Abstract
Gossypin, a flavone originally isolated from Hibiscus vitifolius, has been shown to suppress angiogenesis, inflammation, and carcinogenesis. The mechanisms of these activities, however, are unknown. Because nuclear factor-κB (NF-κB) is associated with inflammation, carcinogenesis, hyperproliferation, invasion, and angiogenesis, we hypothesized that gossypin mediates its effects through modulation of NF-κB activation. In the present study, we demonstrate that gossypin (and not gossypetin, an aglycone analog) inhibited NF-κB activation induced by inflammatory stimuli and carcinogens. Constitutive NF-κB activation in tumor cells was also inhibited by this flavone. Inhibition of IκBα kinase by gossypin led to the suppression of IκBα phosphorylation and degradation, p65 nuclear translocation, and NF-κB-regulated gene expression. This, in turn, led to the down-regulation of gene products involved in cell survival (IAP2, XIAP, Bcl-2, Bcl-xL, survivin, and antiFas-associated death domain–like interleukin-1β–converting enzyme-inhibitory protein), proliferation (c-myc, cyclin D1, and cyclooxygenase-2), angiogenesis (vascular endothelial growth factor), and invasion (matrix metalloprotease-9). Suppression of these gene products by gossypin enhanced apoptosis induced by tumor necrosis factor and chemotherapeutic agents, suppressed tumor necrosis factor–induced cellular invasion, abrogated receptor activator of NF-κB ligand–induced osteoclastogenesis, and vascular endothelial growth factor–induced migration of human umbilical vein endothelial cells. Overall, our results demonstrate that gossypin inhibits the NF-κB activation pathway, which may explain its role in the suppression of inflammation, carcinogenesis, and angiogenesis.
Introduction
Modern targeted therapies for cancer are associated with substantial nonspecific toxicities, high costs, and highly limited efficacy. Alternate treatments that are devoid of such shortcomings are therefore needed. Traditionally known anticancer agents have been in use for centuries, but their active components and molecular targets have not been well defined. One potential agent is gossypin (3,5,8,3′,4′-pentahydroxy-7-O-glucosyl flavone), which was originally isolated from Hibiscus vitifolius (tropical rose mallow; also called Japa or Karupatti) and H. furcatus (Panchavam in Malayalam). The extracts of these plants are traditionally used for the treatment of diabetes, jaundice, and inflammation.1,2 The very limited information available on this flavone indicates that it exhibits potent antioxidant activity,3,4 suppresses b amyloid-induced toxicity,5 mediates antinociception by modulating the γ-amino butyric acid system,6,7 protects against carbon tetrachloride-induced toxicity in rat hepatocytes,8 and protects against bis(2-choloroethyl)sulfide-induced dermal toxicity.9 Gossypin also has anti-inflammatory activity because it prevented carrageenin-induced paw edema in mice by inhibiting arachidonic acid metabolism.10
Gossypin has been found to inhibit cell proliferation in L929, HT29, and K562 tumor cell lines in culture. In mice, it inhibited the growth of Ehrlich's ascites carcinoma and inhibited angiogenesis.4 It also exhibited anticarcinogenic activity against DMBA/croton oil-induced papilloma in rodents.4 Gossypin's antitumoral effects have been partly ascribed to its ability to inhibit topoisomerase I and II.4 The exact mechanisms of its antiinflammatory and anticarcinogenic properties, however, are not fully understood.
Nuclear factor-κB (NF-κB) is an inducible transcription factor for genes involved in cell survival, adhesion, inflammation, differentiation, and growth. It regulates the genes that are critical in the early and late stages of aggressive cancers, including cyclin D1, apoptosis-suppressor proteins such as Bcl-2 and Bcl-xL, and genes required for metastasis and angiogenesis such as matrix metalloprotease (MMP) and vascular endothelial growth factor (VEGF).11
Because NF-κB activation is common to both inflammation and cancer, we hypothesized that gossypin's anti-inflammatory, anticarcinogenic, and anti-angiogenic properties result from the inhibition of NF-κB and NF-κB-regulated gene expression. Gossypin inhibited NF-κB activation induced by different agents, inhibited NF-κB-regulated gene expression, abrogated receptor activator of NF-κB ligand (RANKL)-induced osteoclastogenesis, and VEGF-induced migration of human umbilical vein endothelial cells (HUVEC). It also potentiated chemotherapeutic agent-induced apoptosis.
Materials and methods
Reagents
Fifty mM solutions of gossypin and gossypetin (Sigma Aldrich, St. Louis, MO) were prepared in dimethyl sulfoxide; stored as small aliquots at −20°C, and then thawed and diluted, as needed, in cell culture medium. Bacteria-derived human recombinant tumor necrosis factor (TNF), purified to homogeneity with a specific activity of 5 × 107 units/mg, was provided by Genentech (South San Francisco, CA). Cigarette smoke condensate, prepared as described previously,12 was supplied by Dr. G. Gairola (University of Kentucky, Lexington, KY). Phorbol myristate acetate (PMA), okadaic acid, lipopolysaccharide (LPS), H2O2, Taxol, medium 199, amphotericin B, gelatin, and HEPES were obtained from Sigma. Penicillin, streptomycin, RPMI 1640, Iscove modified Dulbecco medium, Dulbecco modified Eagle medium (DMEM), DMEM/F-12, minimum essential medium (MEM) alpha, and fetal bovine serum were obtained from Invitrogen (Grand Island, NY). Antib actin was obtained from Sigma-Aldrich. Antip65, antip50, antiIκBα, antipoly(ADP-ribose) polymerase (PARP), anti-intercellular adhesion molecule-1, matrix metalloproteinase (MMP)-9, cyclin D1, c-myc, bcl-2, bclxL, and anti-inhibitor of apoptosis protein (IAP) 2 were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Anticyclooxygenase (COX)-2 was obtained from BD Biosciences (San Diego, CA). Phosphospecific antiIκBα (Ser32) and antip65 (Ser536) were purchased from Cell Signaling (Beverly, MA). AntiIκBα kinase (IKK)-α, antiIKK-β, and antiFas-associated death domain–like interleukin-1b–converting enzyme-inhibitory protein (FLIP) antibodies were provided by Imgenex (San Diego, CA), and VEGF was obtained from R&D System, (Minneapolis, MN).
Cell lines
The human cell lines KBM-5 (chronic myeloid leukemia), A293 (embryonic kidney carcinoma), H1299 (lung adenocarcinoma), U266 (multiple myeloma), 253JBV (bladder cancer), and BxPC3 (pancreatic cancer) and the murine monocytic cell line RAW 264.7 were obtained from American Type Culture Collection (Manassas, VA). Cells were cultured as follows: KBM-5, Iscove modified Dulbecco medium with 15% fetal bovine serum; H1299 and U266, RPMI 1640; A293 and BxPC3, DMEM; and RAW 264.7, DMEM/F-12 supplemented with 10% fetal bovine serum. Culture media were supplemented with 100 units/mL penicillin and 100 μg/mL streptomycin. Human umbilical vein endothelial cells were a gift from XingLi Wang in the Michael E. DeBakey Department of Surgery, Baylor College of Medicine (Houston, TX). Human umbilical vein endothelial cells were cultured in M199 containing 100 mg/L heparin, 15 mMol/L Hepes, penicillin (50 IU/L), streptomycin (50 mg/L), and 20% fetal calf serum in a 5% CO2/air atmosphere.13
Electrophoretic mobility shift assay
To determine NF-κB activation, we used electrophoretic mobility shift assays (EMSAs) as described previously.14 In brief, nuclear extracts prepared from TNF-treated cells were incubated with 32P-end-labeled 45-mer double-stranded NF-κB oligonucleotide (15 μg protein with 16 fmol DNA) from the HIV long terminal repeat 5′-TTGTTACAAGGGACTTTCCGCTGGGGACTTTCCAGGGAGGCGTGG-3′ (boldface indicates NF-κB binding sites) for 30 minutes at 37°C. The DNA-protein complex that formed was separated from the free oligonucleotide on 6.6% native polyacrylamide gels. A double-stranded mutated oligonucleotide, 5′-TTGTTACAACTCACTTTCCGCTGCTCACTTTCCAGGGAGGCGTGG-3′, was used to evaluate the specificity of NF-κB binding to the DNA. The specificity was also determined through competition with the unlabeled oligonucleotide. The dried gels were visualized, and the radioactive bands were quantitated with a Storm 220 PhosphorImager (Amersham Biosciences, Piscataway, NJ) using ImageQuant software (Molecular Dynamics, Sunnyvale, CA)
Western blot analysis
To determine the levels of protein expression in the cytoplasm and nucleus, we fractionated extracts with sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), as described previously.15 The proteins were then electrotransferred to nitrocellulose membranes, blotted with each antibody, and the expression of proteins were detected with an enhanced chemiluminescence reagent (Amersham Biosciences). We quantitated the bands with National Institutes of Health imaging software (Bethesda, MD).
IκBα kinase assay
To determine the effects of gossypin on TNF-induced IKK activation, we used the IKK assay, as described previously.16 In brief, to determine the total amounts of IKK-α and IKK-β in each sample, we resolved 50 μg whole-cell protein by 7.5% SDS-PAGE, electrotransferred it to a nitrocellulose membrane, and blotted it with anti-IKK-α or anti-IKK-β antibodies.
NF-κB-dependent reporter gene expression assay
We performed an NF-κB-dependent reporter gene expression assay, as previously described.17 The effects of gossypin on TNF-dependent, TNF receptor (TNFR)-dependent, TNFR-associated death domain (TRADD)-dependent, TNFR-associated factor 2 (TRAF2)-dependent, NF-κB-inducing kinase (NIK)-dependent, IKK-β-dependent, TAK-1-dependent, TAB-1-dependent, and p65-induced NF-κB-dependent reporter gene transcriptions were analyzed by the secretory alkaline phosphatase (SEAP) assay, as described previously.17
Immunocytochemical analysis of NF-κB p65 localization
The effects of gossypin on p65 nuclear translocation were evaluated by an immunocytochemical analysis, as described in the Cytotoxicity Assay Section.15
Live/dead assay
To determine membrane permeability, we used the live/dead assay (Molecular Probes, Eugene, OR), which measures intracellular esterase activity and plasma membrane integrity. This assay was performed as described in the Cytotoxicity Assay Section.18
Cytotoxicity assay
The effects of gossypin on the cytotoxicity of TNF and chemotherapeutic agents were determined by the 3-(4,5-dimethylthiazole-2-yl)-2,5-diphenyltetrazolium bromide uptake method, as described previously.19 After staining, the slides were mounted using mounting media (Sigma Aldrich, USA), examined under a fluorescent microscope (CFWN 10 × 1.5 oil objective; Lapshot-2; Nikon, Tokyo, Japan), and the photographs were taken by using Photometrix Coolsnap CF color camera (Nikon, Lewisville, TX) using Metamorph version 4.6.5 software (Universal Imaging, Downingtown, PA, USA).
Annexin V assay
An early indicator of apoptosis is the rapid translocation and accumulation of the membrane phospholipid phosphtidylserine from the cytoplasmic interface of membrane to the extracellular surface. This loss of membrane asymmetry can be detected by using the binding properties of annexin V. To identify apoptosis, we used an annexin V antibody, which was conjugated with the fluorescein isothiocynate fluorescence dye. Briefly, cells were preincubated with gossypin, treated with TNF for 16 hours at 37°C, and subjected to annexin V staining. The cells were washed in phosphate-buffered saline, resuspended in 100 ml of binding buffer containing a fluorescein isothiocynate conjugated anti-annexin V antibody, and then analyzed with a flow cytometer (FACS Caliber; BD Biosciences).
Terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling assay
We also determined cytotoxicity using the terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) method, which is used to evaluate DNA strand breaks during apoptosis using an in situ cell death detection reagent. This assay was performed as described previously.20
Invasion assay
Extracellular matrix invasion is a crucial step in tumor metastasis. Therefore, we determined the effect of gossypin with an invasion assay, as described previously.20 A Matrigel basement membrane matrix extracted from the Engelbreth-Holm-Swarm mouse tumor (BD Biosciences) was reconstituted and used for this assay.
Osteoclast differentiation assay
We performed an osteoclast differentiation assay, as described previously.21 In brief, primary bone marrow cells from C57BL/6J mice (6-week-old) were isolated as described.22 These cells and RAW 264.7 cells were cultured in 24-well dishes at a density of 104 per well and allowed to adhere overnight. They were then exposed to RANKL, with or without gossypin, for different numbers of days and examined for osteoclast formation.
Human umbilical vein endothelial cell wound-healing/ migration assay
To determine the ability of gossypin to modulate the VEGF-induced HUVEC migration, a wound-healing/migration assay was performed.23 Cells were allowed to grow to confluency on six-well plates precoated with gelatin and washed with phosphate-buffered saline. Monolayers of cells were wounded by scratching with 1-mL pipette tip and washed 3 times with phosphate-buffered saline. Fresh medium was added with VEGF (4 ng/mL) along with different concentrations of gossypin, incubated for 24 hours, and pictures were taken using a Nikon digital camera.
Results
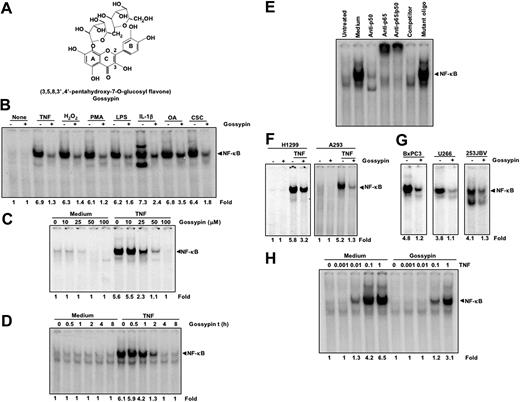
We evaluated the effects of gossypin on the NF-κB activation pathway as induced by various carcinogens and inflammatory stimuli. We also determined its effects on NF-κB-regulated gene expression and NF-κB-mediated cellular responses. The TNF-induced NF-κB activation pathway has been well characterized. Moreover, TNF is the most potent proinflammatory cytokine and the most potent inducer of the NF-κB activation pathway. Thus, we determined gossypin's effects on TNF-induced NF-κB activation. The structure of gossypin is shown in Figure 1A. Previous studies have shown that the antioxidant activity of this flavonoid requires an o-dihydroxy structure in the B ring, C2, C3 double bonds in conjugation with the C4 keto group in the C ring, and C3, C5 OH groups with C4 oxo function.3
Gossypin inhibits both constitutive and inducible NF-κB activation. (A) Structure of gossypin. (B) Gossypin blocked NF-κB activation induced by TNF, H2O2, PMA, Lipopolysaccaride (LPS), interleukin-1β (IL-1β), okadaic acid (OA), cigarette smoke condensate (CSC). KBM-5 cells (2 × 106) were preincubated for 30 minutes at 37°C with 50 μM gossypin and then treated with TNF (0.1 nmol/L, 30 minutes), H2O2 (500 μM, 2 hours), PMA (25 ng/mL, 1 hour), LPS (100 ng/mL, 2 hours), IL-1β (100 ng/mL, 30 minutes), OA (500 nM, 4 hours), CSC (10 μg/mL, 1 hour). Nuclear extracts were prepared and assayed for NF-κB activation using EMSA. (C) Gossypin suppressed TNF-induced NF-κB in a dose-dependent manner. Human myeloid leukemia KBM-5 cells (2 × 106) were incubated with different concentrations of gossypin for 2 hours and then treated with 0.1 nmol/L TNF for 30 minutes. Nuclear extracts were prepared and assayed for NF-κB activation by EMSA. (D) Gossypin suppressed TNF-induced NF-κB in a time-dependent manner. Human myeloid leukemia KBM-5 (2 × 106) cells were incubated with 50 μM gossypin for the indicated time periods and then treated with 0.1 nmol/L TNF for 30 minutes. Nuclear extracts were then prepared and assayed for NF-κB activation by EMSA. (E) NF-κB consists of p50 and p65 subunits, and its binding to DNA is specific. Nuclear extracts from KBM-5 cells (2 × 106 cells/mL) left untreated or treated with 0.1 nm/L TNF were incubated at 37°C for 30 minutes with different antibodies or unlabeled NF-κB oligonucleotide probes and then tested for NF-κB activation, as described in “Materials and methods.” (F) Gossypin blocked TNF-induced NF-κB activation in human lung adenocarcinoma H1299 cells and embryonic kidney A293 cells that had been preincubated with 50 μM gossypin for 2 hours and 0.1 nmol/L TNF for 30 minutes. (G) Gossypin suppressed constitutive activation of NF-κB in BxPC3, U266, and 253JBV cells. Cells were incubated with 50 μM gossypin for 2 hours. Nuclear extracts were then prepared and assayed for NF-κB activation by EMSA. (H) Gossypin blocked various concentrations of TNF-induced NF-κB activation.
Gossypin inhibits both constitutive and inducible NF-κB activation. (A) Structure of gossypin. (B) Gossypin blocked NF-κB activation induced by TNF, H2O2, PMA, Lipopolysaccaride (LPS), interleukin-1β (IL-1β), okadaic acid (OA), cigarette smoke condensate (CSC). KBM-5 cells (2 × 106) were preincubated for 30 minutes at 37°C with 50 μM gossypin and then treated with TNF (0.1 nmol/L, 30 minutes), H2O2 (500 μM, 2 hours), PMA (25 ng/mL, 1 hour), LPS (100 ng/mL, 2 hours), IL-1β (100 ng/mL, 30 minutes), OA (500 nM, 4 hours), CSC (10 μg/mL, 1 hour). Nuclear extracts were prepared and assayed for NF-κB activation using EMSA. (C) Gossypin suppressed TNF-induced NF-κB in a dose-dependent manner. Human myeloid leukemia KBM-5 cells (2 × 106) were incubated with different concentrations of gossypin for 2 hours and then treated with 0.1 nmol/L TNF for 30 minutes. Nuclear extracts were prepared and assayed for NF-κB activation by EMSA. (D) Gossypin suppressed TNF-induced NF-κB in a time-dependent manner. Human myeloid leukemia KBM-5 (2 × 106) cells were incubated with 50 μM gossypin for the indicated time periods and then treated with 0.1 nmol/L TNF for 30 minutes. Nuclear extracts were then prepared and assayed for NF-κB activation by EMSA. (E) NF-κB consists of p50 and p65 subunits, and its binding to DNA is specific. Nuclear extracts from KBM-5 cells (2 × 106 cells/mL) left untreated or treated with 0.1 nm/L TNF were incubated at 37°C for 30 minutes with different antibodies or unlabeled NF-κB oligonucleotide probes and then tested for NF-κB activation, as described in “Materials and methods.” (F) Gossypin blocked TNF-induced NF-κB activation in human lung adenocarcinoma H1299 cells and embryonic kidney A293 cells that had been preincubated with 50 μM gossypin for 2 hours and 0.1 nmol/L TNF for 30 minutes. (G) Gossypin suppressed constitutive activation of NF-κB in BxPC3, U266, and 253JBV cells. Cells were incubated with 50 μM gossypin for 2 hours. Nuclear extracts were then prepared and assayed for NF-κB activation by EMSA. (H) Gossypin blocked various concentrations of TNF-induced NF-κB activation.
Gossypin inhibited NF-κB activation induced by various agents
TNF, interleukin-1b, H2O2, PMA, LPS, okadaic acid, and cigarette smoke condensate are potent activators of NF-κB12,15,24,25 ; we therefore determined gossypin's effects on NF-κB activation using these agents. All agents activated NF-κB in human KBM-5 cells, as determined by DNA binding assays, and incubation with gossypin suppressed this activation (Figure 1B). Cells were fully viable at this concentration and exposure time. These results suggest that gossypin acts at a step in the NF-κB activation pathway that is common to all these agents.
Inhibition of NF-κB activation by gossypin was dose-dependent
TNF is one of the most potent activators of NF-κB, and the mechanism of NF-κB activation has been well established; thus, we determined gossypin's effects on TNF-induced NF-κB activation in myeloid leukemia (KBM-5) cells. Gossypin suppressed TNF-induced NF-κB activation in a dose-dependent manner (Figure 1C).
Suppression of NF-κB activation by gossypin was time-dependent
To determine the minimum time of exposure required for gossypin to inhibit TNF-mediated NF-κB activation, cells were exposed to the inhibitor for 30 minutes or 1, 2, 4, or 8 hours and then treated with TNF for 30 minutes. As shown in Figure 1D, gossypin started to inhibit TNF-induced NF-κB activation after only 1 hour. After 4 hours, TNF-induced NF-κB activation was completely suppressed. Under these conditions, gossypin alone had no effect on NF-κB activation.
Inhibition of NF-κB activation by gossypin was specific and consisted of p50 and p65 subunits
To determine the composition of the NF-κB band, nuclear extracts were incubated with anti-p50, anti-p65, unlabeled oligonucleotides, or mutated oligonucleotides. As shown in Figure 1E, both antibodies supershifted the band, indicating that the composition of the band was p50 and p65. Competition of the band with wild-type oligonucleotides but not mutant oligonucleotides showed that the band was specific.
Inhibition of NF-κB activation by gossypin was not cell type-specific
Because the signal transduction pathway mediated by NF-κB may be distinct in different cell types, we also determined whether gossypin blocked TNF-induced NF-κB activation in H1299 and A293 cells (Figure 1F). Gossypin inhibited TNF-induced NF-κB activation in these epithelial cells, indicating that gossypin-induced suppression of NF-κB activation is not cell type-specific.
Gossypin also suppressed constitutive NF-κB activation
Most tumor cells express constitutively active NF-κB,26 although the mechanism of constitutive activation is not well understood. The cell lines BxPC3 (pancreatic cancer), U266 (multiple myeloma), and 253JBV (bladder cancer) are known to express constitutively active NF-κB. Treatment with gossypin suppressed constitutive NF-κB activation in these cells (Figure 1G).
Gossypin was a potent inhibitor of NF-κB activation
We treated cells with various concentrations of TNF for 30 minutes, with or without gossypin, and then used EMSA to assess for NF-κB activation (Figure 1H). Gossypin abolished TNF-induced NF-κB activation even when exposed to TNF at a concentration of 10 nmol/L.
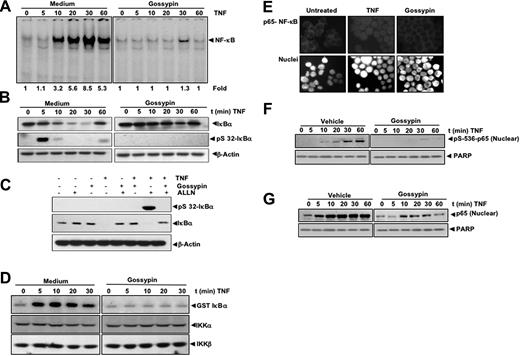
Gossypin inhibits tumor necrosis factor-dependent IκBα degradation
We next determined how gossypin inhibits TNF-induced NF-κB activation. Cells were exposed to gossypin for 2 hours and then treated with TNF for different periods of time. Nuclear extracts were then prepared and analyzed for NF-κB; cytoplasmic extracts were prepared and analyzed for IκBα. As shown in Figure 2A, gossypin completely inhibited TNF-induced NF-κB activation. Gossypin also suppressed TNF-induced IκBα degradation as determined by Western blot analysis (Figure 2B).
Gossypin inhibits TNF-induced NF-κB activation, IκBα degradation, IκBα phosphorylation, IKK activation, p65 nuclear translocation, and p65 phosphorylation. (A) Gossypin inhibited time-dependent TNF-induced activation of NF-κB. KBM-5 (2 × 106) cells were preincubated with 50 μM gossypin for 2 hours. They were then treated with 0.1 nmol/L TNF for the indicated times and analyzed for NF-κB activation by EMSA. (B) Effects of gossypin on TNF-induced degradation of IκBα. KBM-5 cells (2 × 106) were preincubated with 50 μM gossypin for 2 hours for the indicated times. Cytoplasmic extracts were prepared, fractionated by 10% SDS-PAGE, and electrotransferred to a nitrocellulose membrane. A Western blot analysis was performed using anti- IκBα. (C) Gossypin suppressed TNF-induced phosphorylation of IκBα. KBM-5 cells (2 × 106) were preincubated with 50 μM gossypin for 2 hours. N-acetyl-Leu-Leu-norleucinal was added, and the cells were treated with 0.1 nmol/L TNF for 15 minutes. Cytoplasmic extracts were prepared, fractionated by 10% SDS-PAGE, and electrotransferred to a nitrocellulose membrane. A Western blot analysis was performed using antiphospho-specific IκBα and anti-IκBα. (D) Gossypin suppressed the TNF-induced activation of IKK. KBM-5 cells were pretreated with 50 μM gossypin for 2 hours and then treated with 1 nmol/L TNF for the indicated times. Whole-cell extracts were immunoprecipitated with an antibody against IKK-α and analyzed by immune complex kinase assay, as described in “Materials and methods.” To determine the effect of gossypin on the level of IKK proteins, whole-cell extracts were fractionated by SDS-PAGE and examined by Western blot analysis using anti-IKK-α and anti-IKK-β antibodies. (E) Immunocytochemical analysis of p65 localization. KBM-5 cells (2 × 106) were preincubated with 50 μM gossypin for 2 hours, TNF for 15 minutes, and subjected to immunocytochemical analysis, as described in Materials and methods. (F,G) Effects of gossypin on TNF-induced phosphorylation of p65. KBM-5 cells (2 × 106) were preincubated with 50 μM gossypin for 2 hours and then treated with 0.1 nmol/L TNF for the indicated times. Nuclear extracts were prepared, fractionated by 10% SDS-PAGE, and electrotransferred to a nitrocellulose membrane. A Western blot analysis was performed using phosphospecific p65.
Gossypin inhibits TNF-induced NF-κB activation, IκBα degradation, IκBα phosphorylation, IKK activation, p65 nuclear translocation, and p65 phosphorylation. (A) Gossypin inhibited time-dependent TNF-induced activation of NF-κB. KBM-5 (2 × 106) cells were preincubated with 50 μM gossypin for 2 hours. They were then treated with 0.1 nmol/L TNF for the indicated times and analyzed for NF-κB activation by EMSA. (B) Effects of gossypin on TNF-induced degradation of IκBα. KBM-5 cells (2 × 106) were preincubated with 50 μM gossypin for 2 hours for the indicated times. Cytoplasmic extracts were prepared, fractionated by 10% SDS-PAGE, and electrotransferred to a nitrocellulose membrane. A Western blot analysis was performed using anti- IκBα. (C) Gossypin suppressed TNF-induced phosphorylation of IκBα. KBM-5 cells (2 × 106) were preincubated with 50 μM gossypin for 2 hours. N-acetyl-Leu-Leu-norleucinal was added, and the cells were treated with 0.1 nmol/L TNF for 15 minutes. Cytoplasmic extracts were prepared, fractionated by 10% SDS-PAGE, and electrotransferred to a nitrocellulose membrane. A Western blot analysis was performed using antiphospho-specific IκBα and anti-IκBα. (D) Gossypin suppressed the TNF-induced activation of IKK. KBM-5 cells were pretreated with 50 μM gossypin for 2 hours and then treated with 1 nmol/L TNF for the indicated times. Whole-cell extracts were immunoprecipitated with an antibody against IKK-α and analyzed by immune complex kinase assay, as described in “Materials and methods.” To determine the effect of gossypin on the level of IKK proteins, whole-cell extracts were fractionated by SDS-PAGE and examined by Western blot analysis using anti-IKK-α and anti-IKK-β antibodies. (E) Immunocytochemical analysis of p65 localization. KBM-5 cells (2 × 106) were preincubated with 50 μM gossypin for 2 hours, TNF for 15 minutes, and subjected to immunocytochemical analysis, as described in Materials and methods. (F,G) Effects of gossypin on TNF-induced phosphorylation of p65. KBM-5 cells (2 × 106) were preincubated with 50 μM gossypin for 2 hours and then treated with 0.1 nmol/L TNF for the indicated times. Nuclear extracts were prepared, fractionated by 10% SDS-PAGE, and electrotransferred to a nitrocellulose membrane. A Western blot analysis was performed using phosphospecific p65.
Gossypin inhibited tumor necrosis factor-dependent IκBα phosphorylation
IκBα phosphorylation is needed for IκBα degradation; thus, we determined whether gossypin modulated IκBα phosphorylation. Because TNF-induced phosphorylation of IκBα leads to its rapid degradation, we blocked IκBα degradation with the proteasome inhibitor N-acetyl-Leu-Leu-norleucinal. We performed a Western blot analysis using an antibody that is specific for the serine-phosphorylated form of IκBα and found that gossypin suppressed TNF-induced phosphorylation of IκBα (Figure 2C).
Gossypin inhibited tumor necrosis factor-induced IκBα kinase activation
IKK is required for TNF-induced phosphorylation of IκBα and for the phosphorylation of p65.27 Because gossypin inhibited the phosphorylation of IκBα, we determined its effects on TNF-induced IKK activation. Immune complex kinase assays showed that gossypin suppressed TNF-induced IKK activation (Figure 2D). Neither TNF nor gossypin affected the expression of IKK proteins.
Gossypin inhibited tumor necrosis factor-induced p65 nuclear translocation
We performed an immunocytochemical analysis to determine whether gossypin modulates TNF-induced p65 nuclear translocation. Gossypin suppressed the TNF-induced nuclear translocation of p65 (Figure 2E).
Gossypin inhibited tumor necrosis factor-induced p65 phosphorylation
p65 phosphorylation is required for the transactivation of NF-κB. We used phosphospecific anti-p65 (Ser 536) antibody to determine whether gossypin modulates p65 phosphorylation. As shown in Figure 2F and G, TNF induced p65 phosphorylation; this was substantially inhibited by gossypin. This finding was related to suppression of p65 nuclear translocation and was confirmed by an immunocytochemical analysis.
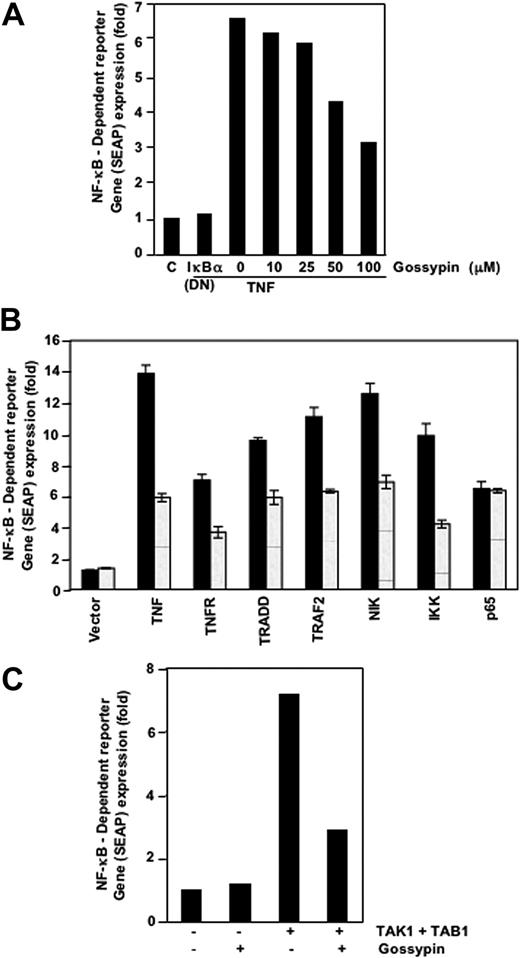
Gossypin suppressed TNF-induced NF-κB-dependent reporter gene expression.
Because DNA binding is not always related to NF-κB-dependent gene transcription,28 we determined the effects of gossypin on TNF-induced reporter activity. Cells were transiently transfected with the NF-κB-regulated SEAP reporter construct, incubated with gossypin, and stimulated with TNF. TNF induced NF-κB reporter activity; this activity was substantially diminished by gossypin (Figure 3A). These results suggest that gossypin inhibits TNF-induced gene expression. In addition, DN-IκBα plasmid suppressed TNF-induced reporter activity, indicating its specificity.
Gossypin inhibited the TNF-induced expression of the NF-κB-dependent genes TNFR1, TRADD, TRAF2, NIK, IKK-β, TAK-1, and TAB-1. (A-C) A293 cells were transiently transfected with NF-κB-containing SEAP reporter gene plasmid, alone or with the indicated plasmids, for 24 hours. After transfection, cells were washed and treated with 50 μM gossypin for 2 hours. To evaluate the effects of TNF treatment, we treated cells with 0.1 nmol/L TNF for an additional 24 hours. The supernatants of the culture medium were assayed for SEAP activity, as described in “Materials and methods.”
Gossypin inhibited the TNF-induced expression of the NF-κB-dependent genes TNFR1, TRADD, TRAF2, NIK, IKK-β, TAK-1, and TAB-1. (A-C) A293 cells were transiently transfected with NF-κB-containing SEAP reporter gene plasmid, alone or with the indicated plasmids, for 24 hours. After transfection, cells were washed and treated with 50 μM gossypin for 2 hours. To evaluate the effects of TNF treatment, we treated cells with 0.1 nmol/L TNF for an additional 24 hours. The supernatants of the culture medium were assayed for SEAP activity, as described in “Materials and methods.”
Gossypin inhibited NF-κB activation induced by TNFR1, TRADD, TRAF2, NIK, and IKK
TNF-induced NF-κB activation is mediated through sequential interaction of TNFR and TRADD, TRAF2, NIK, and IKK, resulting in phosphorylation of IκBα.29 We transiently transfected cells with the NF-κB-regulated SEAP reporter construct and TNFR1-, TRADD-, TRAF2-, NIK-, IKK-β-, or p65-expressing plasmids and treated them with gossypin and then monitored them for NF-κB-dependent SEAP expression. Gossypin suppressed NF-κB activation induced by TNFR1, TRADD, TRAF2, NIK, and IKK-β but not that induced by p65 (Figure 3B).
Gossypin inhibited TAK-1-induced NF-κB activation
TNF-induced NF-κB activation may require the recruitment of TAK-1, an upstream kinase to IKK.30,31 Thus, we determined whether gossypin inhibits this kinase. TAK-1 activated NF-κB activity, and gossypin inhibited TAK-1-and TAB-1-mediated NF-κB activation (Figure 3C). These results suggest that gossypin acts upstream of IKK.
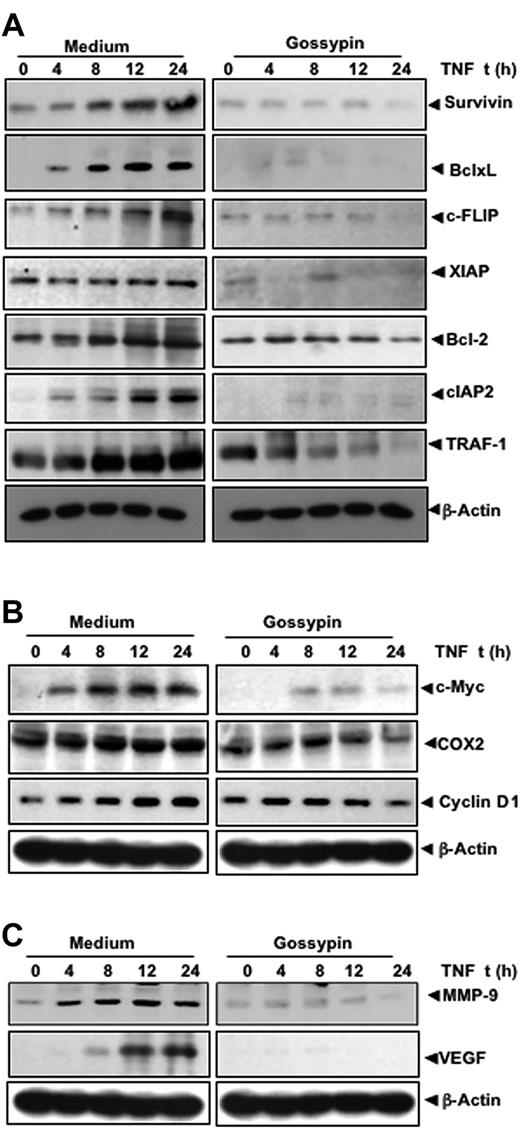
Gossypin inhibited tumor necrosis factor-induced NF-κB-regulated antiapoptotic gene products
NF-κB is key to the survival of tumor cells, because its activation induces the expression of antiapoptotic gene products. Because gossypin inhibited TNF-induced NF-κB activation, we hypothesized that it would also inhibit TNF-induced antiapoptotic gene products such as survivin, Bcl-xL, c-FLIP, XIAP, Bcl-2, cIAP2, and TRAF-1, all known to be regulated by NF-κB.31–37 The results of our Western blot analysis show that gossypin inhibited the expression of all these proteins (Figure 4A).
Gossypin inhibits TNF-induced expression of NF-κB–regulated gene products. KBM-5 cells were preincubated with 50 μM gossypin for 2 hours and 1 nmol/L TNF for the indicated times. Whole-cell extracts were prepared and analyzed by Western blot analysis using the indicated antibodies.
Gossypin inhibits TNF-induced expression of NF-κB–regulated gene products. KBM-5 cells were preincubated with 50 μM gossypin for 2 hours and 1 nmol/L TNF for the indicated times. Whole-cell extracts were prepared and analyzed by Western blot analysis using the indicated antibodies.
Gossypin inhibited tumor necrosis factor-induced expression of proliferative gene products
NF-κB activation has been shown to regulate the expression of COX-2, cyclin D1, and c-myc38–40 involved in the proliferation of various tumor cells. Therefore, we determined whether the expression of these gene products is modulated by gossypin. As shown in Figure 4B, gossypin suppressed the TNF-induced expression of all these gene products.
Gossypin suppressed tumor necrosis factor-induced matrix metalloproteinase-9 and vascular endothelial growth factor expression
MMP-9 involved in tumor cell invasion and metastasis is regulated by NF-κB.41 A Western blot analysis showed that gossypin inhibited TNF-induced MMP-9 expression (Figure 4C). VEGF is the most potent angiogenic factor, and recent studies have found that its expression is regulated by NF-κB.42 Therefore, we determined the effect of gossypin on TNF-induced VEGF expression. TNF-induced VEGF and gossypin inhibited TNF-induced VEGF expression (Figure 4C).
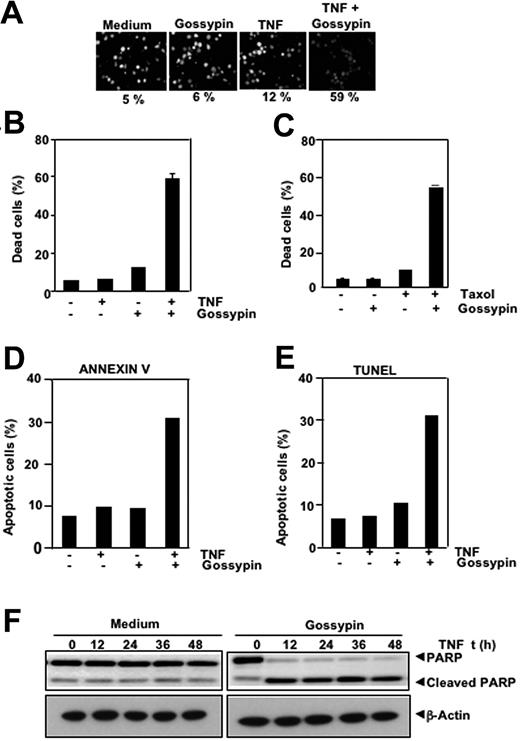
Gossypin potentiated apoptosis induced by TNF and chemotherapeutic agents
NF-κB activation can inhibit TNF and chemotherapeutic agents induced apoptosis through the expression of antiapoptotic gene products shown.43–45 Therefore, we determined whether gossypin enhances apoptosis induced by TNF and other cytotoxic agents. We used different methods to measure different aspects of apoptosis. We performed a live/dead assay, which measures cell membrane permeability, and found that gossypin up-regulated TNF-induced cytotoxicity from 5% to 59% (Figure 5A). The results of the 3-(4,5-dimethylthiazole-2-yl)-2,5-diphenyltetrazolium bromide method, which measures mitochondrial activity, showed that gossypin potentiates the cytotoxic activity of TNF (Figure 5B) and of Taxol (Figure 5C). TUNEL and annexin staining also showed that TNF-induced apoptosis was enhanced by incubation with gossypin (Figure 5D, E). We performed a caspase-3-activated PARP cleavage assay and found that gossypin had a dramatic effect on TNF-induced PARP cleavage (Figure 5F). The results of all these assays suggest that gossypin enhanced cytotoxicity by enhancing the apoptotic effects of TNF and Taxol.
Gossypin potentiates the apoptotic effects of TNF and chemotherapeutic agents. (A,B) Live/dead assay results indicate that gossypin upregulated TNF-induced cytotoxicity. KBM-5 cells were preincubated with 50 μM gossypin for 2 hours and then treated with 1 nmol/L TNF for 24 hours. Cells were stained with live/dead assay reagent for 30 minutes and then analyzed with a fluorescence microscope, as described in “Materials and methods.” (C) Gossypin enhanced Taxol-induced cytotoxicity. KBM-5 cells were pretreated with 50 μM gossypin and then incubated with 3 nmol/L of Taxol for 24 hours. Thereafter, cell viability was analyzed by the 3-(4,5-dimethylthiazole-2-yl)-2,5-diphenyltetrazolium bromide method, as described in Materials and methods. (D) Cells were pretreated with 50 μM gossypin for 2 hours and then incubated with 1 nM TNF for 16 hours. Cells were incubated with anti-annexin V Ab conjugated with FITC and then analyzed with a flow cytometer to identify early apoptotic effects. (E) TUNEL staining shows that TNF-induced apoptosis was enhanced by incubation with gossypin. KBM-5 cells were preincubated with 50 μM gossypin for 2 hours and then treated with 1 nmol/L TNF for 16 hours. Cells were fixed, stained with TUNEL assay reagent, and analyzed by flow cytometry, as described in Materials and methods. (F) Gossypin potentiated TNF-induced apoptosis. KBM-5 cells were preincubated with 50 μM gossypin for 2 hours and then treated with 1 nmol/L TNF for the indicated times. Whole-cell extracts were prepared, subjected to SDS-PAGE, and blotted with anti-PARP antibody.
Gossypin potentiates the apoptotic effects of TNF and chemotherapeutic agents. (A,B) Live/dead assay results indicate that gossypin upregulated TNF-induced cytotoxicity. KBM-5 cells were preincubated with 50 μM gossypin for 2 hours and then treated with 1 nmol/L TNF for 24 hours. Cells were stained with live/dead assay reagent for 30 minutes and then analyzed with a fluorescence microscope, as described in “Materials and methods.” (C) Gossypin enhanced Taxol-induced cytotoxicity. KBM-5 cells were pretreated with 50 μM gossypin and then incubated with 3 nmol/L of Taxol for 24 hours. Thereafter, cell viability was analyzed by the 3-(4,5-dimethylthiazole-2-yl)-2,5-diphenyltetrazolium bromide method, as described in Materials and methods. (D) Cells were pretreated with 50 μM gossypin for 2 hours and then incubated with 1 nM TNF for 16 hours. Cells were incubated with anti-annexin V Ab conjugated with FITC and then analyzed with a flow cytometer to identify early apoptotic effects. (E) TUNEL staining shows that TNF-induced apoptosis was enhanced by incubation with gossypin. KBM-5 cells were preincubated with 50 μM gossypin for 2 hours and then treated with 1 nmol/L TNF for 16 hours. Cells were fixed, stained with TUNEL assay reagent, and analyzed by flow cytometry, as described in Materials and methods. (F) Gossypin potentiated TNF-induced apoptosis. KBM-5 cells were preincubated with 50 μM gossypin for 2 hours and then treated with 1 nmol/L TNF for the indicated times. Whole-cell extracts were prepared, subjected to SDS-PAGE, and blotted with anti-PARP antibody.
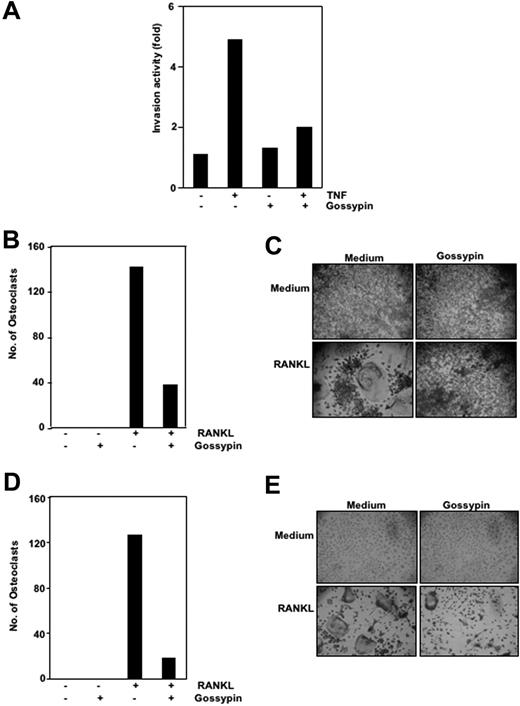
Gossypin suppressed tumor necrosis factor-induced invasion activity
Matrix metalloproteinases have been found to provide critical assistance to tumor cells during metastasis. Because gossypin was found to suppress TNF-induced MMP-9 expression, we determined its effects on TNF-induced invasion. For this experiment, we seeded H1299 cells in the top chamber of a Matrigel invasion chamber in the absence of serum. Cells were incubated with TNF, with or without gossypin, for 24 hours. As shown in Figure 6A, gossypin suppressed TNF-induced cell invasion activity.
Gossypin inhibits TNF-induced invasion and RANKL-induced osteoclastogenesis. (A) Gossypin suppressed TNF-induced invasion. H1299 cells were seeded in the top of a Matrigel invasion chamber overnight in the absence of serum, preincubated with 50 μM gossypin for 2 hours, treated with 1 nmol/L TNF for 24 hours in the presence of 1% serum, and then subjected to an invasion assay, as described in “Materials and methods.” (B and C) Gossypin inhibited RANKL-induced osteoclastogenesis. RAW 264.7 cells were incubated alone or with RANKL (5 nmol/L), with or without 50 μM gossypin, for 5 days and stained for TRAP expression. (B,D) Multinucleated (three nuclei) osteoclasts were counted. (C) TRAP-positive cells were photographed (10× 0.25 objective, TMS, Nikon, Tokyo, Japan; Coolpix 950 color camera, Nikon, Lewisville, TX). Original magnification, ×100. (D,E) Gossypin inhibited RANKL-induced osteoclastogenesis. Mouse (C57BL/6J) primary bone marrow cells were incubated alone or with RANKL (5 nmol/L), with or without 50 μM gossypin, for 3 days and stained for TRAP expression. (D) Multinucleated (three nuclei) osteoclasts were counted. (E) TRAP-positive cells were photographed. Original magnification, ×100.
Gossypin inhibits TNF-induced invasion and RANKL-induced osteoclastogenesis. (A) Gossypin suppressed TNF-induced invasion. H1299 cells were seeded in the top of a Matrigel invasion chamber overnight in the absence of serum, preincubated with 50 μM gossypin for 2 hours, treated with 1 nmol/L TNF for 24 hours in the presence of 1% serum, and then subjected to an invasion assay, as described in “Materials and methods.” (B and C) Gossypin inhibited RANKL-induced osteoclastogenesis. RAW 264.7 cells were incubated alone or with RANKL (5 nmol/L), with or without 50 μM gossypin, for 5 days and stained for TRAP expression. (B,D) Multinucleated (three nuclei) osteoclasts were counted. (C) TRAP-positive cells were photographed (10× 0.25 objective, TMS, Nikon, Tokyo, Japan; Coolpix 950 color camera, Nikon, Lewisville, TX). Original magnification, ×100. (D,E) Gossypin inhibited RANKL-induced osteoclastogenesis. Mouse (C57BL/6J) primary bone marrow cells were incubated alone or with RANKL (5 nmol/L), with or without 50 μM gossypin, for 3 days and stained for TRAP expression. (D) Multinucleated (three nuclei) osteoclasts were counted. (E) TRAP-positive cells were photographed. Original magnification, ×100.
Gossypin inhibited RANKL-induced osteoclastogenesis
RANKL was recently implicated as a major mediator of bone resorption.46 Thus, agents that suppress RANKL signaling may inhibit bone resorption or osteoclastogenesis. To determine the effect of gossypin on osteoclastogenesis, we incubated RAW 264.7 cells with 5 μmol/mL gossypin in the presence of RANKL and allowed them to grow and differentiate into osteoclasts. Figures 6B and 6C illustrate that RANKL-induced osteoclast differentiation and gossypin substantially decreased it. A 5-μmol/mL concentration of gossypin was sufficient to reduce osteoclastogenesis by more than 65%. Under these conditions, the cells remained fully viable (data not shown).
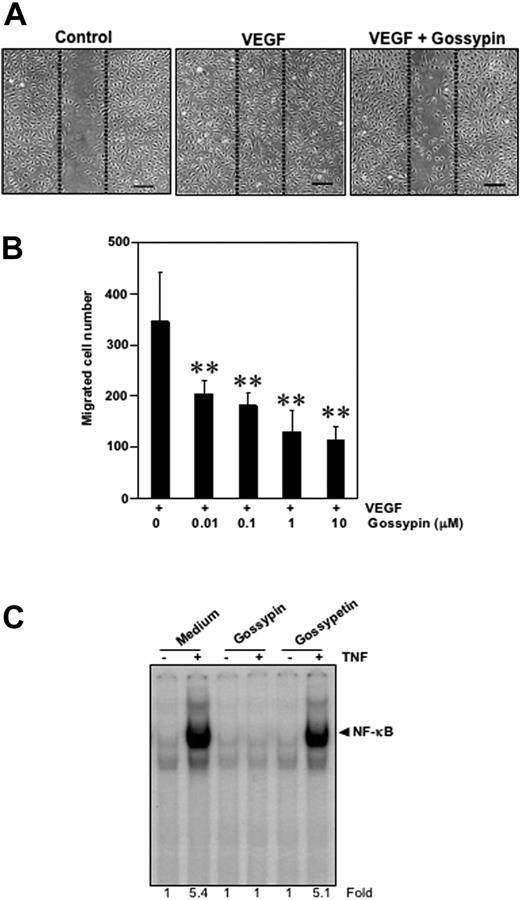
Effect of gossypin on human umbilical vein endothelial cell migration
To examine the potential effect of gossypin on migration, we performed the wound-healing/migration assay. The HUVECs migrated into the wounded area in response to VEGF stimulation and this migration was strongly inhibited by gossypin in a dose-dependent manner over the range of 0.01-10 μM (Figure 7A,B).
Gossypin inhibits the VEGF-induced migration of human umbilical vein vascular endothelial cells. (A) Wound-healing/migration. HUVECs were plated, scraped, and incubated in medium with 4 ng/mL VEGF in the presence or absence of various concentrations (0.01-10 μM) of gossypin. The cells migrated into the scraped area. Representative photomicrographs of untreated control cells (left) and cells treated with VEGF (center) or VEGF plus gossypin (right) are shown. Dotted lines indicate the initial scraping, scale bar = 200 μm. (B) Quantitative measurement of cell migration. Data are expressed as the migrated cells number in the VEGF-treated cultures. The data shown are representative of three independent experiments. **P < 0.01 versus VEGF-treated control. (C) Comparison between the activity of gossypin and its aglycone-gossypetin for TNF-induced NF-κB activation. Human myeloid leukemia KBM-5 (2 × 106) cells were incubated with 50 μM gossypin and goosypetin for 2 hours and then treated with 0.1 nmol/L TNF for 30 minutes. Nuclear extracts were then prepared and analyzed for NF-κB activation by EMSA.
Gossypin inhibits the VEGF-induced migration of human umbilical vein vascular endothelial cells. (A) Wound-healing/migration. HUVECs were plated, scraped, and incubated in medium with 4 ng/mL VEGF in the presence or absence of various concentrations (0.01-10 μM) of gossypin. The cells migrated into the scraped area. Representative photomicrographs of untreated control cells (left) and cells treated with VEGF (center) or VEGF plus gossypin (right) are shown. Dotted lines indicate the initial scraping, scale bar = 200 μm. (B) Quantitative measurement of cell migration. Data are expressed as the migrated cells number in the VEGF-treated cultures. The data shown are representative of three independent experiments. **P < 0.01 versus VEGF-treated control. (C) Comparison between the activity of gossypin and its aglycone-gossypetin for TNF-induced NF-κB activation. Human myeloid leukemia KBM-5 (2 × 106) cells were incubated with 50 μM gossypin and goosypetin for 2 hours and then treated with 0.1 nmol/L TNF for 30 minutes. Nuclear extracts were then prepared and analyzed for NF-κB activation by EMSA.
Gossypetin lacks the ability to suppress NF-κB activation
We also examined the effect of gossypetin, an aglycone analog of gossypin, on TNF induced NF-κB activation. The results showed that under the conditions gossypin completely suppressed TNF-induced NF-κB inhibition, gossypetin had no effect (Figure 7C), thus indicating the importance of carbohydrate moiety.
Discussion
The present study was aimed at evaluating the role of gossypin in NF-κB activation and NF-κB-regulated gene expression, apoptosis, invasion, and osteoclastogenesis. Gossypin, but not gossypetin, inhibited NF-κB activation induced by different carcinogens and proinflammatory molecules and inhibited constitutive NF-κB expression. Gossypin inhibited NF-κB by suppressing TAK-1-mediated IKK activation, leading to the inhibition of IκBα phosphorylation and degradation, p65 phosphorylation and translocation, and NF-κB-mediated gene transcription. Gossypin also inhibited the NF-κB-regulated gene products involved in suppressing apoptosis, proliferation, invasion, and angiogenesis, leading to apoptosis potentiation induced by TNF and Taxol. Gossypin's suppression of NF-κB activation was also associated with inhibition of TNF-induced invasion and RANKL-induced osteoclastogenesis. Gossypin also suppressed VEGF-induced HUVEC migration.
To our knowledge, ours is the first study to demonstrate that gossypin can suppress NF-κB activation induced by inflammatory stimuli such as TNF, interleukin-1β, and LPS, prooxidants such as H2O2 and carcinogens such as okadaic acid, tumor promoter, and cigarette smoke. Gossypin's suppression of these agents suggests that it acts at a step that is common to all these agents. We found that gossypin inhibited NF-κB activation by inhibiting IKK activation, which led to the suppression of IκBα phosphorylation, p65 phosphorylation, and nuclear translocation. The suppression of IKK by gossypin was found to result from the inhibition of upstream regulatory kinases of IKK such as TAK-1.31 In the present study, we found that gossypin suppressed TAK-1-mediated NF-κB activation. However, whether suppression of NF-κB activation by other stimuli also occurs through inhibition of TAK-1 is unclear. Besides inducible NF-κB activation, we found that gossypin also suppressed the constitutive activation of NF-κB commonly seen in a wide variety of tumors.47–53
Gossypin has been shown to exhibit antioxidant activity.3 It is possible that gossypin mediates its effects on NF-κB activation by quenching oxygen free radicals. However, this is unlikely because most free radical quenchers are not efficient at blocking NF-κB activation.54 Moreover, there are other reports that suggest reactive oxygen species not to play a role in NF-κB activation.55 Our current studies, however, cannot rule out the suppression of NF-κB activation through the antioxidant effects of gossypin.
We found that the expression of several gene products involved in the proliferation of tumor cells was suppressed by gossypin. Three of these products, cyclin D1, c-myc, and COX-2, are essential for cellular proliferation and survival.56–58 Expression of the c-myc oncoprotein prevents cell-cycle arrest in response to growth-inhibitory signals, differentiation stimuli, and mitogen withdrawal. Moreover, myc activation in quiescent cells is sufficient to induce cell cycle entry in the absence of growth factors. Cyclin D1 exercises powerful control over the mechanisms that regulate the mitotic cell cycle. Our results indicate that gossypin inhibits the TNF-induced expression of these two genes, suggesting that it is a potent inhibitor of the cell cycle. The suppression of tumor cell proliferation by gossypin may therefore be linked to the inhibition of expression of these gene products. The expression of most antiapoptotic gene products was also suppressed by this flavone. This activity is consistent with gossypin's potentiation of apoptosis induced by TNF and chemotherapeutic agents and suggests that gossypin may be clinically useful.
Our results showed that gossypin inhibited the MMP-9 expression and TNF-induced invasive activity of H1299 cells, suggesting that gossypin not only blocks primary tumor development, but also malignant progression, as previously published.4 In our study, gossypin also substantially inhibited TNF-induced COX-2 expression.
We found that gossypin inhibited TNF-induced VEGF expression in vitro, further showing that gossypin has antiangiogenic activity as reported earlier in Dalton's lymphoma ascites-induced solid tumor-bearing animals.4
Osteoclastogenesis is a process of bone loss commonly associated with aging and cancer. RANKL has been shown to play a critical role in osteoclastogenesis. We found that gossypin inhibited RANKL-induced osteoclastogenesis both in mouse primary bone marrow cells and RAW 264.7 cells in vitro. Overall, our results suggest that the antiproliferative, proapoptotic, anti-invasive, anti-angiogenic, and anti-osteoclastogenic effects of gossypin may result from the suppression of NF-κB and NF-κB-regulated gene products. Studies in animals are needed to validate these findings.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Ann Sutton for a careful review of the manuscript and Dr. Bryant G. Darney and Ambily Gopinathan for providing mouse primary bone marrow cells.
This work was supported by the Clayton Foundation for Research and by the National Institutes of Health Cancer Center Core Grant CA 1 672. B.B.A. is a Ransom Horne Professor of Cancer Research.
National Institutes of Health
Authorship
Contribution: A.B.K., A.S.N., K.S.A., M.K.P., and X.Y. conducted all the experiments; M.L. and B.B.A. supervised and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Bharat B. Aggarwal, Cytokine Research Laboratory, Department of Experimental Therapeutics, Unit 143, The University of Texas M. D. Anderson Cancer Center, 1515 Holcombe Boulevard, Houston, TX 77030; e-mail:aggarwal@mdanderson.org.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal