Abstract
Therapeutic options for chronic myelogenous leukemia (CML) resistant to 400 to 600 mg imatinib are limited. Escalating imatinib doses may overcome resistance. Dasatinib, a significantly more potent inhibitor of BCR-ABL, is safe and effective in this population. Patients with imatinib-resistant chronic-phase (CP) CML were randomized 2:1 to 140 mg dasatinib (n = 101) or 800 mg imatinib (n = 49). With a median follow up of 15 months, complete hematologic responses were observed in 93% and 82% of patients receiving dasatinib and high-dose imatinib (P = .034), respectively. Dasatinib resulted in higher major cytogenetic response rates (52%) than high-dose imatinib (33%) (P = .023); this included complete cytogenetic response in 40% and 16% (P = .004). Major molecular responses were also more frequent with dasatinib (16% versus 4%; P = 0.038). Treatment failure (hazard ratio [HR], 0.16; P < .001) and progression-free survival (HR, 0.14; P < .001) both favored dasatinib. Superficial edema (42% versus 15%) and fluid retention (45% versus 30%) were more prevalent with imatinib; pleural effusion was more common with dasatinib (17% versus 0%). Grade 3 to 4 nonhematologic toxicity was minimal. Cytopenias were more frequent and severe with dasatinib. Dasatinib represents a safe and effective therapy for CP-CML resistant to conventional imatinib doses with improved cytogenetic and molecular response rates and progression-free survival relative to high-dose imatinib.
Introduction
Recent data from an international, randomized study indicate that approximately 30% of the 553 newly diagnosed patients with chronic-phase (CP) chronic myelogenous leukemia (CML) treated with imatinib who were enrolled had discontinued therapy after 5 years of follow up because of unsatisfactory therapeutic effects or toxicity.1 Resistance to imatinib (400-600 mg) is a well-recognized problem for patients with CP-CML,1–6 and effective therapeutic options for such patients are limited. Escalating the dose of imatinib to 800 mg per day can overcome some of these cases of resistance,2,7–9 but the resulting responses are short in duration and tolerability of high-dose imatinib continues to be an issue.10 These data highlight the need for effective alternative treatments for this patient population.
Dasatinib (SPRYCEL, formerly BMS-354825; Bristol-Myers Squibb, New York), a novel, oral, multitargeted kinase inhibitor of BCR-ABL, SRC, c-KIT, ephrin, and PDGFR-β, has been shown to be safe and effective in patients with CML.11–18 Dasatinib was recently approved in the United States and the European Union for use in patients with chronic, accelerated, or blast phases of CML or Philadelphia-chromosome-positive (Ph+) acute lymphoblastic leukemia with resistance or intolerance to prior therapy, including imatinib. In vitro, dasatinib possesses 325-fold greater potency than imatinib in inhibiting Bcr-Abl kinase.19 Unlike imatinib and its derivative, nilotinib (AMN107), dasatinib can bind to both the active and inactive conformation of the ABL kinase domain.19–21 Because dasatinib has less stringent binding requirements than imatinib, dasatinib is active against many imatinib-resistant kinase domain mutations of BCR-ABL. In cell-line models, dasatinib inhibited (within a narrow concentration range similar to that required to block wild-type BCR-ABL) all but one BCR-ABL mutation conferring imatinib resistance that have been tested to date. This may account, at least in part, for the greater efficacy reported with dasatinib in vitro,22,23 although dasatinib's ability to inhibit other kinases may also contribute.24 Durable hematologic and cytogenetic responses have been demonstrated for dasatinib in a number of open-label phase II clinical studies,13–16 including a population of patients with CP-CML.15
We present the relative efficacy and safety data from a randomized trial of dasatinib and high-dose imatinib in patients with CP-CML after failure on imatinib 400 to 600 mg daily.
Patients, materials, and methods
Patients and trial design
Patients with CP-CML with primary or acquired resistance to conventional doses of imatinib (400-600 mg) were enrolled in this randomized, international, open-label, phase II study. The definition of CP-CML was consistent with that previously reported.18 Primary resistance to imatinib was defined as a lack of complete hematologic response (CHR) after 3 months of imatinib treatment, a lack of any cytogenetic response after 6 months of treatment, or a lack of a major cytogenetic response (MCyR) (Ph+ cells >35%) after 12 months of treatment. Relapse after a hematologic response or MCyR was considered as secondary or acquired resistance.
Patients had to be at least 18 years of age and have adequate hepatic and renal function. Patients who had received imatinib in the 7 days before the study were ineligible, as were patients who had received imatinib at doses in excess of 600 mg per day. All patients were dasatinib-naïve. To avoid potential bias, patients with known specific BCR-ABL mutations (with high resistance to imatinib: L248V, G250E, Q252H/R, Y253H/F, E255K/V, T315I/D, F317L, and H396P/R) before study entry were excluded.
Patients were randomized on a 2:1 basis to receive either 140 mg dasatinib (70 mg twice daily) or 800 mg imatinib (400 mg twice daily). This schema was proposed because it was believed that the safety and efficacy profile of high-dose imatinib were already well characterized. Crossover to the alternate treatment was permitted after confirmed progression (ie, progression to accelerated-phase or blast-phase CML, loss of CHR or MCyR, or increasing white blood cell count), lack of MCyR at the week 12 cytogenetic evaluation, or intolerance (grade 3-4 nonhematologic toxicity or hematologic toxicity requiring multiple dose modifications). Doses of dasatinib could be escalated to 180 mg for patients with inadequate response at 12 weeks or progression or reduced to 100 or 80 mg for patients experiencing toxicity. Reduction of the imatinib dose for toxicity to 600 mg was permitted for patients who had not previously received 600 mg imatinib.
Evaluations consisted of weekly blood counts for the first 12 weeks of treatment and every 2 weeks thereafter. Cytogenetic response was evaluated through bone marrow aspirates every 12 weeks.
The study was conducted in accordance with the Declaration of Helsinki and was approved by the Institutional Review Board/Ethics Committee at each participating center. All patients provided written informed consent in accordance with the Declaration of Helsinki.
Efficacy assessments
The rate of MCyR at week 12 was the primary end point for the short-term phase of this study. Secondary end points included rates of MCyR and CHR at any time before crossover, duration of MCyR and CHR, and time to MCyR and CHR before crossover; these end points were also evaluated postcrossover. Definitions of response and treatment outcomes were as previously described.18 Time to treatment failure was defined as the time from randomization to progression or end of treatment (lack of response, study drug intolerance, or off treatment for any reason); subjects still on treatment were censored as of their last day of dosing. Progression v survival (PFS) was defined as the time from randomization until disease progression (defined above), death, or discontinuation of treatment because of progression prior to crossover.
With longer-term follow up, the rate of complete cytogenetic response (CCyR) is a more significant and clinically relevant end point, and a correlation exists between increased depth of CCyR and improved survival. This parameter provides a more rigorous evaluation of the 2 treatments and is consistent with the ultimate goal of therapy being to induce cytogenetic remission.
Response rates are provided with their exact 95% confidence interval (CI). Treatment differences were analyzed retrospectively using the Agresti-Min exact test, and the corresponding 95% CIs are provided. Time to treatment failure was analyzed using Kaplan-Meier methodology and an unstratified log rank test; the hazard ratio was computed using a Cox proportional hazard model.
All analyses, unless otherwise noted, were based on results before crossover.
Safety analysis
Adverse events (AEs) were assessed continuously and were graded according to the NCI Common Terminology Criteria for Adverse Events (CTCAE) Version 3.0. As part of the evaluation of these safety data, specific focus was given to cases of myelosuppression and fluid retention.
Analysis of mutational status
Sequence of BCR-ABL genes was evaluated to determine the presence and site of point mutations.
Study responsibilities
The study was designed by academic investigators in conjunction with representatives from the sponsor, Bristol-Myers Squibb. Both parties contributed to the collection and analysis of the data.
Results
In total, 150 patients whose disease was resistant to imatinib were enrolled between February and November 2005 from 58 centers in 23 countries; 101 were randomized to receive dasatinib and 49 to treatment with high-dose imatinib.
Baseline patient characteristics
Patient characteristics were well balanced at baseline (Table 1) with one exception; approximately twice as many patients in the dasatinib treatment arm (45%) had a BCR-ABL mutation as in the high-dose imatinib group (22%). Patients recruited to the study had a long history of CML and were extensively pretreated. Median age was 51 years (range, 24-85 years) and median time from initial diagnosis of CML was 59 months (range, 6-166 months). Approximately two-thirds of patients had received treatment with 600 mg imatinib per day, and the duration of imatinib therapy had exceeded 3 years for 40% of patients. Major cytogenetic responses to previous imatinib therapy before progression had been observed in 28% of patients.
Baseline characteristics of the patients
| Characteristic . | Dasatinib (n = 101) . | High-dose imatinib (n = 49) . |
|---|---|---|
| Age, y | ||
| Median | 51 | 51 |
| Range | 24-85 | 24-80 |
| Male, no. (%) | 53 (52) | 22 (45) |
| Disease history, duration in months, median (range) | 64 (6-166) | 52 (14-133) |
| History of imatinib therapy | ||
| Max imatinib dose >400 mg/day; no. (%) | 65 (64) | 35 (71) |
| Duration of prior imatinib therapy, no. (%) | ||
| Less than 1 year | 12 (12) | 5 (10) |
| 1-3 years | 44 (44) | 29 (59) |
| 3 years or more | 45 (45) | 15 (31) |
| Prior response to imatinib, no. (%) | ||
| CHR | 93 (92) | 47 (96) |
| CCyR | 15 (15) | 4 (8) |
| PCyR | 13 (13) | 10 (20) |
| Reason for imatinib resistance,* no. (%) | ||
| Loss of MCyR | 21 (21) | 14 (29) |
| Loss of CHR | 24 (24) | 15 (31) |
| Increasing white blood cell count | 4 (4) | 2 (4) |
| No CHR after 3 months | 3 (3) | 2 (4) |
| No CyR after 6 months | 39 (39) | 16 (33) |
| No MCyR after 12 months | 39 (39) | 24 (49) |
| Treatment history, no. (%) | ||
| Hydroxyurea/anagrelide | 97 (96) | 46 (94) |
| Interferon-α | 74 (73) | 33 (67) |
| Chemotherapy | 39 (39) | 18 (37) |
| Bone marrow transplant | 7 (7) | 2 (4) |
| Hematologic analysis | ||
| WBC count, × 109/L | ||
| Median | 7.5 | 7.4 |
| Range | 2-153 | 2-133 |
| WBC count, 20 × 109/L or more, % | 11 | 14 |
| Platelet count, × 109/L | ||
| Median | 256 | 248 |
| Range | 55-1903 | 80-2318 |
| Mutation status, BCR-ABL mutation; n/N (%) | 41/92 (45) | 11/46 (24) |
| Response status, no. (%) | ||
| Patients in CHR at entry | 51 (50) | 27 (55) |
| Patients in MCyR at entry | 6 (6) | 0 |
| Characteristic . | Dasatinib (n = 101) . | High-dose imatinib (n = 49) . |
|---|---|---|
| Age, y | ||
| Median | 51 | 51 |
| Range | 24-85 | 24-80 |
| Male, no. (%) | 53 (52) | 22 (45) |
| Disease history, duration in months, median (range) | 64 (6-166) | 52 (14-133) |
| History of imatinib therapy | ||
| Max imatinib dose >400 mg/day; no. (%) | 65 (64) | 35 (71) |
| Duration of prior imatinib therapy, no. (%) | ||
| Less than 1 year | 12 (12) | 5 (10) |
| 1-3 years | 44 (44) | 29 (59) |
| 3 years or more | 45 (45) | 15 (31) |
| Prior response to imatinib, no. (%) | ||
| CHR | 93 (92) | 47 (96) |
| CCyR | 15 (15) | 4 (8) |
| PCyR | 13 (13) | 10 (20) |
| Reason for imatinib resistance,* no. (%) | ||
| Loss of MCyR | 21 (21) | 14 (29) |
| Loss of CHR | 24 (24) | 15 (31) |
| Increasing white blood cell count | 4 (4) | 2 (4) |
| No CHR after 3 months | 3 (3) | 2 (4) |
| No CyR after 6 months | 39 (39) | 16 (33) |
| No MCyR after 12 months | 39 (39) | 24 (49) |
| Treatment history, no. (%) | ||
| Hydroxyurea/anagrelide | 97 (96) | 46 (94) |
| Interferon-α | 74 (73) | 33 (67) |
| Chemotherapy | 39 (39) | 18 (37) |
| Bone marrow transplant | 7 (7) | 2 (4) |
| Hematologic analysis | ||
| WBC count, × 109/L | ||
| Median | 7.5 | 7.4 |
| Range | 2-153 | 2-133 |
| WBC count, 20 × 109/L or more, % | 11 | 14 |
| Platelet count, × 109/L | ||
| Median | 256 | 248 |
| Range | 55-1903 | 80-2318 |
| Mutation status, BCR-ABL mutation; n/N (%) | 41/92 (45) | 11/46 (24) |
| Response status, no. (%) | ||
| Patients in CHR at entry | 51 (50) | 27 (55) |
| Patients in MCyR at entry | 6 (6) | 0 |
CCyR indicates complete cytogenetic response; CHR, complete hematologic response; CyR, cytogenetic response; MCyR, major cytogenetic response; PCyR, partial cytogenetic response; WBC, white blood cells; n/N, datum number/number of patients treated.
Patients may have more than one reason for imatinib resistance.
Treatment duration, dosing information, and patient disposition
Treatment duration (calculated from the first dose to the last dose of study drug) was considerably longer for patients receiving dasatinib; the median duration of therapy was 13.7 months (range, 0.2-19.3 months) for patients treated with dasatinib and 3.1 months (range, 0.2-15.6 months) for the high-dose imatinib group. Median average daily doses of dasatinib and imatinib were 103 mg (range, 38-175 mg) and 796 mg (range, 358-800 mg), respectively. For patients who remained on their initially allocated treatment (ie, dasatinib or high-dose imatinib), the median duration of therapy for the dasatinib treatment group was 14.1 months (range, 11.0-19.3 months) and for patients receiving high-dose imatinib was 13.1 months (range, 12.4-14.9 months).
With a median follow up of 15 months (range, 1-21 months), significantly more patients had discontinued treatment with high-dose imatinib than with dasatinib (P < .0001); 72% of patients (73 of 101) randomized to dasatinib remained on their allocated initial therapy, whereas 18% of patients (9 of 49) continued to receive high-dose imatinib. Reasons for discontinuation of high-dose imatinib were primarily as a result of a lack of response or disease progression (30 of 49 [61%]) or drug intolerance (9 of 49 [18%]), most often attributable to nonhematologic toxicity, whereas discontinuation of dasatinib was mainly attributable to drug intolerance (16 of 101 [16%]) or disease progression (5 of 101 [5%]). Of the 68 patients who discontinued treatment, 54 crossed over to the alternate treatment; 15 patients initially randomized to dasatinib subsequently received high-dose imatinib, whereas 39 patients randomized to high-dose imatinib crossed over to dasatinib. Fourteen patients (13 from the dasatinib treatment group and one receiving high-dose imatinib) discontinued treatment permanently and did not crossover; reasons for discontinuation for these 13 dasatinib-treated patients were study drug toxicity (6), patient request (4), and disease progression (3), whereas the single patient receiving high-dose imatinib who discontinued but failed to crossover was withdrawn as a result of noncompliance.
Rates of hematologic, cytogenetic, and molecular response
Treatment with dasatinib resulted in more favorable rates of CHR than high-dose imatinib therapy. A CHR was achieved by 94 of 101 patients (93%) treated with dasatinib and 40 of 49 patients (82%) treated with high-dose imatinib (treatment difference, 11%; 95% CI, -0.7% to 25.2%; P = .034) (Table 2). CHRs were present at the time of entry into the study for 51 patients (50%) randomized to dasatinib and in 27 patients assigned to high-dose imatinib therapy (Table 1). Among dasatinib-treated patients, CHRs were maintained by all 51 (100%) of these patients and were attained by 43 of 50 patients (86%). Twenty-four of 27 patients (89%) treated with high-dose imatinib maintained their response, whereas 16 of 22 patients (73%) attained a CHR.
Treatment response
| Response . | Initial treatment . | Crossover treatment . | ||||||
|---|---|---|---|---|---|---|---|---|
| Dasatinib; N = 101 . | High-dose imatinib; N = 49 . | From high-dose imatinib to dasatinib; N = 39* . | From dasatinib to high-dose imatinib; N = 15* . | |||||
| % . | 95% CI . | % . | 95% CI . | % . | 95% CI . | % . | 95% CI . | |
| Complete hematologic response | 93 | 86.2-97.2† | 82 | 68.0-91.2 | 92 | 79.1-98.4‡ | 53 | 26.6-78.7 |
| Cytogenetic response | ||||||||
| Major | 52 | 42.3-62.5§ | 33 | 19.9-47.5 | 45 | 28.6-61.7‖ | 15 | 1.9-45.4 |
| Complete | 40 | 30.0-49.8¶ | 16 | 7.3-29.7 | 29 | 15.4-45.9# | 0 | 0-25.7 |
| Partial | 13 | 7.0-21.0 | 16 | 7.3-29.7 | 16 | 6.0-31.3 | 15 | 1.9-45.4 |
| Major molecular response | 16 | 9.3-24.4** | 4 | 0.5-14.0 | NA | NA | NA | NA |
| Response . | Initial treatment . | Crossover treatment . | ||||||
|---|---|---|---|---|---|---|---|---|
| Dasatinib; N = 101 . | High-dose imatinib; N = 49 . | From high-dose imatinib to dasatinib; N = 39* . | From dasatinib to high-dose imatinib; N = 15* . | |||||
| % . | 95% CI . | % . | 95% CI . | % . | 95% CI . | % . | 95% CI . | |
| Complete hematologic response | 93 | 86.2-97.2† | 82 | 68.0-91.2 | 92 | 79.1-98.4‡ | 53 | 26.6-78.7 |
| Cytogenetic response | ||||||||
| Major | 52 | 42.3-62.5§ | 33 | 19.9-47.5 | 45 | 28.6-61.7‖ | 15 | 1.9-45.4 |
| Complete | 40 | 30.0-49.8¶ | 16 | 7.3-29.7 | 29 | 15.4-45.9# | 0 | 0-25.7 |
| Partial | 13 | 7.0-21.0 | 16 | 7.3-29.7 | 16 | 6.0-31.3 | 15 | 1.9-45.4 |
| Major molecular response | 16 | 9.3-24.4** | 4 | 0.5-14.0 | NA | NA | NA | NA |
CI indicates confidence interval; NA, not applicable.
Fifty-one patients were evaluable for cytogenetic response postcrossover (dasatinib, 38; imatinib, 13). Statistically significant differences between treatments:
P = .034.
P = .001.
P = .023.
P = .063.
P = .004.
P = .031.
P = .038.
Dasatinib therapy was also associated with greater cytogenetic response rates than high-dose imatinib. At 12 weeks, MCyR was achieved in 36 of 101 patients (36%) treated with dasatinib and 14 of 49 patients (29%) receiving treatment with high-dose imatinib (Table 2; P = .40). The proportion of patients achieving a CCyR was significantly higher for the dasatinib treatment group (22 of 101 patients [22%]) than for the high-dose imatinib group, in which 4 of 49 patients (8%) attained a CCyR at 12 weeks (treatment difference, 14%; 95% CI, 0.6% to 24.8%; P = .041).
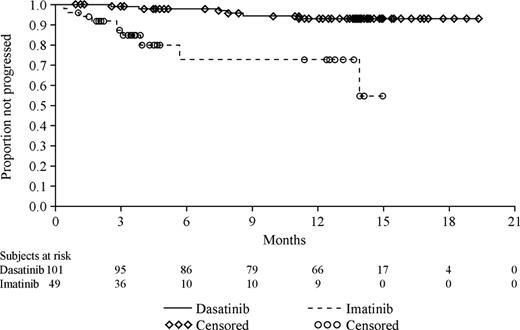
With further follow-up (median follow-up, 15 months), rates of MCyR increased to 52% and 33%, respectively, for patients receiving treatment with dasatinib and high-dose imatinib (Table 2), corresponding to a statistically significant treatment difference of 20% (95% CI, 2.6% to 35.3%; P = .023). Of note, CCyRs were also observed in a significantly higher proportion of dasatinib-treated patients (40%) than patients receiving high-dose imatinib (16%) (treatment difference, 23%; 95% CI, 7.7% to 36.5%; P = .004). The degree of improvement in cytogenetic response from baseline is summarized in Table 3; the proportion of patients for whom this improvement was from 96% to 100% Ph+ cells (ie, no cytogenetic response at baseline) to MCyR was 3-fold higher for patients receiving dasatinib therapy. Of note, responses seen with dasatinib therapy also proved to be durable (Figure 1).
Degree of improvement in cytogenetics from baseline (pretreatment cytogenetic response status)
| Pretreatment cytogenetic response status . | Number (%) of patients . | |||||||
|---|---|---|---|---|---|---|---|---|
| Dasatinib; n = 101 . | High-dose imatinib; n = 49 . | |||||||
| Complete/partial . | Minor/minimal . | None . | Undetermined . | Complete/partial . | Minor/minimal . | None . | Undetermined . | |
| Complete/partial | 5 (5) | 0 | 0 | 1 (1) | 0 | 0 | 0 | 0 |
| Minor/minimal | 18 (18) | 4 (4) | 2 (2) | 2 (2) | 10 (20) | 1 (2) | 4 (8) | 2 (4) |
| None | 29 (29) | 15 (15) | 22 (22) | 0 | 5 (10) | 3 (6) | 16 (33) | 2 (4) |
| Undetermined | 1 (1) | 2 (2) | 0 | 0 | 1 (2) | 3 (6) | 2 (4) | 0 |
| Pretreatment cytogenetic response status . | Number (%) of patients . | |||||||
|---|---|---|---|---|---|---|---|---|
| Dasatinib; n = 101 . | High-dose imatinib; n = 49 . | |||||||
| Complete/partial . | Minor/minimal . | None . | Undetermined . | Complete/partial . | Minor/minimal . | None . | Undetermined . | |
| Complete/partial | 5 (5) | 0 | 0 | 1 (1) | 0 | 0 | 0 | 0 |
| Minor/minimal | 18 (18) | 4 (4) | 2 (2) | 2 (2) | 10 (20) | 1 (2) | 4 (8) | 2 (4) |
| None | 29 (29) | 15 (15) | 22 (22) | 0 | 5 (10) | 3 (6) | 16 (33) | 2 (4) |
| Undetermined | 1 (1) | 2 (2) | 0 | 0 | 1 (2) | 3 (6) | 2 (4) | 0 |
Cytogenetic responses were calculated from the percentage of Ph+ cells in metaphase and defined as complete (0%), partial (1-35%), minor (36-65%), minimal (66-95%), none (≥96%), or undetermined.
Major molecular responses were reported for 16 of 101 patients (16%) receiving dasatinib therapy and for 2 of 49 patients (4%) receiving high-dose imatinib; this difference was statistically significant (treatment difference, 12%; 95% CI, 0.8% to 21.8%; P = .038).
Prognostic factors.
Major cytogenetic response rates were consistently higher for dasatinib in all subgroups of prognostic interest based on demographic and baseline characteristics.
In subgroups with the highest resistance to imatinib (no cytogenetic response with prior imatinib and prior imatinib doses of 600 mg per day), the difference between dasatinib and high-dose imatinib was also evident and was statistically significant (Table 4). In patients with no prior cytogenetic response to imatinib, 19 of 39 patients (49%) were able to achieve clinically important MCyRs with dasatinib; with dose escalation of imatinib MCyR was attained in one of 15 patients (7%) (P = .006). The majority of these MCyRs in the dasatinib group were complete with a CCyR rate of 31%; the MCyR in the high-dose imatinib arm was also a CCyR (7%). In the subgroup that received prior imatinib doses of 600 mg per day, the MCyR rates were 49% and 24%, respectively (P = .015); the majority of these responses were CCyRs (38% and 6%, respectively). Rates of MCyR also favored dasatinib in the subgroup that received prior imatinib therapy at 400-mg per day doses (dasatinib 58%; high-dose imatinib 53%), although the difference was not significant.
Treatment response in groups of prognostic interest
| Subpopulations . | Patients with MCyR . | Patients with CCyR . | ||||||
|---|---|---|---|---|---|---|---|---|
| Dasatinib . | High-dose imatinib . | Dasatinib . | High-dose imatinib . | |||||
| n/N (%) . | 95% CI . | n/N (%) . | 95% CI . | n/N (%) . | 95% CI . | n/N (%) . | 95% CI . | |
| Prior chemotherapy | 15/39 (38) | 23.4-55.4 | 4/18 (22) | 6.4-47.6 | 12/39 (31) | 17.0-47.6 | 1/18 (6) | 0.1-27.3 |
| Prior stem cell transplant | 3/7 (43) | 9.9-81.6 | 1/2 (50) | 1.3-98.7 | 3/7 (43) | 9.9-81.6 | 0/2 | 0.0-84.2 |
| Imatinib 600 mg/day | 31/63 (49) | 36.4-62.1* | 8/34 (24) | 10.7-41.2 | 24/63 (38) | 26.1-51.2‡ | 2/34 (6) | 0.7-19.7 |
| No prior CHR with imatinib | 4/8 (50) | 15.7-84.3 | 1/2 (50) | 1.3-98.7 | 3/8 (38) | 8.5-75.5 | 1/2 (50) | 1.3-98.7 |
| No prior CyR with imatinib | 19/39 (49) | 32.4-65.2† | 1/15 (7) | 0.2-31.9 | 12/39 (31) | 17.0-47.6 | 1/15 (7) | 0.2-31.9 |
| BCR-ABL mutation | 19/41 (46) | 30.7-62.6 | 3/11 (27) | 6.0-61.0 | 13/41 (32) | 18.1-48.1 | 2/11 (18) | 2.3-51.8 |
| Subpopulations . | Patients with MCyR . | Patients with CCyR . | ||||||
|---|---|---|---|---|---|---|---|---|
| Dasatinib . | High-dose imatinib . | Dasatinib . | High-dose imatinib . | |||||
| n/N (%) . | 95% CI . | n/N (%) . | 95% CI . | n/N (%) . | 95% CI . | n/N (%) . | 95% CI . | |
| Prior chemotherapy | 15/39 (38) | 23.4-55.4 | 4/18 (22) | 6.4-47.6 | 12/39 (31) | 17.0-47.6 | 1/18 (6) | 0.1-27.3 |
| Prior stem cell transplant | 3/7 (43) | 9.9-81.6 | 1/2 (50) | 1.3-98.7 | 3/7 (43) | 9.9-81.6 | 0/2 | 0.0-84.2 |
| Imatinib 600 mg/day | 31/63 (49) | 36.4-62.1* | 8/34 (24) | 10.7-41.2 | 24/63 (38) | 26.1-51.2‡ | 2/34 (6) | 0.7-19.7 |
| No prior CHR with imatinib | 4/8 (50) | 15.7-84.3 | 1/2 (50) | 1.3-98.7 | 3/8 (38) | 8.5-75.5 | 1/2 (50) | 1.3-98.7 |
| No prior CyR with imatinib | 19/39 (49) | 32.4-65.2† | 1/15 (7) | 0.2-31.9 | 12/39 (31) | 17.0-47.6 | 1/15 (7) | 0.2-31.9 |
| BCR-ABL mutation | 19/41 (46) | 30.7-62.6 | 3/11 (27) | 6.0-61.0 | 13/41 (32) | 18.1-48.1 | 2/11 (18) | 2.3-51.8 |
CCyR indicates complete cytogenetic response; CHR, complete hematologic response; CI, confidence interval; CyR, cytogenetic response; MCyR, major cytogenetic response; n/N, numbers of patients with a response/number of patients treated.
Statistically significant differences between treatments:
P = .015;
P = .006;
P = .0006.
Of the 52 patients (38%) with imatinib-resistant BCR-ABL mutations detected at baseline, 19 of 41 patients (46%) treated with dasatinib subsequently achieved a MCyR versus 3 of 11 patients (27%) receiving high-dose imatinib (P = .282) (Tables 4 and 5).
Efficacy by baseline BCR-ABL mutational analysis
| . | Total* n/N (%) . | Patients with MCyR n/N (%) . | Patients with CHR n/N (%) . | |||
|---|---|---|---|---|---|---|
| Dasatinib; N = 92 . | High-dose imatinib; N = 46 . | Dasatinib . | High-dose imatinib . | Dasatinib . | High-dose imatinib . | |
| No mutations | 51 (55) | 35 (76) | 28/51 (55) | 12/35 (34) | 50/51 (98) | 32/35 (91) |
| Any mutation | 41 (45) | 11 (24) | 19/41 (46) | 3/11 (27) | 36/41 (88) | 6/11 (55) |
| Mutation associated with ≥5-fold increase in imatinib resistance | 25 (27) | 7 (15) | 12/25 (48) | 1/7 (14) | 21/25 (84) | 2/7 (29) |
| Specific BCR-ABL point mutations† | ||||||
| L248V | 2 | 0 | 1 | NA | 2 | NA |
| I418V | 1 | 1 | 1 | 1 | 1 | 1 |
| M351T (IC50: 880 nmol/L)‡ | 6 | 1 | 2 | 0 | 6 | 1 |
| F317L (IC50: 1050 nmol/L)‡ | 4 | 0 | 1 | NA | 4 | NA |
| G250E (IC50: 1350 nmol/L)‡ | 7 | 0 | 4 | NA | 6 | NA |
| H396R (IC50: 1750 nmol/L)‡ | 3 | 0 | 1 | NA | 2 | NA |
| F359V (IC50: 1825 nmol/L)‡ | 4 | 2 | 1 | 1 | 4 | 1 |
| M244V (IC50: 2000 nmol/L)‡ | 9 | 3 | 5 | 0 | 8 | 1 |
| Y253H (IC50: >6400 nmol/L)‡ | 1 | 1 | 0 | 0 | 0 | 0 |
| T315l (IC50: >6400 nmol/L)‡ | 3 | 0 | 1 | NA | 1 | NA |
| P-loop (amino acids 248 to 256) | 10 (11) | 2 (4) | 5/10 (50) | 0/2 | 8/10 (80) | 0/2 |
| A-loop (amino acids 379 to 398) | 8 (9) | 0 | 4/8 (50) | NA | 7/8 (88) | NA |
| Other regions | 24 (26) | 9 (20) | 10/24 (42) | 3/9 (33) | 21/24 (88) | 6/9 (67) |
| . | Total* n/N (%) . | Patients with MCyR n/N (%) . | Patients with CHR n/N (%) . | |||
|---|---|---|---|---|---|---|
| Dasatinib; N = 92 . | High-dose imatinib; N = 46 . | Dasatinib . | High-dose imatinib . | Dasatinib . | High-dose imatinib . | |
| No mutations | 51 (55) | 35 (76) | 28/51 (55) | 12/35 (34) | 50/51 (98) | 32/35 (91) |
| Any mutation | 41 (45) | 11 (24) | 19/41 (46) | 3/11 (27) | 36/41 (88) | 6/11 (55) |
| Mutation associated with ≥5-fold increase in imatinib resistance | 25 (27) | 7 (15) | 12/25 (48) | 1/7 (14) | 21/25 (84) | 2/7 (29) |
| Specific BCR-ABL point mutations† | ||||||
| L248V | 2 | 0 | 1 | NA | 2 | NA |
| I418V | 1 | 1 | 1 | 1 | 1 | 1 |
| M351T (IC50: 880 nmol/L)‡ | 6 | 1 | 2 | 0 | 6 | 1 |
| F317L (IC50: 1050 nmol/L)‡ | 4 | 0 | 1 | NA | 4 | NA |
| G250E (IC50: 1350 nmol/L)‡ | 7 | 0 | 4 | NA | 6 | NA |
| H396R (IC50: 1750 nmol/L)‡ | 3 | 0 | 1 | NA | 2 | NA |
| F359V (IC50: 1825 nmol/L)‡ | 4 | 2 | 1 | 1 | 4 | 1 |
| M244V (IC50: 2000 nmol/L)‡ | 9 | 3 | 5 | 0 | 8 | 1 |
| Y253H (IC50: >6400 nmol/L)‡ | 1 | 1 | 0 | 0 | 0 | 0 |
| T315l (IC50: >6400 nmol/L)‡ | 3 | 0 | 1 | NA | 1 | NA |
| P-loop (amino acids 248 to 256) | 10 (11) | 2 (4) | 5/10 (50) | 0/2 | 8/10 (80) | 0/2 |
| A-loop (amino acids 379 to 398) | 8 (9) | 0 | 4/8 (50) | NA | 7/8 (88) | NA |
| Other regions | 24 (26) | 9 (20) | 10/24 (42) | 3/9 (33) | 21/24 (88) | 6/9 (67) |
n/N, number responding/number treated; CHR, complete hematologic response; MCyR, major cytogenetic response; NA, not applicable.
Twelve of 150 patients had no mutational analysis performed at baseline (dasatinib, 9; high-dose imatinib, 3).
Reported in 2 or more patients.
Cellular IC50 values taken from O'Hare et al, 2005.
Postcrossover analyses.
As a result of the trial design, 54 patients (15 initially randomized to treatment with dasatinib and 39 originally randomized to high-dose imatinib) crossed over to the alternate therapy.
Median time to crossover was 28 weeks (range, 1-56 weeks) for patients initially receiving dasatinib and 13 weeks (range, 1-68 weeks) for patients treated with high-dose imatinib. The median duration of treatment postcrossover was 48 weeks (range, 3-76 weeks) for the 39 patients who crossed over to dasatinib and 16 weeks (range, 4-52 weeks) for the 15 patients who went on to receive high-dose imatinib. Postcrossover, 17 of the 38 evaluable patients (45%; 95% CI, 28.6% to 61.7%) who received dasatinib subsequently achieved a MCyR after an initial treatment failure with imatinib. In contrast, 2 of the 13 evaluable patients (15%; 95% CI, 1.9% to 45.4%) who were originally randomized to dasatinib and who crossed over to high-dose imatinib attained a MCyR (P = .063).
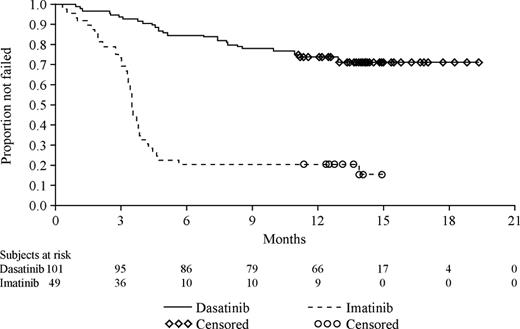
Time to treatment failure
The Kaplan-Meier curves of time to treatment failure illustrate that patients receiving high-dose imatinib therapy failed their respective treatment earlier than patients who received dasatinib (Figure 2). Treatment failure was documented at 6 months for 15 of 101 patients (15%) in the dasatinib treatment group and 37 of 49 patients (76%) randomized to high-dose imatinib. With a median follow up of 15 months, failure was documented for 28% of patients randomized to dasatinib treatment and in 82% of patients randomized to high-dose imatinib (Figure 2). The median time to treatment failure was not reached for dasatinib and was 3.5 months (95% CI, 3.3 to 3.8 months) for high-dose imatinib. This difference between the 2 treatment groups was highly statistically significant and represented an 84% relative risk reduction in favor of the dasatinib cohort (hazard ratio, 0.16; 95% CI, 0.10 to 0.26; P < .001).
Progression-free survival
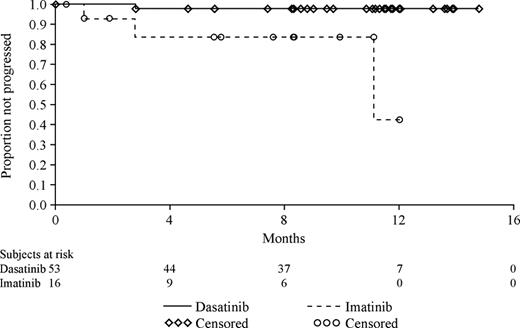
Progression-free survival also favored dasatinib therapy (Figure 3) with a highly statistically significant difference and a corresponding risk reduction of 86% relative to high-dose imatinib (hazard ratio, 0.14; 95% CI, 0.05 to 0.40; P < .001).
Safety and tolerability
The safety and tolerability of dasatinib and high-dose imatinib were consistent with the known safety profiles of these 2 agents derived from previous studies. The majority of AEs in both treatment groups were mild or moderate in intensity and resolved spontaneously or with supportive care. Although the respective drug profiles differ, grade 3 or 4 nonhematologic toxicity in this study was minimal in both treatment groups (Table 6)
Treatment-related nonhematologic adverse events (%) reported by 10% or more of patients from either treatment group
| Adverse event . | Dasatinib; n = 101 . | High-dose imatinib; n = 49 . | ||
|---|---|---|---|---|
| Any grade . | Grade 3-4* . | Any grade . | Grade 3-4* . | |
| Fluid retention | 30 | 5 | 45 | 0 |
| Superficial edema | 15 | 0 | 42 | 0 |
| Edema peripheral | 10 | 0 | 20 | 0 |
| Face edema | 4 | 0 | 10 | 0 |
| Pleural effusion | 17 | 4 | 0 | 0 |
| Other nonhematologic events | ||||
| Diarrhea | 35 | 2 | 29 | 2 |
| Fatigue | 30 | 2 | 22 | 4 |
| Headache | 25 | 2 | 10 | 2 |
| Nausea | 24 | 0 | 33 | 0 |
| Dyspnea | 21 | 4 | 4 | 0 |
| Rash | 17 | 0 | 14 | 0 |
| Pyrexia | 14 | 0 | 10 | 0 |
| Anorexia | 13 | 0 | 8 | 0 |
| Asthenia | 13 | 0 | 4 | 0 |
| Vomiting | 9 | 0 | 24 | 0 |
| Pain in extremity | 7 | 0 | 10 | 2 |
| Weight increased | 5 | 0 | 10 | 0 |
| Muscle spasms | 2 | 0 | 12 | 0 |
| Adverse event . | Dasatinib; n = 101 . | High-dose imatinib; n = 49 . | ||
|---|---|---|---|---|
| Any grade . | Grade 3-4* . | Any grade . | Grade 3-4* . | |
| Fluid retention | 30 | 5 | 45 | 0 |
| Superficial edema | 15 | 0 | 42 | 0 |
| Edema peripheral | 10 | 0 | 20 | 0 |
| Face edema | 4 | 0 | 10 | 0 |
| Pleural effusion | 17 | 4 | 0 | 0 |
| Other nonhematologic events | ||||
| Diarrhea | 35 | 2 | 29 | 2 |
| Fatigue | 30 | 2 | 22 | 4 |
| Headache | 25 | 2 | 10 | 2 |
| Nausea | 24 | 0 | 33 | 0 |
| Dyspnea | 21 | 4 | 4 | 0 |
| Rash | 17 | 0 | 14 | 0 |
| Pyrexia | 14 | 0 | 10 | 0 |
| Anorexia | 13 | 0 | 8 | 0 |
| Asthenia | 13 | 0 | 4 | 0 |
| Vomiting | 9 | 0 | 24 | 0 |
| Pain in extremity | 7 | 0 | 10 | 2 |
| Weight increased | 5 | 0 | 10 | 0 |
| Muscle spasms | 2 | 0 | 12 | 0 |
No grade 4 adverse event was seen in either treatment group.
As a result of the higher discontinuation rate from high-dose imatinib (AEs leading to discontinuation were neutropenia [2 patients]; and thrombocytopenia, abdominal pain, diarrhea, vomiting, fatigue, pain, joint effusion, and blister [all one patient each]), median exposure to imatinib and dasatinib differed. This imbalance in treatment exposure needs to be taken into consideration when comparing AE incidence rates between the 2 groups.
The incidence of superficial edema or fluid retention (all grades) was less common in patients receiving dasatinib therapy (15% and 30%, respectively) than in imatinib-treated patients (42% and 45%, respectively). Pleural effusion was diagnosed in 17% of dasatinib-treated patients and 0% in the high-dose imatinib group; no grade 4 pleural effusions were reported and 4 grade 3 cases were observed (4%). Pleural effusions were successfully managed with dasatinib dose interruption, diuretics, and/or pulse steroid therapy. Cytopenias were more common and more severe in the dasatinib treatment group (grade 3-4 neutropenia: 61% versus 39%; grade 3-4 thrombocytopenia: 56% versus 14%); these were reversible and manageable with dose adjustments. Transfusions of packed red blood cells (23% versus 12%) and platelets (14% versus 0%) were more frequently required among patients receiving dasatinib therapy.
Dose interruptions were required in 84 patients (83%) receiving dasatinib therapy (primarily attributable to hematologic toxicity [61%]), dose reductions were noted in 67 patients (66%), and dose escalations were introduced for 33 patients (33%). In patients treated with high-dose imatinib, dose interruptions were necessary in 16 patients (33%) and dose reduction was required in 5 patients (10%). The difference in dose reductions was also impacted by the fact that reduction of the imatinib dose for toxicity to 600 mg was permitted only for patients who had not previously received 600 mg imatinib.
Discussion
Treatment of patients with CP-CML resistant to conventional imatinib doses is challenging. Treatment options are limited and have mostly been targeted at maintaining normal blood counts without achieving cytogenetic response. Despite limitations with the use of high-dose imatinib, this has become a widely investigated option for patients resistant to conventional imatinib doses. Clinical data to support this strategy are limited,2,7–9 especially if the patient has a BCR-ABL mutation or has acquired resistance to conventional doses of imatinib. Because the potency of BCR-ABL inhibition is associated with obtaining deeper responses in patients who respond to high-dose imatinib, one would predict that the increased potency of dasatinib would be the primary reason why a higher response rate could be expected in this patient population. Durable hematologic and cytogenetic responses have previously been demonstrated for dasatinib in imatinib-resistant or -intolerant CP-CML in an open-label study accruing 387 patients (START-C trial). Interim data from the first 186 patients showed a MCyR rate of 45%15 ; the MCyR rate was 31% in the imatinib-resistant patients (n = 127), and responses were also documented in patients who never achieved a cytogenetic response with imatinib.15
Results of the current study, in which dasatinib was evaluated relative to imatinib, suggest that dasatinib is more effective than high-dose imatinib in this CP-CML setting in patients who are resistant to conventional doses of imatinib. With a median follow up of 15 months, PFS was significantly prolonged with dasatinib therapy. Rates of MCyR favored dasatinib with a statistically significant 20% absolute difference between treatments. This difference between the 2 treatment groups was more evident in terms of CCyR, in which dasatinib was associated with a statistically significant >2-fold increase in response rates relative to high-dose imatinib. Molecular response rates also favored dasatinib and this difference was also statistically significant.
Dasatinib also proved to be the better therapeutic option across a number of subgroups. Phase II data predicted that high-dose imatinib would be ineffective in patients with no prior cytogenetic response to the conventional lower doses of imatinib therapy; this was also our finding. In contrast, dasatinib achieved a MCyR rate approaching 50%. Similarly, limited activity for high-dose imatinib would be expected in patients who failed or progressed on doses of 600 mg per day. Again, dasatinib proved to be the superior therapeutic option for this patient population. Conversely, in subgroups only minimally resistant to imatinib, little difference would be expected or was noted between the 2 treatment groups. The magnitude of the difference observed was reduced for patients who had failed or progressed on 400 mg imatinib per day. One might question whether the control arm, largely constituting patients who had received imatinib doses of 600 mg per day before entry, was particularly challenging. Patients with CP-CML who had developed resistance while being treated with doses of 400 to 600 mg per day of imatinib were eligible for the trial and there was no way of knowing in advance that approximately two-thirds of these patients would have received 600-mg doses before entry.
Treatment with dasatinib was effective in patients who were also clearly resistant to high-dose imatinib. Previously, prognoses for such patients have been extremely poor. In light of these findings, further consideration on the optimal timing for initiating dasatinib therapy in patients developing resistance to imatinib is warranted.
Several previous studies with imatinib have shown the prognostic significance of achieving early responses to treatment.1,25,26 With the incidence of imatinib resistance increasing, the earlier use of dasatinib could prove beneficial by promoting an early response, and thereby potentially improving the prognosis for the patient, and/or by avoiding the development of treatment resistance.
Several additional important observations were noted. The rate of cytogenetic response increased over time for both treatment groups, but this was more rapid for patients receiving dasatinib as opposed to high-dose imatinib (with CCyR rates of 40% and 16%, respectively). Of those who achieved a major cytogenetic response (high-dose imatinib, 16 patients; dasatinib, 53 patients), there were 3 progressions in the high-dose imatinib treatment group and 1 progression in the dasatinib group.
The AE profile seen for patients treated with dasatinib was in line with previous phase I and II studies, although the overall evaluation noted a number of differences between dasatinib and high-dose imatinib. Dasatinib was associated with a greater degree of myelosuppression, in particular thrombocytopenia. The greater potency of dasatinib may contribute to this more profound myelosuppression attributable to rapid clearance of BCR-ABL expressing malignant hematopoietic cells. Unlike imatinib, dasatinib is not a substrate of the p-glycoprotein pump, and therefore higher concentrations of dasatinib might be achieved in hematopoietic progenitor cells. In patients who experienced myelosuppression, recovery occurred after brief dose interruptions or reductions, occasionally requiring transfusions.
Although fluid retention was more common with imatinib, its presentation was different, consisting primarily of superficial edema in patients receiving imatinib and pleural effusion with dasatinib. The underlying mechanism of the pleural effusion seen with dasatinib is unknown; the current favored hypothesis is inhibition of platelet-derived growth factor receptors. Early identification, temporary interruption of treatment, diuretic and/or pulse steroid use, and subsequent dasatinib dose reduction led to resolution in all cases.
Patient selection and treatment duration may have influenced the distribution and severity of the AEs reported. All patients had received pretreatment with imatinib, most at the 600-mg dose for extended periods of time. All patients had tolerated these doses, and therefore intolerance to high-dose imatinib was not to be expected with the accompanying increase in dose exposure in this study. Overall, it had been anticipated that the safety profile would favor treatment with high-dose imatinib because these patients were already known to tolerate imatinib, albeit at lower doses.
Many of the other events reported with dasatinib therapy could be attributed to either the underlying disease or concurrent medical conditions. Most events reported were grades 1 or 2 and resolved spontaneously. The low discontinuation rate as a result of drug intolerance from the dasatinib treatment group relative to the imatinib cohort and high rates of response postcrossover imply that dasatinib is both well tolerated and effective in this patient population.
Finally, the study demonstrated a key difference in the benefit-risk profile of the 2 drugs, favoring dasatinib therapy over high-dose imatinib. Clear differentiation was evident in terms of the time to treatment failure (Figure 2), a composite end point that incorporates both efficacy (lack of response, progression at any time) and safety end points (crossover for intolerance, treatment discontinuation), and provides a global assessment of the therapeutic index. Dasatinib was associated with a significant prolongation in the time to treatment failure relative to high-dose imatinib, illustrating the benefit of treatment with dasatinib in this group of patients.
This randomized study confirmed that treatment with dasatinib results in early and complete cytogenetic responses in patients with CP-CML resistant to imatinib at conventional doses of 400 to 600 mg. Dasatinib represents a safe and effective therapy for patients with CP-CML resistant to conventional doses of imatinib with improved cytogenetic and molecular response rates and progression-free survival relative to high-dose imatinib (800 mg). Based on these data, dasatinib appears to be more active than high-dose imatinib for patients who experience imatinib failure.
The online version of this manuscript contains a data supplement.
An Inside Blood analysis of this article appears at the front of this article.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by research funding from Bristol-Myers Squibb.
Authorship
Contribution: H.K. designed and performed research and analyzed data; R.P., N.H., P.R., J.H., S.J., N.K., T.M., A.S., A.H., A.Z., A.G., J.R., and T.H. performed research; T.R. performed research and analyzed data; A.C. analyzed the data and wrote the paper; and N.S. designed and performed research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: All authors received financial support from Bristol-Myers Squibb. A.C. is an employee of Bristol-Myers Squibb; T.R. has received research funding from Bristol-Myers Squibb and T.H. has received research funding from Novartis.
Correspondence: Hagop Kantarjian, MD, Department of Leukemia, Unit 428, University of Texas M.D. Anderson Cancer Center, PO Box 301402, Houston, TX 77230-1402; e-mail: hkantarj@mdanderson.org.