The management of patients with chronic-phase CML refractory to standard doses of imatinib mesylate includes dose escalation, allogeneic transplantation, or a switch to a new BCR/ABL inhibitor. In this issue of Blood, Kantarjian and colleagues present the results of a multicenter trial demonstrating that, for highly refractory patients, treatment with dasatinib is superior to dose escalation of imatinib mesylate.
In the short period of time since the initial trials in the late 1990s, imatinib mesylate (Gleevec; Novartis, Basel, Switzerland) has revolutionized the treatment of chronic myelogenous leukemia (CML) and created a model for the development of other so-called targeted therapies. In contrast to the mixed results with putatively targeted treatments in more genetically complex malignancies, imatinib mesylate has been so effective because the single mutation producing the chimeric BCR/ABL protein is sufficient to produce the clinical features of CML.
The starting dose of imatinib mesylate in chronic phase is 400 mg per day with no adjustments for patient weight. With 5 years of follow-up, 87% of the 553 patients who received imatinib mesylate as initial treatment on the International Randomized Study of Interferon and STI571 trial achieved complete cytogenetic remission (CCyR) at some time, with progression-free survival of 83% and overall survival of 89%; 69% remain on imatinib mesylate. The depth of response improves over time, with decreases in BCR/ABL transcript number as assessed by serial quantitative polymerase chain reaction (PCR).1 Studies are in progress to assess whether higher doses of imatinib or the newer, more potent tyrosine kinase inhibitors, nilitonib (Tasigna; Novartis) and dasatinib (Sprycel; Bristol-Meyers-Squibb, New York, NY), could improve upon these results, possibly permitting discontinuation of therapy in patients who become consistently PCR negative.
In the interim, there remain some patients who require changes after initial imatinib mesylate treatment. In this issue, Kantarjian and colleagues describe a phase 2 study in which chronic-phase patients refractory to 400 g or 600 g imatinib mesylate were randomized to receive dasatinib or a dose increase of imatinib mesylate to 400 mg twice a day. There is actually little information, from either the IRIS trial or elsewhere, on the results of dose increase from 400 mg to 800 mg, although some retrospective studies have suggested that responses are suboptimal.
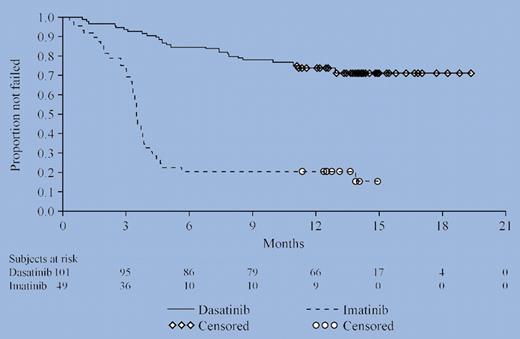
Randomized phase 2 trials are generally underpowered and not intended to be definitive tests of hypotheses, although there is a recent tendency to use this design as a poor man's phase 3 trial, hoping for substantive differences, as was noted in this study. The overall response rate and time to treatment failure (see figure) were strikingly superior in the dasatinib recipients, although the duration of benefit is not known because of the brief follow-up period. Certainly, it is not surprising that dasatinib treatment was better than an imatinib dose escalation of only 200 mg, particularly since the patients in this study had long-standing, rather resistant CML characterized by hematologic or cytogenetic relapses, with only 28% having ever achieved a major cytogenetic remission. Of note, however, is the fact that the superiority of dasatinib was less apparent in patients who had previously received only 400 mg imatinib mesylate.
Time to treatment failure. See the complete figure in the article beginning on page5143.
Time to treatment failure. See the complete figure in the article beginning on page5143.
This study does not address patients with more subtle suggestions of treatment failure to 400 mg imatinib mesylate. Of concern is the temptation to switch therapy for patients in CCyR who have had less than what has been termed a major molecular response1 or who have had raised BCR/ABL transcript levels with serial monitoring. The cytogenetic relapse rate remains extremely low in patients in CCyR, and we are only beginning to learn how to react to these more sensitive measurements of residual disease.2 Before succumbing to the new disorder of “PCRitis,” it is important to remember that the treatment of chronic phase is a marathon, not a sprint. Currently, there is inadequate standardization of results of PCR assays among different laboratories in the United States, and fluctuation in values is common. If increasing transcript values are confirmed repeatedly in patients still in CCyR, an initial trial with a dose increase to 800 mg imatinib mesylate is reasonable, switching to dasatinib if no response is noted. If BCR/ABL mutations known to be resistant to the available tyrosine kinase inhibitors are detected, allogeneic transplantation should also be considered if there is cytogenetic relapse.
Conflict-of-interest disclosure: The author has received grant support for clinical trials and has served on advisory boards for Novartis and Bristol, Myers, Squibb. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal