Abstract
The addition of antithymocyte globulin (ATG) to a regimen of high-dose cyclophosphamide has been advocated to enhance engraftment after allogeneic bone marrow transplantation (BMT) for severe aplastic anemia (SAA). In a prospective clinical trial, 134 patients were randomly assigned to receive cyclophosphamide alone or in combination with ATG. All patients received T-cell–replete bone marrow from an HLA-matched sibling. With a median follow-up of 6 years, the 5-year probabilities of survival were 74% for the cyclophosphamide alone group and 80% for the cyclophosphamide plus ATG group (P = .44). Graft failure and graft-versus-host disease (GVHD) rates were similar in both groups. With the survival rates achieved, this study is not adequately powered to detect significant differences between the 2 treatment groups. In conclusion, the results of allogeneic BMT for SAA have improved over time related to advances in supportive care. The addition of ATG to the preparative regimen did not significantly improve the outcome.
Introduction
Bone marrow transplantation (BMT) is an effective treatment for patients with severe aplastic anemia (SAA),1,2 but graft rejection remains a significant problem especially in patients who have been heavily transfused.2–7 Cyclophosphamide, 200 mg/kg, has been commonly used as the preparative regimen.2 Graft failure rates are relatively high at approximately 15%.3 The addition of total lymphoid or total body irradiation to cyclophosphamide reduced the risk of rejection to less than 5%,8–10 but rates of graft-versus-host disease (GVHD), interstitial pneumonia, and secondary malignancies were higher and survival was not improved.11–13
Storb et al14 first reported the use of cyclophosphamide and antithymocyte globulin (ATG) as a successful preparative regimen for second transplantations after initial graft rejection. A subsequent uncontrolled study reported improved engraftment and extended survival in 90% of patients receiving this regimen for their first transplantation.15 It is unclear whether the use of ATG or improvement in supportive care was responsible for the improved outcome. Therefore, a prospective randomized controlled trial was conducted by the International Bone Marrow Transplant Registry to compare outcomes after cyclophosphamide alone versus cyclophosphamide plus ATG as the preparative regimen for allogeneic BMT from an HLA-matched sibling donor.
Patients, materials, and methods
Eligibility criteria
Patients had to have SAA (marrow cellularity < 20%) and 2 of the following criteria: absolute neutrophil count (ANC) no greater than 0.5 × 109/L; platelet counts no greater than 20 × 109/L; and reticulocyte count no greater than 50 × 109/L, be no older than 60 years age, and have an HLA-matched sibling donor. Exclusion criteria included a clonal cytogenetic abnormality, myelodysplasia, paroxysmal nocturnal hemoglobinuria, congenital aplastic anemia, Fanconi anemia, HIV seropositivity, uncontrolled infection, serum bilirubin level greater than 3 times or creatinine level more than 2 times the upper limit of normal, abnormal cardiac ejection fraction, or pregnancy. The institutional review board of all participating institutions approved the protocol, and all patients (or a legal guardian) provided written informed consent, in accordance with the Declaration of Helsinki. Patients were randomly assigned centrally and stratified by age (< 20 and ≥ 20 years), history of red blood cell transfusion, and prior ATG therapy. The randomization method consisted of an adaptive-biased coin design to avoid overrepresentation of patients in any given strata.
Treatment plan
Patients in the cyclophosphamide alone group received 50 mg/(kg · d) intravenously on days −5 to −2. Those patients randomly assigned to cyclophosphamide plus ATG received the identical cyclophosphamide dosing plus equine ATG (ATGAM; Upjohn, Kalamazoo, MI; or Lymphoglobulin Merieux; Laboratories Pasteur Merieux, Lyon, France) at 30 mg/kg on days −5 to −3. GVHD prophylaxis consisted of cyclosporine and methotrexate.
End points
The primary end point was survival. Secondary end points included graft failure and acute and chronic GVHD. The date of neutrophil recovery was scored as the first of 3 consecutive days with ANC 0.5 × 109/L or greater. Graft failure was scored as primary failure if ANC was less than 0.5 × 109/L on day 28 or secondary failure if there was neutrophil recovery followed by an otherwise unexplained fall to less than 0.5 × 109/L for 3 or more days.
Statistical methods
Our goal was to determine whether the addition of ATG to cyclophosphamide would improve survival at 1 year from 65% to 85% and lower the rate of graft rejection from the anticipated 15% to less than 5%. The study planned to accrue 224 patients, randomly assigned equally to both treatment groups, and had 90% power at the 0.05 level to detect a 20% difference in overall survival and 80% power to detect a 15% decrease in rate of graft failure.
The probabilities of day 100 mortality and survival were calculated using the Kaplan-Meier estimator. The probabilities of neutrophil and platelet recovery and acute and chronic GVHD were calculated using the cumulative incidence estimator; for these analyses, death without an event was the competing event.16 All P values are 2-sided. All analyses were performed in SAS 9.1 (Carey, NC).
Results and discussion
Patient characteristics are summarized in Table 1. The study was conducted between 1994 and 2001. The study accrued approximately 60% of the targeted enrollment. The Data and Safety Monitoring Board closed the study after an interim analysis showed no statistically significant differences in outcome between the treatment groups and a minimal probability of detecting a significant difference with the remaining accrual. Median follow-up of surviving patients is 6 years. Only one patient was lost to follow-up before the primary end point evaluation time of 1 year.
Characteristics of 130 eligible patients by treatment group
| Variables . | Cy . | Cy + ATG . | P* . | ||
|---|---|---|---|---|---|
| N eval . | N (%) . | N eval . | N (%) . | ||
| No. of patients | 60 | 70 | |||
| No. of centers | 27 | 29 | |||
| Median age, y (range) | 60 | 26 (4-51) | 70 | 23 (1-51) | .11 |
| Age at transplantation | 60 | 70 | .70 | ||
| Younger than 10 y | 5 (8) | 9 (13) | |||
| 10-19 y | 13 (22) | 20 (28) | |||
| 20-29 y | 18 (30) | 20 (28) | |||
| 30-39 y | 13 (22) | 12 (18) | |||
| At least 40 y | 11 (18) | 9 (13) | |||
| Male sex | 60 | 35 (58) | 70 | 46 (66) | .39 |
| Karnofsky score at least 90% | 60 | 32 (53) | 67 | 41 (61) | .37 |
| Prior treatment | 60 | 69 | .99 | ||
| None | 30 (50) | 37 (53) | |||
| ATG + CsA ± other | 4 (7) | 6 (8) | |||
| ATG ± other (not CsA) | 1 (1) | 1 (2) | |||
| CsA ± other (not ATG) | 9 (15) | 11 (16) | |||
| Corticosteroids | 4 (7) | 4 (6) | |||
| Corticosteroids + other | 5 (8) | 5 (7) | |||
| Other† | 7 (12) | 6 (8) | |||
| No. of donor exposures prior to transplantation | 60 | 70 | .04 | ||
| 0 | 3 (5) | 1 (2) | |||
| 1-10 | 20 (33) | 21 (30) | |||
| More than 10 | 30 (50) | 26 (37) | |||
| Transfusions given, unknown no. | 7 (12) | 22 (31) | |||
| Median nucleated cell dose, × 108/kg (range) | 44 | 2.4 (.01-5) | 59 | 2.7 (.01-7) | .82 |
| Donor-recipient sex match | 60 | 69 | .37 | ||
| Male → Male | 21 (35) | 26 (38) | |||
| Male → Female | 15 (25) | 9 (13) | |||
| Female → Male | 14 (23) | 20 (29) | |||
| Female → Female | 10 (17) | 14 (20) | |||
| Donor-recipient CMV status | 53 | 61 | .90 | ||
| Donor +/recipient + | 28 (53) | 29 (47) | |||
| Donor +/recipient − | 10 (19) | 11 (18) | |||
| Donor −/recipient + | 5 (9) | 6 (10) | |||
| Donor −/recipient − | 10 (19) | 15 (25) | |||
| Graft type | 60 | 69 | .56 | ||
| Bone marrow | 58 (96) | 68 (99) | |||
| PBS‡C | 1 (2) | 1 (1) | |||
| BM + PBSC‡ | 1 (2) | 0 | |||
| Median time from Dx to Tx, mo (range) | 60 | 2 (<1-109) | 69 | 2 (<1-70) | .63 |
| Time from Dx to Tx | 60 | 69 | .78 | ||
| Less than 3 mo | 41 (69) | 45 (65) | |||
| 3 to less than 6 mo | 9 (15) | 8 (12) | |||
| 6-12 mo | 5 (8) | 9 (13) | |||
| At least 12 mo | 5 (8) | 7 (10) | |||
| Year of transplantation | 60 | 69 | .65 | ||
| 1994-1995 | 16 (27) | 18 (26) | |||
| 1996-1997 | 23 (38) | 22 (32) | |||
| 1998-1999 | 13 (22) | 14 (20) | |||
| 2000-2001 | 8 (13) | 15 (22) | |||
| GVHD prophylaxis | 60 | 69 | .35 | ||
| CsA + MTX ± other‡§ | 60 (100) | 68 (99) | |||
| None‡ | 0 | 1 (1) | |||
| Total no. of transplantations‖; | 60 | 69 | .12 | ||
| 1 | 53 (88) | 62 (88) | |||
| 2 | 4 (7) | 8 (12) | |||
| More than 2 | 3 (5) | 0 | |||
| Median follow-up of survivors, mo¶ | 77 (5-124) | 75 (22-123) | |||
| Variables . | Cy . | Cy + ATG . | P* . | ||
|---|---|---|---|---|---|
| N eval . | N (%) . | N eval . | N (%) . | ||
| No. of patients | 60 | 70 | |||
| No. of centers | 27 | 29 | |||
| Median age, y (range) | 60 | 26 (4-51) | 70 | 23 (1-51) | .11 |
| Age at transplantation | 60 | 70 | .70 | ||
| Younger than 10 y | 5 (8) | 9 (13) | |||
| 10-19 y | 13 (22) | 20 (28) | |||
| 20-29 y | 18 (30) | 20 (28) | |||
| 30-39 y | 13 (22) | 12 (18) | |||
| At least 40 y | 11 (18) | 9 (13) | |||
| Male sex | 60 | 35 (58) | 70 | 46 (66) | .39 |
| Karnofsky score at least 90% | 60 | 32 (53) | 67 | 41 (61) | .37 |
| Prior treatment | 60 | 69 | .99 | ||
| None | 30 (50) | 37 (53) | |||
| ATG + CsA ± other | 4 (7) | 6 (8) | |||
| ATG ± other (not CsA) | 1 (1) | 1 (2) | |||
| CsA ± other (not ATG) | 9 (15) | 11 (16) | |||
| Corticosteroids | 4 (7) | 4 (6) | |||
| Corticosteroids + other | 5 (8) | 5 (7) | |||
| Other† | 7 (12) | 6 (8) | |||
| No. of donor exposures prior to transplantation | 60 | 70 | .04 | ||
| 0 | 3 (5) | 1 (2) | |||
| 1-10 | 20 (33) | 21 (30) | |||
| More than 10 | 30 (50) | 26 (37) | |||
| Transfusions given, unknown no. | 7 (12) | 22 (31) | |||
| Median nucleated cell dose, × 108/kg (range) | 44 | 2.4 (.01-5) | 59 | 2.7 (.01-7) | .82 |
| Donor-recipient sex match | 60 | 69 | .37 | ||
| Male → Male | 21 (35) | 26 (38) | |||
| Male → Female | 15 (25) | 9 (13) | |||
| Female → Male | 14 (23) | 20 (29) | |||
| Female → Female | 10 (17) | 14 (20) | |||
| Donor-recipient CMV status | 53 | 61 | .90 | ||
| Donor +/recipient + | 28 (53) | 29 (47) | |||
| Donor +/recipient − | 10 (19) | 11 (18) | |||
| Donor −/recipient + | 5 (9) | 6 (10) | |||
| Donor −/recipient − | 10 (19) | 15 (25) | |||
| Graft type | 60 | 69 | .56 | ||
| Bone marrow | 58 (96) | 68 (99) | |||
| PBS‡C | 1 (2) | 1 (1) | |||
| BM + PBSC‡ | 1 (2) | 0 | |||
| Median time from Dx to Tx, mo (range) | 60 | 2 (<1-109) | 69 | 2 (<1-70) | .63 |
| Time from Dx to Tx | 60 | 69 | .78 | ||
| Less than 3 mo | 41 (69) | 45 (65) | |||
| 3 to less than 6 mo | 9 (15) | 8 (12) | |||
| 6-12 mo | 5 (8) | 9 (13) | |||
| At least 12 mo | 5 (8) | 7 (10) | |||
| Year of transplantation | 60 | 69 | .65 | ||
| 1994-1995 | 16 (27) | 18 (26) | |||
| 1996-1997 | 23 (38) | 22 (32) | |||
| 1998-1999 | 13 (22) | 14 (20) | |||
| 2000-2001 | 8 (13) | 15 (22) | |||
| GVHD prophylaxis | 60 | 69 | .35 | ||
| CsA + MTX ± other‡§ | 60 (100) | 68 (99) | |||
| None‡ | 0 | 1 (1) | |||
| Total no. of transplantations‖; | 60 | 69 | .12 | ||
| 1 | 53 (88) | 62 (88) | |||
| 2 | 4 (7) | 8 (12) | |||
| More than 2 | 3 (5) | 0 | |||
| Median follow-up of survivors, mo¶ | 77 (5-124) | 75 (22-123) | |||
One hundred thirty-four recipients from 38 participating transplantation centers were enrolled between 1994 and 2001. Of these, 4 patients were excluded; 2 failed to meet criteria for SAA: hepatitis B seropositive donor (n = 1) and syngeneic donor (n = 1). Among the 130 eligible patients, there were 9 minor protocol violations. These violations included the following: 3 patients received peripheral blood grafts, 4 received growth factor for hematopoietic recovery prior to day +21, 1 patient did not receive GVHD prophylaxis, and 1 patient received tacrolimus and a single dose of methotrexate for GVHD prophylaxis. Of the 130 eligible patients, 60 were randomly assigned to receive cyclophosphamide and 70 to receive cyclophosphamide and antithymocyte globulin. One patient randomly assigned to receive cyclophosphamide and antithymocyte globulin died before transplantation.
Cy indicates cyclophosphamide; eval, evaluable; CsA, cyclosporine; ±, with or without; CMV, cytomegalovirus; PBSC, peripheral blood stem cells; Dx, diagnosis; Tx, transplantation; GVHD, graft-versus-host disease; MTX, methotrexate.
The Mantel-Haenszel test was used for discrete covariates; the Kruskal-Wallis test was used for continuous covariates.
Other types of treatments were cytokines (9), androgens (2), other immune suppression (2).
Protocol violation.
Other GVHD prophylaxis were corticosteroids (10), tacrolimus with only one dose of MTX (1), corticosteroids + ATG (2), ATG (2).
All transplantations on study were the patient's first transplantation.
From date of randomization.
Hematopoietic recovery
Neutrophil and platelet recoveries were similar in both groups. The day 28 probabilities of neutrophil recovery were 78% (95% CI, 67%-88%) for the cyclophosphamide alone group and 81% (95% CI, 71%-89%) for the cyclophosphamide plus ATG group (P = .69). Corresponding probabilities for platelet recovery at day 60 were 93% (95% CI, 86%-98%) and 97% (95% CI, 85%-98%), respectively (P = .90). Among patients receiving cyclophosphamide alone, 3 did not achieve neutrophil recovery and 8 patients had initial recovery followed by a sustained decline. Among recipients of cyclophosphamide plus ATG, 2 did not achieve neutrophil recovery and 9 had initial recovery followed by a sustained decline. The median total nucleated cell dose infused in patients with primary and secondary graft failure did not differ by conditioning regimen, 2.88 × 108/kg in the cyclophosphamide group and 2.03 × 108/kg in the cyclophosphamide plus ATG group. Fifteen of 22 patients with graft failure underwent second BMT from their initial donor (7 and 8 patients from each group). Twelve of these patients are alive with a median follow-up of 54 months from their second transplantations.
Acute and chronic GVHD
Rates of grades 2 to 4 acute GVHD and chronic GVHD were similar in both treatment groups. The day 100 probabilities of grades 2 to 4 acute GVHD were 18% (95% CI, 10%-29%) for the cyclophosphamide group and 11% (95% CI, 6%-22%) for the cyclophosphamide plus ATG group. Corresponding 5-year probabilities of chronic GVHD were 21% (95% CI, 12%-33%) and 32% (95% CI, 21%-44%).
Infections
Forty of 60 patients in the cyclophosphamide alone group and 55 of 68 patients in the cyclophosphamide plus ATG group developed infections after transplantation (P = .07). The proportion of patients with bacterial, viral, and fungal infections was similar in both groups.
Survival
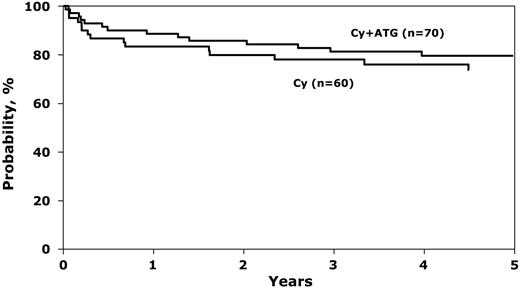
There were no statistically significant differences in survival by treatment group (Figure 1). Day 100 mortality rates were 12% (95% CI, 5%-21%) with cyclophosphamide alone and 7% (95% CI, 2%-14%) with cyclophosphamide and ATG (P = .38). Corresponding probabilities of survival at 1 year were 83% (95% CI, 73%-92%) and 89% (95% CI, 80%-95%), respectively. The excellent 5-year overall probabilities of survival, 74% after cyclophosphamide alone and 80% after cyclophosphamide and ATG, compares favorably with other multicenter studies and may be explained by advances in supportive care.17,18 Causes of death were similar in the 2 groups, primarily graft failure, GVHD, and infection.
Probability of overall survival after BMT for SAA, by conditioning regimen. The 1-year probability of survival was 83% (95% CI, 73%-92%) and 89% (95% CI, 80%-95%) after cyclophosphamide alone and cyclophosphamide plus ATG, respectively (P = .39). Corresponding 5-year probability was 74% (95% CI, 61%-84%) and 80% (95% CI, 69%-88%), P = .44.
Probability of overall survival after BMT for SAA, by conditioning regimen. The 1-year probability of survival was 83% (95% CI, 73%-92%) and 89% (95% CI, 80%-95%) after cyclophosphamide alone and cyclophosphamide plus ATG, respectively (P = .39). Corresponding 5-year probability was 74% (95% CI, 61%-84%) and 80% (95% CI, 69%-88%), P = .44.
With the current sample size, and 1-year survival rate of 83% in the cyclophosphamide group, the study had only a 50% chance of detecting an improvement in 1-year survival to 95% after cyclophosphamide plus ATG. One hundred sixty patients in each group would be required to have 90% power at the 0.05 level to detect this difference. Given the low prevalence of SAA, such a trial is unlikely to be done. In conclusion, the results of allogeneic BMT for SAA have improved over time related to advances in supportive care. The addition of ATG to cyclophosphamide in the preparative regimen did not significantly improve engraftment, GVHD, or survival in this prospective randomized trial.
Authorship
Contribution: R.E.C., J.R.P., B.M.C., J.P.K., and M.M.H. designed the study; W.S.P. and J.P.K. performed the statistical analysis; R.E.C. and M.E. prepared the manuscript; and all authors participated in interpretation of data and approved the final manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Richard E. Champlin, M. D. Anderson Cancer Center, University of Texas, Houston, TX 77030-4095; e-mail: rchampli@mdanderson.org.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by Public Health Service Grant U24-CA76518 from the National Cancer Institute, the National Institute of Allergy and Infectious Diseases, and the National Heart, Lung, and Blood Institute; Office of Naval Research; Health Services Research Administration (DHHS); grants from AABB, Abbott Laboratories; Aetna; AIG Medical Excess; American Red Cross; Amgen; AnorMED; Astellas Pharma US; Berlex Laboratories; Biogen IDEC; Blue Cross and Blue Shield Association; BRT Laboratories; Celgene; Cell Therapeutics; CelMed Biosciences; Cubist Pharmaceuticals; Dynal Biotech; Edwards Lifesciences RMI; Endo Pharmaceuticals; Enzon Pharmaceuticals; ESP Pharma; Gambro BCT; Genzyme; GlaxoSmithKline; Histogenetics; Human Genome Sciences; International Waldenstrom Macroglobulinemia Foundation; Kirin Brewery Company; Ligand Pharmaceuticals; Merck; Millennium Pharmaceuticals; Miller Pharmacal Group; Milliman USA; Miltenyi Biotec; National Center for Biotechnology Information; National Leukemia Research Association; National Marrow Donor Program; Nektar Therapeutics; NeoRx; Novartis Pharmaceuticals; Novo Nordisk Pharmaceuticals; Ortho Biotech; Osiris Therapeutics; Pall Medical; Pfizer; Pharmion; Protein Design Labs; QOL Medical; Roche Laboratories; StemCyte; Stemco Biomedical; StemSoft Software; SuperGen; Sysmex; The Marrow Foundation; THERAKOS, Johnson & Johnson; University of Colorado Cord Blood Bank; Valeant Pharmaceuticals; ViaCell; ViraCor Laboratories; W. B. Saunders Mosby Churchill; Wellpoint; Zelos Therapeutics; and an anonymous donation to the Medical College of Wisconsin.
The views expressed in this article do not reflect the official policy or position of the National Institute of Health, the Department of the Navy, the Department of Defense, or any other agency of the US government.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal