Abstract
Disease relapse sometimes occurs after acute promyelocytic leukemia (APL) therapy with all-trans retinoic acid (ATRA). Among the diagnostic parameters predicting relapse, heterogeneity in the in vitro differentiation rate of blasts is an independent factor. To identify biologic networks involved in resistance, we conducted pharmacogenomic studies in APL blasts displaying distinct ATRA sensitivities. Although the expression profiles of genes invested in differentiation were similarly modulated in low- and high-sensitive blasts, low-sensitive cells showed higher levels of transcription of ATRA-target genes, transcriptional regulators, chromatin remodelers, and transcription factors. In opposition, only high-sensitive blasts expressed the CYP26A1 gene, encoding the p450 cytochrome which is known to be involved in retinoic acid catabolism. In NB4 cells, ATRA treatment activates a novel signaling pathway, whereby interleukin-8 stimulates the expression of the homeobox transcription factor HOXA10v2, an effective enhancer of CYP26A1 transcription. These data were corroborated in primary APL cells, as maturation levels correlated with CYP26A1 expression. Treatment with a retinoic acid metabolism blocking agent (RAMBA) results in high-nucleoplasmic concentrations of retinoid and growth of NB4-resistant subclones. Hence, for APL blasts associated with poor prognosis, the low CYP26A1 expression may explain high risk of resistance installation, by increased retinoid pressure. Pharmacogenomic profiles of genes involved in retinoid acid metabolism may help to optimize anticancer therapies, including retinoids.
Introduction
Hematopoiesis can be described as a balance between proliferation and differentiation. In 90% of acute promyelocytic leukemia (APL) cases, the t(15;17) translocation and consequent expression of the PML (promyelocytic leukemia protein)/RARα (retinoic acid receptor α) fusion protein blocks differentiation at the promyelocytic stage. RARα belongs to the nuclear receptor superfamily and functions as a ligand-dependent transcription factor, acting to establish the granulocytic lineage. Under normal conditions, binding of the ligand (all-trans retinoic acid, or ATRA) to the receptor provokes a conformational change in the receptor resulting in the disruption of a co-repression complex, recruitment of coactivators, binding to the retinoic acid response element (RARE), and finally the transcription of ATRA-dependent genes.1 The PML portion of the PML/RARα fusion protein has a high affinity for the co-repression complex, preventing RARα transcriptional activity. To induce complete remission in a high proportion of patients, a pharmacologic dose of ATRA is required to destabilize this interaction and reestablish the differentiation program. Combining ATRA with chemotherapy results in a significant prolongation of disease-free and overall survival.2–6
Despite the general efficacy of ATRA-based therapy, 10% to 20% of patients with APL still relapse. Several mechanisms have been proposed to explain the involved retinoid resistance arising during cancer therapy. With the exception of primary resistance, which is extremely rare in PML/RARα cells,7,8 APL relapse has been suggested to be related to various processes observed in cell lines. These include variations in nuclear ATRA concentrations due to, for example, cytoplasm retinoid sequestration,9,10 increased intracellular metabolism,11 alterations in ATRA transcriptional efficiency via mutations in the PML/RARα,12–15 increased binding affinity for the corepressor,16 or an absence of PML/RARα proteasomal degradation.17–20
We have previously identified the existence of heterogeneity in the sensitivity of APL cells to ATRA-induced differentiation at diagnosis and have shown it to be an independent prognostic factor for disease-free survival in the APL93 trial.21 These data suggested that low-sensitive blasts may harbor residual leukemic clones that serve as a basis for disease relapse. The precise processes involved in the differential sensitivities of blasts to ATRA and in the establishment of resistance are, however, still unclear, with only mutations in the ligand-binding domain of PML/RARα having been identified in relapsing patients to date.22–24 To explore the biologic networks involved in ATRA sensitivity, we decided to analyze the pharmacogenomic profiles of APL blasts showing distinct responses to ATRA in the hopes of identifying new resistance-related processes.
By analyzing relevant differentiation markers selected by the Serial Analysis of Gene Expression (SAGE) method in the NB4 cell line model, we observed that both low- and high-sensitive blasts displayed similar gene expression profiles in response to ATRA. However, genes encoding p450-cytochrome enzymes, which are involved in the metabolism of ATRA, were differentially regulated. Low levels of transcription of the gene encoding the CYP26A1 cytochrome were particularly observed following the differentiation of low-sensitive APL blasts.
Because the CYP26A1 gene was regulated differently than other retinoid-induced targets tested, the mechanism underlying its regulation appeared to be more complex than previously proposed.25,26 In particular, it was impossible to ascribe CYP26A1 transcriptional activation to the simple substrate-driven feedback mechanism that generally regulates the intracellular concentrations of retinoids. We thus reassessed retinoic acid metabolism with respect to the sensitivity and differentiation of APL cells in response to ATRA. The present work identifies a novel retinoic acid signaling pathway that involves interleukin 8 (IL-8) and a homeobox transcription factor (HOX10v2). When this pathway is inactive, the resulting low-transcription level of the CYP26A1 gene results in high-intracellular concentrations of ATRA, potentially leading to the selection of resistant clones. These data strongly suggest that specific pharmacogenomic profiles of ATRA sensitivity may help identify patients at risk of relapse for whom adapted regimens may be necessary.
Materials and methods
Cell culture, differentiation, and reagents
The NB4 promyelocytic cell line (DSMZ, Braunschweig, Germany) was cultured in RPMI 1640 (Invitrogen, Cergy Pontoise, France) containing 10% decomplemented FBS (Dutscher, Brumath, France). The chemical agents used for the differentiation were all-trans retinoic acid (ATRA; Sigma-Aldrich, Gillingham, Untied Kingdom) and 4-azolyl-retinoic acid (VN/14-1),27 a retinoic acid metabolism blocking agent (RAMBA) kindly provided by Dr Vincent C.O. Njar (University of Maryland, College Park, MD). ATRA catabolites were extracted from the culture medium of NB4 cells after 24 hours of differentiation, following ethyl acetate extraction.28
Extracellular proteins, secreted into the culture medium from either proliferative or differentiated NB4 cells (4 × 106), were retinoid deprived and concentrated (20 ×) via a protein purification column (MacroSep 10 kDa Omega; Pall, Ann Arbor, MI). Extracts were albumin depleted (Qiagen, Courtabeuf, France) and injected into a size exclusion liquid chromatography column (Superdex 200; Amersham Biosciences, Chalfont St Giles, United Kingdom). Following elution with PBS, protein fragmentation was examined by electrophoresis (12% SDS–polyacrylamide gel electrophoresis [PAGE]) and by transfer onto nitrocellulose membranes, colored by red Ponceau. All fractions were tested with the human interleukin-8 (IL-8) Enzyme Immunosorbent Assay (Calbiochem, Nottingham, United Kingdom). To test the impact of chemokines on specific HOXA10v2 transcription, either 50 μL of the 20 × concentrates or 500μL of each eluted fraction was added to proliferative NB4 cells for 12 hours. The recombinant IL-8 (R&D Systems, Abingdon, United Kingdom) was tested over 12 hours at a final concentration of 25 ng/mL, alone or in combination with ATRA (0.1 μM). The CXCR2 receptor was blocked following incubation with 200 nM specific inhibitor SB225002 (Calbiochem). Antagonist was added for 15 minutes before incubation with ATRA (0.1 μM) or protein extracts for 12 hours.
In the resistance studies, NB4 cells were treated 4 times, either with 1 μM ATRA or with 1 μM RAMBA, with a 3-day–spacing administration regime. An equivalent dose of DMSO was added to untreated controls. Residual cells after 12 days of treatment were individualized by serial dilution into 96-well plates. To assess the percentage of resistant growth clones, statistical analysis was performed on 100 clones for each condition.
SAGE library processing
Libraries were constructed using a modified SAGE procedure with the Sau3AI anchoring enzyme (GATC). mRNA was extracted from untreated NB4 cells (UnT) and from cells induced to differentiate by a 48-hour exposure to 1 μM ATRA (Diff). Automatic tag detection, tag-to-gene mapping, and differential gene expression analyses were performed with software dedicated to SAGE data mining (Skuld-Tech, Montpellier, France).29,30 A total of 38 668 tags were sequenced for the UnT- and Diff-libraries (19 005 and 19 663 tags, respectively), identifying 10 818 different tags. SAGE data are available at GEO (http://www.ncbi.nlm.nih.gov/geo/) under accession nos. GSM151619 and GSM151622, which correspond to SAGE data of the untreated and ATRA-treated NB4 cells. NB4 libraries were also compared with the previously described granulocytic Sau3AI SAGE library, whose construction required polymorpho-nuclear neutrophil (PMN) purification from 12 different donors.31
In vitro culture of APL patient samples
Blood samples were obtained from patients with newly diagnosed APL, after informed consent (APL2000 trial) was obtained, in accordance with the Declaration of Helsinki, and before therapeutical administration of ATRA. Approval was obtained from the CNRS, INSERM, and Ministère de La Recherche institutional review board for these studies. Mononuclear fractions from blood samples containing more than 90% blastic cells were separated by Ficoll-Hypaque density gradient and cultured at a concentration of 1 × 106/mL, with or without 0.1 μM ATRA.9 Differentiation was assessed by a Nitroblue tetrazolium (NBt) test after 3 and 6 days of treatment. Heterogeneity in the in vitro differentiation rates of APL blasts at day 3 was used as a factor in the overall therapeutic efficiency, because this parameter has been described to reflect the in vivo heterogeneous outcome.21
RNA extraction, reverse transcription, and high-throughput real-time PCR
RNA was extracted following cesium chloride precipitation. RNA quality was monitored using the 2100-Bioanalyzer (Agilent Technologies, Waldronn, Germany) and quantified using a spectrophotometer (NanoDrop Technologies, Wilmington, DE). Reverse transcription was performed with random primers (High-capacity cDNA Archive kit; Applied Biosystems, Courtaboeuf, France), using 1 μg total RNA. By mining the SAGE data, 95 transcripts showing (or not) previously described significant variation following NB4 cell differentiation were selected for high-throughput real-time polymerase chain reaction (PCR) analysis on microfluidic cards, using the ABI7900HT system (Applied Biosystems). This technology provides reproducibility because all PCRs are performed with the same cDNA sample mix (100 ng). Analysis of the relative quantity gene expression (RQ) data were performed using the 2−ΔΔCt method.32 Transcriptional modulation (Log10 RQ) was calculated by comparing blasts treated for 3 days with ATRA (0.1 μM) with an untreated control. Standard error (SE) was measured using a control performed in triplicate. For normalization, GAPDH was selected as the calibration transcript. Data were collected and analyzed with Sequence Detector Software (SDS2.2; Applied Biosystems). By mining the NB4 data, the reliability of SAGE and the high-throughput real-time PCR was evaluated. Among the significant markers selected from the SAGE data (P < .01), 95% displayed significant modulation when tested on microfluidic cards.
Specific PCR, cloning, and transient transfection
All primers used in this work are given in Figure S1 (available on the Blood website; see the Supplemental Materials link at the top of the online article). Real-time PCR was performed with a Light Cycler (Roche Diagnostics, Meylan, France). Standard error (SE) was measured for each relative quantity of PCR product (n = 3). Data were analyzed with the Student t test. In the figures, asterisks indicate P values lower than 0.05 compared with the ATRA control. All amplicons were confirmed by sequencing (Genome Express, Meylan, France). Both the HOXA1 (NM_005522) and HOXA10v2 (variant 2; NM_153715) open reading frames were cloned into the pcDNA4-His/Max TOPO expression vector (Invitrogen). Homeobox vectors were transfected by nucleofection into NB4 cells (Amaxa Biosystems, Gaithersburg, MD) or by calcium-salt precipitation into Cos-7 cells. After transgenic expression, ATRA was administered at a final concentration of 0.1 μM. For NB4 cells, ATRA was administered for 6 hours after 1 day of expression. For Cos-7 cells, ATRA was added for one day, after 48 hours of expression. Because Cos-7 cells expressed low levels of the retinoid receptors, homeobox vectors were transfected with or without both RARα and RXRβ expression vectors.
Small-interfering RNA transfection
Small-interfering RNA (siRNA) was composed of double-stranded RNA oligonucleotides (MWG) designed to induce HOXA10 transcript variant 2 silencing. The sequence of the HOXA10v2 siRNA used was 5′-AGAGCAGCACGGUACAUUA-3′ (sense) and 5′-UAAUGUACCGUGCUGCUCU-3′ (antisense). Negative control siRNA (Silencer no. 1; Ambion, Huntingdon, United Kingdom) was used to demonstrate that the transfection had no nonspecific effects on gene expression. Two concentrations of siRNA (5 or 25 nM) were transfected into NB4 cells with the Hiperfert reagent according to the manufacturer's instructions (Qiagen). Two days after siRNA transfection, ATRA was administered for 6 hours at a final concentration of 0.1 μM.
Metabolites and HPLC
NB4 cells (4 × 106) were incubated in 20 mL of the appropriate medium with ATRA or RAMBA (1 μM each). Cells were separated from the culture medium by spinning at 2000g. Cytoplasm was dissociated from nucleoplasm by incubating cells (3 minutes at 4°C) in the following buffer: 20 mM HEPES, 10 mM potassium acetate, 1.5 mM magnesium acetate, and 0.5% NP40. Following a 10-minute spin at 10 000g, the cytoplasm-containing supernatant was removed, and the nucleus-containing pellet was burst in PBS buffer with 0.5% SDS. Retinoids were extracted from the culture medium, the cytoplasm, and the nucleoplasm fractions by adding an equal volume of ethyl acetate.28 Following a spin at 5000g, the organic phase was extracted and evaporated under a vacuum pump. Dry residues were reconstituted in 200 μL methanol and injected into a reverse-phase high-performance liquid chromatography (HPLC) system composed of a Waters 600 chromatograph (pump and controller) and a C18 reversed-phase column (5 μm, 4.6 mm × 250 mm; Symmetry; Waters, Milford, MA). Absorbance was measured at 340 nm on a UV detector set (Waters 2487). Elution was achieved using a linear gradient as follows: after equilibration in 65% methanol and 35% ammonium acetate buffer (1% in water), methanol was increased to 100% within 35 minutes at a flow rate of 1 mL/minute.
Protein extraction and Western blots
For Western blots, cells (2 × 106 cells) were lysed in 20 μL Laemmli buffer and incubated for 30 minutes at 37°C with 0.1 U/μL bensonase (Sigma, Poole, United Kingdom). Proteins were separated on a 10% SDS-PAGE and transferred to a nitrocellulose membrane. PML/RARα proteins were revealed by an anti-RARα antibody (9α9A6; 1/10 000), kindly provided by Cecile Rochette-Egly (IGBMC, Strasbourg, France). After blotting with an HRP-conjugated secondary antibody, chemiluminescence activity was revealed using the enhanced chemiluminescence (ECL) Advance Western blot detection kit (Amersham Biosciences).
Results
Mining SAGE libraries to select hallmarks of APL differentiation
Serial Analysis of Gene Expression (SAGE) was applied to the APL model. Both proliferative and 48-hour–differentiated NB4 cell libraries were constructed and compared with neutrophil data.31 By mining gene expression, we identified 2 clusters related to the granulocytic phenotype: 48 down-regulated genes (Figure 1A) and 35 up-regulated ones (Figure 1B). In addition, 75 retinoid-induced genes were distinctly clustered, because they were only detectable during the differentiation of NB4 cells (Figure 1C). Clusters were graphically displayed to highlight major changes involving cell-specific functions (Figure S2).
Transcriptional activities of APL blasts displaying different sensitivities to ATRA in vitro. Within the subset of SAGE tags that matched genes with described functions and that were registered according to the HuGO nomenclature, the abundances of SAGE tags from a proliferative NB4 library (UnT) were compared with their respective levels in both ATRA-treated NB4 (Diff) and polymorpho-nuclear neutrophil (PMN) libraries. Variations (P values) were calculated between proliferative NB4 cells and cells induced to differentiate by a 48-hour exposure to 1 μM ATRA (Diff/UnT). The proliferative NB4 library was also compared with the granulocytes (PMN/UnT). By mining NB4 gene expression data relative to the granulocytic phenotype, 48 underexpressed genes (A) were separated from 35 up-regulated genes (B). A cluster of 75 retinoid-induced genes that were barely detectable in the granulocyte but clearly induced by retinoid during NB4 cell differentiation (C). Heterogeneity at day 3 in the differentiation of 3 APL blasts (NBt test) exerting distinct sensitivities to ATRA (D). Data from the Relative Quantity gene expression (RQ) analysis. Transcriptional modulation (Log10 RQ) was calculated for blasts treated for 3 days with ATRA (0.1 μM) in comparison with an untreated control. Clusters of retinoid-responsive genes (E), granulocytic markers (F), chromatin remodelers, transcription factors, and coregulators (G). Genes involved in retinoic acid metabolism and the inflammatory response to maturation (H).
Transcriptional activities of APL blasts displaying different sensitivities to ATRA in vitro. Within the subset of SAGE tags that matched genes with described functions and that were registered according to the HuGO nomenclature, the abundances of SAGE tags from a proliferative NB4 library (UnT) were compared with their respective levels in both ATRA-treated NB4 (Diff) and polymorpho-nuclear neutrophil (PMN) libraries. Variations (P values) were calculated between proliferative NB4 cells and cells induced to differentiate by a 48-hour exposure to 1 μM ATRA (Diff/UnT). The proliferative NB4 library was also compared with the granulocytes (PMN/UnT). By mining NB4 gene expression data relative to the granulocytic phenotype, 48 underexpressed genes (A) were separated from 35 up-regulated genes (B). A cluster of 75 retinoid-induced genes that were barely detectable in the granulocyte but clearly induced by retinoid during NB4 cell differentiation (C). Heterogeneity at day 3 in the differentiation of 3 APL blasts (NBt test) exerting distinct sensitivities to ATRA (D). Data from the Relative Quantity gene expression (RQ) analysis. Transcriptional modulation (Log10 RQ) was calculated for blasts treated for 3 days with ATRA (0.1 μM) in comparison with an untreated control. Clusters of retinoid-responsive genes (E), granulocytic markers (F), chromatin remodelers, transcription factors, and coregulators (G). Genes involved in retinoic acid metabolism and the inflammatory response to maturation (H).
To conduct the pharmacogenomic studies of APL blasts, markers were selected from different classes encompassing multiple genes involved in pathways directly mediating the effects of retinoids. The selected markers included both previously described genes and genes that were unexplored with respect to APL differentiation. Markers were thus selected that were related to different mechanisms potentially involved in the establishment of resistance, including cytoplasmic retinoid metabolism, retinoid sequestration, expression of downstream ATRA-target genes, chromatin remodeling, transcription factors, and co-regulators of nuclear receptor function.
Selection of APL blasts predisposed to distinct sensitivities to ATRA
When APL blasts were treated with an ATRA concentration of 0.1 μM to identify blasts having differing sensitivies,9 heterogeneity in the in vitro differentiation rate was identified as a novel factor in the in vivo therapeutic efficiency.21 Patients whose leukemic cells presented a delay in maturation at day 3 (ie, < 50% differentiated cells) were associated with a poor prognosis, whereas, conversely, patients whose blast maturation rate was rapid (ie, more than 50% differentiated cells) showed significantly superior disease-free survival in therapy.
To define the genomic profiles of the APL blasts, pharmacogenomic studies were carried out on 3 blast populations showing distinct sensitivities to ATRA at day 3 (Figure 1D). Blasts from patient 1 were highly sensitive (Pt1, 50%). Blasts from patient 2 presented an intermediate sensitivity (Pt2, 30%). Finally, patient 3 was associated with a poor prognosis, because the leukemic cell population showed a delay in its maturation (Pt3, 10%). Nevertheless, the maturation rate of the low-sensitive leukemic cells in this patient accelerated considerably, reaching the same level of maturation as observed in high-sensitive blasts by day 6 (Pt1, 77%; Pt2, 76%; Pt3, 89%).
Increased gene transcription in APL blasts displaying a delay in differentiation
Pharmacogenomic profiles were assessed by high-throughput real-time PCR to define the genomic profiles of APL blasts showing differential ATRA sensitivities. No differences were detected in the basal transcription of untreated blastic populations (data not shown). When treated with ATRA, all blasts displayed a transcriptional response to the retinoid at day 3 (Figure 1E-H). Modulation predominantly involved the up-regulation of genes (> 60%), with a minority being repressed (< 10%). Interestingly, for up-regulated genes, the transcriptional production was higher in low-sensitive blasts. Specifically, the mean transcriptional level was 2.3 ± 1.4 (P < .05) and 4.1 ± 2.2 (P < .05) times higher in Pt2 and Pt3 blasts, respectively, than in Pt1 cells. Low-sensitive cells showed higher transcription levels of ATRA-target genes (Figure 1E) as well as of genes encoding proteins with specific functions in the granulocyte (Figure 1F). Low-sensitive cells also expressed higher amounts of transcriptional regulators (Figure 1G). This latter finding was consistent with the observed enhanced transcriptional activity of low-sensitive blasts, as the expressed genes included chromatin remodelers (HDAC3, 10, and 11), co-regulators (NCOA3, CRABP2), and transcription factors (CREG, STAT6, SPI1, and RAM2).
Transcription of cytochromes involved in retinoic acid metabolism versus regulation of other retinoid-inducible genes
Few genes (< 5%) were expressed at lower levels in low-sensitive cells. Interestingly, genes encoding the p450-cytochrome family 26 showed a unique differential expression pattern, as CYP26 genes were almost exclusively expressed by high-sensitive blasts (Figure 1H). As these cytochrome genes encode enzymes that are involved in ATRA degradation and thus act to regulate intracellular concentrations of retinoid,25,26 the predominant expression of CYP26 genes in high-sensitive cells offered an explanation as to why the global transcriptional rate was lower in these cells. Among the different genes of the CYP26 family, CYP26A1 was expressed at a significantly higher level than CYP26B1, suggesting that this gene may play an important role in the regulation of ATRA metabolism in this setting. The regulation of CYP26A1 expression by selective RARα and RXR agonists has been described in promyelocytic NB4 leukemic cells.33 However, because the CYP26A1 gene was modulated differently than other retinoid-inducible targets (Figure 2A), its transcriptional activation seemed to be more complex and difficult to ascribe to the previously described substrate-driven feedback mechanism that regulates intracellular concentrations of ATRA.34 Further, the promoter of the CYP26A1 gene is atypical, with conserved TAAT elements flanking the retinoic acid response element (RARE).35 Because such consensus sequences have been previously shown to form the core recognition element for homeobox (Hox) transcription factors in other promoters,36 we next investigated whether Hox proteins participate in the transcriptional regulation of CYP26A1.
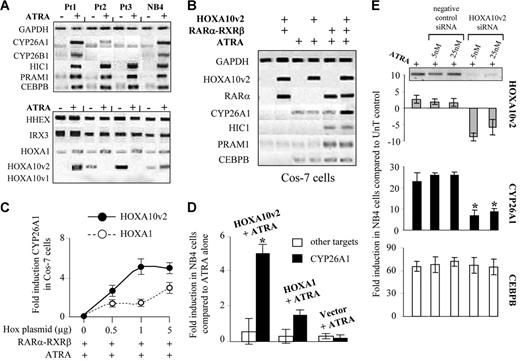
Enhancement of CYP26A1 transcription by a specific homeobox factor. (A) Semiquantitative reverse transcriptase PCR following the treatment of 3 APL blast populations showing distinct sensitivities to ATRA and following the differentiation of NB4 cells. Electrophoresis results of retinoid-inducible targets (top) and homeobox transcription factors (bottom). (B) Specific enhancement of CYP26A1 transcription in Cos-7 cells following the expression of homeobox factors. Electrophoresis results of individual or cotransfection of 1 μg of both RARα and RXRβ plasmids along with 1 μg of the HOXA10v2 plasmid. (C) Fold induction observed with distinct doses of the HOXA10v2 or HOXA1 plasmids in Cos-7 cells. (D) Induction of CYP26A1 transcription in NB4 cells following the nucleofection of either HOXA10v2 or HOXA1 plasmid. The specificity of the enhancement of the cytochrome was confirmed by measuring the modulation of other retinoid-induced targets (cumulative means for HIC1, PRAM1, and CEBPB). *P < .05 compared with ATRA alone. (E) Specific down-regulation of CYP26A1 was observed after siRNA transfection that induced HOXA10v2 transcript silencing. *P < .05 compared with ATRA control. (C-E) Error bars represent the standard error (n = 2).
Enhancement of CYP26A1 transcription by a specific homeobox factor. (A) Semiquantitative reverse transcriptase PCR following the treatment of 3 APL blast populations showing distinct sensitivities to ATRA and following the differentiation of NB4 cells. Electrophoresis results of retinoid-inducible targets (top) and homeobox transcription factors (bottom). (B) Specific enhancement of CYP26A1 transcription in Cos-7 cells following the expression of homeobox factors. Electrophoresis results of individual or cotransfection of 1 μg of both RARα and RXRβ plasmids along with 1 μg of the HOXA10v2 plasmid. (C) Fold induction observed with distinct doses of the HOXA10v2 or HOXA1 plasmids in Cos-7 cells. (D) Induction of CYP26A1 transcription in NB4 cells following the nucleofection of either HOXA10v2 or HOXA1 plasmid. The specificity of the enhancement of the cytochrome was confirmed by measuring the modulation of other retinoid-induced targets (cumulative means for HIC1, PRAM1, and CEBPB). *P < .05 compared with ATRA alone. (E) Specific down-regulation of CYP26A1 was observed after siRNA transfection that induced HOXA10v2 transcript silencing. *P < .05 compared with ATRA control. (C-E) Error bars represent the standard error (n = 2).
A homeobox transcription factor as enhancer of RA-mediated CYP26A1 gene expression
Among the Hox transcripts screened in our SAGE libraries, only variant 2 of the HOXA10 gene (HOXA10v2) had an expression pattern correlated with the cytochrome modulation, whereas the variation of other Hox transcripts was similar in all treated blasts (Figure 2A). Both CYP26A1 and HOXA10v2 transcripts were coinduced in Pt1 blasts. Further, after 3 days of treatment of both Pt2 and Pt3 blasts, when CYP26A1 transcription levels were low, HOXA10v2 expression was also reduced.
To determine the mechanism underlying the control of CYP26A1 expression, we investigated whether HOXA10v2 may act as a homeobox transcription factor that directly participates in the transcription of CYP26A1. Although the homeobox factor was unable to induce the expression of the cytochrome by itself, it strongly enhanced the induction of CYP26A1 in Cos-7 cells when transcription was primed by the RARα and RXR retinoid receptors (Figure 2B). In contrast to other well-known ATRA-targets (CEBPB, HIC1, and PRAM1), the transcription of CYP26A1 was specifically induced by HOXA10v2, indicating that this factor may play an important role in enhancing retinoic acid metabolism. Also, although another Hox factor (HOXA1) had no effect, even a low dose of the HOXA10v2 factor was sufficient to significantly increase the level of CYP26A1 transcription (Figure 2C). The specific enhancement of CYP26A1 transcription was confirmed by nucleofection of the HOXA10v2 plasmid into NB4 cells (Figure 2D). Moreover, the use of small-interfering RNA to induce HOXA10v2 transcript silencing in NB4 cells specifically reduced CYP26A1 expression (Figure 2E).
APL blast sensitivity correlates with the transcriptional rate of CYP26A1
In NB4 cells, HOXA10v2 thus appears to act as an enhancer that specifically regulates CYP26A1 transcription but not that of other ATRA-inducible genes. This observation was corroborated by analyzing 7 APL cells showing varying sensitivities to ATRA at the time of diagnosis (Figure 3A), while the maturation level at day 3 correlated with both HOXA10v2 and CYP26A1, and other retinoid-inducible genes were expressed at constant levels in both low- and high-sensitive blasts (Figure 3B). Whereas homeobox genes are frequently targeted by retinoids and play significant roles in both normal and deregulated hematopoiesis,37,38 the HOXA10 gene was not detected as a direct retinoid target in our studies (test with cycloheximide, data not shown). It was thus possible that another retinoid-related signaling pathway was involved in the transcriptional regulation of CYP26A1. For instance, chemokine production has been linked to ATRA sensitivity,39–41 we consequently investigated the possibility that maturation events play a role in enhancing ATRA metabolism.
Correlation between CYP26A1 transcriptional activity and the in vitro blasts sensitivity to ATRA. Heterogeneity in the in vitro differentiation rates of 7 populations of APL blasts displaying varying sensitivities to ATRA. (A) Results of the NBt test performed at days 3 and 6. Error bars represent the standard error (n = 2). (B) Positive correlation between blasts sensitivity at day 3 and the transcriptional level of CYP26A1 and HOXA10v2. Transcriptional modulation (Log10) was calculated between ATRA-treated and untreated blasts.
Correlation between CYP26A1 transcriptional activity and the in vitro blasts sensitivity to ATRA. Heterogeneity in the in vitro differentiation rates of 7 populations of APL blasts displaying varying sensitivities to ATRA. (A) Results of the NBt test performed at days 3 and 6. Error bars represent the standard error (n = 2). (B) Positive correlation between blasts sensitivity at day 3 and the transcriptional level of CYP26A1 and HOXA10v2. Transcriptional modulation (Log10) was calculated between ATRA-treated and untreated blasts.
RA-mediated production of interleukin-8 enhances HOXA10v2 expression
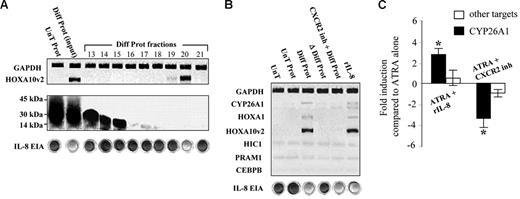
The NB4 cell line represents an ideal model for identifying specific actors in the regulation of retinoid metabolism. Chemokines are known to be secreted into the culture medium following the RA-induced differentiation of NB4 cells. Accordingly, NB4 cells were cultured in the presence of ATRA, and extracellular proteins were retinoid deprived and concentrated by purification column. The administration of the resulting extracts to proliferative NB4 cells induced the transcription of HOXA10v2 messenger RNA (Figure 4A). The protein extract was then fractionated using size exclusion chromatography, and only one eluted fraction, composed of low molecular weight proteins, was found to be able to activate transcription of variant 2 of the HOXA10 gene. Screening 33 potential secreted proteins based on their transcription in the SAGE data identified interleukin-8 (IL-8), which has a molecular weight of 11 kDa, as the most relevant candidate. The single activating fraction was thus tested for the presence of IL-8 using an enzyme immunosorbent assay and was found to indeed contain the protein. In addition, the administration of recombinant IL-8 was observed to induce expression of the HOXA10v2 factor, and inhibiting the IL-8 receptor CXCR2 using an antagonist (SB225002) prior to the administration of the protein extract reduced HOXA10v2 transcript level (Figure 4B). These results supported the hypothesis that the secretion of IL-8 following ATRA treatment leads to the modulation of HOXA10v2. When the IL-8 receptor CXCR2 was inhibited before the administration of ATRA, CYP26A1 transcription was repressed by more than 3-fold. Moreover, whereas the addition of the chemokine alone was unable to induce CYP26A1 expression, the cytochrome transcription was increased by 3-fold when IL-8 was added along with ATRA (Figure 4C). These results obtained using the APL cell line model thus support the hypothesis that IL-8 plays a role in enhancing retinoid metabolism.
Interleukin-8 induces CYP26A1 expression by enhancing transcription of the HOXA10v2 factor. (A) Transcription of the HOXA10v2 factor following the administration of proteins secreted into the culture medium by differentiated NB4 cells (Diff Prot). Results following the administration of proteins secreted by untreated NB4 cells (UnT Prot). HOXA10v2 induction with fractions obtained by the size exclusion liquid chromatography of Diff Prot (Diff Prot fractions). Elutions were evaluated by transferring onto nitrocellulose membranes, staining with red Ponceau, and testing with the human IL-8 enzyme immunosorbent assay (cupules) prior to their administration to proliferative NB4 cells for 12 hours. (B) Results following protein inactivation by boiling the protein extract for 15 minutes at 100°C (Δ Diff Prot) or following the earlier inhibition of the IL-8 receptor (CXCR2) with 200 nM of the SB225002 antagonist (CXCR2 inh + Diff Prot). Results following the addition of 25 ng/mL recombinant interleukin-8 (rIL-8). Because CYP26A1 expression required the activation of the retinoid signal, NB4 cells were treated with ATRA (0.1 μM), either with the previous inhibition of the IL-8 signal with 200 nM of the SB225 002 antagonist (ATRA + CXCR2 inh) or following the addition of rIL-8 at a final dose of 25 ng/mL (ATRA + rIL-8). (C) Results of the CYP26A1 transcriptional modulation measured by real-time PCR. *P < .05 compared with ATRA control. The specificity of the enhancement of CYP26A1 transcription was verified by measuring the modulation of other retinoid-induced targets (cumulative means for HIC1, PRAM1, and CEBPB). Error bars represent the standard error (n = 3).
Interleukin-8 induces CYP26A1 expression by enhancing transcription of the HOXA10v2 factor. (A) Transcription of the HOXA10v2 factor following the administration of proteins secreted into the culture medium by differentiated NB4 cells (Diff Prot). Results following the administration of proteins secreted by untreated NB4 cells (UnT Prot). HOXA10v2 induction with fractions obtained by the size exclusion liquid chromatography of Diff Prot (Diff Prot fractions). Elutions were evaluated by transferring onto nitrocellulose membranes, staining with red Ponceau, and testing with the human IL-8 enzyme immunosorbent assay (cupules) prior to their administration to proliferative NB4 cells for 12 hours. (B) Results following protein inactivation by boiling the protein extract for 15 minutes at 100°C (Δ Diff Prot) or following the earlier inhibition of the IL-8 receptor (CXCR2) with 200 nM of the SB225002 antagonist (CXCR2 inh + Diff Prot). Results following the addition of 25 ng/mL recombinant interleukin-8 (rIL-8). Because CYP26A1 expression required the activation of the retinoid signal, NB4 cells were treated with ATRA (0.1 μM), either with the previous inhibition of the IL-8 signal with 200 nM of the SB225 002 antagonist (ATRA + CXCR2 inh) or following the addition of rIL-8 at a final dose of 25 ng/mL (ATRA + rIL-8). (C) Results of the CYP26A1 transcriptional modulation measured by real-time PCR. *P < .05 compared with ATRA control. The specificity of the enhancement of CYP26A1 transcription was verified by measuring the modulation of other retinoid-induced targets (cumulative means for HIC1, PRAM1, and CEBPB). Error bars represent the standard error (n = 3).
Disrupting cytoplasmic ATRA metabolism leads to retinoid accumulation in the nucleus and increased transcriptional activity
Because low levels of CYP26A1 transcription were exclusively observed in blasts showing low sensitivity and associated with the poorest prognosis, we reasoned that low expression of this cytochrome may be a potent cause of ATRA resistance. We thus investigated the effects of inhibiting retinoid metabolism in the NB4 cell model.
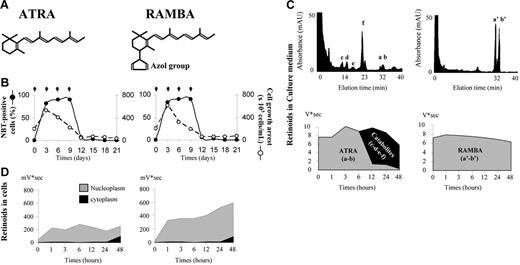
Intracellular ATRA metabolism in target cells occurs primarily by oxidation in a reaction involving membrane-bound microsomal CYP26 enzymes.42 4-Azolyl retinoic acid, an analog of ATRA (Figure 5A), is a retinoic acid metabolism blocking agent (RAMBA). The azole group makes RAMBA a specific and potent inhibitor of CYP26 cytochromes,27 without affecting cell growth or differentiation (Figure 5B). ATRA metabolism was monitored by high-pressure liquid chromatography (HPLC).28 A metabolic switch was observed to typically occur between 6 and 12 hours after ATRA treatment of NB4 cells, with the generation of oxidative polar metabolites observed exclusively in the culture medium (Figure 5C). Catabolites were never observed in the intracellular compartment. The metabolism was almost complete after 24 hours (> 95%), with the predominant hydroxylated form being 4,6 epoxy-ATRA (> 80%). Moreover, as previously reported,11 the nuclear level of ATRA remained relatively constant throughout the differentiation process (Figure 5D). We suggest that extracellular effluxes of catabolites play an important role in stabilizing nucleoplasmic levels of ATRA. In contrast, metabolism could not occur in the RAMBA-treated NB4 cells (Figure 5C), resulting in retinoid accumulation in their nuclei (Figure 5D). The consequences of this increase in nuclear retinoid levels were next addressed in terms of transcriptional activity.
Retinoid modulation following the differentiation of the NB4 cell line using either ATRA or RAMBA. (A) ATRA and RAMBA chemical molecules. (B) Efficiency of differentiation (•) and cell growth inhibition (○) after treatment (black arrows) of NB4 cells with 1 μM of either ATRA or RAMBA. (C) Retinoid modulation in NB4 culture medium performed with gradient reversed-phase HPLC. Chromatogram of retinoids extracted from the culture medium after 24 hours of treatment with either ATRA or RAMBA (upper graphs). Retinoid modulation in the culture medium following NB4 cell differentiation kinetics by treatment with either ATRA or RAMBA (lower graphs). As the azole group makes RAMBA a specific and potent inhibitor of CYP26 p450-cytochromes, metabolism was only observed following ATRA treatment. Hydroxylated products were more rapidly eluted than parental isoforms. 13-cis RA (a), 13-cis RAMBA (a′), their stereoisomers ATRA (b) and RAMBA (b′). The identified metabolites were 4-oxo-ATRA (c), 4-hydroxy-ATRA (d), 18-hydroxy-ATRA (e), and 5,6-epoxy-ATRA (f). (D) Retinoid modulation in cytoplasm and nucleoplasm following NB4 cell differentiation.
Retinoid modulation following the differentiation of the NB4 cell line using either ATRA or RAMBA. (A) ATRA and RAMBA chemical molecules. (B) Efficiency of differentiation (•) and cell growth inhibition (○) after treatment (black arrows) of NB4 cells with 1 μM of either ATRA or RAMBA. (C) Retinoid modulation in NB4 culture medium performed with gradient reversed-phase HPLC. Chromatogram of retinoids extracted from the culture medium after 24 hours of treatment with either ATRA or RAMBA (upper graphs). Retinoid modulation in the culture medium following NB4 cell differentiation kinetics by treatment with either ATRA or RAMBA (lower graphs). As the azole group makes RAMBA a specific and potent inhibitor of CYP26 p450-cytochromes, metabolism was only observed following ATRA treatment. Hydroxylated products were more rapidly eluted than parental isoforms. 13-cis RA (a), 13-cis RAMBA (a′), their stereoisomers ATRA (b) and RAMBA (b′). The identified metabolites were 4-oxo-ATRA (c), 4-hydroxy-ATRA (d), 18-hydroxy-ATRA (e), and 5,6-epoxy-ATRA (f). (D) Retinoid modulation in cytoplasm and nucleoplasm following NB4 cell differentiation.
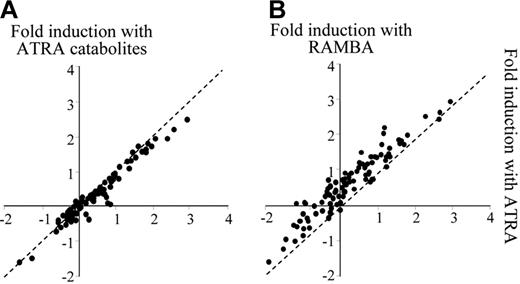
All ATRA metabolite forms, tested separately, have been shown to be active retinoids for APL differentiation, albeit with lower activity than ATRA.28 Although the greater activity of ATRA relative to its hydroxylated forms was the basis of the belief that clinical therapy would be more effective with ATRA,43 the exogenous application of ATRA catabolites produced de novo by NB4 cells was still able to reduce the transcriptional activity (Figure 6A). Conversely, blocking ATRA metabolism with RAMBA resulted in the nuclear accumulation of retinoids, leading to an increase in the transcriptional activity (Figure 6B). The effects of this elevated exposure to ATRA were then investigated in terms of the resistance outcome.
Treatment by ATRA or RAMBA leads to distinct transcriptional efficiencies. Transcriptional activity in response to ATRA was compared with the activity in the presence of either ATRA catabolites (A) or RAMBA (B). Transcriptional fold induction (Log10) was measured after 48 hours of treatment with 1 μM of each retinoid.
Treatment by ATRA or RAMBA leads to distinct transcriptional efficiencies. Transcriptional activity in response to ATRA was compared with the activity in the presence of either ATRA catabolites (A) or RAMBA (B). Transcriptional fold induction (Log10) was measured after 48 hours of treatment with 1 μM of each retinoid.
Retinoid accumulation in the nucleus increases the growth of resistant subclones
The hypothesis that cellular APL metabolism is a factor in the acquisition of resistance was based on the observation that the CYP26 enzyme protects cells from an elevated exposure to active retinoids. To determine whether ATRA metabolism was important for preventing resistance, retinoid acid metabolism was blocked in NB4 cells with RAMBA (1 μM), and the cells were compared with ATRA-treated (1 μM) and untreated controls.
ATRA and RAMBA were equally effective in terms of cell growth inhibition and differentiation (Figure 5B). Residual cells were then individualized at day 12 by clonal dilution. Although no cell growth was observed in the ATRA-treated cells, 7% of the RAMBA-treated cells were resistant (Figure 7A-B). All of the resistant clones grew within 25 to 35 days and were confirmed to be refractory to ATRA-induced differentiation (Figure 7C). The PML/RARα protein was always detected in the resistant clones (Figure 7D), suggesting that the resistant cells originated from the NB4 cell pool. In conclusion, these results support the hypothesis that deficient ATRA metabolism in NB4 cells can increase drug-selective pressure on retinoid-resistant subclones, present in the cell population at the beginning of the differentiation process.
Inhibition of ATRA metabolism leads to high growth of NB4-resistant subclones. Percentage of resistant NB4 subclone growth following treatment with either ATRA (A) or RAMBA (B). Statistical analysis was performed on 100 clones for each condition. (C) RAMBA-treated subclones showing growth were confirmed to be resistant to ATRA using the NBt test. Error bars represent the standard error (n = 7). (D) Western blot revealing the presence of the PML/RARα protein in 3 RAMBA-treated resistant clones.
Inhibition of ATRA metabolism leads to high growth of NB4-resistant subclones. Percentage of resistant NB4 subclone growth following treatment with either ATRA (A) or RAMBA (B). Statistical analysis was performed on 100 clones for each condition. (C) RAMBA-treated subclones showing growth were confirmed to be resistant to ATRA using the NBt test. Error bars represent the standard error (n = 7). (D) Western blot revealing the presence of the PML/RARα protein in 3 RAMBA-treated resistant clones.
Discussion
All-trans retinoic acid induces complete remission in more than 80% of patients with acute promyelocytic leukemia. However, reduced retinoid sensitivity can develop in a subtype of leukemic cells present at diagnosis, resulting in disease relapse. Although a detailed understanding of how APL cells can develop reduced sensitivity to ATRA remains elusive, several possible mechanisms have been proposed.44,45 In the present work, we have characterized a new process involving the low expression of CYP26 cytochromes as a potent cause of the selection of clones with differential RA-sensitivities.
In an earlier study, close monitoring of APL blasts differentiation in vitro revealed that the time-dependent terminal differentiation of blasts from the leukemic cell population of a given patient followed a distinct pattern that was correlated with disease-free survival and relapse in the APL93 trial.21 Although in all samples more than 80% of the blast population achieved terminal differentiation by day 6 of culture with ATRA, significant heterogeneity was observed at day 3. Patients with a leukemic population that was slow to differentiate at day 3 had a lower disease-free survival rate than those patients whose samples had more than 50% differentiated cells. Relapse was thus correlated with reduced ATRA responsiveness in a subset of APL cells at diagnosis. To identify novel biologic networks potentially involved in this altered differentiation process, we carried out pharmacogenomic studies of APL blasts displaying distinct sensitivities to ATRA. Although genes involved in retinoid-associated pathways were globally expressed in both low- and high-sensitive blasts, transcription of the CYP26A1 gene, which encodes an enzyme specifically involved in retinoic acid metabolism, was restricted to high-sensitive treated blasts. We propose that the metabolism of ATRA was not activated in low-sensitive clones and hypothesize that diminished ATRA metabolic potency may lead to an increase in subcellular retinoid concentrations. Such increased ATRA levels may explain the enhanced transcription of retinoid targets that was observed in low-sensitive blasts.
In promyelocytic leukemic cells, CYP26A1 has been described as a retinoid-inducible gene.33 CYP26A1 expression has also been described as being involved in a substrate-driven feedback mechanism that acts to regulate intracellular retinoid concentrations.34 According to this view, CYP26A1 transcription should be particularly active in low-sensitive, ATRA-treated cells, similar to other retinoid-inducible genes. In fact, we found that this cytochrome was not induced in low-sensitive cells, although it was in high-sensitive ones. Therefore, the process controlling the cytoplasmic CYP26A1-mediated regulation of ATRA appears to be more complex than a simple feedback loop, where the incoming ATRA would induce the expression of the cytochrome. As 2 core TAAT recognition elements, which are specific for homeobox proteins, flank the RARE response element in the CYP26A1 promoter,35 we first investigated whether homeobox factors participate in the transcriptional regulation of CYP26A1.
In APL blasts treated by ATRA, only a transcriptional variant of the HOXA10 gene (HOXA10v2) presented an expression pattern that was correlated with the modulation of CYP26A1. In contrast to typical homeobox genes, this variant results from the alternative transcription of the HOXA10 gene and encodes a small protein consisting mostly of the homeodomain region. Although this variant is expressed in a variety of hematopoietic cell types, its biologic function remains poorly defined.46 In promyelocytic leukemic cells, CYP26A1 has been described as a retinoid-inducible gene that is regulated by the RARα and RXR receptors.33 Although the HOXA10v2 factor was unable to induce the expression of the cytochrome by itself, we found it to be an effective enhancer of the retinoic acid receptors that specifically induce CYP26A1 transcription, but not of other RA-inducible genes. The importance of the TAAT elements that flank the RARE in the CYP26A1 promoter, and whether HOXA10v2 factor interacts with these sequences, are both issues that remain to be investigated. Also, although HOXA10v2 and retinoid receptors participate together in the regulation of the CYP26A1 promoter, the nature of this interaction remains to be explored.
Although the HOXA10 gene was not detected as a direct retinoid target, another signaling pathway may be involved in the transcriptional regulation of CYP26A1. As increased CYP26A1 transcription was specifically observed in high-sensitive blasts, we addressed the possibility that maturation events play a role in enhancing ATRA metabolism. This hypothesis was indeed corroborated, as the maturation level was closely correlated with the transcriptional rate of CYP26A1 during the in vitro treatment of primary APL cells. In addition, chemokine production has previously been linked to ATRA sensitivity and is thought to be involved in the leukocyte activation events that occur along with differentiation.39–41 Moreover, in NB4 cells, we obtained evidence that a novel signaling pathway is activated in APL cells on ATRA treatment, whereby interleukin-8 activates CYP26A1 transcription by inducing the specific transactivation of the HOXA10v2 factor.
The balance between influx and efflux rates determined the effective cellular uptake of retinoid in target cells. Metabolism occurred through microsomal membrane-bound p450 cytochromes and involved the hydroxylation of the ligand and the extracellular efflux of catabolite forms42 that are impoverished in their differentiation efficiency.28 We demonstrated that this metabolism is critical for regulating ATRA levels in the nucleus and alleviating transcriptional activity. In NB4 cells, inhibiting the retinoic acid metabolism pathway with a retinoic acid metabolism blocking agent (RAMBA) increased the exposure of the cells to active retinoid in the nucleus, leading to a profound increase in transcriptional activity. In addition, perturbing retinoic acid metabolism increased the growth of resistant NB4 subclones.
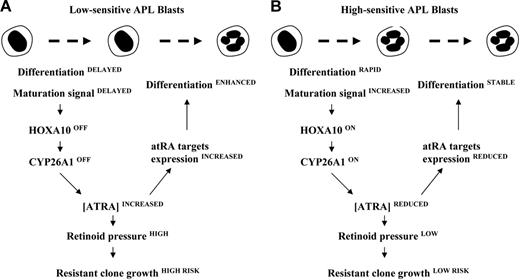
Our results strongly support the hypothesis that effective retinoid therapy requires the transient activation of the retinoid signal. Metabolism needs to be initiated following the cell response to ATRA. The present study has identified a novel retinoic acid signaling pathway, involving maturation signals and a homeobox transcription factor that enhances the metabolism process. A model is presented in Figure 8 that depicts a mechanism of action for the development of resistance in low-sensitive APL cells. Nevertheless, the pathways that are activated by chemokines to induce transcription of the HOXA10v2 factor remain to be determined, and new, additional maturation stimuli that act to enhance retinoid metabolism may yet be discovered.
A proposed model for the mechanism of action underlying low expression of the CYP26 cytochrome as a potent cause of relapse. The hypothesis that the growth of APL-resistant blasts is provoked by an increased exposure to active retinoids is based on the observation that, by studying the kinetics of differentiation, cytochromes involved in ATRA metabolism are differently activated between low- (A) and high-sensitive (B) APL cells.
A proposed model for the mechanism of action underlying low expression of the CYP26 cytochrome as a potent cause of relapse. The hypothesis that the growth of APL-resistant blasts is provoked by an increased exposure to active retinoids is based on the observation that, by studying the kinetics of differentiation, cytochromes involved in ATRA metabolism are differently activated between low- (A) and high-sensitive (B) APL cells.
Resistant subclones proliferated after 3 weeks of treatment with RAMBA, whereas the growth of resistant APL cell lines generally requires continuous selective pressure with retinoid for several months.47 In this study, we characterized the drug dynamics that underlie retinoid selection pressure and that could be the key factor in the emergence of resistant subclones. We hypothesize that reductions in the strength of ATRA metabolism prevent the normal efflux of retinoid, inevitably provoking an elevation in the nuclear retinoid level. The resulting increased exposure to active retinoid leads to the rapid selection of an APL cell population that is insensitive.23,24 Because the PML/RARα protein was still expressed in RAMBA-resistant clones, the features underlying their insensitivity still need to be explored. Possible explanations for their resistance include that the clones harbor a mutation in the PML/RARα region that is related to the RA effects,24,48 or that they contain another perturbation in the multifactorial nuclear complex that regulates gene expression.
The administration of ATRA is commonly the recommended approach for APL therapy, and the drug is given orally with several doses for a set amount of time, usually for 1 or 2 years of administration. Moreover, RAMBA is counted among the relevant drugs that could be used to delay the in vivo retinoid metabolism, in particular cases of elevated physiologic retinoid degradation. In our studies, low transcription of the CYP26A1 gene involved in the ATRA catabolism was only observed in blasts from patients with the most unfavorable diagnosis. In addition, in NB4 cell line model, the inhibition of catabolism induced the growth of a large number of resistant subclones. Although our pharmacogenomic results need to be confirmed in larger studies, they strongly support the notion that ATRA catabolism needs to be initiated following the cell response and suggest the hypothesis that therapy with high-dose administration of ATRA or with unmetabolizable drugs might have the harmful consequence of exposing cells to continuous selective pressure, which dramatically accelerates the growth of APL-resistant clones. Management by discontinuing the administration of ATRA could be an appropriate solution for controlling intracellular levels of the drug. Interestingly, intermittent treatment with a time interval for pulses ATRA therapy has been found to be effective at maintaining long-term remission in a clinical pilot study.49 Our results corroborate this idea and suggest that clinically pulses of ATRA maintenance would be useful, especially for patients associated to unfavorable prognosis. At last, based on the use of markers related to retinoic acid metabolism, the ultimate objective will be to detect individual genomic variations and personalize treatments according to the specific characteristics of each patient with APL.
Authorship
Contribution: R.Q. designed research and performed research, analyzed data, and wrote the paper. A.B. designed research and performed research, analyzed data, and provided critical revision of the manuscript. B.C. contributed to the in vitro culture of APL patient samples and treatments. G.B. performed the granulocytic SAGE library construction. L.M. and D.P. contributed to the development of analytical tools for SAGE data analysis. J.M., C.C., and T.C. designed research, analyzed data, and provided critical revision of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Thérèse Commes, Groupe d'Etude des Transcriptomes, Institut de génétique humaine, UPR CNRS 1142, 141 rue de la Cardonille, 34395 Montpellier, France; e-mail: commes@univ-montp2.fr.
Presented at the 47th annual meeting of the American Society of Hematology, Atlanta, GA, December 12, 2005.50
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
We thank Dr Stamatis Varsamos and Peter Follette for comments and insight concerning this manuscript. We thank Applied Biosystems (Courtaboeuf, France) for the ABI7900HT apparatus graciously made available to us and the Genopole LR for sequencing SAGE tags. We thank Claude Bonfils for discussions concerning ATRA metabolism analyses, Bernard Romestand for help with the size exclusion chromatography, and Joël Martin for the Cos-7 cell transfection protocol.
This work was supported by grants from the Laurette Fugain association, the Centre National pour la Recherche Scientifique, and the Ligue Régionale Contre Le Cancer Languedoc-Roussillon, and by scholarships from the Ministère Français de la Recherche (A.B.) and from the Ligue Régionale Contre Le Cancer Languedoc-Roussillon (A.B.).




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal