Abstract
The FIP1L1-PDGFRA fusion gene is a recurrent molecular lesion in eosinophilia-associated myeloproliferative disorders, predicting a favorable response to imatinib mesylate. To investigate its prevalence, 376 patients with persistent unexplained hypereosinophilia were screened by the United Kingdom reference laboratory, revealing 40 positive cases (11%). To determine response kinetics following imatinib, real-time quantitative–polymerase chain reaction (RQ-PCR) assays were developed and evaluated in samples accrued from across the European LeukemiaNet. The FIP1L1-PDGFRA fusion transcript was detected at a sensitivity of 1 in 105 in serial dilution of the EOL-1 cell line. Normalized FIP1L1-PDGFRA transcript levels in patient samples prior to imatinib varied by almost 3 logs. Serial monitoring was undertaken in patients with a high level of FIP1L1-PDGFRA expression prior to initiation of imatinib (100 mg/d-400 mg/d). Overall, 11 of 11 evaluable patients achieved at least a 3-log reduction in FIP1L1-PDGFRA fusion transcripts relative to the pretreatment level within 12 months, with achievement of molecular remission in 9 of 11 (assay sensitivities 1 in 103-105). In 2 patients, withdrawal of imatinib was followed by a rapid rise in FIP1L1-PDGFRA transcript levels. Overall, these data are consistent with the exquisite sensitivity of the FIP1L1-PDGFRα fusion to imatinib, as compared with BCR-ABL, and underline the importance of RQ-PCR monitoring to guide management using molecularly targeted therapies.
Introduction
A significant breakthrough in the understanding of idiopathic hypereosinophilic syndrome and eosinophilia-associated myeloproliferative disorders came with the discovery by Cools et al1 of the fusion between genes encoding Fip 1–like 1 (FIP1L1) and platelet-derived growth factor receptor alpha (PDGFRA) due to a cytogenetically cryptic interstitial deletion involving chromosome 4q12. Importantly, presence of the FIP1L1-PDGFRA fusion gene has been shown to predict a dramatic response to imatinib mesylate, with rapid normalization of peripheral eosinophil counts; indeed, such responses have been achieved with relatively low doses (ie, 50 mg/d-200 mg/d) as compared with those routinely employed for treatment of BCR-ABL+ disease (400 mg).1–4 While the original study reported the presence of the FIP1L1-PDGFRA fusion in 9 of 16 (56%) cases analyzed,1 frequencies of this lesion have varied markedly in subsequent series, ranging from 3% to 56%.1–6 This may reflect differing levels of stringency in the diagnosis of idiopathic hypereosinophilic syndrome (HES) and other eosinophilia-associated hematologic malignancies, but may also be compounded by difficulties in establishing a molecular diagnosis. The latter relates to the considerable heterogeneity in breakpoints within the FIP1L1 locus, variable mechanisms leading to formation of an in-frame fusion product involving use of cryptic splice sites, in addition to the marked alternative splicing between FIP1L1 exons.1,7,8 FIP1L1-PDGFRA transcripts can be difficult to detect by single-step reverse transcriptase–polymerase chain reaction (RT-PCR) and in many instances nested PCR is required for reliable identification of the fusion.9 This implies that the level of fusion gene expression may be relatively low in this disease and indeed, fluorescence in situ hybridization (FISH) has suggested that the proportion of cells harboring the FIP1L1-PDGFRA fusion varies considerably between cases.10,11
In the present study we determined the frequency of the FIP1L1-PDGFRA fusion in a large cohort of patients referred for molecular analysis to the United Kingdom national reference laboratory over a 4-year period for investigation of persistent unexplained hypereosinophilia. In order to provide a tool to enhance our understanding of the biology of this disease and its response to molecularly targeted therapy, real-time quantitative PCR (RQ-PCR) assays were developed within the European LeukemiaNet (http://www.leukemia-net.org) and applied to patients treated with imatinib.
Materials and methods
Analysis of leukemia samples by real-time quantitative PCR undertaken at Guy's Hospital London was subject to Local Research Ethics Committee approval.
Reverse transcriptase–polymerase chain reaction (RT-PCR)
RNA was extracted from peripheral blood (PB) or bone marrow (BM) mononuclear cells and complementary DNA (cDNA) generated using random hexamers as described.12 Nested PCR was performed using published primer sets.1 PCR products were sequenced as described13 to establish FIP1L1-PDGFRA fusion junctions.
Real-time quantitative reverse transcriptase–polymerase chain reaction (RQ-PCR)
Primers and probes for detection of FIP1L1-PDGFRA were designed using Primer Express software (Applied Biosystems, Warrington, United Kingdom) (Table 1). Assays were undertaken using standardized protocols established by the Europe Against Cancer (EAC) group.12 Assays were confirmed in all cases to be RNA specific by checking for absence of amplification due to contaminating genomic DNA in the “no RT” control, as described.14 To normalize FIP1L1-PDGFRA transcript levels, standardized EAC control gene assays for the Abelson (ABL) and β2-microglobulin (B2M) genes were used.15 All FIP1L1-PDGFRA assays satisfied EAC selection criteria, with a ΔRn of at least 1.0 in diagnostic material. FIP1L1-PDGFRA expression was normalized to the expression of each of the control genes using the difference in Ct value (ΔCt) method, as described,16 since the marked heterogeneity in FIP1L1-PDGFRA breakpoints renders absolute quantification against a plasmid standard curve impracticable. Using this approach, a 1-log difference in fusion gene expression was equivalent to a difference in cycle threshold (Ct) value of 3.5, according to the slope of the standard curve generated using serial dilution of the FIP1L1-PDGFRA+ EOL-1 cell line in HL60 cells. Assay sensitivities for PCR negative follow-up samples were determined according to the ΔCt between FIP1L1-PDGFRA and the control gene in the pretreatment sample and the Ct value of the control genes in any given follow-up sample, taking a Ct value of 40 as the detection limit for the fusion gene, as described by Pallisgaard et al.16 All RQ-PCR assays were run using the ABI7700 platform (Applied Biosystems) in triplicate wells using a threshold of 0.05; amplification for FIP1L1-PDGFRA in at least 2 of 3 wells with a Ct value of 40 or less was required to define a result as “PCR positive” in accordance with EAC criteria.12 For follow-up samples testing negative for the FIP1L1-PDGFRA fusion, only those affording a sensitivity of at least 1 in 103 based on pretreatment expression level of FIP1L1-PDFGRA and an adequate level of control gene expression were considered evaluable for assessment of minimal residual disease (MRD).
Primer and probe sequences for real-time quantitative PCR detection of FIP1L1-PDGFRA fusion transcripts
| Primer/Probe . | 5′-3′ sequence . |
|---|---|
| FIP1L1ex9-F | GCAGAGATCCAAGATGGCAGAT |
| FIP1L1ex10-F | TTGTTCAAGACTGGGCTTCCA |
| FIP1L1ex11-F | ATATGGGAGGGCCGAATCA |
| FIP1L1ex12-F | GGGCAAATGAGAACAGCAACA |
| FIP1L1ex13-F | ACTGCTCCACCTCTGATTCCA |
| PDGFRA-R1 | CAAGACCCGACCAAGCACTAGT |
| PDGFRA-Pr1 | FAM-CTGCCTTATGACTCAAGATGGGAGTTTCCAA-TAMRA |
| Primer/Probe . | 5′-3′ sequence . |
|---|---|
| FIP1L1ex9-F | GCAGAGATCCAAGATGGCAGAT |
| FIP1L1ex10-F | TTGTTCAAGACTGGGCTTCCA |
| FIP1L1ex11-F | ATATGGGAGGGCCGAATCA |
| FIP1L1ex12-F | GGGCAAATGAGAACAGCAACA |
| FIP1L1ex13-F | ACTGCTCCACCTCTGATTCCA |
| PDGFRA-R1 | CAAGACCCGACCAAGCACTAGT |
| PDGFRA-Pr1 | FAM-CTGCCTTATGACTCAAGATGGGAGTTTCCAA-TAMRA |
Patient samples
FIP1L1-PDGFRA fusion status was determined by nested RT-PCR in PB or BM samples from 376 patients referred for investigation of persistent unexplained eosinophilia to the United Kingdom national reference laboratory at the Wessex Regional Genetics Laboratory, Salisbury. Full blood count data from time of sampling were available from 105 cases. In order to design RQ-PCR assays suitable for amplifying the FIP1L1-PDGFRA fusion, sequence information from cDNA breakpoint junction regions of 43 cases identified from laboratories participating in the MRD workpackage (Workpackage 12) of the European LeukemiaNet (ELN) was taken into account.8 Serial monitoring was undertaken by RQ-PCR in ELN cases identified to have relatively high levels of FIP1L1-PDGFRA expression (as defined in the preceding section of “Materials and methods”) and treated with imatinib, at doses ranging between 100 mg/d and 400 mg/d. All patients provided informed consent for molecular analyses, in accordance with the Declaration of Helsinki.
Statistical analyses
Correlations between continuous variables were calculated using the Pearson correlation coefficient. Comparison between 2 groups of continuous variables were performed using the nonparametric Mann-Whitney U test. Significance was set at P < .05.
Results
Incidence of FIP1L1-PDGFRA
Molecular screening for the FIP1L1-PDGFRA fusion was undertaken by nested RT-PCR in a series of 376 patients with idiopathic HES, as defined by World Health Organization criteria,17 or persistent unexplained eosinophilia. Forty (11%) were identified as having an underlying FIP1L1-PDGFRA fusion. Cases with the fusion had a significantly higher absolute blood eosinophil count (median 6.6 × 109/L, range 0.2 × 109/L-44.6 × 109/L vs median 1.9 × 109/L, range 1.6 × 109/L-27 × 109/L; P = .002) and eosinophil percentage (median 50%, range 5.1%-85% vs median 18.2%, range 0.1%-85%; P < .001); however, there was no significant difference in total WBC (median 12 × 109/L, range 1.9 × 109/L-72.6 × 109/L vs median 11.7 × 109/L, range 5.2 × 109/L-44 ×109/L; P = .5).
Breakpoint analysis in FIP1L1-PDGFRA+ cases
Breakpoint junctions were sequenced from PCR-amplified cDNA in a series of 43 FIP1L1-PDGFRA+ leukemias collected from ELN centers. In all cases breakpoints occurred within exon 12 of PDGFRA, whereas breakpoints were dispersed across the FIP1L1 locus occurring between exons 9 and 13. All fusion transcripts were found to be in frame and involved use of cryptic splice sites within PDGFRA exon 12 (defined as type A breakpoint) or within intronic sequence of FIP1L1 (type B). In the former, FIP1L1 and PDGFRA exonic sequences were directly apposed at the cDNA fusion junction, whereas in the latter they were separated by a stretch of intervening nucleotides (2 bp-76 bp) derived from intronic regions of FIP1L1.
Development of RQ-PCR assays for the FIP1L1-PDGFRA fusion
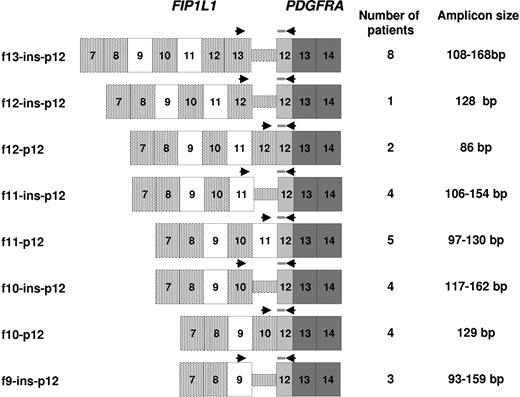
Initially, an RQ-PCR assay was designed to amplify the FIP1L1-PDGFRA fusion transcript in the EOL-1 cell line,18,19 locating the probe (PDGFRA-Pr1) within PDGFRA exon 12. The reverse primer (PDGFRA-R1) was designed to span the PDGFRA exon 12/exon 13 cDNA junction in order to render the assay RNA-specific, with the forward primer (FIP1L1ex12-F) located in FIP1L1 exon 12 (Table 1). The fusion transcript was found to be expressed at a comparable level to ABL in EOL-1 cells (ΔCtFIP1L1-PDGFRA-ABL: −1) and could be detected at a sensitivity of 1 in 105 as determined by serial dilution within HL60 cells. Breakpoint analysis in the series of 43 ELN cases indicated that the probe and reverse primer designed for amplification of the EOL-1 breakpoint junction could be used to detect fusion gene junctions in 33 of 43 cases (77%). In an attempt to design an assay suitable for detecting all identified breakpoints, a probe and primer located within PDGFRA exon 13 were designed; however, this assay performed poorly and failed to satisfy EAC criteria (data not shown). In order to amplify FIP1L1-PDGFRA transcripts in primary patient samples, a series of forward primers (FIP1L1ex9-F, FIP1L1ex10-F, FIP1L1ex11-F, FIP1L1ex12-F, and FIP1L1ex13-F) were designed to be used in conjunction with the common probe and reverse primer (Figure 1, Table 1).
Strategy for detection of FIP1L1-PDGFRA fusion transcripts in CEL patients with heterogeneous breakpoint patterns. An appropriate forward primer (Table 1) was used in conjunction with the common probe (PDGFRA-Pr1) and reverse primer (PDGFRA-R1) according to the identified breakpoint location. The number of patients analyzed with any given assay is shown, together with the respective size of the amplicon.
Strategy for detection of FIP1L1-PDGFRA fusion transcripts in CEL patients with heterogeneous breakpoint patterns. An appropriate forward primer (Table 1) was used in conjunction with the common probe (PDGFRA-Pr1) and reverse primer (PDGFRA-R1) according to the identified breakpoint location. The number of patients analyzed with any given assay is shown, together with the respective size of the amplicon.
The relative expression of the FIP1L1-PDGFRA transcript was established in pre-imatinib samples from 31 ELN-derived patients with chronic eosinophilic leukemia (CEL). In the majority of patients FIP1L1-PDGFRA expression was comparable to that of ABL, but expression level varied markedly, with a 1000-fold range across the whole group (median ΔCtFIP1L1-PDGFRA-ABL: 2.3; range, −0.3-10.3). There was no evidence of correlation between the relative level of FIP1L1-PDGFRA expression and WBC (r = −0.10; P = .6), absolute eosinophil count (r = −0.13; P = .5), eosinophil percentage (r = −0.13; P = .5) or the proportion of cells harboring the 4q12 deletion (r = −0.22; P = .4), as determined by interphase FISH for the CHIC2 locus, which lies between FIP1L1 and PDGFRA on 4q12.10,20 Moreover, the observed variability in FIP1L1-PDGFRA expression could not be accounted for by treatment (steroids alone [n = 4], hydroxyurea (HU) alone [n = 2], HU+steroids [n = 3], HU+α-interferon [n = 1], α-interferon alone [n = 1]) at the time of molecular analysis (untreated [n = 20]: median ΔCtFIP1L1-PDGFRA-ABL: 2.1; range, −0.3-10.3 vs treated [n = 11]: median ΔCtFIP1L1-PDGFRA-ABL: 2.6; range, 0.3-7.7; P = .6). The relatively low level of fusion transcript expression observed in some cases may account for why the FIP1L1-PDGFRA fusion is often difficult to detect by single round RT-PCR, requiring a nested RT-PCR approach.9 Only a few paired BM and PB samples were available for RQ-PCR analysis; however, fusion transcript expression levels appeared comparable between the 2 sample types (ΔCtFIP1L1-PDGFRA-B2M: PB [n = 22] median 12.4 [8.6-18.8], BM [n = 12] median 11.6 [5.2-18.6]; ΔCtFIP1L1-PDGFRA-ABL: PB median 1.95 [−0.3-9.8], BM median 2.8 [0.7-10.3]).
Kinetics of molecular response to imatinib
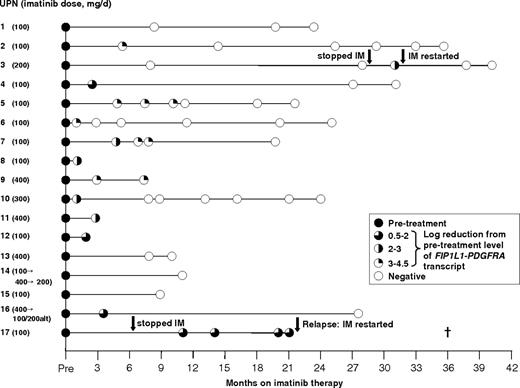
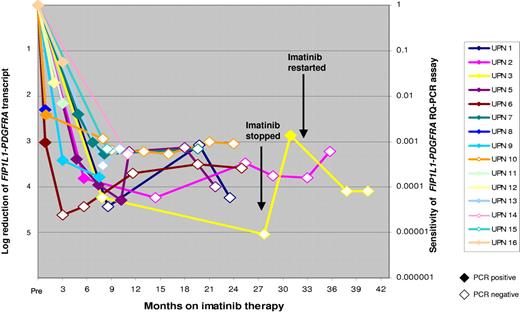
In order to investigate the kinetics of molecular response to imatinib, analysis was restricted to 17 cases of CEL derived from across the ELN with the highest pretreatment levels of FIP1L1-PDGFRA expression, enabling MRD detection with assay sensitivities of at least 1 in 1000 (generally ∼ 1 in 104). Patients received 100 mg/d to 400 mg/d imatinib as indicated in Figure 2, with a median follow-up of 21 months (range, 0.9-40.4 months). Overall, 11 of 11 evaluable patients achieved at least a 3-log reduction in FIP1L1-PDGFRA fusion transcripts relative to the pretreatment level within 12 months; achievement of complete molecular remission at assay sensitivities ranging between 1 in 103 and 105 (Figure 3) was documented in 9 patients. In 2 cases (UPN 3, UPN 17), withdrawal of imatinib was followed by a rapid rise in FIP1L1-PDGFRA transcript levels (Figure 3, UPN 3). In UPN 3, a further molecular remission was documented and sustained following reinstatement of imatinib (200 mg/d; Figure 3). In the remaining patient, further imatinib therapy (100 mg/d) led to normalization of the eosinophil count; however, the patient died as a result of severe cardiac involvement that had been apparent prior to initial imatinib therapy.
PCR profiles of patients with CEL following imatinib therapy. Dose of imatinib for each patient is shown on the left. Analysis was restricted to patients with sufficiently high levels of FIP1L1-PDGFRA expression to allow detection of MRD at a sensitivity of at least 1 in 1000. Patients treated and maintained on imatinib achieved molecular complete remission, whereas discontinuation of imatinib in 2 patients (UPN 3, UPN 17) was associated with rapid disease progression. UPN indicates unique patient number; alt, alternate day dosing schedule; IM, imatinib.
PCR profiles of patients with CEL following imatinib therapy. Dose of imatinib for each patient is shown on the left. Analysis was restricted to patients with sufficiently high levels of FIP1L1-PDGFRA expression to allow detection of MRD at a sensitivity of at least 1 in 1000. Patients treated and maintained on imatinib achieved molecular complete remission, whereas discontinuation of imatinib in 2 patients (UPN 3, UPN 17) was associated with rapid disease progression. UPN indicates unique patient number; alt, alternate day dosing schedule; IM, imatinib.
Kinetics of molecular response to imatinib therapy. The rate of decline of FIP1L1-PDGFRA fusion transcripts normalized to the ABL gene following start of imatinib therapy is shown. PCR-positive results are depicted as colored data points, with log reduction as compared with pretreatment level marked on Y-axis on left. PCR-negative results are depicted as white data points, with the sensitivity for each respective PCR negative sample shown on the right-hand axis. In patient UPN 3, discontinuation of imatinib was followed by a rise in fusion transcript level associated with molecular relapse; a second molecular remission was obtained following reinstitution of imatinib. UPN indicates unique patient number.
Kinetics of molecular response to imatinib therapy. The rate of decline of FIP1L1-PDGFRA fusion transcripts normalized to the ABL gene following start of imatinib therapy is shown. PCR-positive results are depicted as colored data points, with log reduction as compared with pretreatment level marked on Y-axis on left. PCR-negative results are depicted as white data points, with the sensitivity for each respective PCR negative sample shown on the right-hand axis. In patient UPN 3, discontinuation of imatinib was followed by a rise in fusion transcript level associated with molecular relapse; a second molecular remission was obtained following reinstitution of imatinib. UPN indicates unique patient number.
Discussion
The incidence of the FIP1L1-PDGFRA fusion among patients with persistent unexplained hypereosinophilia is not firmly established; in the original study by Cools et al,1 more than half the 16 patients studied were found to have an underlying FIP1L1-PDGFRA fusion. Most subsequent studies, including the present one, indicate that this abnormality is detected in around 10% to 15% of patients.1–3,5,6 Previous studies have highlighted the marked male preponderance in this molecular subtype of leukemia; in addition, marrow fibrosis, increased mast cells, and elevated serum tryptase levels in the presence of eosinophilia indicate those cases most likely to harbor the FIP1L1-PDGFRA fusion gene.4,5,10,21,22 Differences in the incidences between studies are also likely to reflect methodologic issues such as relatively small sample sizes, differing levels of stringency in the diagnosis of idiopathic hypereosinophilic syndrome, or other eosinophilia-associated hematologic malignancies, or technical issues such as the small size of the clone in some cases, the marked heterogeneity of FIP1L1 breakpoints and the relatively low level of fusion gene expression highlighted by the present study. The latter may also account for the limitations in reliably detecting the FIP1L1-PDGFRA fusion using single-round RT-PCR.9
Previous studies have underlined the importance of identifying the FIP1L1-PDGFRA fusion, since it predicts a favorable response to molecularly targeted therapy with imatinib. While presence of the FIP1L1-PDGFRA fusion can be inferred by use of a FISH approach looking for evidence of deletion of the CHIC2 locus,10,20 we also advocate the use of nested RT-PCR as a confirmatory test to determine breakpoint location, which is a prerequisite for the subsequent monitoring of treatment response by quantitative PCR. Indeed, in our experience nested RT-PCR provides a more reliable screening test, since the percentage of cells with loss of CHIC2 probe signal by interphase FISH was no higher than background levels found in normal controls (ie, 10%),20 in 8 of 16 (50%) cases with FIP1L1-PDGFRA–associated CEL (median % cells with CHIC2 deletion 10.2%, range 0%-79%) in which both methodologies were used in parallel. We successfully designed RQ-PCR assays for detection of the FIP1L1-PDGFRA fusion, using a common probe and reverse primer, which when used in conjunction with a limited range of forward primers could be applied to the majority of patients. Analysis of the remaining patients would necessitate the design of patient-specific assays.
The potential value of RQ-PCR to monitor treatment responses to imatinib has been amply demonstrated in the context of chronic-phase chronic myeloid leukemia (CML). In the IRIS study, molecular response was found to be a key independent predictor of outcome.23 Patients failing to achieve a major molecular response (MMR), defined as at least a 3-log reduction in BCR-ABL transcript level below a predefined baseline level by 12 months, predicted an increased risk of disease progression and poorer outcome.23 Moreover, serial monitoring has highlighted the potential for RQ-PCR assays to identify patients who although showing an initial molecular response to therapy, subsequently exhibit a rise in BCR-ABL transcript levels, which commonly reflects the emergence of an imatinib-resistant subclone. This enables appropriate and timely treatment intervention; the nature of which is dependent on the identified mutation (reviewed in Hughes et al24 ). While the prognostic significance of the kinetics of molecular response in FIP1L1-PDGFRA–associated leukemia is uncertain, the potential value of molecular monitoring in this disease has been highlighted by reports of disease progression due to the emergence of an imatinib-resistant clone with a T674I mutation in the PDGFRα moiety of the fusion protein.1,25 However, this mutant retains sensitivity to other tyrosine kinase inhibitors, like PKC412,1 nilotinib,26 or sorafenib,27 which could provide potential treatment options in such a scenario. The frequency of emergence of imatinib resistance in FIP1L1-PDGFRA+ disease is currently unclear; we observed no molecular or hematologic relapses among patients maintained on therapy, albeit at relatively short follow-up.
MRD assessment of patients with chronic-phase CML has shown that the kinetics of molecular response to imatinib are relatively slow, with only approximately 40% of patients achieving an MMR by 12 months.23 Moreover, achievement of complete molecular remission is only very rarely observed.23 This is considered to reflect the persistence of a population of quiescent BCR-ABL+ stem cells that are resistant to imatinib.28 In contrast, our study documenting the kinetics of molecular response of FIP1L1-PDGFRA–associated leukemia to imatinib revealed a more dramatic decline in transcript levels, with all evaluable patients achieving at least a 3-log reduction in fusion transcripts as compared with the pretreatment level. This included patients receiving low-dose imatinib at 100 mg/d or 200 mg/d. This may reflect the greater sensitivity of FIP1L1-PDGFRα to this agent observed in in vitro assays, as compared with BCR-ABL.1 Indeed, in marked contrast to the experience in CML, we found that imatinib induced complete molecular remissions in patients with FIP1L1-PDGFRA+ CEL, as determined by RQ-PCR (and nested RT-PCR, data not shown). In some instances this may reflect the relative insensitivity of PCR assays for the FIP1L1-PDGFRA fusion, as compared with that for BCR-ABL due to the relatively low level of fusion transcript expression observed in a significant proportion of CEL cases. Nevertheless, PCR negativity for the FIP1L1-PDGFRA fusion at a sensitivity of at least 1 in 105 was observed in one patient treated with 200 mg/d imatinib. This raised the possibility that the stem cell compartment in CEL, in contrast to that in CML, may be sensitive to imatinib. However, this may not be the case, since we observed a rapid rise in FIP1L1-PDGFRA transcripts in both patients with CEL who discontinued imatinib therapy. Others have reported clinical relapse under similar circumstances.4 This is reminiscent of the situation in CML and suggests that imatinib should be continued long-term in patients with FIP1L1-PDGFRA+ CEL. Overall, these data are consistent with the exquisite sensitivity of the FIP1L1-PDGFRα fusion to imatinib, as compared with BCR-ABL, and emphasizes the importance of RQ-PCR monitoring to guide management using molecularly targeted therapies.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the European LeukemiaNet within the 6th European Framework Program for Research and Technology Development.
D.G. and N.C.P.C. are grateful to the Leukaemia Research Fund of Great Britain for grant support. A.R. and C.W. were supported by the Deutsche José Carreras Leukämie-Stiftung e.V. (grant no. DJCLS R06/02). We acknowledge Robert Hills for assistance with statistical analyses, Mariano Rocchi for provision of CHIC2 probe for FISH analyses, and Abida Awan and Sarah Daly for help with preparation of the figures. We also wish to thank Samir Agrawal, Barbara Bain, Peter Campbell, Jamie Cavenagh, Tanya Cranfield, Dominic Culligan, Peter Cumber, Andrew Duncombe, Helen Dignum, Roger Evely, Mark Gompels, Claire Harrison, Sunil Handa, Aloysius Ho, Paul Kelsey, Stuart Laidlaw, Jackie Martin, Mary Frances McMullin, Pradeep Mehrotra, Sandrine Meyer, Gareth Morgan, John Murphy, Lisa Newton, Deepti Radia, Beverley Robertson, Tomasz Sacha, Shafeek Salim, Abdul Shlebak, Penny Stableforth, John Van de Pette, Natasha Wiles, Barrie Woodcock, Arshi Yasmin, and John Yin for provision of clinical information concerning their patients.
Authorship
Contribution: J.V.J. designed and undertook real-time quantitative PCR assays and analyzed the data under the supervision of E.S. and D.G.; J.S. and K.W. undertook molecular screen for the FIP1L1-PDGFRA fusion under the supervision of N.C.P.C.; D.C., E.G., G. Metzgeroth, P.E., H.P., C.W., E.O., and C.R.-L. undertook molecular characterization of FIP1L1-PDGFRA transcripts under the supervision of G.S., A.H., A.R., R.H., G. Martinelli, and C.P.; M.R. and F.B.-B. undertook analysis of clinical data; A.R. and D.G. designed the study, with contributions from J.A. and G. Martinelli. The manuscript was written by D.G. and J.V.J., with contributions from A.H., A.R., N.C.P.C., and R.H. The project was performed within the European LeukemiaNet, led by R.H.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
A complete list of the members of the European LeukemiaNet appears as Document S1 (available on the Blood website; see the Supplemental Materials link at the top of the online article).
Correspondence: David Grimwade, Cancer Genetics Laboratory, Department of Medical & Molecular Genetics, 8th Fl, Guy's Tower, Guy's Hospital, London, SE1 9RT United Kingdom; e-mail: david.grimwade@genetics.kcl.ac.uk.