIM and DSM, respectively. Proteins were precipitated, separated, and immunoblotted for CD38. (C) CD38+ tonsillar B cells and L-CD31+ or control L-mock fibroblasts were mixed at a 10:1 ratio, cocentrifuged, and incubated at 37°C for the indicated time. After stopping the experiment, CD38 or CD81 molecules were visualized by an indirect method using a Texas Red–GαMIg. Heteroconjugates were identified by visual observation under an Olympus 1 × 71 confocal microscope at 60 × magnification. Images are representative of 6 independent experiments.
CD38 engagement is followed by raft translocation. (A) CD38 molecules were stained with the AT-1 antibody followed by a Texas Red–GαMIg. The cells were then moved to 37°C for 40 minutes before stopping the experiment and staining for the GM1 ganglioside and CD81 using FITC-labeled reagents. (B) Tonsillar B cells were treated with the agonistic anti-CD38 antibody followed by a goat anti–mouse IgG used as a cross-linker for 5 minutes. After treatment, cells were lysed with Brij98 and the different membrane fractions separated by ultracentrifugation. Fractions 2 to 4 and 8 to 9 were pooled together and considered DIM and DSM, respectively. Proteins were precipitated, separated, and immunoblotted for CD38. (C) CD38+ tonsillar B cells and L-CD31+ or control L-mock fibroblasts were mixed at a 10:1 ratio, cocentrifuged, and incubated at 37°C for the indicated time. After stopping the experiment, CD38 or CD81 molecules were visualized by an indirect method using a Texas Red–GαMIg. Heteroconjugates were identified by visual observation under an Olympus 1 × 71 confocal microscope at 60 × magnification. Images are representative of 6 independent experiments.
Co-capping of CD38 and CD19 in B cells
| Cap . | Co-Cap . | # caps . | % co-caps . | |
|---|---|---|---|---|
| −MβCD . | +MβCD . | |||
| CD38 | CD81 | 83 | 77 | 20 |
| CD19 | 77 | 79 | 22 | |
| HLA Class I | 84 | 12 | 14 | |
| CD19 | CD38 | 84 | 81 | 22 |
| CD81 | CD38 | 87 | 73 | 16 |
| Cap . | Co-Cap . | # caps . | % co-caps . | |
|---|---|---|---|---|
| −MβCD . | +MβCD . | |||
| CD38 | CD81 | 83 | 77 | 20 |
| CD19 | 77 | 79 | 22 | |
| HLA Class I | 84 | 12 | 14 | |
| CD19 | CD38 | 84 | 81 | 22 |
| CD81 | CD38 | 87 | 73 | 16 |
These results were confirmed biochemically by fractioning the B-cell membranes after antibody ligation: DIM and DSM fractions were pooled, proteins were precipitated, and CD38 expression was checked by Western blot. CD38 engagement is followed by a significant decrease in the DSM pool of the molecule in tonsillar B lymphocytes as well as in the B-cell lines tested (Figure 2B). Conversely, the vast majority of CD45 molecules are maintained in the DSM (not shown).
Lastly, the events observed following CD38 ligation with an agonistic antibody were reproduced by using L-CD31+ cells to engage CD38, mimicking physiological conditions. Exposure of purified tonsillar B cells (Figure 2C) or representative B-cell lines (not shown) to the cell surface–expressed CD31 ligand is followed by a marked redistribution of CD38 molecules to the contact areas after 10 minutes (Figure 2C). The effect is specific, with no relocalization being observed upon interaction of tonsillar B lymphocytes and L-mock cells (Figure 2C). CD81 molecules analyzed in parallel show similar behavior, suggesting that lipid rafts are localized in the zone of interaction (Figure 2C). After 30 minutes, the conjugates become more stable, with the fibroblasts starting to adhere firmly to the plastic and surrounded by B cells in a rosettelike fashion. At this stage, CD38 and CD81 molecules reacquire a homogeneous surface expression pattern, indicating that the reorganization events on the membrane are highly dynamic and unstable (Figure 2C).
Taken together, these experiments indicate that CD38 ligation by agonistic antibodies and the cell-bound ligand induces the molecule to enter the raft areas. Dynamic ins and outs of selected areas of the membrane by CD38 appear to be a necessary condition for signal transduction.
CD38/CD31 cross-talk induces lateral association of CD38 and CD19 within lipid rafts
According to our working hypothesis, the transduction of a CD38-mediated signal relies on lateral interactions with other surface receptors. The existence of large supramolecular complexes has been shown in T,16 NK,43 and myeloid lineages.11,22 Furthermore, CD38 has been shown to be laterally associated with CD19 and with surface Ig in normal15 and CLL33 B lymphocytes.
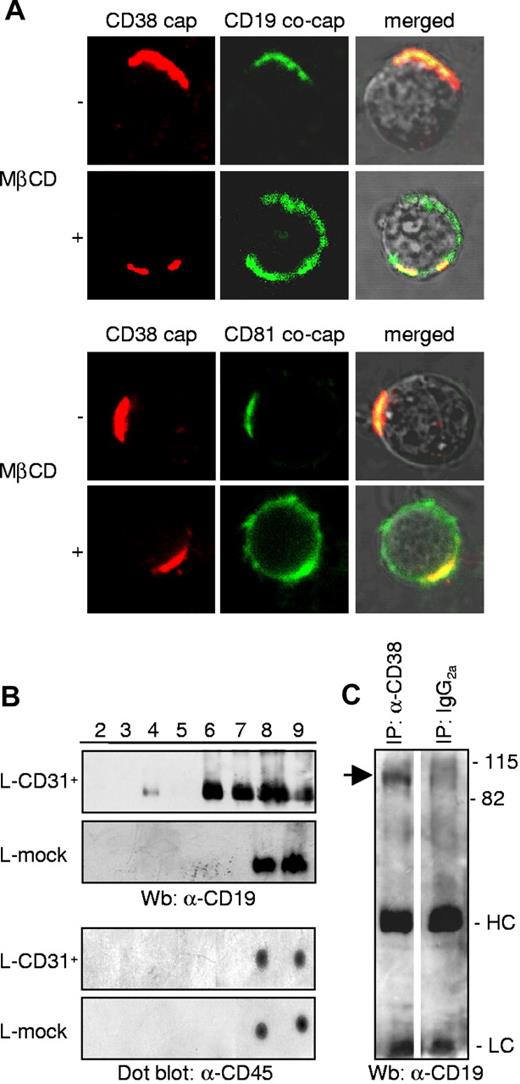
The membrane relationship between CD38 and CD19 was confirmed in tonsillar B lymphocytes and in all the cell lines tested (Figure 3A; Table 2) using cocapping experiments. CD19 molecules are invariably found in CD38 caps and vice versa, regardless of the differentiation step or stage of tumor transformation (Figure 3A). HLA class I and II molecules were used as controls (not shown). Moreover, the interaction proved to be sensitive to cholesterol depletion, as shown by the lack of cocapping when cells were preincubated with MβCD. This finding suggests that the CD38/CD19 association takes place within lipid rafts. Confirming this assumption, the association between CD38 and CD81 is also perturbed in the presence of MβCD (Figure 3A; Table 2).
CD38/CD31 cross-talk induces CD38/CD19 lateral association within lipid rafts. (A) Representative images of cocapping experiments between CD38 and CD19 or CD81 in tonsillar B lymphocytes. When indicated, cells were pretreated with MβCD, a cholesterol-chelating agent used to disrupt the rafts. Images were taken at 60 × magnification. (B) CD38+ B cells and L-CD31+ transfectants were interacted for 5 minutes, before lysis with Brij98 and membrane separation by ultracentrifugation. CD19 molecules were highlighted by Western blotting, while CD45 was detected by means of a dot blot using the same lysates. (C) Nalm-6 B cells were treated with anti-CD38 antibody (10 minutes, on ice) followed by a DαMIgG (10 minutes, on ice) and incubated at 37°C for 5 minutes. Lysates were then immunoprecipitated using the anti-CD38 OKT10 antibody, or an irrelevant isotype-matched antibody. CD19 molecules were visualized by Western blotting.
CD38/CD31 cross-talk induces CD38/CD19 lateral association within lipid rafts. (A) Representative images of cocapping experiments between CD38 and CD19 or CD81 in tonsillar B lymphocytes. When indicated, cells were pretreated with MβCD, a cholesterol-chelating agent used to disrupt the rafts. Images were taken at 60 × magnification. (B) CD38+ B cells and L-CD31+ transfectants were interacted for 5 minutes, before lysis with Brij98 and membrane separation by ultracentrifugation. CD19 molecules were highlighted by Western blotting, while CD45 was detected by means of a dot blot using the same lysates. (C) Nalm-6 B cells were treated with anti-CD38 antibody (10 minutes, on ice) followed by a DαMIgG (10 minutes, on ice) and incubated at 37°C for 5 minutes. Lysates were then immunoprecipitated using the anti-CD38 OKT10 antibody, or an irrelevant isotype-matched antibody. CD19 molecules were visualized by Western blotting.
These experiments indicate that the CD38/CD19 complex is formed, at least in large part, within lipid rafts as a functional response to CD38 ligation. The same results were obtained after juxtaposing CD38 and the CD31 ligand. A 5-minute interaction between L-CD31+ fibroblasts and tonsillar B lymphocytes is followed by marked redistribution of CD19 molecules, with a pronounced amount entering fraction 4 of the DIM and a significant proportion moving out of the DSM into the intermediate fractions (Figure 3B). This experimental approach mimics what is expected to happen in vivo and overcomes the potential for false readings due to the presence of large amounts of agonistic and cross-linking antibodies. The same interaction conducted with the control fibroblasts does not affect CD19 compartmentalization (Figure 3B). Moreover, the rearrangement is specific for CD19, with no modification of CD79β (not shown). The nonraft resident molecule CD45 is also unaffected by the interaction (Figure 3B).
Coimmunoprecipitation experiments confirmed the association between CD38 and CD19 in response to CD38 engagement. An agonistic anti-CD38 antibody was used to ligate the cells and the CD38 molecule was immunoprecipitated using an antibody specific for a different epitope. The precipitated proteins were then analyzed by means of SDS-PAGE and Western blotting with anti-CD19 antibody. As can be seen in Figure 3C, CD19 is present in the CD38 immunoprecipitates. No CD19 was found in the immunoprecipitates in control conditions, where an isotype-matched irrelevant antibody was used.
CD38 and CD19 are part of a supramolecular functional complex in human B cells
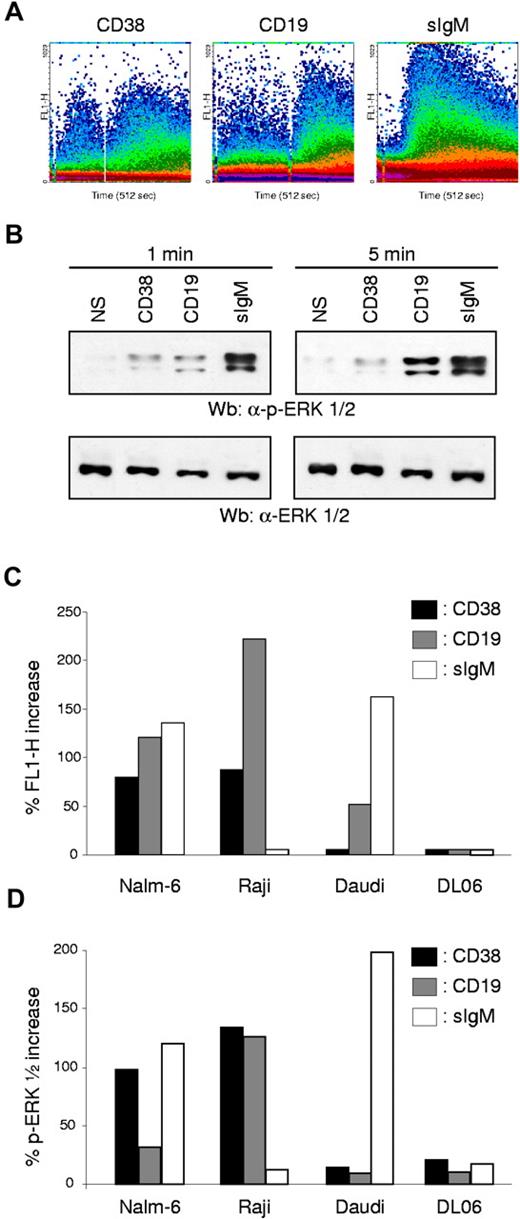
The last issue to be addressed concerns the functional relationship between CD38 and CD19. We first explored the ability of CD38 to perform as a signaling molecule in B cells. Intracellular Ca2+ signaling and ERK1/2 phosphorylation were used as rapid and efficient means of detecting the onset of a signaling pathway. The results indicate that CD38 cross-linking induces Ca2+ fluxes in normal human tonsillar B cells (Figure 4A). As previously reported in other models, the appearance of a CD38-mediated Ca2+ flux is dependent on the presence of a cross-linking antibody, and likely reflects the need for intense clusterization of the molecule. Taking into account previous results, it appears that the second antibody may simply recruit the CD38 molecules into the DIM, or facilitate association with CD19, or both. Finally, only a minor subset of tonsillar B cells seems to be responsive to CD38, suggesting that the effect is linked to a specific differentiation step, at least in normal populations. CD19 and sIgM ligations were assayed in parallel and as expected provided potent signals. CD19-mediated Ca2+ fluxes also benefited from cross-linking (Figure 4A). A polyclonal reagent was used to activate sIgM: the Ca2+ wave recorded indicates a very rapid spike followed by a progressive decline in intracellular Ca2+ levels, in keeping with previously reported experiments.44
CD38 and CD19 are part of a supramolecular functional complex in human B cells. CD38 ligation in human B cells is followed by Ca2+ fluxes (A) and by ERK1/2 phosphorylation (B). CD19- and sIgM-mediated signals were comparatively assayed. Tonsillar B lymphocytes were labeled with Fluo 3-AM, washed, and analyzed continuously using a FACSort. The primary antibody was added 10 seconds after beginning the analysis and was followed by a cross-linker antibody (for anti-CD38 and anti-CD19) after approximately 2 to 300 seconds. ERK1/2 phosphorylation was checked 1 and 5 minutes after receptor ligation by using specific reagents in a Western blot system. (C-D) Ca2+ fluxes and phosphorylation of ERK1/2 proteins induced by CD38, CD19, and sIgM ligation were checked in a panel of cell lines using the same experimental approaches as in (B). In both cases, bars represent the percentage of increase over background levels, obtained after incubating the cells with an irrelevant antibody.
CD38 and CD19 are part of a supramolecular functional complex in human B cells. CD38 ligation in human B cells is followed by Ca2+ fluxes (A) and by ERK1/2 phosphorylation (B). CD19- and sIgM-mediated signals were comparatively assayed. Tonsillar B lymphocytes were labeled with Fluo 3-AM, washed, and analyzed continuously using a FACSort. The primary antibody was added 10 seconds after beginning the analysis and was followed by a cross-linker antibody (for anti-CD38 and anti-CD19) after approximately 2 to 300 seconds. ERK1/2 phosphorylation was checked 1 and 5 minutes after receptor ligation by using specific reagents in a Western blot system. (C-D) Ca2+ fluxes and phosphorylation of ERK1/2 proteins induced by CD38, CD19, and sIgM ligation were checked in a panel of cell lines using the same experimental approaches as in (B). In both cases, bars represent the percentage of increase over background levels, obtained after incubating the cells with an irrelevant antibody.
CD38 ligation was also followed by phosphorylation of ERK1/2 proteins, starting 1 minute after receptor engagement and continuing for 5 minutes. CD19 ligation also led to ERK1/2 phosphorylation, with markedly different kinetics, starting 1 minute after engagement and sharply increasing after 5 minutes. In line with the Ca2+ flux data, sIgM induced substantial ERK1/2 phosphorylation.
Next, we turned to CD38-mediated Ca2+ fluxes and ERK1/2 phosphorylation in a panel of B-cell lines, namely Nalm-6, Raji, Daudi, Namalwa, and DL06. The results indicate that CD38 is able to selectively mobilize Ca2+ in Nalm-6 and Raji cell lines, while it is ineffective in all the others, regardless of their differentiation stage (Figure 4C). In the same cell lines, engagement of CD38 is also followed by ERK1/2 phosphorylation (Figure 4D). To gather information providing a link between CD38- and CD19-mediated functions, the signaling properties of CD19 were analyzed in parallel in the same cell targets. The results indicate that CD19 ligation strongly mobilizes intracellular Ca2+ and induces ERK1/2 phosphorylation in Nalm-6 and Raji, the same cells that respond to CD38 signals (Figure 4C-D). Instead, no signals were observed in Daudi and Namalwa lines. The profile recorded upon sIgM ligation was markedly different, with prominent fluxes in Nalm-6, Namalwa, and Daudi cells. No Ca2+ fluxes or ERK1/2 phosphorylation was observed after ligation of sIgM in Raji cells (Figure 4C-D).
Collectively, these results suggest that CD38 can deliver signals only when CD19 is present and functionally active. No correlation between the presence and functional activities of sIgM was highlighted, at least in the experimental models adopted. Consistently, CD38 was unable to deliver signals in the plasma cell–derived line DL06 (CD38+/CD19−/sIgM−).
CD38 enzymatic activities are independent of signaling properties
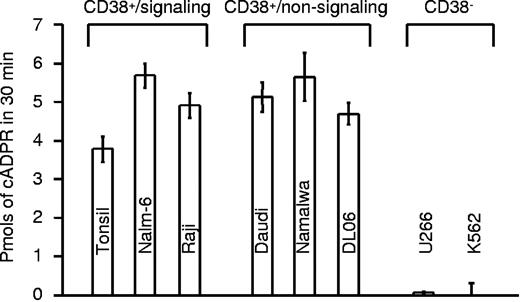
It has previously been postulated that the calcium-mobilizing metabolites produced by CD38 may regulate its receptor-based activities. To test this hypothesis, we measured the enzymatic functions of CD38 in normal human B cells and in the panel of B-cell lines used in the present study. All CD38+ cells analyzed were able to catalyze the conversion of NAD to cADPR, as measured by the radioreceptor assay in sea urchin egg homogenates (Figure 5). cADPR generation appeared to be a function of the quantity of CD38 present on the cell surface, but independent of the signaling properties of the molecules. Expression of CD19 proved to be irrelevant for CD38-mediated generation of cADPR, as indicated by the strong signal observed in the DL06 line. Similarly, lipid raft disruption did not significantly affect CD38 enzymatic activities (not shown). K562 and U266 were used as CD38− cell lines and did not generate cADPR, further confirming that CD38 is the main extracellular ADP ribosyl cyclase in these cells (Figure 5).
CD38 enzymatic activities are independent of signaling properties. Production of cADPR was assayed in a panel of normal B cells and cell lines using a radioreceptor assay. Results are presented as picomoles of cADPR produced in 30 minutes. The cells are divided in signaling and nonsignaling as inferred from the experiments described in Figure 4C. U266 and K562 are CD38low/− and were used as control. Error bars represent standard deviation derived from 3 independent measurements.
CD38 enzymatic activities are independent of signaling properties. Production of cADPR was assayed in a panel of normal B cells and cell lines using a radioreceptor assay. Results are presented as picomoles of cADPR produced in 30 minutes. The cells are divided in signaling and nonsignaling as inferred from the experiments described in Figure 4C. U266 and K562 are CD38low/− and were used as control. Error bars represent standard deviation derived from 3 independent measurements.
Localization in lipid microdomains and association with CD19 are necessary for CD38 signals
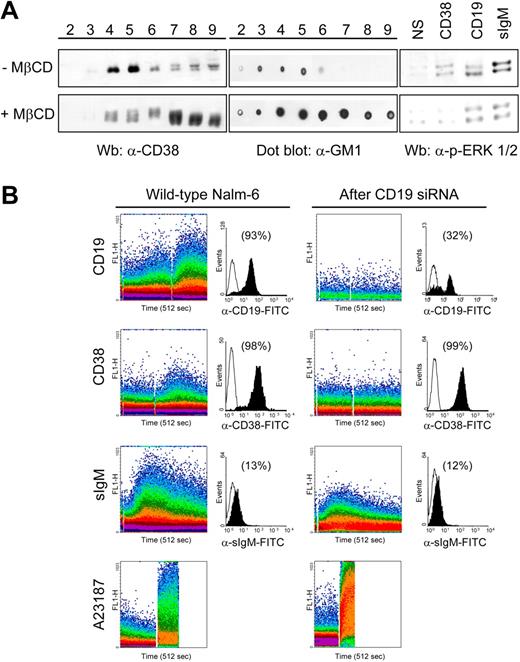
It can be concluded from the results described so far that CD38 receptor activity may be regulated through localization in lipid microdomains and functional interaction with CD19. The contribution of lipid raft integrity to CD38-mediated signals was confirmed by loss of signal transduction after MβCD treatment of the cells. This condition resulted in cholesterol depletion with relocalization of CD38 molecules outside lipid microdomains. Similarly, the GM1 ganglioside lost its privileged association with rafts (Figure 6A). Under these experimental conditions, ligation of CD38 did not trigger ERK1/2 phosphorylation and gave the expected decrease of CD19- and sIgM-mediated signals. Further, the existence of a functional link between CD38 and CD19 was demonstrated by silencing CD19 in cells expressing this mRNA and then testing for the CD38 receptor functions. The cell line adopted was Nalm-6, in virtue of its high levels of functional CD38 and CD19. CD19 expression was switched off by using siRNA, yielding approximately 70% reduction of surface protein (Figure 6B). The reduced expression was accompanied by the disappearance of CD19-mediated Ca2+ fluxes. Conversely, the treatment did not influence the surface expressions of CD38 and of sIgM molecules. However, CD38 signaling was completely inhibited following CD19 silencing, while sIgM was unchanged in both intensity and length. Collectively, these results suggest that the localization in specialized membrane areas as well as the ratio between the 2 molecules are early key elements in constructing the signaling transmission mechanism.
Localization in lipid microdomains and association with CD19 are necessary for CD38 signals. (A) Nalm-6 cells were treated with 10 mM MβCD (30 minutes, 37°C) prior to lysis and sucrose gradient centrifugation. Aliquots of the 8 recovered fractions were separated on a 10% SDS-PAGE and immunoblotted with anti-CD38 antibody. The localization of the GM1 ganglioside was checked with a dot blot. Untreated cells were used as the control. Loss of lipid microdomain integrity is followed by redistribution of CD38 molecules (left panel) and by impaired CD38 signaling (right panel), as witnessed by lack of ERK1/2 phosphorylation. (B) CD38-mediated Ca2+ fluxes were studied in Nalm-6 cells before and after CD19 silencing. sIgM Ca2+ signals were checked as control and were not significantly affected by CD19 siRNA treatment. The black histograms represent cell surface expression of the indicated molecules before and after CD19 siRNA treatment, plotted against an isotype-matched control (open profiles). Percentages refer to the number of positive cells. The Ca2+ ionophore A23187 was used to check the efficient loading of the cells.
Localization in lipid microdomains and association with CD19 are necessary for CD38 signals. (A) Nalm-6 cells were treated with 10 mM MβCD (30 minutes, 37°C) prior to lysis and sucrose gradient centrifugation. Aliquots of the 8 recovered fractions were separated on a 10% SDS-PAGE and immunoblotted with anti-CD38 antibody. The localization of the GM1 ganglioside was checked with a dot blot. Untreated cells were used as the control. Loss of lipid microdomain integrity is followed by redistribution of CD38 molecules (left panel) and by impaired CD38 signaling (right panel), as witnessed by lack of ERK1/2 phosphorylation. (B) CD38-mediated Ca2+ fluxes were studied in Nalm-6 cells before and after CD19 silencing. sIgM Ca2+ signals were checked as control and were not significantly affected by CD19 siRNA treatment. The black histograms represent cell surface expression of the indicated molecules before and after CD19 siRNA treatment, plotted against an isotype-matched control (open profiles). Percentages refer to the number of positive cells. The Ca2+ ionophore A23187 was used to check the efficient loading of the cells.
Discussion
The starting point of this study was an attempt to validate in normal human B cells and B-cell line models the observations inferred from studying a specific neoplastic transformation of this lineage, where CD38 is known to play a role in determining the prognosis. Indeed, the presence of the molecule on the surface of the CLL cell not only marks a subset of patients with an unfavorable prognosis, but also seems to trigger a chain of events eventually leading to cell proliferation, refractoriness to conventional therapies, and acquisition of other negative prognostic markers.23 CD38 interacts with CD31 expressed by nurselike cells in this specific disease model and fits the profile of a receptor transducing positive signals.25
Now the question is whether the signaling properties of CD38 are unique to CLL, whether they are exacerbated as a consequence of the neoplastic transformation, or whether they represent a normal activation pathway of human B cells. These questions were answered by turning to normal mature B cells to confirm the receptorial hypothesis of CD38 in this model, updating some seminal results from the 1990s, mainly focused on B-cell precursors.8
The results indicate that CD38 is a receptor and that CD38-mediated signals are tightly regulated at 3 distinct levels. The first concerns the structural organization of CD38, which is clearly divided into monomeric and dimeric forms. This finding confirms in a human model previous observations from murine cells,37 and predictions inferred from the crystal structures of the human protein38 and of Aplysia ADP-ribosyl cyclase.45 The dimeric structure is apparent only when cells are lysed using a mild detergent and the membranes overlaid on a sucrose gradient, suggesting that the dimer is more sensitive to solubilization than the monomer, and raising the question as to whether the dimer derives from membrane assembly of 2 monomers. The latter hypothesis is also indirectly confirmed by the results of pulse-chase experiments (T.V., unpublished results), which indicate that CD38 is constantly observed as a single chain during cytoplasmic transit.
A second level of regulation is based on a dynamic localization of CD38 molecules within the plasma membrane in lipid microdomains. The third level of control is determined by lateral associations with other proteins, namely with the CD19/CD81 complex.
The first 2 points were confirmed by monitoring the location of CD38 inside or outside the membrane lipid rafts in resting conditions and during activation. The results allow us to derive a model where the functional requisites (ie, signal transduction) accompany a translocation from the nonraft membrane fractions to the rafts. In the experimental conditions adopted, resting conditions are marked by a predominance of the molecule in nonraft areas, with about one third located in the rafts. One can speculate that approximately 30% represents the result of a constitutive activation that has gone undetected: this is plausible especially given the use of tonsillar B cells, which can be considered partially activated, whereas the other cell models are all of neoplastic origin and are thus constitutively activated. In line with these considerations, the rafts in cell lines contained generally more CD38 monomers than did tonsillar B cells; however, serum deprivation does not apparently affect CD38 localization (not shown). Another possible interpretation is that the raft-resident fraction of CD38 represents a ready-to-use pool of the molecule. Conversely, the fraction that resides outside the rafts is the one that transiently associates with CD19 and enters lipid microdomains upon receptor engagement via antibodies or via CD31. A third possibility is that membrane compartmentalization is a means of discriminating between the enzymatic and receptorial functions attributed to CD38. Indeed, the 2 molecular species are regulated independently, with the large majority of the approximately 90-kDa dimer constitutively outside lipid rafts and not significantly modulated during activation. This could mean that the dimeric state is a locked form of the molecule, stored outside the functional domains, and protected from external interactions. When CD31 (or the surrogate antibody) binds, the bivalve structure opens, and the monomeric form is ready for association with CD19. This model is indirectly confirmed by the crystal structure of CD38, which suggests that the cavity formed by the conjunction of 2 CD38 monomers is the site of interaction with CD31.38
In any case, raft localization alone is not enough to activate signal transduction via CD38. Our results indicate that lateral association with CD19 or—more precisely—with the CD19 supramolecular complex is another necessary condition for CD38-mediated signals. Evidence derived through the use of antibodies and cross-linkers indicates that CD19 relocalizes completely to the CD38 cap areas. Even more importantly, since it may reflect a physiological condition, the CD31 ligand expressed by murine transfectants induces relocalization of CD19 to the contact areas. A biochemical approach confirms that substantial quantities of CD19 molecules shift from the nonraft areas into the intermediate and raft fractions of the membrane. This behavior indicates that relocalization is a tightly regulated and highly dynamic event, also reflecting the fact that the adhesion between CD38 and CD31 is of selectin type (ie, they are weak interactions as opposed to the binding potency of antibody ligands).26 The CD38/CD19 complex is dependent on conservation of the membrane structure: the addition of MβCD, a chelator of cholesterol, completely abolishes the association, also supporting the results obtained using ODG. However, it is unlikely that 2 molecules are free to float around on the membrane alone; it is more reasonable to surmise that the cytoskeleton participates in this event46 and that other molecules may be appropriate candidates to join this supramolecular complex. The CD19/CD81 complex is part of the so-called “tetraspan web,”47 which comprises different molecules, including β1 integrins such as VLA-4 and VLA-5. This latter point is particularly relevant because of the prognostic value of CD49d in CLL.48,49
Raft localization and association with the CD19 complex are prerequisites for CD38-mediated signals in tonsillar B cells and in continuous lines. Consistently, the panel of cell lines may be functionally distinguished on the basis of its ability to transduce signals following CD19 ligation. Ca2+ fluxes and ERK1/2 phosphorylation induced upon CD38 ligation are seen only in the CD19-responsive lines Nalm-6 and Raji. No CD38-mediated signals are detected in CD19-unresponsive lines, irrespective of the presence of both molecules on the surface. In further agreement with this model, the plasma cell–like line DL06 (CD19−, but highly CD38+) fails to respond to CD38 ligation. Differences in the B population were also seen in tonsillar B cells, where only a discrete subset was shown to be responsive. Lastly, silencing of CD19 directly impacts on the ability of CD38 to mediate Ca2+ fluxes, while leaving surface expression unmodified.
The results obtained confirm that CD38 is a receptor not only in CLL cells but also in a significant subset of mature B cells and selected cell lines. A critical issue in this context is whether the products generated by CD38 as an enzyme are involved in the modulation of the receptorial activity. Our inability to identify a correlation between the production of cADPR, the second messenger generated from NAD, and the receptorial functions supports the hypothesis that CD38 is a pleiotropic molecule, whose behavior as a receptor is independent from its enzymatic functions.
In conclusion, the evidence gathered so far indicates that CD38 and CD19 make up (or are part of) a signaling complex in human B cells. The attempt to identify other partners modulating CD38 functions is ongoing.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC ection 1734.
Acknowledgments
This work was supported by grants from the Chronic Lymphocytic Leukemia Global Research Foundation (CLL-GRF; S.D.), the Associazione Italiana Ricerca Cancro (AIRC; S.D. and F.M.), PRIN (S.D., A.A.G., and F.M.), the University of Turin (S.D. and F.M.), and the Regione Piemonte (A.A.G. and F.M.). The Fondazione Internazionale Ricerche Medicina Sperimentale (FIRMS) and Compagnia di SanPaolo also provided financial contributions.
Thanks are given to Dr R. Villela (Hospital Clinic, Barcelona, Spain) for making the anti-CD81 antibody I.33.22 available, to Dr A. Enrico (Regina Margherita Hospital, Torino, Italy) for providing tonsil samples, and to Dr M. Zubiaur (CSIC, Armilla, Spain) for critically reading the paper. Technical assistance from F. Cottino is also gratefully acknowledged.
Authorship
Contribution: S.D. designed the experiments, performed confocal microscopy and Ca2+ signaling experiments, and wrote the paper together with F.M.; T.V. performed biochemical and confocal microscopy experiments, and contributed to preparing figures; R.B. performed the enzymatic assays and prepared the relevant figure; L.B. provided samples and discussed the results; P.O. performed cell surface staining and FACS analyses; A.A.G. performed the enzymatic assays and discussed the results; F.M. designed the experiments and wrote the paper together with S.D.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Silvia Deaglio or Fabio Malavasi, Department of Genetics, Biology and Biochemistry, University of Torino Medical School, via Santena 19, 10126 Torino, Italy; e-mail: silvia.deglio@unito.it or fabio.malavasi@unito.it.