Abstract
Iron overload could be a significant contributor to treatment-related mortality (TRM) for patients with hematologic malignancies undergoing hematopoietic stem cell transplantation (HSCT). We studied 590 patients who underwent myeloablative allogeneic HSCT at our institution, and on whom a pretransplantation serum ferritin was available. An elevated pretransplantation serum ferritin level was strongly associated with lower overall and disease-free survival. Subgroup multivariable analyses demonstrated that this association was restricted to patients with acute leukemia or myelodysplastic syndrome (MDS); in the latter group, the inferior survival was attributable to a significant increase in TRM. There was also a trend toward an increased risk of veno-occlusive disease in patients with high ferritin. Our results argue that iron overload plays an important role in transplantation outcome for patients with acute leukemia or MDS, as it does in thalassemia. They also suggest future prospective trials to examine the potential benefit of chelation therapy in this setting.
Introduction
Iron overload is an important adverse prognostic factor for patients with thalassemia undergoing hematopoietic stem cell transplantation (HSCT).1–4 This may also hold true for patients who undergo transplantation for hematologic malignancies.5 Indeed, those patients are at increased risk of iron overload, from their often high transfusion load, or possibly from the procedure itself.6–8 Recent studies have suggested a link between iron overload and posttransplantation liver toxicity (including chronic liver disease and veno-occlusive disease),9–11 infectious susceptibility,12 and even survival in a small study of HSCT patients.13 We report here a large retrospective study to determine the impact of iron overload on mortality after allogeneic HSCT in patients with hematologic malignancies
Materials and methods
We studied 922 consecutive adult patients with hematologic malignancies who underwent allogeneic HSCT with myeloablative conditioning at the Dana-Farber/Brigham and Women's Hospital transplantation program between 1997 and 2005. A pretransplantation ferritin level (drawn within 3 months preceding transplantation) was available for 590 (64%) of the 922 patients. Patients underwent transplantation under several treatment and investigational protocols over the 9-year period covered by this study. Conditioning regimens consisted of cyclophosphamide plus total body irradiation (14 Gy total in 14 fractions) or busulfan (16 mg/kg by mouth or 12.8 mg/kg intravenously total in divided doses). Graft-versus-host disease (GVHD) prophylaxis regimens consisted mostly of a combination of calcineurin inhibitor and methotrexate, with or without steroids; tacrolimus plus sirolimus, with or without low-dose methotrexate; and T-cell depletion. Statistical methods used here are described elsewhere.14 All statistical analyses were done using SAS 9.1 (SAS Institute, Cary, NC) and R (version 2.3.1).
Institutional review board approval was obtained from the Office for the Protection of Research Subjects (OPRS) at Dana-Farber/Harvard Cancer Center to perform this study in accordance with the Declaration of Helsinki.
Results and discussion
Patient characteristics
We reviewed the records of 922 adult patients who underwent allogeneic HSCT with myeloablative conditioning at our institution. We used pretransplantation serum ferritin as a surrogate marker of iron burden at the time of transplantation. Although ferritin is clearly not an ideal measure for total body iron burden,15 it is a useful noninvasive surrogate, based on the low cost and ease of its measurement, its correlation with directly measured liver iron content,16 and its established clinical relevance in other settings.17 Of the 922 patients, 590 (64%) had an available pretransplantation serum ferritin. The overall survival (OS) of these 590 patients did not differ significantly from that of the 332 patients without an available baseline ferritin (log-rank P = .5). Their demographic and clinical characteristics are shown in Table 1. Median follow-up for survivors was 34 months. Subsequent analyses were restricted to the 543 patients with well-represented diseases: chronic myelogenous leukemia (CML), acute myeloid leukemia (AML), myelodysplastic syndrome (MDS), acute lymphoblastic leukemia (ALL), and non-Hodgkin lymphoma (NHL). Median ferritin in this group was 930 ng/mL (range, < 5 ng/mL to 16 400 ng/mL). The percentage of patients with elevated (> 300 ng/mL) serum ferritin varied significantly between CML (25%), NHL (71%), MDS (88%), and acute leukemia (97%) (all pairwise P < .01).
Patient baseline characteristics
| Characteristic . | Value . |
|---|---|
| Number of patients analyzed | 590 |
| Median age, y (range) | 42 (18-68) |
| Disease | |
| CML | 154 (26) |
| AML | 144 (24) |
| MDS | 103 (17) |
| RA/RARS/RCMD | 35 (6) |
| RAEB | 26 (4) |
| AML from MDS | 42 (7) |
| ALL | 74 (13) |
| NHL | 68 (12) |
| MPD | 17 (3) |
| CLL | 14 (2) |
| HD | 6 (1) |
| Multiple myeloma | 4 (1) |
| CMML | 4 (1) |
| LGL leukemia | 1 (0) |
| NK leukemia | 1 (0) |
| Stage | |
| Untreated | 74 (13) |
| CR1 | 222 (38) |
| Second or later CR | 66 (11) |
| PR | 61 (10) |
| Induction failure | 85 (14) |
| Relapse | 82 (14) |
| Graft source | |
| Bone marrow | 317 (54) |
| Peripheral blood | 269 (46) |
| Cord blood | 4 (1) |
| Match | |
| MRD | 270 (46) |
| MUD | 242 (41) |
| Mismatched | 78 (13) |
| Conditioning | |
| Cy/TBI | 567 (96) |
| Bu/Cy | 22 (4) |
| Other | 1 (0) |
| GVHD prophylaxis | |
| CnI + Mtx ± steroids | 281 (48) |
| CnI + Siro ± Mtx | 198 (34) |
| TCD | 68 (12) |
| Other | 43 (7) |
| Male recipient/female donor | 122 (21) |
| CMV seropositivity | |
| Recipient | 244 (41) |
| Donor | 221 (37) |
| Characteristic . | Value . |
|---|---|
| Number of patients analyzed | 590 |
| Median age, y (range) | 42 (18-68) |
| Disease | |
| CML | 154 (26) |
| AML | 144 (24) |
| MDS | 103 (17) |
| RA/RARS/RCMD | 35 (6) |
| RAEB | 26 (4) |
| AML from MDS | 42 (7) |
| ALL | 74 (13) |
| NHL | 68 (12) |
| MPD | 17 (3) |
| CLL | 14 (2) |
| HD | 6 (1) |
| Multiple myeloma | 4 (1) |
| CMML | 4 (1) |
| LGL leukemia | 1 (0) |
| NK leukemia | 1 (0) |
| Stage | |
| Untreated | 74 (13) |
| CR1 | 222 (38) |
| Second or later CR | 66 (11) |
| PR | 61 (10) |
| Induction failure | 85 (14) |
| Relapse | 82 (14) |
| Graft source | |
| Bone marrow | 317 (54) |
| Peripheral blood | 269 (46) |
| Cord blood | 4 (1) |
| Match | |
| MRD | 270 (46) |
| MUD | 242 (41) |
| Mismatched | 78 (13) |
| Conditioning | |
| Cy/TBI | 567 (96) |
| Bu/Cy | 22 (4) |
| Other | 1 (0) |
| GVHD prophylaxis | |
| CnI + Mtx ± steroids | 281 (48) |
| CnI + Siro ± Mtx | 198 (34) |
| TCD | 68 (12) |
| Other | 43 (7) |
| Male recipient/female donor | 122 (21) |
| CMV seropositivity | |
| Recipient | 244 (41) |
| Donor | 221 (37) |
Values indicate number of patients (%) unless otherwise indicated. Percentages may not add to 100 because of rounding.
CML indicates chronic myelogenous leukemia; AML, acute myeloid leukemia; MDS, myelodysplastic syndrome; RA, refractory anemia; RARS, refractory anemia with ringed sideroblasts; RCMD, refractory cytopenia with multilineage dysplasia; RAEB, refractory anemia with excess blasts; ALL, acute lymphoblastic leukemia; NHL, non-Hodgkin lymphoma; MPD, myeloproliferative disease excluding CML and CMML; CLL, chronic lymphocytic leukemia; HD, Hodgkin disease; CMML, chronic myelomonocytic leukemia; LGL, large granular lymphocyte; NK, natural killer cell; CR, complete remission; PR, partial remission; MRD, matched related donor; MUD, matched unrelated donor; Cy, cyclophosphamide; TBI, total body irradiation; Bu, busulfan; GVHD, graft-versus-host disease; CnI, calcineurin inhibitor (cyclosporine or tacrolimus); Mtx, methotrexate; ±, with or without; Siro, sirolimus; TCD, T-cell depletion; and CMV, cytomegalovirus.
Survival, relapse, and treatment-related mortality
There was a strong relationship between pretransplantation ferritin and survival. The 5-year OS for patients with pretransplantation ferritin in the first quartile (0 ng/mL-231 ng/mL) was 54%; (95% confidence interval [CI], 45%-63%); in the second quartile (232 ng/mL-930 ng/mL), 50%; (95% CI, 41%-59%); in the third quartile (931 ng/mL-2034 ng/mL), 37%; 95% CI, 27%-46%); and in the fourth quartile (> 2034 ng/mL), 27%; (95% CI, 18%-36%) (P < .001). The 5-year disease-free survival (DFS) rates, from lowest to highest quartile, were 43% (95% CI, 33%-53%), 44% (95% CI, 35%-54%), 34% (95% CI, 24%-43%), and 27% (95% CI, 19%-36%) (P < .001).
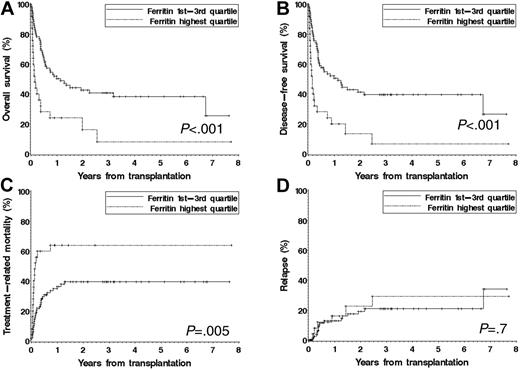
We performed subgroup analyses for each of 4 disease groups (acute leukemia, MDS, NHL, and CML). Within each group, we built proportional hazards models using pretransplantation serum ferritin, all of the covariates in Table 1, as well as cytogenetic risk group for patients with AML, MDS, or ALL; history of prior transplantation; and therapy-related disease (for AML and MDS). For patients with MDS or acute leukemia, an elevated pretransplantation serum ferritin was independently associated with significantly inferior survival. For patients with MDS, the hazard ratio (HR) for mortality associated with a ferritin in the top quartile (≥ 2515 ng/mL) was 2.6 (P = .003). For patients with acute leukemia, the corresponding HR (for ferritin ≥ 2640 ng/mL) was 1.6 (P = .031). In contrast, there was no independent effect of an elevated pretransplantation ferritin on survival for patients with CML or NHL. We also built, within each disease group, competing risks regression models18 for cumulative incidence of relapse and TRM. A pretransplantation serum ferritin in the highest quartile was associated with a significantly increased TRM for patients with MDS (HR = 3.2, P = .002), but not with an increased risk of relapse (HR = 0.8, P = .8). For the other disease groups, the effect of ferritin on TRM and relapse was not statistically significant. Figure 1 displays the outcome of patients with MDS stratified by pretransplantation ferritin.
Outcome of patients with MDS stratified by pretransplantation ferritin level. Patients are stratified using the fourth quartile (ferritin > 2515 ng/mL) versus the lower 3 quartiles. (A) Overall survival. (B) Disease-free survival. (C) Cumulative incidence of treatment-related mortality. (D) Cumulative incidence of relapse.
Outcome of patients with MDS stratified by pretransplantation ferritin level. Patients are stratified using the fourth quartile (ferritin > 2515 ng/mL) versus the lower 3 quartiles. (A) Overall survival. (B) Disease-free survival. (C) Cumulative incidence of treatment-related mortality. (D) Cumulative incidence of relapse.
Role of albumin
The issue most likely to confound the relationship between serum ferritin and iron overload in our study is the role of ferritin as an acute-phase reactant. We have attempted to account for this by including pretransplantation serum albumin in the models. Albumin is a negative acute-phase reactant; therefore, if the relationship between serum ferritin and mortality that we observed were mostly dependent on acute-phase issues, we would expect that the inclusion of albumin in the multivariable models would diminish the prognostic impact of ferritin. We repeated all of our analyses with the addition of a term for albumin under 40 g/L. In all cases, the impact of ferritin on outcome was unchanged by the inclusion of albumin in the model; therefore, the prognostic effect of serum ferritin is unlikely to depend substantially on acute-phase issues.
Hyperferritinemia and transplantation complications
We performed logistic regression analyses for veno-occlusive disease (VOD) of the liver, using as covariates conditioning regimen, elevated liver function tests at the time of admission for transplantation, and hepatitis B and C serostatus. In this model, a pretransplantation ferritin in the top quartile was associated with a borderline significant increase in the risk of VOD (odds ratio = 1.7, 95% CI 1.0 to 2.9, P = .054).
In logistic regression analyses for acute GVHD, using as other covariates age, HLA match, GVHD prophylaxis regimen, graft source, CMV serostatus, sex, and conditioning regimen, hyperferritinemia was not associated with an increased risk of acute GVHD or acute liver GVHD.
Conclusions
Our study lends strong credence to the idea that iron overload is frequent and deleterious in patients with acute leukemia or MDS undergoing myeloablative HSCT, acknowledging the limitations inherent in a retrospective, single-institution study. Our results have several implications. First, they establish a new important and independent prognostic marker for patients with acute leukemia or MDS undergoing myeloablative allogeneic HSCT. They also justify prospective studies examining the role of iron overload using more direct measurement methods (eg, MRI). Such studies could address the relationship between pretransplantation transfusions and iron overload within each disease group. More importantly, they suggest a possible role for iron chelation therapy in the pre- or posttransplantation setting. Given the absolute difference of 37% in 5-year OS for patients with MDS between the highest and lowest ferritin quartiles, judicious chelation therapy could lead to significant improvement in transplantation outcomes for this group of patients.
Authorship
Contribution: P.A. designed and performed the research, analyzed the data, and wrote the paper; H.T.K. analyzed the data and edited the paper; C.S.C., V.T.H., J.K., E.P.A., and R.J.S. collected data and edited the paper; and J.H.A. designed the research, collected data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Philippe Armand, Dana-Farber Cancer Institute, 44 Binney St, Boston, MA 02115; e-mail: parmand@partners.org.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgment
This work was funded in part by grant P01 HL070 149 from the National Heart, Lung, and Blood Institute.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal