Abstract
Murine models of bone marrow transplantation were used to study the mechanisms governing the activation of donor lymphocyte infusions (DLIs) manifesting as lymphohematopoietic graft-versus-host (LH-GVH) and graft-versus-leukemia (GVL) reactivities. We demonstrate here that established mixed chimerism influences the potency of DLI-mediated alloreactivity only in the MHC-mismatched but not MHC-matched setting. In the MHC-matched setting, high levels (≥ 40%) of residual host chimerism correlated negatively with DLI-mediated alloreactivity irrespective of the timing of their administration, the donor's previous sensitization to host antigens, or the level of residual host APCs. In vivo administration of Toll-like receptor (TLR) ligands was required to maximize DLI-mediated LH-GVH and GVL reactivities in chimeras with low levels (≤ 15%) of residual host chimerism. In contrast, coadministration of DLI with antigen-presenting cell (APC) activators was insufficient to augment their LH-GVH response in the presence of high levels of host chimerism unless the host's T cells were transiently depleted. Together, these results show the cardinal influence of donor-host incompatibility on DLI-mediated GVH responses and suggest that in MHC-matched chimeras, the induction of optimal alloreactivity requires not only donor T cells and host APCs but also TLR ligands and in the presence of high levels of host chimerism depletion of host T cells.
Introduction
The ability of donor lymphocyte infusions (DLIs) to induce complete remissions of chronic myeloid leukemia in patients relapsing after allogeneic bone marrow transplantation (alloBMT) has provided the most direct clinical evidence for the existence of the T cell-mediated graft-versus-leukemia (GVL) effect.1,2 Unfortunately, the efficacy of DLI in the treatment of other relapsed hematologic malignancies after alloBMT is much lower. Although several different approaches to separate the GVL effect from graft-versus-host disease (GVHD) have achieved some clinical success,3–8 the underlying immunologic mechanisms of the improved outcomes remain poorly defined.9 An understanding of the factors and mechanisms that govern the DLI-derived T cell-mediated immune responses may be critical for both decreasing GVHD and optimizing their antitumor efficacy.
The induction of allogeneic T-cell responses requires an encounter between lymphocytes and antigen-presenting cells (APCs) in secondary lymphoid tissues.10,11 The critical role of host-versus-donor APCs in initiating or sustaining GVHD and the GVL effect in mice immediately after conditioning has been a focus of recent extensive studies led by several groups of investigators.12–17 However, interactions between T cells and APCs late after transplantation are less well understood and in part may differ from those immediately after conditioning, because DLI-mediated graft-versus-host (GVH) and GVL reactivities can be achieved without GVHD.15,18 This is because reconstituted donor-derived and residual host-derived dendritic cells (DCs) may be present to varying degrees, the conditioning-induced inflammatory response has subsided, tolerance may have been established, and immunoregulatory networks may exist.18–20 These factors may have a profound influence on the ability of T cells infused late after transplantation to mediate the GVL effect or GVHD or both. We demonstrate here that the contribution of these factors in governing DLI-derived T cell-mediated alloimmune responses in the MHC-matched and MHC-mismatched settings differs and that the mechanisms underlying them can be therapeutically explored for augmenting the GVH and GVL reactivities mediated by the adoptively transferred donor T cells.
Materials and methods
Mice
C57BL/6 (B6; H-2b), B6.SJL (H-2b; CD45.1), and BALB/cAnNCr (H-2d; CD45.2) mice were obtained from the National Cancer Institute (Frederick, MD). B10.D2 (H-2d H2-T18c Hc1/nSnJ), B6.PL-Thy1a/CyJ (H-2b; Thy1.1), and C3H.SW-H2b/SnJ (H-2b; CD45.2) mice were obtained from Jackson Laboratories (Bar Harbor, ME). B10.D2-Thy1.1 (H-2d; Thy1.1), BALB/c-Thy1.1 (H-2d; Thy1.1), and BALB/c-CD45.1 (H-2d CD45.1; F12 generation) strains were propagated in our facility. Animals were maintained in microisolator cages and were approximately 8 to 12 weeks of age at the time of transplantation. All experiments were performed in accordance with protocols approved by the Animal Care and Use Committee of the Johns Hopkins University.
Hematopoietic cell transplantation, DLI, and CFSE labeling
Animals were lethally irradiated (B6: 900 cGy, BALB/c: 850 cGy) in a 137Cs irradiator (Gammacell 40; Atomic Energy of Canada, Ottawa, ON, Canada) at an exposure rate of approximately 73 cGy/min. “Mixed” chimeras were constructed by reconstituting lethally irradiated recipients with mixture of T cell-depleted (TCD) donor (107) and host (5 × 106) bone marrow (BM) cells, whereas “full” chimeras were constructed by reconstituting lethally irradiated recipients with TCD donor (107-1.5 × 107) BM cells only, unless otherwise noted.15,21 TCD of BM was performed as previously described.22 DLI consisted of 2 × 107 donor splenocytes, unless otherwise noted. CFSE (Molecular Probes, Eugene, OR) labeling of DLI was performed as previously described.23 For presensitization of donors against BALB/c alloantigens, B10.D2 mice were either primed intraperitoneally with BALB/c splenocytes or received full-thickness skin grafts.24,25 The stimulatory and control CpG oligodeoxynucleotides (ODNs) used in this study were CpG1826 (5′-TCCATGACGTTCCTGACGTT3′) and CpG1982 (5′-TCCAGGACTTCTCTCAGGTT3′), respectively.26 CpG ODNs (Oligos Etc, Wilsonville, OR) were administered intraperitoneally in a dose of 150 μg/mouse on each of days 0, +3 and +7 after DLI. Imiquimod (Aldara, 3M Pharmaceuticals, St Paul, MN) was applied topically at a dose of 6.25 mg on each of days −3 to −1 prior to DLI on shaved the chest/abdomen and on day of DLI administration on the back. Anti-CD25 monoclonal antibody (mAb; PC61; 0.5 mg/dose) and anti-Thy1.2 mAb (30H12; 1 mg/dose) were administered on days −7 and −4 before DLI as previously described.19,27,28
Isolation of DCs from spleen, lymph nodes, and epidermal sheets
Harvested spleens and cutaneous lymph nodes LNs (CLNs: pooled submandibular, cervical, brachial, and axillary nodes) were incubated with collagenase D (400 U/mL; Roche, Indianapolis, IN) for 60 and 30 minutes, respectively. The CD11c+ fraction was isolated using CD11c Microbeads (Miltenyi Biotec, Auburn, CA) according to the manufacturer's protocol. Epidermal sheets were cultured in the presence of GM-CSF and TNF-α and prepared for fluorescence-activated cell sorting (FACS) analysis as described previously.29
Ex vivo generation and maturation of DCs
BM-derived DCs were generated ex vivo in the presence of GM-CSF and IL-4 and matured for 24 hours with lipopolysaccharide (LPS) from Escherichia coli serotype 055:B5 (Sigma-Aldrich, St Louis, MO).30 DC maturation status was confirmed by flow cytometry.
Flow cytometric analysis
Spleens, LNs, or peripheral blood were obtained and prepared for flow cytometry as previously described.22 The mAbs used were biotinylated or fluorochrome-conjugated anti-CD45.1, anti–H-2b, anti-Ly9.1, anti-CD90.1 (Thy1.1), anti–interferon-γ (IFN-γ), anti–IL-2, rat anti–mouse IgG1, rat anti–mouse IgG2b, anti-CD11c, anti-CD4, and anti-CD8 (BD Biosciences, San Diego, CA). Data were acquired on FACSCalibur flow cytometer (BD Biosciences) and were analyzed using FlowJo software (Tree Star, Ashland, OR). To determine IFN-γ production, cells were stimulated with 4α-phorbol-12-myristate-13-acetate (PMA; 3 ng/mL) and ionomycin (500 ng/mL; Sigma-Aldrich) for 5 hours in culture in the presence of GolgiStop (BD Biosciences). Thereafter cells were stained according to the manufacturer's protocol (CytoFix/CytoPerm kit, BD Biosciences).
Statistical analysis
Data were analyzed using PRISM software (version 4.0; GraphPad, San Diego, CA). Values are presented as the mean ± SEM. The statistical differences were calculated using Student 2-tailed t test. A value of P below .05 was considered statistically significant.
Results
The residual host chimerism influences the potency of DLI-mediated GVH reactivity only in the MHC-mismatched but not MHC-matched setting
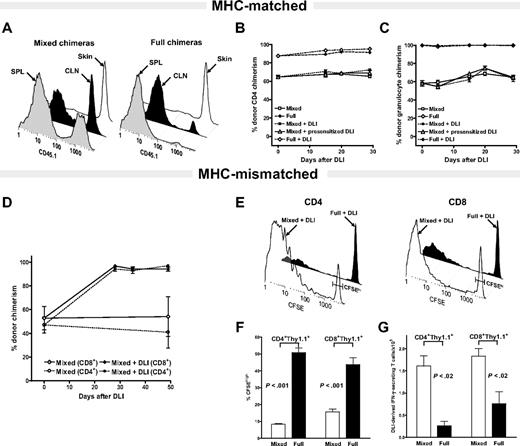
To determine the role of residual host APCs on the DLI-mediated LH-GVH response in the MHC- matched and MHC-mismatched setting, “mixed” and “full” chimeras with a different amounts of residual host chimerism were constructed.15,21 To distinguish the origin of DCs in MHC-matched, mHAg-mismatched setting we relied on the differential expression of alleles of CD45, the common leukocyte antigen. As depicted in Figure 1A, 27.5% ± 0.88% and 42.83% ± 1.87% of splenic and CLNs CD11c+ DCs in B10.D2 + BALBc-CD45.1→BALB/c-CD45.1 mixed and 2.3% ± 0.84% and 10.17% ± 2.97% of splenic and CLNs CD11c+ DCs in B10.D2→BALB/c-CD45.1 full chimeras, respectively, were host-derived (P < .05 for both spleen and CLNs CD11c+ DCs; mixed versus full). The persistence of 98.56% ± 1.19% and 93.96% ± 2.16% of residual host CD11c+ DCs in the skin of mixed and full MHC-matched, mHAg-mismatched chimeras, respectively, is consistent with our recent findings suggesting different levels of donor DC chimerism in skin versus lymphoid tissues.29
The level of residual host CD11c+ DCs does not influence the DLI-mediated LH-GVH response in MHC-matched in contrast to MHC-mismatched chimeras. (A-C) MHC-matched setting. (A) DC chimerism analysis of 8-week-old B10.D2 + BALB/c-CD45.1→BALB/c-CD45.1 mixed and B10.D2→BALB/c-CD45.1 full chimeras. Representative histograms show differing levels of residual host DC chimerism in the spleen (SPL) and cutaneous lymph nodes (CLNs) of mixed versus full chimeras and near complete persistence of host-derived CD11c+ DCs in the skin of both groups. Data represent results of 2 independent experiments. (B-C) Serial analysis of multilineage chimerism in peripheral blood after DLI administration. The 8-week-old B10.D2 + BALB/c-CD45.1→BALB/c-CD45.1 mixed chimeras received nothing (□; n = 5), 2 × 107 splenocytes from naive B10.D2 donor mice (▪; n = 5) or the same dose of DLI from B10.D2 mice presensitized to host antigens (▵; n = 5). 8-week-old of B10.D2→BALB/c-CD45.1 full chimeras received nothing (⋄; n = 5) or 2 × 107 splenocytes from the same naive donor source (♦; n = 5). Changes in donor CD4+ T-cell (B) and granulocyte chimerism (C) were monitored by serial tail bleeding and flow cytometric analysis using fluorochrome-conjugated antibodies against Gr-1, CD4, and CD45.1. Data are presented as a mean percentage of donor chimerism ± SEM and represent one of 2 independent experiments. (D-G) MHC-mismatched setting. At 8 weeks after alloBMT MHC-mismatched B6 + BALB/c→BALB/c mixed chimeras received nothing (open symbols) or DLI (filled symbols) in the form of 2 × 107 splenocytes from donor B6 mice. (D) Changes in CD4+ (□, ▪) and CD8+ (⋄, ♦) T-cell chimerism were determined in peripheral blood by flow cytometry after staining with anti–H-2b–, anti-CD4–, and anti-CD8–specific antibodies. Data are presented as a mean percentage of donor chimerism ± SEM (n = 5 mice/group). (E) At 8 weeks after alloBMT 2 × 107 CFSE-labeled splenocytes from B6.PL-Thy1a mice were transferred to B6 + BALB/c→BALB/c mixed and B6→BALB/c full donor chimeras. Representative CFSE profiles of gated DLI-derived CD4+Thy1.1+ and CD8+Thy1.1+ T cells analyzed using anti-Thy1.1–, anti-CD4–, and anti-CD8–specific antibodies on day 5 after adoptive transfer. Gating used to delineate unproliferated CFSEhi from fast proliferating DLI-derived T-cells is indicated. (F) Percentages of gated CFSEhi DLI-derived CD4+ and CD8+ T cells in spleens of mixed and full chimeras on day +5 after DLI. (G) Absolute numbers of IFN-γ–producing DLI-derived T cells in the spleens of mixed and full MHC-mismatched chimeras on day +5 following DLI (after brief ex vivo stimulation). Data shown represent 1 of 3 independent experiments (mean ± SEM; n = 3-4 mice/group).
The level of residual host CD11c+ DCs does not influence the DLI-mediated LH-GVH response in MHC-matched in contrast to MHC-mismatched chimeras. (A-C) MHC-matched setting. (A) DC chimerism analysis of 8-week-old B10.D2 + BALB/c-CD45.1→BALB/c-CD45.1 mixed and B10.D2→BALB/c-CD45.1 full chimeras. Representative histograms show differing levels of residual host DC chimerism in the spleen (SPL) and cutaneous lymph nodes (CLNs) of mixed versus full chimeras and near complete persistence of host-derived CD11c+ DCs in the skin of both groups. Data represent results of 2 independent experiments. (B-C) Serial analysis of multilineage chimerism in peripheral blood after DLI administration. The 8-week-old B10.D2 + BALB/c-CD45.1→BALB/c-CD45.1 mixed chimeras received nothing (□; n = 5), 2 × 107 splenocytes from naive B10.D2 donor mice (▪; n = 5) or the same dose of DLI from B10.D2 mice presensitized to host antigens (▵; n = 5). 8-week-old of B10.D2→BALB/c-CD45.1 full chimeras received nothing (⋄; n = 5) or 2 × 107 splenocytes from the same naive donor source (♦; n = 5). Changes in donor CD4+ T-cell (B) and granulocyte chimerism (C) were monitored by serial tail bleeding and flow cytometric analysis using fluorochrome-conjugated antibodies against Gr-1, CD4, and CD45.1. Data are presented as a mean percentage of donor chimerism ± SEM and represent one of 2 independent experiments. (D-G) MHC-mismatched setting. At 8 weeks after alloBMT MHC-mismatched B6 + BALB/c→BALB/c mixed chimeras received nothing (open symbols) or DLI (filled symbols) in the form of 2 × 107 splenocytes from donor B6 mice. (D) Changes in CD4+ (□, ▪) and CD8+ (⋄, ♦) T-cell chimerism were determined in peripheral blood by flow cytometry after staining with anti–H-2b–, anti-CD4–, and anti-CD8–specific antibodies. Data are presented as a mean percentage of donor chimerism ± SEM (n = 5 mice/group). (E) At 8 weeks after alloBMT 2 × 107 CFSE-labeled splenocytes from B6.PL-Thy1a mice were transferred to B6 + BALB/c→BALB/c mixed and B6→BALB/c full donor chimeras. Representative CFSE profiles of gated DLI-derived CD4+Thy1.1+ and CD8+Thy1.1+ T cells analyzed using anti-Thy1.1–, anti-CD4–, and anti-CD8–specific antibodies on day 5 after adoptive transfer. Gating used to delineate unproliferated CFSEhi from fast proliferating DLI-derived T-cells is indicated. (F) Percentages of gated CFSEhi DLI-derived CD4+ and CD8+ T cells in spleens of mixed and full chimeras on day +5 after DLI. (G) Absolute numbers of IFN-γ–producing DLI-derived T cells in the spleens of mixed and full MHC-mismatched chimeras on day +5 following DLI (after brief ex vivo stimulation). Data shown represent 1 of 3 independent experiments (mean ± SEM; n = 3-4 mice/group).
Having characterized the level of residual host CD11c+ DC persistence in MHC-matched, mHAg-mismatched setting, 8-week-old mixed and full chimeras received DLIs in the form of 2 × 107 donor splenocytes. Changes in donor multilineage chimerism reflective of DLI-mediated lymphohematopoietic graft-versus-host (LH-GVH) reactivity were measured in peripheral blood, spleen, and skin based on the differential expression of the CD45.1 marker. Surprisingly, administration of DLIs to the mixed chimeras containing large amounts of residual host CD11c+ DCs had only minimal effect in augmenting donor T-cell and myeloid chimerism (Figure 1B-C), or increasing donor CD11c+ DC chimerism in the spleen or skin (Figure S1, available on the Blood website; see the Supplemental Figure link at the top of the online article); administration of 2 times higher dose of donor splenocytes (data not shown) or presensitized DLI-derived T cells had only a minor effect on increasing donor multilineage chimerism (Figures 1B-C and S1). Despite the presence of residual host DCs in the CLNs and skin, DLI administration to the full chimeras was also ineffective in inducing an LH-GVH response capable of eradicating approximately 15% of residual host T cells (Figure 1B). Failure of DLI to significantly augment donor chimerism was observed in multiple experiments in which changes of donor chimerism were monitored for 100 days or longer after DLI administration.
The inability of MHC-matched, mHAg-mismatched DLIs to convert mixed to full donor chimeras in the presence of a large amount of residual host CD11c+ DCs prompted us to examine how the level of residual host APCs influences the DLI-mediated LH-GVH responses in the MHC-mismatched setting. First, we confirmed that administration of the same dose of 2 × 107 donor splenocytes to the B6 + BALB/c→BALB/c MHC-mismatched mixed chimeras results in rapid conversion to full donor chimerism (Figure 1D).15,22 Second, we examined the fate of the DLI-derived T cells in B6 + BALB/c→BALB/c mixed and B6→BALB/c full chimeras. As DLI, we used CFSE-labeled splenocytes from B6.PL-Thy1a mice, which differ from the donor and host in the expression of the CD90.1 marker on T cells. DLI-derived T-cell proliferation and their accumulation in the spleens of chimeras were determined at days +5 and +15 after adoptive transfer. At day +5 following DLI, the overall percentage of undivided CFSEhi DLI-derived CD4+ and CD8+ T cells was markedly lower in mixed versus full chimeras (P < .001, Figure 1E-F; P < .02 day +15, data not shown). Consistent with in vivo proliferation, the absolute number of DLI-derived CD4+ T cells that accumulated in spleen, including those producing IFN-γ, was markedly higher in mixed chimeras on days +5 and +15 (P < .005; Figure 1G and data not shown). The same was observed in CD4+ T cells producing IFN-γ (P < .02; Figure 1G). The absolute number of DLI-derived CD8+ T cells was also higher in mixed chimeras on day +15 (P < .05, data not shown), whereas the absolute number of IFN-γ–secreting adoptively transferred CD8+ T cells was significantly higher in mixed chimeras at both time points (P < .02; Figure 1G and data not shown).
Taken together, LH-GVH reactivity following administration of DLI to established mixed chimeras is dependent primarily on reactivity to MHC antigens rather than mHAgs. Thus, a paradigm established in the MHC-mismatched setting suggesting that DLIs mediate superior alloreactivity in mixed compared to full allogeneic chimeras may be irrelevant after MHC-matched, mHAg-mismatched allografting, an observation consistent with clinical studies.31,32 Because transplantation of HLA-matched allogeneic stem cells is by far the most common graft source for treatment of hematologic malignancies in humans, we focused next on exploring the mechanisms and factors governing the anti-mHAg responses of adoptively transferred donor T cells in the clinically relevant MHC-matched, mHAg-mismatched setting.
DLI-derived T-cell proliferation and expansion in MHC-matched, mHAg-mismatched chimeras is not influenced by the level of residual host CD11c+ DCs
Given the importance of in vivo proliferation, clonal expansion and production of IFN-γ by the adoptively transferred T cells in MHC-mismatched chimeras, we next characterized the same end points in the MHC-matched chimeras with varying amounts of residual host CD11c+ DCs. Because MHC-matched, mHAg-mismatched DLI-derived T cells may exhibit less prominent alloantigen-driven in vivo proliferation that may be confounded with lymphopenia-driven proliferation, we contrasted the in vivo fate of adoptively transferred T cells in allogeneic with those in the syngeneic chimeras. As DLIs, we used CFSE-labeled splenocytes from mice that are congenic to the donors but differ in the expression of the CD90.1 marker. Consistent with previous studies,33,34 we found that adoptively transferred T cells can be separated into 3 populations based on their proliferative activity: (1) nonproliferating (CFSEhi), (2) slowly proliferating (CFSEslow), and (3) fast proliferating (CFSEfast) cells (Figure 2A). Interestingly, the overall division kinetics of DLI-derived CD4+ T cells determined as a percentage of unproliferated CFSEhi T cells was not statistically different between mixed and full chimeras (Figure 2B; similar results were obtained with CD8+ T cells). However, DLI-derived T-cell proliferation in both sets of allogeneic chimeras was faster than in syngeneic chimeras as determined by a lower percentage of CFSEhi cells (P < .05 for both CD4+ and CD8+; Figure 2B and data not shown). Consistent with in vivo proliferation data, the absolute number of DLI-derived T cells and those producing the IFN-γ was similar in both mixed and full chimeras (Table 1). Conversely, presensitized DLI-derived CD8+ T cells in mixed (P < .05) and both CD4+ and CD8+ T cells in full (P < .05 for CD4 and CD8) chimeras divided faster than naive DLI-derived T cells (data not shown). Despite the more prominent in vivo proliferation of presensitized CD8+ T cells in the both sets of chimeras (data not shown) the total number of DLI-derived CD8+ T cells was statistically higher only in full but not in the mixed chimeras (Table 1; P < .05). This prominent expansion of the presensitized DLI-derived T cells in full but not mixed chimeras was associated with a rapid conversion to 100% donor T-cell chimerism (P < .01, Figure 2C versus Figure 1B-C).
Timing of DLI administration but not the level of residual donor chimerism influences the fate of adoptively transferred T cells in MHC-matched, mHAg-mismatched chimeras. (A-B) Splenocytes (2 × 107) from naive or presensitized B10.D2-Thy1.1 mice were CFSE-labeled before transfer to 8-week-old B10.D2→BALBc-CD45.1 full and BALB/c-CD45.1 + B10.D2→ BALB/c-CD45.1 mixed allogeneic chimeras. The same dose of CFSE-labeled splenocytes from BALB/cThy1.1+ mice was transferred to 8-week-old syngeneic BALB/c→BALB/c chimeras. Adoptively transferred T cells were analyzed using anti-Thy1.1–, anti-CD4–, and anti-CD8–specific antibodies. (A) Representative CFSE profiles of DLI-derived CD8+ T cells (day +29 after DLI) and gating used to delineate in vivo unproliferated CFSEhi from slow-proliferating CFSEslow and fast-proliferating CFSEfast DLI-derived T cells in allogeneic and syngeneic chimeras. (B) CFSE dilution of DLI-derived CD4+ T cells in the spleen of the 8-week-old mixed (▪) and full (♦) chimeras on days +5, +14, and +28 after DLI administration was contrasted with adoptively transferred DLI-derived T cells in syngeneic chimeras (▾). Data are presented as a mean percentage of CFSEhi CD4+Thy1.1+ ± SEM and represent one of 2 independent experiments (n = 3-4 mice/group/time point). *P < .05 (▪ versus ▾) and *P < .05 (♦] versus ▾). (C) Rapid conversion to full donor T-cell chimerism in peripheral blood of full chimeras that received presensitized DLI. Changes in donor T-cell chimerism were determined by serial tail bleeding and staining for Ly9.1 and lineage-specific markers (mean percentage donor chimerism ± SEM; n = 5 mice/group). *P < .001 (CD4+; ▪ versus □) and #P < .01 (CD8+; ♦ versus ⋄). (D-E) Timing of DLI but not transfer of host CD11c+ DCs influences clonal expansion of IFN-γ–secreting DLI-derived T cells. B10.D2→BALB/c full chimeras received DLI from B10.D2-Thy1.1 mice, 4 (▪) or 8 (♦) weeks after alloBMT. Additional groups of 4- and 8-week-old full chimeras received with DLI 2 × 106 host CD11c+ DCs (▵, ○). Absolute numbers of IFN-γ–secreting DLI-derived CD4+ Thy1.1+ (D) and CD8+Thy1.1+ (E) T cells were determined in spleens on days +5, +14, and +28 after DLI. Mean ± SEM is shown; n ≥ 3 mice/time point and group. *P < .05 (▪, ▵ versus ♦; for both CD4+ Thy1.1+ and CD8+Thy1.1+). (F) Transferred host derived CD11c+ DCs persist in MHC-matched, mHAg-mismatched chimeras. A total of 2 × 106 splenic CD11c+ DCs from BALB/c-CD45.1 mice and 2 × 107 splenocytes from the B10.D2-Thy1.1 mice were injected intravenously to 4-week-old B10.D2→BALB/c chimeras. On days +1, +7, and +28 after transfer, the presence of host-derived CD11c+ DCs in the BM, CLNs, mesenteric LNs (MLNs), and SPL was determined by flow cytometry using anti-CD11c– and anti-CD45.1–specific antibodies. The absolute number of host-derived CD11c+ DCs that had homed to BM, CLNs, MLNs, and SPL was calculated by multiplying the percentage of CD45.1+CD11c+ DCs by the total number of cells.
Timing of DLI administration but not the level of residual donor chimerism influences the fate of adoptively transferred T cells in MHC-matched, mHAg-mismatched chimeras. (A-B) Splenocytes (2 × 107) from naive or presensitized B10.D2-Thy1.1 mice were CFSE-labeled before transfer to 8-week-old B10.D2→BALBc-CD45.1 full and BALB/c-CD45.1 + B10.D2→ BALB/c-CD45.1 mixed allogeneic chimeras. The same dose of CFSE-labeled splenocytes from BALB/cThy1.1+ mice was transferred to 8-week-old syngeneic BALB/c→BALB/c chimeras. Adoptively transferred T cells were analyzed using anti-Thy1.1–, anti-CD4–, and anti-CD8–specific antibodies. (A) Representative CFSE profiles of DLI-derived CD8+ T cells (day +29 after DLI) and gating used to delineate in vivo unproliferated CFSEhi from slow-proliferating CFSEslow and fast-proliferating CFSEfast DLI-derived T cells in allogeneic and syngeneic chimeras. (B) CFSE dilution of DLI-derived CD4+ T cells in the spleen of the 8-week-old mixed (▪) and full (♦) chimeras on days +5, +14, and +28 after DLI administration was contrasted with adoptively transferred DLI-derived T cells in syngeneic chimeras (▾). Data are presented as a mean percentage of CFSEhi CD4+Thy1.1+ ± SEM and represent one of 2 independent experiments (n = 3-4 mice/group/time point). *P < .05 (▪ versus ▾) and *P < .05 (♦] versus ▾). (C) Rapid conversion to full donor T-cell chimerism in peripheral blood of full chimeras that received presensitized DLI. Changes in donor T-cell chimerism were determined by serial tail bleeding and staining for Ly9.1 and lineage-specific markers (mean percentage donor chimerism ± SEM; n = 5 mice/group). *P < .001 (CD4+; ▪ versus □) and #P < .01 (CD8+; ♦ versus ⋄). (D-E) Timing of DLI but not transfer of host CD11c+ DCs influences clonal expansion of IFN-γ–secreting DLI-derived T cells. B10.D2→BALB/c full chimeras received DLI from B10.D2-Thy1.1 mice, 4 (▪) or 8 (♦) weeks after alloBMT. Additional groups of 4- and 8-week-old full chimeras received with DLI 2 × 106 host CD11c+ DCs (▵, ○). Absolute numbers of IFN-γ–secreting DLI-derived CD4+ Thy1.1+ (D) and CD8+Thy1.1+ (E) T cells were determined in spleens on days +5, +14, and +28 after DLI. Mean ± SEM is shown; n ≥ 3 mice/time point and group. *P < .05 (▪, ▵ versus ♦; for both CD4+ Thy1.1+ and CD8+Thy1.1+). (F) Transferred host derived CD11c+ DCs persist in MHC-matched, mHAg-mismatched chimeras. A total of 2 × 106 splenic CD11c+ DCs from BALB/c-CD45.1 mice and 2 × 107 splenocytes from the B10.D2-Thy1.1 mice were injected intravenously to 4-week-old B10.D2→BALB/c chimeras. On days +1, +7, and +28 after transfer, the presence of host-derived CD11c+ DCs in the BM, CLNs, mesenteric LNs (MLNs), and SPL was determined by flow cytometry using anti-CD11c– and anti-CD45.1–specific antibodies. The absolute number of host-derived CD11c+ DCs that had homed to BM, CLNs, MLNs, and SPL was calculated by multiplying the percentage of CD45.1+CD11c+ DCs by the total number of cells.
DLI-derived T-cell expansion and production of IFN-γ in MHC-matched, mHAg-mismatched chimeras is not influenced by the amount of residual host APCs
| Chimera and time of DLI . | Source of DLI . | Day 5 . | Day 12 . | Day 29 . | |||
|---|---|---|---|---|---|---|---|
| CD4+ . | CD8+ . | CD4+ . | CD8+ . | CD4+ . | CD8+ . | ||
| T-cell expansion, 8 wk, cells/spleen × 105 | |||||||
| B10.D2+BALB/c→BALB/c | Naive | 2.0 ± 0.02 | 1.1 ± 0.01 | 2.5 ± 0.1 | 1.7 ± 0.07 | 1.5 ± 0.04 | 1.2 ± 0.02 |
| B10.D2→BALB/c | Naive | 1.7 ± 0.03 | 1.0 ± 0.01 | 2.9 ± 0.15 | 2.4 ± 0.11 | 1.4 ± 0.02 | 1.5 ± 0.04 |
| B10.D2+BALB/c→BALB/c | Presensitized | 2.3 ± 0.02 | 1.3 ± 0.02 | 2.0 ± 0.08 | 1.4 ± 0.04 | 1.9 ± 0.01 | 1.5 ± 0.06 |
| B10.D2→BALB/c | Presensitized | 7.1 ± 0.07 | 3.8 ± 0.06 | 5.9 ± 0.17* | 42.5 ± 1.3* | 3.7 ± 0.18 | 7.6 ± 0.35* |
| IFN-γ production, 8 wk, cells/spleen × 104 | |||||||
| B10.D2+BALB/c→BALB/c | Naive | 1.78 ± 0.3 | 0.31 ± 0.23 | 1.19 ± 0.32 | 1.79 ± 0.88 | 0.81 ± 0.28 | 0.48 ± 0.17 |
| B10.D2→BALB/c | Naive | 1.63 ± 0.1 | 0.26 ± 0.08 | 2.23 ± 1.31 | 1.99 ± 0.95 | 0.89 ± 0.22 | 1.15 ± 0.71 |
| Chimera and time of DLI . | Source of DLI . | Day 5 . | Day 12 . | Day 29 . | |||
|---|---|---|---|---|---|---|---|
| CD4+ . | CD8+ . | CD4+ . | CD8+ . | CD4+ . | CD8+ . | ||
| T-cell expansion, 8 wk, cells/spleen × 105 | |||||||
| B10.D2+BALB/c→BALB/c | Naive | 2.0 ± 0.02 | 1.1 ± 0.01 | 2.5 ± 0.1 | 1.7 ± 0.07 | 1.5 ± 0.04 | 1.2 ± 0.02 |
| B10.D2→BALB/c | Naive | 1.7 ± 0.03 | 1.0 ± 0.01 | 2.9 ± 0.15 | 2.4 ± 0.11 | 1.4 ± 0.02 | 1.5 ± 0.04 |
| B10.D2+BALB/c→BALB/c | Presensitized | 2.3 ± 0.02 | 1.3 ± 0.02 | 2.0 ± 0.08 | 1.4 ± 0.04 | 1.9 ± 0.01 | 1.5 ± 0.06 |
| B10.D2→BALB/c | Presensitized | 7.1 ± 0.07 | 3.8 ± 0.06 | 5.9 ± 0.17* | 42.5 ± 1.3* | 3.7 ± 0.18 | 7.6 ± 0.35* |
| IFN-γ production, 8 wk, cells/spleen × 104 | |||||||
| B10.D2+BALB/c→BALB/c | Naive | 1.78 ± 0.3 | 0.31 ± 0.23 | 1.19 ± 0.32 | 1.79 ± 0.88 | 0.81 ± 0.28 | 0.48 ± 0.17 |
| B10.D2→BALB/c | Naive | 1.63 ± 0.1 | 0.26 ± 0.08 | 2.23 ± 1.31 | 1.99 ± 0.95 | 0.89 ± 0.22 | 1.15 ± 0.71 |
The absolute number of DLI-derived Thy 1.1+ T cells was calculated on days +5, +12, and +29 after DLI (2 × 107 B10.D2-Thy1.1 splenocytes) by multiplying the total number of viable cells by the percentages of gated CD4+ or CD8+ T cells coexpressing CD90.1 marker, respectively (n=3–4 chimeras/time point and group).
P<.05; between the B10.D2→BALB/c group that received presensitized DLI versus other groups.
Timing of DLI administration is a critical factor governing the DLI-mediated LH-GVH reactivity in MHC-matched, mHAg-mismatched chimeras
The failure of the naive DLI to eradicate residual host T cells in 8-week-old full chimeras (Figure 2C) is in direct contrast to the original studies conducted by Johnson et al,18,35 showing that DLI administration 3 weeks after MHC-matched, mHAg-mismatched alloBMT results in conversion to full donor T-cell chimerism. We hypothesized that different outcome is most likely the result of more vigorous clonal expansion and higher IFN-γ production by DLI-derived T cells in early in contrast to the late (> 8-week-old) chimeras. To test this hypothesis in vivo, we compared the expansion of DLI-derived T cells in B10.D2→BALB/c full chimeras that received DLI early (3-4 weeks) or late (8-12 weeks) after alloBMT. We found a significantly higher expansion of DLI-derived T cells in chimeras that received DLI early versus late after alloBMT (P < .05, data not shown) consistent with our recent findings in B6→C3H.SW, MHC-matched, mHAg-mismatched model.29 In addition, significantly higher numbers of DLI-derived T cells secreted IFN-γ in 4- versus 8-week-old chimeras (P < .05; Figure 2D-E). This observation of different potency of DLI-mediated LH-GVH response in early versus late chimeras may also explain the diminishing DLI-mediated GVL effect associated with their delayed administration.36
To rule out the possibility that a higher amount of host DCs may be responsible for more potent DLI-mediated LH-GVH response in early chimeras, we conducted an adoptive transfer experiment in which 2 × 106 BALB/c-CD45.1 CD11c+ DCs were coadministered with DLI from B10.D2-Thy1.1 mice to the 4- and 8-week-old B10.D2→BALB/c full chimeras. Administration of one spleen equivalent of CD11c+ DCs had only a minor effect on augmenting DLI-derived expansion and production of IFN-γ (data not shown and Figure 2D-E). The inability of transferred host DCs to prominently influence DLI-mediated alloimmune responses was not a result of their rejection by donor T cells or failure to home to primary and secondary lymphoid tissues, because their presence can be detected for at least 3 weeks in the BM and spleen of the chimeras (Figure 2F). Thus, in MHC-matched setting other factors rather than amount of host DCs influence the superior DLI-mediated alloreactivity in early chimeras. Next we examined requirements governing the DLI-mediated alloreactivity in early and late full versus mixed chimeras.
In vivo activation of APCs with TLR ligands augments the potency of DLI in early MHC-matched, mHAg-mismatched full chimeras
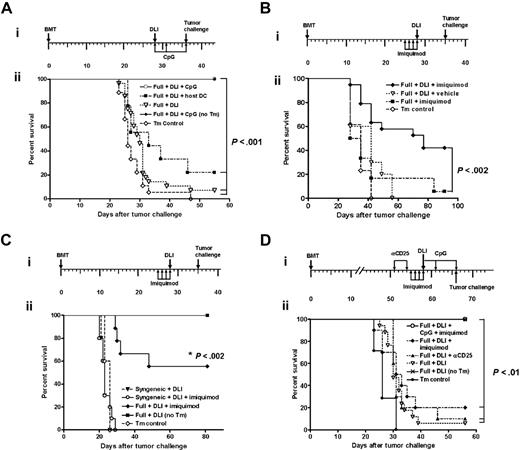
Because the priming of naive T cells depends on the maturation status of APCs,37,38 we hypothesized that the potency of DLI-mediated alloreactivity could be influenced by the activation status of APCs that they encounter in the chimeras. To test our hypothesis, we focused on 2 approaches: (1) to administer ex vivo–matured DCs or (2) to activate APCs in vivo. In testing the hypothesis, to exclude the possibility that observations made using a B10.D2→BALB/c donor/recipient pair are model dependent we conducted the next set of experiments using an established MHC-matched, mHAg-mismatched C3H.SW→B6 model. The coadministration of ex vivo–matured host or donor-derived DCs with DLI from C3H.SW mice to the 4-week-old C3H.SW→B6 full chimeras did not augment the effector function of the adoptively transferred T cells, measured as kinetics of conversion to full donor chimerism (Figure 3A). We next focused on the in vivo activation of DCs. Immunostimulatory CpG ODNs are known to activate DCs through the TLR9, enhance IL-12 production by DCs, promote induction of IFN-γ–secreting cytotoxic effector cells, and possibly prevent the premature death of adoptively transferred T cells.39–43 As such, 4-week-old C3H.SW→B6 full chimeras received DLI alone or with 3 doses of CpG1826 ODNs administered intraperitoneally on days 0, +3, and +7 after DLI. Administration of CpG1826 (but not control CpG1982) augmented kinetics of conversion to full donor CD8+ T-cell chimerism in comparison to the groups that received DLI alone or with ex vivo–matured host DCs (P < .001; Figure 3A), suggesting that the magnitude of the DLI-mediated GVH response was influenced by the in vivo activation of APCs. To determine whether that effect can be mediated by other TLR ligands, we used imiquimod, a known synthetic ligand for TLR7.44 Imiquimod is presumed to act by inducing the secretion of proinflammatory cytokines such as IFN-α, TNF-α, IL-6, and IL-12 in the skin.45,46 This locally generated cytokine milieu induces emigration of skin-derived DCs (in this model majority of the skin-derived CD11c+ DCs are host-derived; Durakovic et al29 ) and biases toward a Th1 cell-mediated immune response, with the generation of cytotoxic effectors.47 Imiquimod was topically administered to the shaved skin (approximately 1 sq in) of the C3H.SW→B6 chimeras for 3 days prior to and on the day of the DLI administration. The conversion to full donor CD8+ T-cell chimerism occurred only in chimeras that received imiquimod with DLI (P < .001; Figure 3B).
In vivo activation of APCs with TLR agonists but not transfer of ex vivo–matured CD11c+ DCs augments the effector function of adoptively transferred T cells. (A-B) Effects of TLR agonists on DLI-mediated LH-GVH reactivity in 4-week-old chimeras. (Ai) Experimental schema; (ii) groups of 4-week-old C3H.SW→B6 full chimeras (constructed using non-TCD BM; n = 8-10 mice/group) received nothing, CpG1826 ODNs, 2 × 107 splenocytes from C3H.SW mice, 2 × 107 splenocytes from C3H.SW mice plus 2 injections of 1.5 × 106 ex vivo–generated host or donor-derived BM DCs administered intraperitoneally or intravenously on days 0 and +7, or DLI plus CpG1826 ODNs, as schematized. Changes in donor CD8+ chimerism were measured longitudinally in peripheral blood (mean ± SEM). *P < .001 for all groups versus mice that received DLI plus CpG1826 ODNs. The low levels of donor T-cell chimerism prior to adoptive transfer represent the lag time required to achieve equilibrium of donor chimerism in blood in contrast to spleen in the same model.29 (Bi) Experimental schema; (ii) groups of 4-week-old C3H.SW→B6 full chimeras (constructed using non-TCD BM; n = 8 mice/group) were left untreated, treated with imiquimod, received DLI in the form of 2 × 107 splenocytes from C3H.SW donor together with 2 × 106 splenic CD11c+ DCs, or were pretreated with imiquimod or vehicle followed by DLI, as schematized. Changes in donor CD8+ chimerism determined by serial tail bleeding are presented as a mean percentage of donor chimerism ± SEM. This experiment has been repeated 3 times. *P < .001 for all groups versus mice that received DLI plus imiquimod. (C-H) Topically applied TLR7 agonist directly influences the in vivo activations status of APCs and cellularity in draining CLNs. Imiquimod was applied to 4-week-old C3H.SW→B6.SJL full chimeras once a day for 2 days. The following day, CLNs were retrieved from imiquimod-treated or untreated chimeras and analyzed by flow cytometry. (C) Total numbers of CD11c+ DCs in the CLNs and their origin analyzed with 2-color flow cytometry using anti-CD11c+ and anti-CD45.1 fluorescein-conjugated–specific antibodies. (D) Expression of CD40 and CD86 on the surface of host and donor CD11c+ DCs retrieved from the CLNs of untreated and imiquimod-treated chimeras was analyzed using anti-CD11c–, anti-CD45.1–, anti-CD40–, and anti-CD86–specific antibodies. Data are presented using the FlowJo histogram overlay scaling option “unit distribution” in which area under each curve corresponds to all gated CD11c+ cells. Data show the expression of CD40 and CD86 on donor and residual host derived CD11c+ DCs in imiquimod-treated (solid line) and untreated (filled histogram) chimeras, and isotype control (dotted line). (E-F) The total number of host and donor-derived CD11c+ DCs expressing CD40 and CD86. Values represent the mean ± SEM for combined CLNs per chimera (n = 3-4 mice/condition). (G-H) The origin and total cell number of IKDCs and B cells in CLNs of imiquimod-treated and untreated chimeras was determined by flow cytometry using anti-CD11c–, anti-CD45.1–, anti-CD49b–, and anti-B220–specific antibodies. Values represent the mean ± SEM (n = 3-4 mice/condition).
In vivo activation of APCs with TLR agonists but not transfer of ex vivo–matured CD11c+ DCs augments the effector function of adoptively transferred T cells. (A-B) Effects of TLR agonists on DLI-mediated LH-GVH reactivity in 4-week-old chimeras. (Ai) Experimental schema; (ii) groups of 4-week-old C3H.SW→B6 full chimeras (constructed using non-TCD BM; n = 8-10 mice/group) received nothing, CpG1826 ODNs, 2 × 107 splenocytes from C3H.SW mice, 2 × 107 splenocytes from C3H.SW mice plus 2 injections of 1.5 × 106 ex vivo–generated host or donor-derived BM DCs administered intraperitoneally or intravenously on days 0 and +7, or DLI plus CpG1826 ODNs, as schematized. Changes in donor CD8+ chimerism were measured longitudinally in peripheral blood (mean ± SEM). *P < .001 for all groups versus mice that received DLI plus CpG1826 ODNs. The low levels of donor T-cell chimerism prior to adoptive transfer represent the lag time required to achieve equilibrium of donor chimerism in blood in contrast to spleen in the same model.29 (Bi) Experimental schema; (ii) groups of 4-week-old C3H.SW→B6 full chimeras (constructed using non-TCD BM; n = 8 mice/group) were left untreated, treated with imiquimod, received DLI in the form of 2 × 107 splenocytes from C3H.SW donor together with 2 × 106 splenic CD11c+ DCs, or were pretreated with imiquimod or vehicle followed by DLI, as schematized. Changes in donor CD8+ chimerism determined by serial tail bleeding are presented as a mean percentage of donor chimerism ± SEM. This experiment has been repeated 3 times. *P < .001 for all groups versus mice that received DLI plus imiquimod. (C-H) Topically applied TLR7 agonist directly influences the in vivo activations status of APCs and cellularity in draining CLNs. Imiquimod was applied to 4-week-old C3H.SW→B6.SJL full chimeras once a day for 2 days. The following day, CLNs were retrieved from imiquimod-treated or untreated chimeras and analyzed by flow cytometry. (C) Total numbers of CD11c+ DCs in the CLNs and their origin analyzed with 2-color flow cytometry using anti-CD11c+ and anti-CD45.1 fluorescein-conjugated–specific antibodies. (D) Expression of CD40 and CD86 on the surface of host and donor CD11c+ DCs retrieved from the CLNs of untreated and imiquimod-treated chimeras was analyzed using anti-CD11c–, anti-CD45.1–, anti-CD40–, and anti-CD86–specific antibodies. Data are presented using the FlowJo histogram overlay scaling option “unit distribution” in which area under each curve corresponds to all gated CD11c+ cells. Data show the expression of CD40 and CD86 on donor and residual host derived CD11c+ DCs in imiquimod-treated (solid line) and untreated (filled histogram) chimeras, and isotype control (dotted line). (E-F) The total number of host and donor-derived CD11c+ DCs expressing CD40 and CD86. Values represent the mean ± SEM for combined CLNs per chimera (n = 3-4 mice/condition). (G-H) The origin and total cell number of IKDCs and B cells in CLNs of imiquimod-treated and untreated chimeras was determined by flow cytometry using anti-CD11c–, anti-CD45.1–, anti-CD49b–, and anti-B220–specific antibodies. Values represent the mean ± SEM (n = 3-4 mice/condition).
To directly examine the effect of imiquimod on DCs, we examined CD11c+ DCs from CLNs draining the treated area of skin. We observed that CLN size, total cellularity, and number of both host and donor CD11c+ DCs were significantly higher in the imiquimod-treated 4-week-old C3H.SW→B6.SJL chimeras (Figure 3C). Consistent with our previous findings,29 we found that host-derived CD11c+ DCs, representing mostly epithelium-derived CD11c+ DCs (epDCs), express higher levels of CD40 and CD86 in steady state than donor CD11c+ DCs in untreated chimeras (Figure 3D). On imiquimod treatment, the expression of CD40 and CD86 on both host and donor DCs increased (Figure 3D; P < .05; untreated versus imiquimod-treated, for mean fluorescence intensity [MFI], both CD40 and CD86). The observed effect was also reflected in the increased number of CD11c+ DCs having mature phenotype (P < .005; for both CD40 and CD86; Figure 3E-F). The effect of imiquimod was not restricted to DCs, since recently described IFN-producing killer DCs (IKDCs)48 and host B cells were recruited to the CLNs as well (Figure 3G-H).
Combined-TLR ligation or depletion of host T cells together with TLR activation of DCs is required to augment the DLI-mediated GVH reactivity in late full or early mixed MHC-matched chimeras
We recently reported that administration of imiquimod with DLI to 8-week-old full chimeras augmented the LH-GVH reactivity of DLI-derived T cells; however, the effect was not potent enough to induce complete conversion to full donor chimerism.29 We also found that in 8-week-old chimeras administration of CpG1826 ODNs with DLI is ineffective in inducing conversion to full donor T-cell chimerism (data not shown). Thus, we hypothesized that insufficient DLI-mediated alloreactivity in 8-week-old chimeras despite administration of TLR7 or TLR9 ligand is either the result of developed immunoregulatory networks or the different APC maturation status that the adoptively transferred T cells encounter in late chimeras. Previous studies had shown that CD4+CD25+ cells represent the most potent regulatory T (Treg) cells in the experimental MHC-mismatched DLI model.28,49 To examine their role, 8-week-old C3H.SW→B6 chimeras received DLI with or without anti-CD25 mAb, which transiently reduces CD4+CD25+ T cells (4- to 5-fold; data not shown). This strategy was ineffective in augmenting DLI-mediated LH-GVH response alone or in augmenting the effect of imiquimod (Figure 4A and data not shown). Because synergistic TLR stimulation is known to result in a more potent APC maturation program,50,51 we next administered TLR7 and TLR9 ligands together with DLI to 8-week-old chimeras. Combined TLR activation of DCs augmented LH-GVH reactivity of DLI resulting in complete conversion to full donor T-cell chimerism (P < .005 for CD4 and P < .05 for CD8; Figure 4B). These results strongly support our hypothesis that the activation status of APCs encountered by adoptively transferred T cells can critically influence the DLI-mediated LH-GVH response and that more potent APC activation is required in 8-week-old chimeras. Despite transient weight loss in chimeras treated with both TLR ligands, no obvious clinical signs of GVHD were observed (Figure 4C).
Synergistic triggering of TLR7 and TLR9 or depletion of host T cells together with TLR9 activation is required to augment the DLI-mediated GVH reactivity in late full or early mixed MHC-matched, mHAgs-mismatched chimeras, respectively. (A-C) Effects of TLR agonists on DLI-mediated LH-GVH reactivity in 8-week-old full chimeras (constructed using non-TCD BM). (Ai) Experimental schema; (ii) 8-week-old C3H.SW→B6 full chimeras received either nothing or 2 doses of anti-CD25 mAb on days −7 and −4 prior to receiving DLI. Changes in donor T-cell chimerism were measured longitudinally in peripheral blood based on the differential expression of Ly9.1 on donor C3H.SW (Ly9.1+) and host (Ly9.1−) T cells. Data representing the mean percentage of donor chimerism ± SEM from one experiment are plotted as a function of time after DLI (at least 8-10 mice/group/time point). P =NS. (Bi) Experimental schema; (ii) 8-week-old C3H.SW→B6 full chimeras received either DLI in the form of 2 × 107 C3H.SW splenocytes alone or were pretreated with imiquimod prior to DLI followed by CpG1826 ODNs as per schema. T-cell chimerism was determined by serial tail bleeding and staining for Ly9.1 and lineage-specific markers. Data are presented as the mean percentage of donor chimerism ± SEM (n = 8 mice/group). #P < .005 for CD4+ and *P < .05 for CD8+ T cells in full + DLI versus full + DLI + CpG + imiquimod. (C) Body weight changes of full chimeras that received either DLI alone (▪) or were treated with αCD-25 mAb (▿), imiquimod + anti-CD25 mAb (⋄) or imiquimod + CpG1826 (•). *P < .05 indicates weight change of more than 10% for (• versus all groups only at designated time points). (Di) Experimental schema; (ii-iii) B10.D2-Thy1.1 + BALB/c-CD45.1 (Thy1.2)→BALB/c-CD45.1 mixed chimeras received nothing (▾), DLI in the form of 2 × 107 B10.D2-Thy1.1 splenocytes (⋄), anti-CD25 depleting mAb (□) or anti-Thy1.2 depleting mAb (•) on days −7 and −4 prior to administration of 2 × 107 B10.D2 splenocytes, anti-Thy1.2 mAb, 2 × 107 B10.D2 splenocytes, and CpG1826 ODNs on days 0, +3 and +7 after DLI (▿). Changes in donor CD4+ T-cell (ii) and granulocyte (iii) chimerism were determined by serial tail bleeding as described in Figure 1. P < .05 for mice that received DLI, anti-Thy 1.2 mAb, and CpG1826 CDNs versus other groups. Data represent the average percent chimerism ± SEM plotted as function of time after DLI (at least 5 mice/group/time point).
Synergistic triggering of TLR7 and TLR9 or depletion of host T cells together with TLR9 activation is required to augment the DLI-mediated GVH reactivity in late full or early mixed MHC-matched, mHAgs-mismatched chimeras, respectively. (A-C) Effects of TLR agonists on DLI-mediated LH-GVH reactivity in 8-week-old full chimeras (constructed using non-TCD BM). (Ai) Experimental schema; (ii) 8-week-old C3H.SW→B6 full chimeras received either nothing or 2 doses of anti-CD25 mAb on days −7 and −4 prior to receiving DLI. Changes in donor T-cell chimerism were measured longitudinally in peripheral blood based on the differential expression of Ly9.1 on donor C3H.SW (Ly9.1+) and host (Ly9.1−) T cells. Data representing the mean percentage of donor chimerism ± SEM from one experiment are plotted as a function of time after DLI (at least 8-10 mice/group/time point). P =NS. (Bi) Experimental schema; (ii) 8-week-old C3H.SW→B6 full chimeras received either DLI in the form of 2 × 107 C3H.SW splenocytes alone or were pretreated with imiquimod prior to DLI followed by CpG1826 ODNs as per schema. T-cell chimerism was determined by serial tail bleeding and staining for Ly9.1 and lineage-specific markers. Data are presented as the mean percentage of donor chimerism ± SEM (n = 8 mice/group). #P < .005 for CD4+ and *P < .05 for CD8+ T cells in full + DLI versus full + DLI + CpG + imiquimod. (C) Body weight changes of full chimeras that received either DLI alone (▪) or were treated with αCD-25 mAb (▿), imiquimod + anti-CD25 mAb (⋄) or imiquimod + CpG1826 (•). *P < .05 indicates weight change of more than 10% for (• versus all groups only at designated time points). (Di) Experimental schema; (ii-iii) B10.D2-Thy1.1 + BALB/c-CD45.1 (Thy1.2)→BALB/c-CD45.1 mixed chimeras received nothing (▾), DLI in the form of 2 × 107 B10.D2-Thy1.1 splenocytes (⋄), anti-CD25 depleting mAb (□) or anti-Thy1.2 depleting mAb (•) on days −7 and −4 prior to administration of 2 × 107 B10.D2 splenocytes, anti-Thy1.2 mAb, 2 × 107 B10.D2 splenocytes, and CpG1826 ODNs on days 0, +3 and +7 after DLI (▿). Changes in donor CD4+ T-cell (ii) and granulocyte (iii) chimerism were determined by serial tail bleeding as described in Figure 1. P < .05 for mice that received DLI, anti-Thy 1.2 mAb, and CpG1826 CDNs versus other groups. Data represent the average percent chimerism ± SEM plotted as function of time after DLI (at least 5 mice/group/time point).
We next sought to determine whether the in vivo DC activation influences the DLI-mediated GVH reactivity in MHC-matched mixed chimeras. The 4-week-old B10.D2 + BALB/c→BALB/c mixed chimeras received DLI alone or in combination with CpG1826 ODNs. In contrast to findings with 4-week-old full chimeras, coadministration of DLI with CpG1826 ODNs was ineffective in augmenting the LH-GVH reactivity of DLI in mixed chimeras (data not shown). The inability of DLI-derived T cells to perturb the state of tolerance and induce LH-GVH reactivity despite the presence of large numbers of professional host-derived APCs and coadministration of TLR9 ligand led us to examine the role of suppressive mechanisms in mixed chimeras. As depicted in Figure 4D, administration of anti-CD25 mAb was ineffective in augmenting DLI-mediated LH-GVH reactivity measured as changes in CD4+ and granulocyte chimerism. Because host T cells can limit DLI-mediated alloimmune responses,27 we next selectively depleted them in vivo, using anti-Thy1.2 mAb given to B10.D2-Thy1.1 + BALB/c-CD45.1 (Thy1.2)→BALB/c-CD45.1 mixed chimeras prior to DLI with or without CpG1826 ODNs. The anti-Thy1.2 treatment resulted in near complete conversion to full donor T-cell chimerism. However, a significant increase in donor granulocyte chimerism reflective of LH-GVH response was observed only in anti-Thy1.2-treated chimeras if the DLI was coadministered with CpG1826 ODNs (P < .05; Figure 4D) suggesting the critical need to activate APCs.
In vivo activation of APCs augments GVL reactivity of DLI administered to MHC-matched full donor chimeras
Based on these results, we hypothesized that augmented LH-GVH reactivity of DLI after systemic activation of APCs should translate into better GVL reactivity. Therefore, using a tumor-protection model,15,18,36,52 C3H.SW→B6 chimeras received DLI 4 weeks after alloBMT, followed either by nothing, ex vivo–matured host DCs, or CpG1826 ODNs. Seven days later, all chimeras and control B6 animals (no transplants) received a lethal dose of recipient type C1498 leukemia cells. All B6 mice in the control group that received C1498 leukemia cells died of the disease. Leukemia-free survival of chimeras that received DLI only or with host DCs was improved over the control group (P < .02; Figure 5A). However, administration of CpG1826 ODNs with DLI significantly improved survival of the chimeras over those that received DLI only (P < .001) or DLI coadministered with host DCs (P < .001; Figure 5A). The next series of experiments were designed to test whether imiquimod augments the GVL effect. Consistent with chimerism results presented earlier, the administration of imiquimod with DLI resulted in significantly improved survival of chimeras (P < .002; Figure 5B). From these results we conclude that the combined administration of DLI and in vivo activation of APCs is a highly potent strategy to stimulate GVL reactivity if administered to full chimeras early after MHC-matched alloBMT.
CpG1826 ODNs and imiquimod augment antitumor efficacy of allogeneic adoptive immunotherapy in full MHC-matched, mHAgs-mismatched chimeras. (Ai) Experimental schema; (ii) 4-week-old C3H.SW→B6 full chimeras (constructed using non-TCD BM) received DLI consisting of 2 × 107 C3H.SW splenocytes (▿), DLI plus 2 intraperitoneal injections of 1. 5 × 106 B6 (host)–derived ex vivo–generated BM DCs on days 0 and +7 (▪) or DLI plus CpG1826 on days 0, +3 and +7 (□). B6 mice that did not receive transplants served as a tumor control (Tm Control; ⋄). All animals (n = 9-11 per group) received an intravenous injection of 5 × 104 C1498 cells on day +35. An additional group of 4-week-old C3H.SW→B6 chimeras that received DLI + CpG but no tumor (no TM) served as DLI control (♦). Tumor-free survival was monitored thrice weekly and is plotted as a function of time after tumor inoculation in this and following experiments. Each experimental group was repeated at least twice with similar results. P < .02 (▿ versus ⋄; ▪ versus ▿), P < .001 (□ versus ⋄, ▿, ▪). (Bi) Experimental schema; (ii) 4-week-old C3H.SW→B6 full chimeras (constructed using non-TCD BM) were treated with imiquimod followed by nothing (▪), or DLI consisting of 2 × 107 C3H.SW splenocytes (♦). An additional group of animals was pretreated with vehicle prior to DLI administration (▿). Seven days later, all groups (n = 8-15/group) received an intravenous injection of 5 × 104 C1498 cells. A group of 5 B6 mice without transplants served as tumor control (⋄). This experiment has been repeated twice with similar results. P < .002 (♦ versus ▪). (Ci) Experimental schema; (ii) 4-week-old B6→B6 syngeneic chimeras (constructed using non-TCD BM) received DLI consisting of 2 × 107 B6 splenocytes (▾) or were pretreated with imiquimod prior to DLI administration (○). The 4-week-old C3H.SW→B6 full chimeras (constructed using non-TCD BM) pretreated with imiquimod prior to DLI served as allogeneic GVL control (♦). Seven days later, all groups received an intravenous injection of 5 × 104 C1498 cells. B6 mice that did not receive transplants served as tumor control (⋄). An additional group of 4-week-old C3H.SW→B6 chimeras that received DLI but no tumor served as DLI control (▪). Results of one representative experiment with 8 to 12 animals in each group. P < .002 (♦ versus ⋄, ○, ▾). (D) Synergistic triggering of TLR7 and TLR9 augments DLI-mediated GVL reactivity in 8-week-old full chimeras (constructed using non-TCD BM). (i) Experimental schema; (ii) C3H.SW→B6 chimeras received DLI alone (▿), were pretreated with anti-CD25 mAb on days −7 and −4 prior to DLI (▴), imiquimod on days −3 through +1 prior to DLI (♦) or were pretreated with imiquimod on days −3 through +1 prior to DLI and received CpGs ODN1826 on days 0, +3, and +7 (○). All chimeras were subsequently challenged with 5 × 104 C1498 leukemia cells 1 week after DLI. B6 mice that did not receive transplants served as tumor control (•). An additional group of 8-week-old C3H.SW→B6 chimeras that received DLI but no tumor served as DLI control ( × ). P < .01 (○ versus ▿, ▴, ♦).
CpG1826 ODNs and imiquimod augment antitumor efficacy of allogeneic adoptive immunotherapy in full MHC-matched, mHAgs-mismatched chimeras. (Ai) Experimental schema; (ii) 4-week-old C3H.SW→B6 full chimeras (constructed using non-TCD BM) received DLI consisting of 2 × 107 C3H.SW splenocytes (▿), DLI plus 2 intraperitoneal injections of 1. 5 × 106 B6 (host)–derived ex vivo–generated BM DCs on days 0 and +7 (▪) or DLI plus CpG1826 on days 0, +3 and +7 (□). B6 mice that did not receive transplants served as a tumor control (Tm Control; ⋄). All animals (n = 9-11 per group) received an intravenous injection of 5 × 104 C1498 cells on day +35. An additional group of 4-week-old C3H.SW→B6 chimeras that received DLI + CpG but no tumor (no TM) served as DLI control (♦). Tumor-free survival was monitored thrice weekly and is plotted as a function of time after tumor inoculation in this and following experiments. Each experimental group was repeated at least twice with similar results. P < .02 (▿ versus ⋄; ▪ versus ▿), P < .001 (□ versus ⋄, ▿, ▪). (Bi) Experimental schema; (ii) 4-week-old C3H.SW→B6 full chimeras (constructed using non-TCD BM) were treated with imiquimod followed by nothing (▪), or DLI consisting of 2 × 107 C3H.SW splenocytes (♦). An additional group of animals was pretreated with vehicle prior to DLI administration (▿). Seven days later, all groups (n = 8-15/group) received an intravenous injection of 5 × 104 C1498 cells. A group of 5 B6 mice without transplants served as tumor control (⋄). This experiment has been repeated twice with similar results. P < .002 (♦ versus ▪). (Ci) Experimental schema; (ii) 4-week-old B6→B6 syngeneic chimeras (constructed using non-TCD BM) received DLI consisting of 2 × 107 B6 splenocytes (▾) or were pretreated with imiquimod prior to DLI administration (○). The 4-week-old C3H.SW→B6 full chimeras (constructed using non-TCD BM) pretreated with imiquimod prior to DLI served as allogeneic GVL control (♦). Seven days later, all groups received an intravenous injection of 5 × 104 C1498 cells. B6 mice that did not receive transplants served as tumor control (⋄). An additional group of 4-week-old C3H.SW→B6 chimeras that received DLI but no tumor served as DLI control (▪). Results of one representative experiment with 8 to 12 animals in each group. P < .002 (♦ versus ⋄, ○, ▾). (D) Synergistic triggering of TLR7 and TLR9 augments DLI-mediated GVL reactivity in 8-week-old full chimeras (constructed using non-TCD BM). (i) Experimental schema; (ii) C3H.SW→B6 chimeras received DLI alone (▿), were pretreated with anti-CD25 mAb on days −7 and −4 prior to DLI (▴), imiquimod on days −3 through +1 prior to DLI (♦) or were pretreated with imiquimod on days −3 through +1 prior to DLI and received CpGs ODN1826 on days 0, +3, and +7 (○). All chimeras were subsequently challenged with 5 × 104 C1498 leukemia cells 1 week after DLI. B6 mice that did not receive transplants served as tumor control (•). An additional group of 8-week-old C3H.SW→B6 chimeras that received DLI but no tumor served as DLI control ( × ). P < .01 (○ versus ▿, ▴, ♦).
Because 4-week-old chimeras are still lymphopenic, and therefore, contain stimuli for homeostatic proliferation of adoptively transferred T cells that may contribute to the antitumor immunity,53 we next sought to dissociate the contribution of this mechanism from the GVL effect driven by mHAg disparity. To accomplish this, syngeneic B6→B6 chimeras, constructed after lethal irradiation and transplantation of syngeneic BM cells, received 2 × 107 B6 splenocytes in the form of DLI alone or together with imiquimod followed by the lethal dose of C1498 leukemia. Both groups of syngeneic chimeras rapidly succumbed to the C1498 leukemia in contrast to the allogeneic chimeras that received DLI and imiquimod, thus confirming that donor T cells recognizing host mHAgs were primarily responsible for GVL effect (P < .002; Figure 5C).
To determine whether this strategy is effective in augmenting the GVL response in late chimeras, C3H.SW→B6 full chimeras 8 weeks after alloBMT received DLI and were treated with anti-CD25 mAb or imiquimod alone or with a combination of imiquimod and CpG1826 ODNs. All chimeras were challenged with 5 × 104 C1498 leukemia cells 7 days after DLI. In vivo depletion of CD25+ cells before DLI had no effect, whereas imiquimod provided minimal survival benefit over DLI alone (P = NS; Figure 5D). However, coadministration of imiquimod and CpG1826 ODNs with DLI significantly increased their GVL reactivity (P < .01; Figure 5D). We did not observe any clinical signs of GVHD in CpG1826 ODN-treated and imiquimod-treated 8-week-old chimeras.
Discussion
With the development of reduced-intensity conditioning regimens and more effective strategies for GVHD prophylaxis, attention has increasingly focused on developing strategies to prevent or control progression of the underlying malignancy, the most common cause of failure of alloBMT. Presently, DLI is the treatment of choice for leukemia in relapse after alloBMT.2,9,31 Several studies have endeavored to elucidate the parameters governing the potency of GVH and GVL responses after alloBMT or DLI.12,15–17,54 By and large, these studies point to the critical role for host APCs in initiating GVHD and GVL effects in freshly irradiated MHC-matched chimeras and MHC-mismatched chimeras receiving delayed DLI. The studies reported here, however, show that the presence of residual host APCs, including DCs, is clearly not sufficient for the induction of significant GVH or GVL responses in chimeras after MHC-matched DLI. This observation is consistent with recent clinical studies suggesting that after HLA-matched nonmyeloablative BMT in humans only a third of evaluable patients converted from mixed to full donor chimerism on DLI administration, and patients with donor T-cell chimerism below 50% rarely responded.31,55
Our studies demonstrate that the outcome of DLI is determined by factors intrinsic to the donor T-cell population as well as characteristics of the host that received the transplant. On the T-cell side, a more potent LH-GVH response after MHC-mismatched as compared to MHC-matched DLI can be explained by the higher precursor frequency of alloreactive T cells and possibly a higher proportion of alloreactive memory T cells present in the MHC-alloreactive repertoire.56,57 Similarly, a higher precursor frequency of anti-mHAg-reactive T cells can explain the more potent alloreactivity of DLIs from MHC-matched presensitized donors. From the host side, factors influencing the outcome of adoptive immunotherapy can be divided into those actively resisting the DLI-derived T cells, such as Treg cells, those reflecting the amount of residual host antigen load, and those that act through the activation status of the APCs. Suppressor cells in transplantation tolerance were described over 2 decades ago by Weiden et al58 and Tutschka et al59 in animal models, and by Tsoi et al60 in the humans. Several studies have shown that Treg cells of donor19,28 and host origin27 and myeloid non-T Mac1+ suppressor cells61 can limit the DLI-mediated alloimmune responses in mice. Our studies support the role of host T cells in limiting the DLI-mediated LH-GVH responses in MHC-matched, mHAgs-mismatched mixed chimeras. The second possible mechanism is that donor T cells become “exhausted” by a high dose of disseminated antigen,62–64 even when and perhaps because host DCs are abundant. Therefore, and in agreement with the clinical observations, MHC-matched, mHAg-mismatched DLI-mediated alloimmune responses are most effective if DLI is administered early after conditioning in the presence of low (≤ 15%) but not high (≥ 40%) amounts of residual host chimerism.31,55 The series of experiments performed by Rocha et al65 using a minor antigen-mismatched (H-Y-mismatched) transgenic system supports the latter explanation. Finally, the augmentation of DLI-mediated GVH and GVL responses by administration of TLR agonists raises the possibility that the activation of donor T cells is governed to some extent by the maturation status of host APCs.
Comparisons of the factors characterized in this study with those of previously published studies may help reveal important aspects of DLI-mediated GVH reactions after alloBMT. For example, it has been suggested that MHC-mismatched DLI mediates superior GVL effects in mixed compared to full donor chimeras.15 Although our results confirm that mixed chimerism is predictive of a more powerful DLI-mediated LH-GVH effect (a potential surrogate for GVL reactivity) in the MHC-mismatched setting, they also demonstrate that this is not the case in the MHC-matched mixed chimeras where 40% or more of professional APCs are host derived, and as such, capable of directly presenting host mHAgs to DLI-derived T cells. Billiau at al36 have identified the timing of DLI administration as a critical factor for the induction of optimal LH-GVH and GVL reactions. Our studies extend this finding by showing that the activation status of the APCs rather than amount of residual host DCs is critically important for DLI-mediated alloreactivity in both early and late chimeras. However, whereas topical application or systemic administration of TLR ligands promoted GVHD after MHC-mismatched DLI,42,66 the same treatment combined with MHC-matched DLI was able to induce LH-GVH and GVL reactivities without causing clinical GVHD (Figures 3,Figure 4–5 and Durakovic et al29 ). In contrast, the combination of MHC-matched DLI and TLR ligand administration induced significant GVHD in freshly reirradiated 8-week-old C3H.SW→B6 chimeras (L.L., unpublished observations, May 2006). Therefore, it is likely that the induction of GVHD after MHC-matched DLI requires not only donor T cells, host APCs, and TLR ligands but also tissue damage, which may facilitate the emigration of donor T cells into GVHD target organs.66 Thus, in the absence of tissue damage, activation of host APCs by TLR ligands may be sufficient to promote LH-GVH and GVL reactivities without exacerbating lethal GVHD. The lack of a requirement for fresh tissue damage in the induction of GVHD following the combination of TLR ligand(s) and MHC-mismatched DLI may be explained by the intrinsically more prominent T-cell response to MHC antigens resulting in the higher number of alloreactive T cells capable of migrating to GVHD target organs or the more potent ability of activated host DCs to reprogram MHC-mismatched DLI-derived T cells for circulation in peripheral tissues.57,67–69 Similar mechanisms may also be used to explain the greater incidence of DLI-mediated GVHD seen in the clinic. In particular, several factors may be present at the same time ranging from higher proportion of alloreactive memory T cells in DLI as a result of age, viral, fetal, or other environmental antigenic exposures, to the freely available endogenous and exogenous TLR ligands in the host that received the transplant, as a result of the concomitant infections or damaged epithelial surfaces and tissues caused by chemotherapy given to control relapsed underlying malignancy.
Our finding that TLR ligands augment GVH and GVL reactivity of DLI extends the emerging data suggesting that TLR-mediated signaling is important for T-cell alloimmunity.70 Furthermore, our data clearly show that TLR-augmented GVL reactivity of DLIs administered to established chimeras is mediated primarily by anti-mHAgs responses (Figure 5C.) The inability of ex vivo–matured host DCs to significantly influence the DLI-mediated alloimmune responses is consistent with other studies in a nontransplantation setting showing that in vivo triggering of DC maturation with TLR ligands is a superior strategy for manipulating T-cell responses.71 This sustained signaling may result in a better “maturation” state of DCs due to the known ability of TLRs to up-regulate all 3 categories of positive signals that DCs deliver to T cells: antigen, costimulation, and cytokines.72,73 Finally, our finding that TLR ligands augment the GVL effect of MHC-matched DLI without inducing lethal GVHD is highly relevant for studies after HLA-matched nonmyeloablative allografting in which conversion to complete donor T-cell chimerism, known to be associated with achievement of disease remission, may be delayed,74,75 or for improving the relatively low efficacy of DLI in the treatment of aggressive malignancies, such as acute leukemia, in relapse after HLA-matched alloBMT.
Authorship
Contribution: N.D., V.R, and M.S. performed research and analyzed data; K.B.B. performed research; J.D.P. contributed vital reagents and analytical tools; E.J.F. contributed to data analysis and writing of the paper; and L.L. designed research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
N.D. and V.R contributed equally to this study.
Correspondence: Leo Luznik, Cancer Research Bldg, Rm 290, 1650 Orleans St, Baltimore, MD 21231; e-mail: luznile@jhmi.edu.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this manuscript contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
This work was supported by grant from the Amy Strelzer Manasevit Scholars Program funded by the Marrow Foundation in cooperation with the National Marrow Donor Program, National Cancer Institute (K08 CA89546) and the Susan Komen Foundation (all to L.L.).
We thank Dr H. I. Levitsky for providing us with critical reagents (BALB/c-CD45.1 mice and anti-CD25 mAb).

![Figure 2. Timing of DLI administration but not the level of residual donor chimerism influences the fate of adoptively transferred T cells in MHC-matched, mHAg-mismatched chimeras. (A-B) Splenocytes (2 × 107) from naive or presensitized B10.D2-Thy1.1 mice were CFSE-labeled before transfer to 8-week-old B10.D2→BALBc-CD45.1 full and BALB/c-CD45.1 + B10.D2→ BALB/c-CD45.1 mixed allogeneic chimeras. The same dose of CFSE-labeled splenocytes from BALB/cThy1.1+ mice was transferred to 8-week-old syngeneic BALB/c→BALB/c chimeras. Adoptively transferred T cells were analyzed using anti-Thy1.1–, anti-CD4–, and anti-CD8–specific antibodies. (A) Representative CFSE profiles of DLI-derived CD8+ T cells (day +29 after DLI) and gating used to delineate in vivo unproliferated CFSEhi from slow-proliferating CFSEslow and fast-proliferating CFSEfast DLI-derived T cells in allogeneic and syngeneic chimeras. (B) CFSE dilution of DLI-derived CD4+ T cells in the spleen of the 8-week-old mixed (▪) and full (♦) chimeras on days +5, +14, and +28 after DLI administration was contrasted with adoptively transferred DLI-derived T cells in syngeneic chimeras (▾). Data are presented as a mean percentage of CFSEhi CD4+Thy1.1+ ± SEM and represent one of 2 independent experiments (n = 3-4 mice/group/time point). *P < .05 (▪ versus ▾) and *P < .05 (♦] versus ▾). (C) Rapid conversion to full donor T-cell chimerism in peripheral blood of full chimeras that received presensitized DLI. Changes in donor T-cell chimerism were determined by serial tail bleeding and staining for Ly9.1 and lineage-specific markers (mean percentage donor chimerism ± SEM; n = 5 mice/group). *P < .001 (CD4+; ▪ versus □) and #P < .01 (CD8+; ♦ versus ⋄). (D-E) Timing of DLI but not transfer of host CD11c+ DCs influences clonal expansion of IFN-γ–secreting DLI-derived T cells. B10.D2→BALB/c full chimeras received DLI from B10.D2-Thy1.1 mice, 4 (▪) or 8 (♦) weeks after alloBMT. Additional groups of 4- and 8-week-old full chimeras received with DLI 2 × 106 host CD11c+ DCs (▵, ○). Absolute numbers of IFN-γ–secreting DLI-derived CD4+ Thy1.1+ (D) and CD8+Thy1.1+ (E) T cells were determined in spleens on days +5, +14, and +28 after DLI. Mean ± SEM is shown; n ≥ 3 mice/time point and group. *P < .05 (▪, ▵ versus ♦; for both CD4+ Thy1.1+ and CD8+Thy1.1+). (F) Transferred host derived CD11c+ DCs persist in MHC-matched, mHAg-mismatched chimeras. A total of 2 × 106 splenic CD11c+ DCs from BALB/c-CD45.1 mice and 2 × 107 splenocytes from the B10.D2-Thy1.1 mice were injected intravenously to 4-week-old B10.D2→BALB/c chimeras. On days +1, +7, and +28 after transfer, the presence of host-derived CD11c+ DCs in the BM, CLNs, mesenteric LNs (MLNs), and SPL was determined by flow cytometry using anti-CD11c– and anti-CD45.1–specific antibodies. The absolute number of host-derived CD11c+ DCs that had homed to BM, CLNs, MLNs, and SPL was calculated by multiplying the percentage of CD45.1+CD11c+ DCs by the total number of cells.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/109/10/10.1182_blood-2006-09-048124/4/m_zh80100700930002.jpeg?Expires=1765889053&Signature=ieZ-Nlzww2CT7uJVG3Of2LQQXZlI7f5-Pd~nQr2yL~u~PcQC5OPOD7Oqy7ECvenRO-yYn9q7fJF-lo7U0U5wtnT9RZXRVwR0olkyJE0CI6f5A6FeZicXNFylytYdS~Ayl3tsTPVcJ~p0tC2OWOOL7ow4A8KJ8bfc~S7wRGKpchxqcelGpRfv9rKFWUQ2Tmhrd0Q1T70yXzI3iE6Ij6IXqvyoG5F~FirevVFvFJ5O8ho2AiTBRXvjkeippyMFOqvNNlHjmZWAMVho4jQodeCnZHy4dYXeQ3a0nApxfP0c7bJs1EWqgfYrW77SWnlUgAXSG5aydNjm6zihEwhpn4hZTg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal