Abstract
The distinct combination of homing receptors such as selectins, chemokine receptors, and integrins directs the migration of lymphocytes throughout the body. Upon activation lymphocytes irreversibly switch their set of homing receptors, now guiding them to entirely different destinations. Here we report that exposure of naive B cells to the microenvironment of the peritoneal cavity modulates their migration propensities in the absence of antigenic stimulation. B1 and B2 cells isolated from the peritoneal cavity reenter this compartment more efficiently compared with splenic follicular B cells. Moreover, when kept in the peritoneal cavity splenic follicular B cells gain such increased capability to reenter this compartment. These altered migratory capacities are reflected by an up-regulation of the chemokine receptors CXCR4 and CXCR5 and β7 integrin by the peritoneum-experienced splenic B cells, among which CXCR5 is instrumental in directing B cells into the peritoneal cavity. Moreover, intraperitoneal transfer of plasma blasts favors their migration into the small intestine presumably before class switch recombination occurs, demonstrating that a reconfigured transient migration pattern is not restricted to naive cells. In conclusion, these data demonstrate a hitherto unrecognized role for tissue-specific cues, altering the migratory capacity of B1, naive B2, as well as antigen-experienced B2 cells.
Introduction
Naive B cells continuously migrate through lymphoid compartments. This process is a prerequisite for the efficient induction of humoral immune responses. A considerable amount of research has already been carried out on the molecular factors that underlie these migratory routes and their ability to mediate lymphocyte immigration via high endothelial venules (HEVs). They are described as a cascade of consecutive events known as the multistep model. The distinct steps in this cascade are distinguished by the interaction of homing molecules expressed on the surface of lymphocytes with their ligands present on HEVs.1,2 In case of lymphocyte immigration into peripheral lymph nodes in particular, the interaction of L selectin (CD62L) expressed on lymphocytes with sialyl-LewisX synthesized by HEVs enables the cells to roll along the endothelium. This in turn allows the chemokines CCL19 and CCL21 displayed on the luminal HEV surface to activate the chemokine receptor CCR7 on the rolling lymphocytes, thereby inducing a high-affinity conformation of αL/β2 integrin (LFA-1). Interaction of the high-affinity integrin with its ligands leads to a stable arrest of lymphocytes on the endothelium allowing them to enter the lymph nodes.3
The principal framework of the multistep model appears to adequately describe lymphocyte immigration not only into peripheral lymph nodes but also into other lymphoid and nonlymphoid tissues. However, the particular usage of homing molecules and their ligands varies between different compartments. For example, homing of B cells into Peyer patches (PPs) strongly depends on the chemokine receptor CXCR5 4,5 and β7 integrins,6 whereas homing into skin-draining peripheral lymph nodes seems to be independent of CXCR5.4 Consistently, the CXCR5 ligand CXCL13 and the β7 integrin ligand MAdCAM-1 are both present on HEVs located within PP follicles but not peripheral lymph nodes.4,5
Upon activation B cells differentiate into plasma blasts and profoundly change their repertoire of homing molecules. In this process the plasma blasts are excluded from reentering into secondary lymphoid organs and gain access to effector sites. The specific pattern of homing molecules acquired during activation essentially depends on the site of activation. For example, B cells activated in peripheral lymph nodes will secrete antibodies of the IgG isotype and express CXCR4, CXCR3, and P selectin, allowing them to enter inflammatory sites as well as the bone marrow. In contrast, B cells activated in the PP or the gut-draining mesenteric lymph nodes preferentially secrete IgA and express α4β7 integrin and the chemokine receptor CCR9, targeting these cells to the small intestinal lamina propria.7
In addition to plasma cells differentiated from conventional B cells (B2 cells), B1 cells also contribute to humoral immunity.8 B1 cells are particularly prominent in body cavities such as the peritoneal and pleural cavities but are almost absent in secondary lymphoid organs. They can be phenotypically distinguished from B2 cells by low expression levels of CD23 and IgD but by a high rate of IgM. The major function of B1 cells is the production of natural serum IgM, thereby providing a first-line defense against pathogens. Additionally, B1 cells residing in the peritoneal cavity are able to generate intestinal IgA-secreting plasma cells, although the relevance of this process is still unclear.9–11
The anatomic site of B-cell entry into the peritoneal cavity has been suggested to be the omentum. B cells might enter this bilayered sheet of mesothelial cells directly from the blood across vessels located within lymphocyte-rich follicular structures known as “milky spots”.12 B1 and B2 cells do not irrevocably reside in the peritoneal cavity but are able to leave this compartment, resulting in considerable exchange of lymphocytes between body cavities and secondary lymphoid organs.12–14 Similar to its function in B-cell migration into the peritoneal cavity, the omentum has also been suggested to mediate exit of B cells from this compartment.14 In addition, lymphocytes can exit the peritoneal cavity via afferent lymphatics reaching the draining parathymic lymph nodes.15 Notably, these 2 alternatives might not represent mutually exclusive mechanisms, but their individual contribution might depend on distinct cell subsets as well as on the activation status of the cells.
Migration of B cells into the peritoneal cavity depends on the chemokine CXCL13. CXCL13-deficient mice as well as mice lacking the cognate receptor, CXCR5, exhibit decreased numbers of B cells in the peritoneal cavity, and adoptively transferred B cells fail to enter the peritoneal cavity in CXCL13-deficient recipients.12,16 Toll-like receptor induced down-regulation of integrins, and CD9 has been suggested to initiate B1-cell exit from the peritoneal cavity.14 Furthermore, sphingosine-1-phosphate receptors required for lymphocyte exit from lymph nodes have also been reported to mediate exit of lymphocytes from the peritoneal cavity.17 This suggests that homeostasis of the peritoneal cavity is regulated at the step of immigration into this site as well as exit from the peritoneal cavity.
In this study we demonstrate that a transit of B cells through the peritoneal cavity modifies their migratory capacity. Generally, peritoneal B1 and B2 cells migrate more efficiently into the peritoneal cavity after intravenous transfer compared with splenic B2 cells. Likewise, splenic B2 cells adoptively transferred into the peritoneal cavity gain the ability to reenter this site. Concomitantly, homing molecules expressed by B cells, including CXCR4, CXCR5, and β7 integrin, adjust in response to local cues present in the peritoneal environment. Furthermore, we show that an intraperitoneal transfer of B2 cells leads to a much higher probability to migrate into the small intestine as compared with intravenous transfer. This suggests that the ability of peritoneal B1 cells to migrate into the intestine might be due to environmentally instructed cues rather than to intrinsic properties of the cell.
Materials and methods
Mice
C57BL/6 Ly5.2, C57BL/6 Ly5.1, and CXCR5-deficient mice18 were bred in the Central Animal Facility of Hannover Medical School under specified pathogen-free conditions and were used at the age of 8 to 12 weeks. C57BL/6 mice were additionally purchased from Charles River (Sulzfeld, Germany). CXCR5-deficient mice were backcrossed for 15 generations to C57BL/6 mice. All animal experiments have been performed in accordance with institutional guidelines and have been approved by the local government.
Antibodies
The following antibodies were used: anti-CD18–FITC, anti-CD23–APC, anti-CD23–PE, and anti-IgA–FITC (Caltag, Burlingame, CA), anti-CD45.1–biotin (Chemicon, Temecula, CA), anti-α4β7 integrin, anti-β7 integrin, anti-B220–PE, anti-B220–PerCP, anti-CD21/CD35–FITC, anti-CD29, anti-CD45.1–PE, and anti-IgM–biotin (BD Biosciences, San Jose, CA), and anti-CD19–PE (SouthernBiotech, Birmingham, AL). Anti-B220 (clone TIB146) and anti-IgM (HB88) antibodies were provided by Elisabeth Kremmer (GSF München, Munich, Germany) and conjugated to Cy5 or biotin as recommended by the manufacturer (Amersham, Arlington Heights, IL). Anti-CCR6 (R&D Systems, Minneapolis, MN), anti-CCR5 (clone MC68; kindly provided by M. Mack, University of Regensburg, Germany), anti-CCR9 (clone 7E7 19 ), anti-CXCR4 (clone 2B11 20 ), and anti-CXCR5 (clone 2G8 18 ) were detected using mouse anti–rat-Cy5 (Jackson ImmunoResearch). Anti–human EBNA1 monoclonal antibody (mAb) (E1-BS 1H4) or anti–human CCR7 (clone 3D12-1) was used as an isotype control for anti–mouse CCR5, CCR9, CXCR4, and CXCR5. CCR7 and CCR10 expression was detected by a CCL19-hIgG1 (described previously21 ) or CCL27-hIgG1 fusion protein (produced by O.P. and S.B.) followed by goat anti–human-Cy5 (Jackson ImmunoResearch, West Grove, PA). Biotinylated antibodies were recognized by streptavidin coupled to PerCP (BD Biosciences), Cy3 (Jackson ImmunoResearch), or Alexa405 (Molecular Probes, Eugene, OR).
Cell isolation and flow cytometry
Mice were killed by CO2 inhalation, and lymphoid organs were dissected and used for preparing single-cell suspensions by mincing through a 45 μm nylon mesh. Erythrocytes were lysed when necessary using a buffer containing ammonium chloride (1.7 M), potassium hydrogen carbonate (100 mM), and EDTA (1 mM). Intestinal lamina propria cells were isolated as described previously.22 For isolation of peritoneal cells the peritoneal cavity was flushed with 10 mL ice-cold PBS/3% FCS. Cells were stained with the antibodies described under “Antibodies,” and analysis was performed using a FACSCalibur or an LSRII (BD Biosciences). Data were analyzed using WinList5.0 (Verity Software House) or FlowJo (Tree Star, Ashland, OR).
Immunofluorescence microscopy
Intestines were embedded in OCT on dry ice, and 6 μm cryosections were prepared. Sections were air dried, fixed for 10 minutes in ice-cold acetone, and subsequently stained with antibodies against the indicated markers as described previously.22 Images were visualized using a motorized Axiovert 200M microscope and Axiovision software (Carl Zeiss, Jena, Germany) or an IX81 microscope and analySISD software (Olympus, Hamburg, Germany).
Adoptive transfers
Cells were labeled with 5-(and 6-) carboxyfluorescein diacetate succinimidyl ester (CFSE) or 5-(and 6)-carboxytetramethylrhodamine (TAMRA) (both from Molecular Probes). In brief, 2 × 106 cells per milliliter were preincubated for 30 minutes in RPMI 1640 medium containing 25 mM HEPES at 37°C. CFSE or TAMRA was added to a final concentration of 0.1 μM (CFSE) or 15 μM (TAMRA) for 10 minutes, followed by washing the cells in ice-cold PBS containing 3% FCS. For competitive adoptive transfer experiments, cell suspensions were adjusted to equal numbers of B cells labeled with each fluorochrome. Cell suspensions were injected intraperitoneally and/or into the tail vein of 8- to 12-week-old syngeneic recipients. Selective effects of the fluorescent staining procedure on the migration of cells were excluded by using both combinations of CFSE- and TAMRA-labeled cells throughout. In some experiments the subset composition of B1 and B2 cells in the injected mixtures was determined by flow cytometry, allowing us to calculate the migration efficacy of individual B-cell subsets. In some experiments cells were transferred into mice that were pretreated with 2.5% dextran sulfate sodium (DSS) (MP Biomedicals, Eschwege, Germany) in their drinking water for 4 days before transfer.
ELISPOT assay
Cholera toxin (CT)–specific plasma cells were detected by enzyme-linked immunospot (ELISPOT) assays. In brief, MultiScreen HTS plates (Millipore, Billerica, MA) were coated with 1 μg/mL CT in PBS overnight at 4°C, washed twice with RPMI 1640 medium supplemented with 2% FCS, and blocked for 2 hours at 37°C with RPMI 1640 medium containing 10% FCS. A total of 106 cells per well were seeded in triplicates in a final volume of 100 μL complete RPMI 1640 medium supplemented with 10% FCS, 20 mM HEPES, 100 U/mL penicillin G, and 100 μg/mL streptomycin per well. Cells were incubated at 37°C in 5% CO2 overnight. The plates were washed twice with distilled water followed by 2 washes with PBS/5% FCS. Detection of CT-specific IgA or IgM plasma cells was performed using peroxidase-conjugated anti-IgA antibody (Caltag) or biotinylated anti-IgM antibody (BD Biosciences) followed by peroxidase-labeled streptavidin (Jackson ImmunoResearch) and with AEC substrate (3-amino-9-ethylcarbazole; Sigma, St Louis, MO). Color development was stopped by washing with running water. The plates were examined under a stereomicroscope.
Results
Peritoneal B cells efficiently reenter the peritoneal cavity compared with splenic B cells
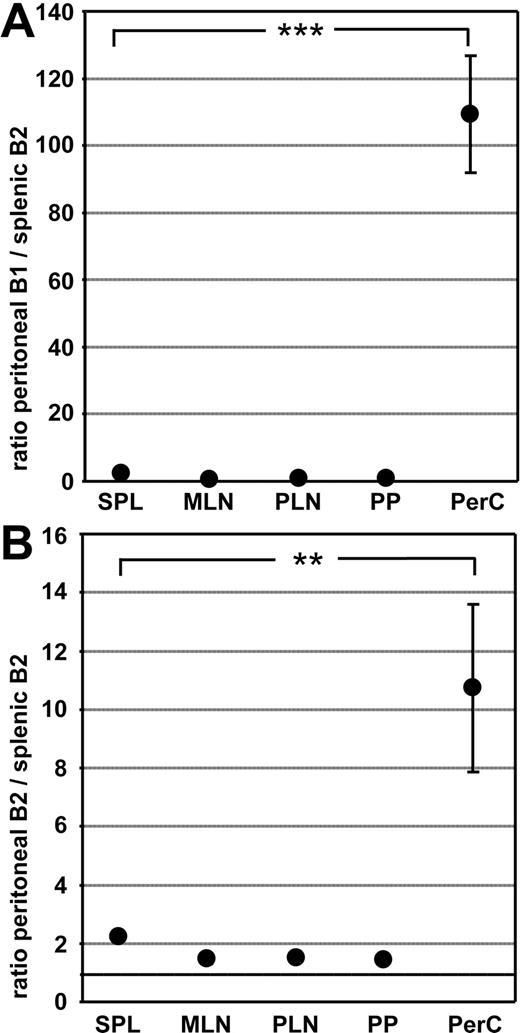
In the mouse the peritoneal cavity harbors a prominent population of B1 cells that can be identified as CD19+B220lowCD23lowCD43+ cells expressing high levels of IgM. However, besides B1 cells, B2 cells (CD19+B220hiCD23hiCD43−) are also present. In contrast with B1 cells, B2 cells rapidly circulate throughout the peritoneal cavity, the spleen, and other lymphoid compartments.12 To compare the migratory properties of peritoneal B1 and B2 cells with splenic B2 cells, we performed competitive adoptive transfer experiments. Peritoneal and splenic B cells were differentially labeled, and mixtures containing equal numbers of B cells were transferred intravenously into syngeneic recipients. One day later the number of transferred cells recovered from various organs was analyzed and their phenotype determined by flow cytometry. The ratios of peritoneal B1 cells to splenic B2 cells and peritoneal B2 cells to splenic B2 cells were calculated and correlated to the ratio of these cell types in the injected mixture. In line with previous studies12,23 we observed that B1 cells isolated from the peritoneal cavity reentered this compartment much more efficiently compared with splenic B2 cells (Figure 1A). Similarly, B1 cells were favored in entering the pleural cavity, whereas no differences were observed in the ratio of peritoneal B1 to splenic B2 cells in any other compartment analyzed (data not shown and Figure 1A). Interestingly, we also found that peritoneal B2 cells entered the peritoneal cavity approximately 10-fold more efficiently compared with splenic B2 cells (Figure 1B). However, no differences were observed between splenic B2 cells and B cells isolated from peripheral or mesenteric lymph nodes in entering the peritoneal cavity (data not shown). These data indicate that B2 cells residing in the peritoneal cavity might use other homing molecules than B2 cells present in secondary lymphoid organs.
Peritoneal B1 and B2 cells efficiently reenter the peritoneal cavity following adoptive intravenous transfer. Peritoneal B cells and splenocytes were differentially labeled with CFSE and TAMRA, and mixtures containing equal numbers of B cells were injected intravenously into wild-type recipients. One day after transfer, the ratio of transferred peritoneal B1 cells to splenic B2 cells (A) and peritoneal B2 cells to splenic B2 cells (B) was determined in different compartments by flow cytometry. Cells were addressed as CD19+B220lowCD23− for B1 cells and CD19+B220highCD23+ for splenic B2 cells as well as peritoneal B2 cells. Circles indicate the mean of 9 mice analyzed in 3 independent experiments performed; error bars, SEM; SPL, spleen; MLN, mesenteric lymph nodes; PLN, peripheral lymph nodes; PP, Peyer patches; PerC, peritoneal cavity. Statistical differences are indicated for the mean values and variances separately as follows: *P < .05; **P < .01; ***P < .001.
Peritoneal B1 and B2 cells efficiently reenter the peritoneal cavity following adoptive intravenous transfer. Peritoneal B cells and splenocytes were differentially labeled with CFSE and TAMRA, and mixtures containing equal numbers of B cells were injected intravenously into wild-type recipients. One day after transfer, the ratio of transferred peritoneal B1 cells to splenic B2 cells (A) and peritoneal B2 cells to splenic B2 cells (B) was determined in different compartments by flow cytometry. Cells were addressed as CD19+B220lowCD23− for B1 cells and CD19+B220highCD23+ for splenic B2 cells as well as peritoneal B2 cells. Circles indicate the mean of 9 mice analyzed in 3 independent experiments performed; error bars, SEM; SPL, spleen; MLN, mesenteric lymph nodes; PLN, peripheral lymph nodes; PP, Peyer patches; PerC, peritoneal cavity. Statistical differences are indicated for the mean values and variances separately as follows: *P < .05; **P < .01; ***P < .001.
CXCR5 is required for peritoneal B cells to enter body cavities
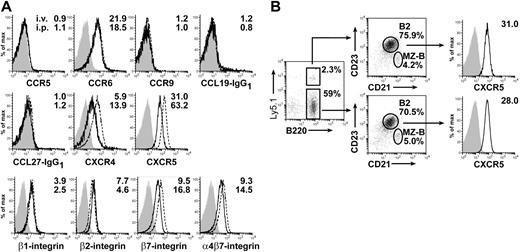
The chemokine CXCL13 plays an important role in the migration of B cells into the peritoneal cavity. B cells fail to enter the peritoneal cavity after intravenous transfer in CXCL13-deficient mice.12 We thus analyzed the expression of the CXCL13 receptor CXCR5 on different B-cell populations. Indeed, we observed that peritoneal B1 cells express higher levels of CXCR5 compared with peritoneal B2 cells (Figure 2A, left).12,24 Furthermore, splenic B-cell populations also differed in their CXCR5 expression with marginal zone B (MZ-B) cells showing a higher CXCR5 level than splenic B2 cells (Figure 2A, right). However, most strikingly, peritoneal B1 and B2 cells consistently showed higher CXCR5 expression compared with all splenic B-cell populations (Figure 2A), suggesting that enhanced CXCR5 expression might favor the efficient migration of B cells into the peritoneal cavity.
CXCR5 is differentially expressed by different B-cell populations and is required for migration of B cells into Peyer patches and the peritoneal cavity. (A) CXCR5 expression was determined for peritoneal B1 cells (CD19+B220lowCD23−), peritoneal B2 cells (CD19+B220highCD23+), splenic MZ-B cells (CD19+CD23lowCD21high), and splenic B2 cells (CD19+CD23+CD21int). Numbers indicate the ratio of the mean fluorescence intensity and the corresponding isotype control staining. Light gray–shaded areas represent isotype controls for the cell populations indicated. (B) The homing efficiency of splenocytes isolated from wild-type and CXCR5-deficient mice was determined by competitive adoptive transfer experiments. One day after adoptive transfer, the ratio of wild-type to CXCR5-deficient cells (circles) was determined in different compartments. Results depicted are representative of 1 of 3 experiments performed with 4 to 6 recipient mice each. Error bars represent the SEM. Statistical differences are indicated for the mean values and variances separately as follows: *P < .05; **P <.01; ***P < .001.
CXCR5 is differentially expressed by different B-cell populations and is required for migration of B cells into Peyer patches and the peritoneal cavity. (A) CXCR5 expression was determined for peritoneal B1 cells (CD19+B220lowCD23−), peritoneal B2 cells (CD19+B220highCD23+), splenic MZ-B cells (CD19+CD23lowCD21high), and splenic B2 cells (CD19+CD23+CD21int). Numbers indicate the ratio of the mean fluorescence intensity and the corresponding isotype control staining. Light gray–shaded areas represent isotype controls for the cell populations indicated. (B) The homing efficiency of splenocytes isolated from wild-type and CXCR5-deficient mice was determined by competitive adoptive transfer experiments. One day after adoptive transfer, the ratio of wild-type to CXCR5-deficient cells (circles) was determined in different compartments. Results depicted are representative of 1 of 3 experiments performed with 4 to 6 recipient mice each. Error bars represent the SEM. Statistical differences are indicated for the mean values and variances separately as follows: *P < .05; **P <.01; ***P < .001.
Comparing the migration efficiency of CXCR5-deficient and wild-type B cells in the same experimental setup as described under “Peritoneal B cells efficiently reenter the peritoneal cavity compared with splenic B cells,” we observed that CXCR5-deficient cells showed severely reduced migration into the peritoneal cavity and PPs (Figure 2B).4 Consistently, it was described previously that CXCR5-deficient mice possess severely reduced numbers of peritoneal B cells.16
Splenic B cells adapt to the environment of the peritoneal cavity by up-regulation of CXCR4, CXCR5, and β7 integrins
As described under “Peritoneal B cells efficiently reenter the peritoneal cavity compared with splenic B cells,” peritoneal B2 cells differ in their migration behavior and CXCR5 expression from splenic B2 cells. Because the B2-cell populations at these 2 different sites represent an interchanging common pool of B2 cells, we speculated whether the environment of the peritoneal cavity might influence the expression of homing molecules on B2 cells. To test this hypothesis we adoptively transferred congenic Ly5.1+ splenocytes intravenously or intraperitoneally into separate wild-type mice. After 2 days the cells were re-collected from the peritoneal cavity and the spleen, and the expression of different chemokine receptors or adhesion molecules was analyzed. We observed that transferred splenic B2 cells, addressed as CD45.1+B220+CD21intCD23hi, after intraperitoneal transfer and re-collected from the peritoneal cavity showed elevated expression of CXCR4, CXCR5, and β7 integrins compared with such cells isolated after intravenous transfer from the recipients' spleens (Figure 3A). In addition, we observed a slight down-regulation of β2 integrins following intraperitoneal transfer. No differences were observed concerning cell surface levels of CCR5, CCR6, CCR7, CCR9, CCR10, and β1 integrins. Moreover, intraperitoneally transferred splenic B2 cells re-collected from the recipients' spleens showed intermediate expression of CXCR5 compared with endogenous splenic B2 cells and transferred cells present in the peritoneal cavity (data not shown). The shifts in the expression levels mentioned above were not caused by experimental manipulation of cells, because intravenously transferred splenic B2 cells did not differ from endogenous splenic B2 cells (Figure 3B).
Residence of B cells in the peritoneal cavity influences their expression of homing molecules. Ly5.1+ splenocytes were injected into congenic Ly5.2+ wild-type recipients either intravenously or intraperitoneally 2 days after adoptive transfer, and cells were isolated from the recipients' spleens in case of intravenously transferred mice or from the peritoneal cavities in case of intraperitoneally transferred mice. (A) Surface expression of different chemokine receptors and adhesion molecules of transferred splenic B2 cells was analyzed by flow cytometry. Solid lines show expression on intravenously transferred cells reisolated from the recipients' spleens, dashed lines show expression on intraperitoneally transferred cells reisolated from the recipients' peritoneal cavities, and shaded areas show isotype control stainings for intravenously transferred cells reisolated from the recipients' spleen. Numbers indicate the ratio of the mean fluorescence intensity and the isotype control staining. (B) Adoptively transferred splenic B2 cells reisolated from the spleen do not differ from endogeneous B2 cells in their CXCR5 expression. Expression of CXCR5 was compared for intravenously transferred and endogeneous (Ly5.1−B220+CD23highCD21int) splenocytes. In the middle panels, numbers indicate the percentages of cells within the corresponding gates. In the right panels, numbers indicate the ratio of the mean fluorescence intensity for CXCR5 staining and the corresponding isotype control staining. Shaded areas show isotype control stainings. Representative data for 3 independent experiments with cells pooled from 4 to 8 mice each are shown.
Residence of B cells in the peritoneal cavity influences their expression of homing molecules. Ly5.1+ splenocytes were injected into congenic Ly5.2+ wild-type recipients either intravenously or intraperitoneally 2 days after adoptive transfer, and cells were isolated from the recipients' spleens in case of intravenously transferred mice or from the peritoneal cavities in case of intraperitoneally transferred mice. (A) Surface expression of different chemokine receptors and adhesion molecules of transferred splenic B2 cells was analyzed by flow cytometry. Solid lines show expression on intravenously transferred cells reisolated from the recipients' spleens, dashed lines show expression on intraperitoneally transferred cells reisolated from the recipients' peritoneal cavities, and shaded areas show isotype control stainings for intravenously transferred cells reisolated from the recipients' spleen. Numbers indicate the ratio of the mean fluorescence intensity and the isotype control staining. (B) Adoptively transferred splenic B2 cells reisolated from the spleen do not differ from endogeneous B2 cells in their CXCR5 expression. Expression of CXCR5 was compared for intravenously transferred and endogeneous (Ly5.1−B220+CD23highCD21int) splenocytes. In the middle panels, numbers indicate the percentages of cells within the corresponding gates. In the right panels, numbers indicate the ratio of the mean fluorescence intensity for CXCR5 staining and the corresponding isotype control staining. Shaded areas show isotype control stainings. Representative data for 3 independent experiments with cells pooled from 4 to 8 mice each are shown.
The peritoneal cavity environment commits naive B cells to enter the peritoneal cavity as well as Peyer patches
Subsequently we investigated whether the phenotypical changes on splenic B2 cells after intraperitoneal transfer translated into changed homing properties of these cells. CD45.1+ splenocytes were transferred intraperitoneally into congenic CD45.2 mice. Cells were reisolated from the peritoneal cavity after 3 hours or 18 hours and fluorescently labeled with CFSE or TAMRA. In parallel splenocytes were isolated from unmanipulated donors and fluorescently labeled discriminatively to the cells isolated from the peritoneal cavity. Mixtures of both cell populations containing equal numbers of splenic B2 cells were transferred intravenously into wild-type recipients. One day later frequencies of differentially labeled cells recovered from various lymphoid organs and the peritoneal cavity were determined. When comparing splenic cells conditioned for 3 hours inside the peritoneal cavity and unmanipulated spleen cells, both cell populations were present at roughly equal numbers in secondary lymphoid organs as well as in the blood. In contrast, splenic B2 cells conditioned in the peritoneal cavity reentered this compartment 1.5-fold more successfully as compared with unmanipulated splenic B2 cells. This effect was even more pronounced when using splenic cells that remained for 18 hours within the peritoneal cavity. In that case, conditioned splenic B2 cells migrated approximately 3-fold more efficiently into the peritoneal cavity compared with unmanipulated splenic B2 cells (Figure 4). In addition to that, we noticed that B cells influenced for 18 hours by the peritoneal environment migrated 1.5-fold more efficiently into the PPs compared with unmanipulated B cells. These effects were not caused by selective enrichment/loss of B-cell populations, because the ratio of MZ and splenic B2 cells present in the peritoneal cavity changed only slightly in favor of B2 cells. In summary, these data show that changes in the expression of homing molecules acquired during residence in the peritoneal cavity are accompanied by altered migratory propensities.
Residence of B cells in the peritoneal cavity enhances their capability to migrate back into the peritoneal cavity. Ly5.1+ splenocytes were injected intraperitoneally into congenic Ly5.2+ wild-type recipients. Three hours (○) or 18 hours (●) after transfer, cells were isolated from the recipients' peritoneal cavity, and their migratory potential was assessed in competitive adoptive transfer experiments compared with freshly isolated wild-type Ly5.1+ splenocytes. The injected mixtures of differentially labeled cells were adjusted to contain equal numbers of Ly5.1+ B cells. One day after transfer, the number of transferred cells influenced by the peritoneal cavity (treated cells) compared with untreated splenocytes was determined by flow cytometry. Data represent the mean of 8 and 4 recipient mice analyzed for 3 hours and 18 hours, respectively. Error bars represent the SEM; PBL, peripheral blood lymphocytes. Statistical differences are indicated for the mean values and variances separately as follows: *P < .05; **P < .01; ***P < .001.
Residence of B cells in the peritoneal cavity enhances their capability to migrate back into the peritoneal cavity. Ly5.1+ splenocytes were injected intraperitoneally into congenic Ly5.2+ wild-type recipients. Three hours (○) or 18 hours (●) after transfer, cells were isolated from the recipients' peritoneal cavity, and their migratory potential was assessed in competitive adoptive transfer experiments compared with freshly isolated wild-type Ly5.1+ splenocytes. The injected mixtures of differentially labeled cells were adjusted to contain equal numbers of Ly5.1+ B cells. One day after transfer, the number of transferred cells influenced by the peritoneal cavity (treated cells) compared with untreated splenocytes was determined by flow cytometry. Data represent the mean of 8 and 4 recipient mice analyzed for 3 hours and 18 hours, respectively. Error bars represent the SEM; PBL, peripheral blood lymphocytes. Statistical differences are indicated for the mean values and variances separately as follows: *P < .05; **P < .01; ***P < .001.
Intraperitoneal adoptive transfer favors migration of B cells into the small intestine
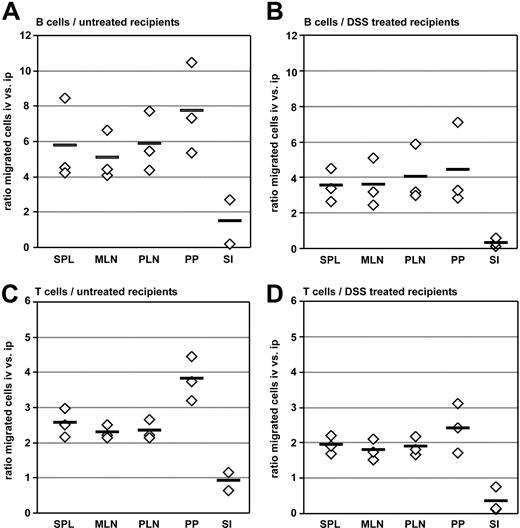
We subsequently analyzed further consequences of altered expression of homing molecules mediated by passage of B cells through the peritoneal cavity. To this aim we compared the ability of lymphocytes to access tissues depending on the route of application (ie, intraperitoneal or intravenous transfer). Equal numbers of differentially labeled splenic B2 cells were injected either intraperitoneally or intravenously into the same recipients. Five days after transfer the frequency of intraperitoneally versus intravenously injected cells was determined in different lymphoid and nonlymphoid organs. In all secondary lymphoid organs analyzed we observed higher numbers of intravenously transferred cells compared with intraperitoneally transferred cells (ratio of intravenously versus intraperitoneally transferred B cells: spleen, 5.7 ± 2.4; mesenteric lymph nodes, 5.1 ± 1.4; peripheral lymph nodes, 5.9 ± 1.7; PPs, 7.7 ± 2.6; 13 mice analyzed, Figure 5A). In contrast, migration of B cells into the small intestine was at least equally efficient for intraperitoneally transferred B cells compared with the intravenous route. Similar results were obtained when analyzing adoptively transferred T cells, although the differences between intraperitoneally and intravenously transferred cells were less pronounced compared with B cells (ratio of intravenously versus intraperitoneally transferred T cells: spleen, 2.6 ± 0.4; mesenteric lymph nodes, 2.3 ± 0.2; peripheral lymph nodes, 2.3 ± 0.3; PPs, 3.8 ± 0.6; small intestine, 0.9 ± 0.4; Figure 5C).
The route of transfer impacts cell migration into secondary lymphoid organs and the small intestine. Splenocytes of C57BL/6 mice were labeled with CFSE or TAMRA, and equal numbers of differentially labeled cells were injected intravenously and intraperitoneally into the same wild-type recipient. Five days after adoptive transfer, lymphoid organs and the small intestine were analyzed for the presence of transferred B cells (A-B) and T cells (C-D) by flow cytometry. Wild-type recipients were either untreated naive mice (A,C) or the recipients received drinking water containing 2.5% dextran sulfate sodium 4 days before adoptive transfer until 1 day after the transfer (B,D). Homing capacities are shown as ratios of intravenously transferred versus intraperitoneally transferred cells present in the respective compartments. ◇, the mean values observed in independent experiments performed with 4 to 5 recipients each; horizontal bars, the mean of all 3 experiments performed; SI, small intestine.
The route of transfer impacts cell migration into secondary lymphoid organs and the small intestine. Splenocytes of C57BL/6 mice were labeled with CFSE or TAMRA, and equal numbers of differentially labeled cells were injected intravenously and intraperitoneally into the same wild-type recipient. Five days after adoptive transfer, lymphoid organs and the small intestine were analyzed for the presence of transferred B cells (A-B) and T cells (C-D) by flow cytometry. Wild-type recipients were either untreated naive mice (A,C) or the recipients received drinking water containing 2.5% dextran sulfate sodium 4 days before adoptive transfer until 1 day after the transfer (B,D). Homing capacities are shown as ratios of intravenously transferred versus intraperitoneally transferred cells present in the respective compartments. ◇, the mean values observed in independent experiments performed with 4 to 5 recipients each; horizontal bars, the mean of all 3 experiments performed; SI, small intestine.
Different adhesion molecules are required for cell entry into tissues under homeostatic and inflammatory conditions. We thus compared the efficiency of intraperitoneally or intravenously transferred cells to access the small intestine under inflammatory conditions. To this aim recipient mice were treated with 2.5% dextran sulfate sodium (DSS) in their drinking water for 4 days before and 1 day after adoptive transfer. However, irrespective of DSS pretreatment, the intraperitoneal route of transfer favored migration of B and T cells into the small intestine to a similar extent compared with untreated recipients (Figure 5B,D). Indeed, in recipient mice exhibiting mild intestinal inflammation the ratio of intraperitoneally to intravenously transferred cells shifted in favor of the intraperitoneal transfer route, although the intravenous transfer was still more efficient compared with the intraperitoneal route (Figure 5B,D).
Intravenously and intraperitoneally transferred plasma blasts differ in their potential to enter the small intestine or the spleen
Employing immunohistology on small intestinal sections stained with anti-B220, anti-CD3, anti-IgM, and anti-IgA, we observed hardly any B220+IgA−CD3− staining cells displaying low expression of IgM in the intestinal lamina propria that would be indicative for naive B cells present in this compartment (data not shown). This suggests that naive B cells generally do not gain access to the murine small intestinal lamina propria but are restricted almost exclusively to follicular structures present in the gut wall such as PPs and solitary intestinal lymphoid tissue.22 In contrast, progeny of naive B cells that are plasma cells are abundantly present in the lamina propria, and besides B2 cells activated in gut-associated lymphoid tissue peritoneal B1 cells also have the potential to seed the intestinal lamina propria and to differentiate into intestinal IgA-secreting plasma cells.9 We thus speculated that activated splenic B2 cells might, similarly to the situation described above under “Intraperitoneal adoptive transfer favors migration of B cells into the small intestine” for naive B cells, gain the ability to home to the small intestine following intraperitoneal transfer. To test this we performed adoptive transfer experiments with activated B cells. Mice were immunized intraperitoneally with cholera toxin (CT), and 5 days later splenocytes were isolated and injected either intraperitoneally or intravenously into recipient mice. The number of CT-specific plasma cells in the spleen and intestinal lamina propria cell preparations was determined by ELISPOT assay 7 days later. We observed that after intravenous transfer 2- to 3-fold more plasma cells secreting CT-specific IgM were present in the spleen compared with intraperitoneally transferred recipients (Figure 6A). In contrast, following intraperitoneal transfer the number of plasma cells secreting CT-specific IgA in the small intestine was slightly higher compared with intravenously transferred mice (Figure 6B). We did not observe CT-specific plasma cells of IgM class in the intestine after intravenous transfer, and only few CT-specific IgA-secreting cells were present after intraperitoneal transfer in the spleen (data not shown). These observations indicate that, in addition to shaping the migratory properties of naive B cells, the peritoneal microenvironment might also affect the fate of activated B cells present at this site.
The route of transfer determines the fate of differentiating plasma blasts. (A-B) Five days after intraperitoneal immunization with CT, splenocytes were isolated and adoptively transferred into individual recipients either by intravenous or intraperitoneal injection. Seven days after adoptive transfer, the number of adoptively transferred CT-specific antibody-secreting cells (ASC) present in the recipients' spleens and small intestinal lamina propria was determined by ELISPOT assay. Bars depict the mean number of CT-specific IgM-secreting cells in the spleen (A) and CT-specific IgA-secreting ASCs in the lamina propria (B) observed in 6 mice in 2 independent experiments. (C-D) Ly5.1+ wild-type splenocytes were injected either intraperitoneally (▩) or intravenously (□) into separate congenic Ly5.2+ recipients. One day (C) and 5 days (D) after transfer, the recipients' intestines were embedded as “Swiss rolls,” and the number of transferred cells present in the lamina propria was determined by immunofluorescence microscopy. Sections were stained with anti-Ly5.1, anti-IgM, and anti-IgA antibodies. The frequency of cells is expressed as number of cells observed per millimeter of length of the intestine analyzed. For each time point and route of transfer, at least 20 cm of intestine obtained from 3 independent recipients was examined. Error bars represent SEM.
The route of transfer determines the fate of differentiating plasma blasts. (A-B) Five days after intraperitoneal immunization with CT, splenocytes were isolated and adoptively transferred into individual recipients either by intravenous or intraperitoneal injection. Seven days after adoptive transfer, the number of adoptively transferred CT-specific antibody-secreting cells (ASC) present in the recipients' spleens and small intestinal lamina propria was determined by ELISPOT assay. Bars depict the mean number of CT-specific IgM-secreting cells in the spleen (A) and CT-specific IgA-secreting ASCs in the lamina propria (B) observed in 6 mice in 2 independent experiments. (C-D) Ly5.1+ wild-type splenocytes were injected either intraperitoneally (▩) or intravenously (□) into separate congenic Ly5.2+ recipients. One day (C) and 5 days (D) after transfer, the recipients' intestines were embedded as “Swiss rolls,” and the number of transferred cells present in the lamina propria was determined by immunofluorescence microscopy. Sections were stained with anti-Ly5.1, anti-IgM, and anti-IgA antibodies. The frequency of cells is expressed as number of cells observed per millimeter of length of the intestine analyzed. For each time point and route of transfer, at least 20 cm of intestine obtained from 3 independent recipients was examined. Error bars represent SEM.
IgM-secreting plasma cells migrate into the small intestinal lamina propria after intraperitoneal but not intravenous transfer of splenic B cells
To further corroborate these findings we aimed to follow the fate of activated B cells transferred intraperitoneally versus intravenously more closely. However, analyzing intestinal lamina propria cells by flow cytometry is generally hampered by the presence of multiple B-cell–rich lymphoid follicles called solitary intestinal lymphoid tissue,22 and lamina propria–derived cell suspensions will generally be contaminated with such cells. We thus employed immunofluorescence microscopy to unequivocally locate transferred B cells in the lamina propria of recipient mice. Splenocytes isolated from untreated Ly5.1+ mice were adoptively transferred either intraperitoneally or intravenously into congenic Ly5.2+ recipients. Notably, such cell suspensions in addition to the vast majority of naive B cells will also contain a small number of activated B cells that might gain access to the intestinal lamina propria. Donor Ly5.1+ cells were enumerated in the recipients' lamina propria 1, 2, and 5 days after transfer. According to their expression of IgM and IgA, donor cells were further classified into 3 categories of IgMhighIgA−, IgM−IgAhigh, or IgMhighIgAhigh cells. Signals obtained with anti-IgM and anti-IgA antibodies were generally very bright and located to the cytoplasma, suggesting that these cells are almost exclusively plasma cells (data not shown). In line with this we noted that IgA+ cells did not express B220, whereas some IgM+ cells showed a low expression of this marker (data not shown). When comparing the 2 transfer routes, we observed that after intraperitoneal transfer slightly higher overall numbers of plasma cells were present in the small intestinal lamina propria after 1 and 5 days (Figure 6C-D). However, profound differences between both routes of transfer were detected when comparing the phenotypes of plasma cells. One and 5 days after intravenous transfer most of the plasma cells were IgMhighIgAhigh or IgM−IgAhigh, whereas IgMhighIgA− cells were not detectable. In contrast, IgMhighIgA− plasma cells could easily be identified in the lamina propria 1 day after intraperitoneal transfer (Figure 6C), whereas such cells were virtually absent at later time points (Figure 6D and data not shown), suggesting that the IgM single-positive cells present 1 day after intraperitoneal transfer might acquire IgA expression within the lamina propria during the subsequent days. In contrast, intravenously transferred cells already performed switch to IgA before entering the lamina propria (ie, only those plasma cells of IgA+ phenotype will gain access to the lamina propria).
In conclusion, these results demonstrate that the passage of B cells through the peritoneal cavity influences their migratory behavior. B cells modify their expression of homing molecules in the peritoneal cavity, thereby gaining more efficient access to this site upon intravenous transfer. Moreover, intraperitoneally transferred cells efficiently enter the intestinal lamina propria, suggesting that B cells at this site might have a special potential to generate intestinal plasma cells.
Discussion
B1 cells present in the peritoneal cavity of mice efficiently migrate back into this compartment after adoptive transfer into the circulation12,23 (Figure 1A), suggesting that these cells exhibit a distinct pattern of homing molecules facilitating their entry into this compartment. In this study, we show that not only peritoneal B1 cells but also B2 cells present in the peritoneal cavity migrate into the peritoneal cavity more efficiently. Although the molecular mechanisms underlying homing into the peritoneal cavity have not been resolved in detail, it is known that this process depends on the chemokine CXCL13.12 Here, we show that migration of B2 cells into the peritoneal cavity, like that of B1 cells, requires a functional chemokine receptor CXCR5, which is the only known receptor for CXCL13. Consequently, the number of peritoneal B cells is severely reduced in CXCR5-deficient mice.16 Among cells isolated by peritoneal lavage, macrophages but no other peritoneal cells were found to produce CXCL13. Moreover, cells isolated from the mesentery, diaphragm, and the body wall failed to express CXCL13 (Ansel et al,12 Ha et al,14 Ito et al,23 and S. B. and O. P., unpublished observations, March 2003). However, at other body sites CXCL13 is amply produced by some stromal cells, and in the omentum CXCL13 radiation-resistant nonhematopoietic cells have been reported to express CXCL13. Thus, we cannot formally exclude that additional CXCL13-producing cells might contribute to CXCL13 production in the peritoneal cavity. The production of CXCL13 by peritoneal macrophages has been suggested to be instrumental for the recruitment of B1 cells into the peritoneal cavity. In a mouse model of systemic lupus erythematosus a decline in the number of peritoneal macrophages during aging correlates with less efficient migration of B1 cells into the peritoneal cavity after adoptive intravenous transfer.23 Furthermore, we observed that CXCR5-deficient splenic B2 cells are retained less efficiently in the peritoneal cavity after intraperitoneal transfer, suggesting that CXCL13 production by peritoneal macrophages in addition might serve retaining cells in the peritoneal cavity (data not shown).
B-cell homing into PPs resembles homing into the peritoneal cavity in its dependency on CXCR5 signaling. However, both mechanisms are clearly different, because residence of splenic B2 cells in the peritoneal cavity strongly enhances their capacity to reenter this compartment, whereas only a slightly enhanced migration into PPs was observed for such cells. This suggests that CXCR5 signaling is a prerequisite for homing into the peritoneal cavity. Among the homing molecules analyzed, in addition to CXCR5 we also observed up-regulation of CXCR4 and β7 integrins. The CXCR4 ligand CXCL12 is expressed in the omentum, the anatomic site of cell entry into the peritoneal cavity, and thus might be involved in mediating cell entry. However, further experiments are required to investigate the function of these molecules for B-cell homing into the peritoneal cavity. The impact of the peritoneal environment on B cells was also shown in a recent study by Hastings et al. When injected intraperitoneally, splenic B2 cells acquired phenotypic and functional characteristics of B1b cells including increased expression of Mac-1 and CD43 as well as in vitro IgM secretion.25 Thus, residence of B cells in the peritoneal cavity profoundly changes their phenotype and function including their migratory properties in the absence of antigenic stimulation and activation. Importantly, all splenic B2 cells homogeneously shift in their expression of the chemokine receptors CXCR4 and CXCR5 as well as the β7 integrins upon transfer into the peritoneal cavity. Thus, the entire population of B2 cells that mostly consists of naive B cells is equally affected, ruling out that the observed changes in homing receptor expression are a consequence of antigenic stimulation.
In contrast to their efficient migration into the peritoneal cavity, naive B cells are largely excluded from entering the intestinal lamina propria. However, B cells activated in the gut-associated lymphoid tissues rapidly gain the ability to enter this compartment. Performing adoptive transfer experiments with splenic B2 cells isolated from untreated donor mice, we noticed that, similarly to the situation observed for naive B cells reentering the peritoneal cavity, the intraperitoneal transfer also favored migration into the lamina propria compared with the intravenous route. Notably, the number of such transferred cells detectable in the lamina propria did not show a linear increase over time, indicating that only a minor fraction of the transferred cells, most likely representing activated B cells, will seed the intestinal lamina propria. Consistently, we observed that after transfer of splenic B2 cells most cells detectable in the lamina propria showed high expression of IgM and/or IgA, suggesting that these cells are antibody-secreting plasma cells. Thus, in addition to their efficient migration into the peritoneal cavity, B cells transferred into this compartment have an enhanced propensity to enter the small intestinal lamina propria compared with secondary lymphoid organs. These observations are further corroborated by the finding that intraperitoneal transfer of spleen cells isolated from immunized donor mice yielded high numbers of antigen-specific IgA-secreting cells in the intestine compared with intravenously transferred cells. Presumably, such gut-seeking plasma blasts acquired their pattern of homing receptors under the influence of the peritoneal cavity. Regulation of typical homing molecules for gut-seeking plasma blasts includes β7 integrin and the chemokine receptors CCR9 and CCR10.7 Among these molecules only β7 integrin was uniformly up-regulated, whereas expression of both chemokine receptors remained unchanged. However, changes in the expression of these receptors might well go unnoticed, because these would be restricted to a small fraction of all transferred cells. Interestingly, we observed that 1 day after intraperitoneal transfer of splenic B2 cells a significant fraction of transferred cells in the lamina propria expressed IgM but no IgA. Such cells could be detected neither at later time points after intraperitoneal transfer nor after intravenous transfer of cells. In contrast, following intraperitoneal and intravenous transfer plasma blasts staining positive for both IgM and IgA were present. Such double-positive cells most likely represent differentiating plasma blasts that recently underwent class switch recombination to IgA and that still have detectable amounts of IgM in their cytosol. It is thus tempting to speculate that intraperitoneal transfer of activated B cells might favor the generation of gut tropic plasma blasts that migrate to the intestine promptly after transfer and undergo class switch recombination after entering the small intestine. In contrast, intravenous transfer of cells will likewise generate intestinal plasma cells, but these cells will preferentially enter the intestine only after class switch recombination has occurred outside the intestine. This is in line with earlier observations reporting that peritoneal B1 cells might contribute to the pool of intestinal plasma cells and perform class switch recombination in situ.9,26 Data presented here further suggest that the potential of peritoneal B1 cells to generate intestinal plasma cells would not only be attributed intrinsically to the B1 cells but might also stem from cues given by the peritoneal cavity. In conclusion, our data demonstrate that B cells during residence in the peritoneal cavity modify their expression of homing molecules. In particular, naive B cells up-regulate CXCR4, CXCR5, and β7 integrins, thereby gaining the capacity to efficiently reenter the peritoneal cavity in a CXCR5-dependent mechanism. Similarly, B cells activated in the peritoneal cavity are instructed to home to the intestine.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work as supported by Deutsche Forschungsgemeinschaft grant SFB621-A1 (R.F.). We thank G. Bernhardt for critically reading the manuscript.
Authorship
Contribution: S.B. performed the experiments, and R.F. and O.P. designed the study, provided scientific support, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Reinhold Förster; e-mail: foerster.reinhold@mh-hannover.de; or Oliver Pabst, Institute of Immunology, Hannover Medical School, Carl-Neuberg Strasse 1, 30625 Hannover, Germany; e-mail: pabst.oliver@mh-hannover.de.