Abstract
PF-4/CXCL4 is a member of the CXC chemokine family, which is mainly produced by platelets and known for its pleiotropic biological functions. Recently, the proteic product of a nonallelic variant gene of CXCL4 was isolated from human platelets and named as CXCL4L1. CXCL4L1 shows only 4.3% amino acid divergence in the mature protein, but exhibits a 38% amino acid divergence in the signal peptide region. We hypothesized that this may imply a difference in the cell type in which CXCL4L1 is expressed or a difference in its mode of secretion. In different types of transfected cells, CXCL4 and CXCL4L1 exhibited a distinct subcellular localization and a differential regulation of secretion, CXCL4 being stored in secretory granules and released in response to protein kinase C activation, whereas CXCL4L1 was continuously synthesized and secreted through a constitutive pathway. A protein kinase C-regulated CXCL4 secretion was observed also in lymphocytes, a cell type expressing mainly CXCL4 mRNA, whereas smooth muscle cells, which preferentially expressed CXCL4L1, exhibited a constitutive pathway of secretion. These results demonstrate that CXCL4 and CXCL4L1 exhibit a distinct subcellular localization and are secreted in a differentially regulated manner, suggesting distinct roles in inflammatory or homeostatic processes.
Introduction
PF-4/CXCL4 is a member of the CXC chemokine family produced by cells of the megakaryocytic lineage. In megakaryocytes CXCL4 is synthesized, enclosed in vesicles, and transferred to the α granules from which it is secreted following platelet activation.1 More recently, CXCL4 expression was also found in monocytes.2 The first biological function described for CXCL4 is its antiheparin activity, responsible for the important role of CXCL4 in the regulation of coagulation processes.3 In addition, CXCL4 has a role in heparin-induced thrombocytopenia (HIT), a common immune-mediated disorder characterized by an immune response against epitopes within circulating heparin-CXCL4 complexes, which leads to a reduction of circulating platelets counts and is recognized as a risk factor for thromboembolic complications.4,5 Subsequent studies have defined an array of apparently unrelated CXCL4 activities. CXCL4 exhibits antiangiogenic properties in vitro and in vivo and inhibits tumor neovascularization through a variety of mechanisms.6–8 First, CXCL4 is able to interact directly with angiogenic growth factors, such as fibroblast growth factors (FGFs) and vascular endothelial growth factor (VEGF), and inhibits their interaction with the cell surface receptor.6,9 Second, CXCL4 may bind proteoglycans and interfere with the proteoglycan-bystander effect on growth factor activity.10 Furthermore, a cell surface receptor that is expressed by human endothelial cells (ECs) in a cell cycle-dependent manner11 and mediates the antiangiogenic effects of CXCL4 has been recently identified and named as CXCR3-B.12 Besides the antiangiogenic properties, CXCL4 expresses immunomodulatory activities, such as down-regulation of IFN-γ production by type 1 T-helper (Th1) cells13,14 and up-regulation of IL-4, IL-5, and IL-13 in type 2 T-helper (Th2) cells.13 Moreover, CXCL4 influences hematopoiesis, inhibiting megakaryocytopoiesis15 and the proliferation of committed erythroid and granulocyte-macrophage colonies,16 as well as of primitive CD34+ progenitors.17
The human CXCL4 gene appears to be duplicated. Indeed, more than a decade ago a second, highly homologous gene, showing only 2.6% DNA divergence was identified and called PF-4alt18 or PF4-V1.19 More recently, the protein product of the PF-4alt gene was isolated for the first time from thrombin-stimulated human platelets and renamed as CXCL4L1.20 CXCL4L1, compared with CXCL4, is a 30-fold more potent inhibitor of EC chemotaxis in vitro and has more profound effects in blocking angiogenesis in vivo.20 CXCL4L1 shows only 4.3% amino acid divergence in the mature protein, which contains 3 amino acid substitutions in the COOH-terminus, a region known to be critical for CXCL4-heparin interaction.20 However, CXCL4 and CXCL4L1 exhibit a 38% amino acid divergence in the signal peptide region. Previous studies hypothesized that this may imply a difference in the cell type in which CXCL4L1 is expressed or a difference in its mode of secretion.19 However, this hypothesis has not yet been verified.
In this study, by introducing plasmids encoding CXCL4 or CXCL4L1 in heterologous cell systems, we demonstrate that whereas CXCL4 is stored within the cytoplasm and secreted via a regulated pathway in response to protein kinase C (PKC) activation, CXCL4L1 is continuously secreted through a constitutive pathway. Accordingly, human T cells, which preferentially expressed CXCL4, released low levels of this chemokine in basal conditions that was mostly stored in the intracellular compartment. After PMA stimulation, human T cells immediately released high amounts of CXCL4. On the other hand, human vascular smooth muscle cells (VSMCs) that expressed almost exclusively CXCL4L1, constitutively released CXCL4L1 and were unresponsive to PMA stimulation for CXCL4L1 secretion. These results suggest a distinct role for CXCL4 and CXCL4L1 in inflammatory or homeostatic processes, respectively.
Materials and methods
Immunofluorescence and confocal microscopy
Confocal microscopy was performed as previously described.21 Briefly, immunofluorescence was performed on cells cultured on chamber slides fixed in 4% paraformaldehyde for 20 minutes. The following antibodies were used: anti-CXCL4 monoclonal antibody (mAb; R&D Systems, Minneapolis, MN, clone 170106, 7 μg/mL), and anti–VAMP-1/2/3 pAb (Santa Cruz Biotechnology, Santa Cruz, CA, 8 μg/mL). Goat anti–mouse IgG1 Alexa Fluor 488 and goat anti–rabbit IgG Alexa Fluor 546 (1:1000, Molecular Probes, Eugene, OR) were used as secondary antibodies. Bodipy TR C5 ceramide (1:200) was obtained from Molecular Probes. Slides were examined by conventional confocal microscopy on a Zeiss LSM 510 META microscope system (Carl Zeiss, Jena, Germany). Nuclei were counterstained with To-pro-3 (Molecular Probes).
Cell cultures
Human microvascular ECs (HMVECs), human aortic smooth muscle cells (HASMCs), and human coronary smooth muscle cells (HCoSMC) were obtained from Cambrex BioScience (East Rutherford, NJ). HMVECs were cultured in EGM-2 MV-Microvascular Endothelial Cell Medium-2 (Cambrex); HASMCs and HCoSMCs were cultured in SmGM-2-Smooth Muscle Medium-2 (Cambrex). Primary cultures were maintained at 37°C, 5% CO2 and used between passages 4 and 6. Platelets were obtained from whole blood as described.22 HCT-8 (human colonic epithelial cell line), A-704 (human kidney adenocarcinoma cell line), and the HEK-293 EBNA cell line were from European Collection of Cell Cultures (ECACC; Sigma-Aldrich, St Louis, MO). HCT-8 and A-704 cells were cultured in EMEM (Cambrex) 10% FCS (HyClone, Logan, UT); HEK-293 cells were cultured in DMEM (Sigma-Aldrich) 10% FCS. Human samples were collected abiding by the rules of the University of Florence Institutional Review Board for these studies and in accordance with the Declaration of Helsinki.
Isolation and culture of CD3+ and CD14+ cells.
CD3+ T cells and CD14+ were obtained from peripheral blood mononuclear cells (PBMCs) of healthy subjects by fluorescence-activated cell sorting (FACS; FACSAria sorter, BD Biosciences, Milan, Italy), as described.21 Total PBMCs were stained with anti–CD3-APC, anti–CD14-FITC, and anti–CD19-PE mAbs (BD Biosciences). Stained samples were sorted into CD3+ T lymphocytes gating on the lymphocyte populations, accordingly with forward- and side-scattered light signals, and then on CD3+CD14−CD19− and into CD14+ cells gating on the monocyte population, accordingly with forward- and side-scattered light signals, and then on CD3−CD19−CD14+ cells. A total of 106 CD3+ and CD14+ cells, with a purity being consistently more than 99%, were collected as analyzed by the Diva software (BD Biosciences). Immediately after sorting, absence of platelet contamination in cell preparations was verified after staining with anti-GPIIb antibody (BD Biosciences). After sorting, recovered T cells were plated and initially stimulated with PHA (0.1% vol/vol; Gibco Laboratories, Grand Island, NY) and IL-2 (20 U/mL; Eurocetus, Milan, Italy) for 5 days. Cells were then amplified in RPMI 1640 medium (Seromed, Berlin, Germany) supplemented with 2 mM l-glutamine, 1% nonessential amino acids, 1% pyruvate, 2 × 10−5 M 2-ME (all from Gibco Laboratories), 20 U/mL recombinant IL-2, and 10% heat-inactivated FCS. T-cell clones were derived from T-cell cultures by limiting dilution as previously described.23 For monocytes cultures, CD14+ cells were cultured in RPMI 1640 medium supplemented with 10% heat-inactivated FCS for 24 hours.
Real-time quantitative RT-PCR
Total RNA was extracted by using the RNeasy Micro Kit (Qiagen, Hilden, Germany) and treated with DNase I to eliminate possible gDNA contamination.
TaqMan reverse transcription-polymerase chain reaction (RT-PCR) was performed as described elsewhere.13 To specifically detect CXCL4 and CXCL4L1 mRNAs, primers and probes were the following:
CXCL4: probe VIC 5′-ACCTGCAGTGCCTGTGTGTGAAGACC-3′, forward: 5′-TTCTGCGCCTCACGCCCC-3′, reverse: 5′-TGGGACGGACCTGGGAG-3′; CXCL4L1: probe VIC 5′-ACCTGCAGTGCCTGTGTGTGAAGACC-3′, forward: 5′-CGCCACCCGCCAGGAGAT-3′, reverse: 5′-TGGGACGGACCTGGGAG-3′.
The 2 sets of primers displayed similar amplification efficiency and selective specificity.
GPIIIa, GPIIb, and GAPDH quantitation was performed using Assay on Demand kits (Applied Biosystems, Warrington, United Kingdom). GAPDH was used as a housekeeping gene to correct for small differences in cDNA contents. Total mRNA/cDNA (25 ng) was processed in all experiments.
RT-qPCR using Sybrgreen
Total RNA was extracted by TRIzol reagent (Invitrogen, Carlsbad, CA). To eliminate all gDNA, RNA was purified with RNeasy Plus Mini Kit (Qiagen), according to the manufacturer's instructions. Real-time quantitative PCR (qPCR) was performed in triplicate, in a MW3000p thermocycler (Stratagene, Garden Grove, CA) using the SYBRgreen dye (Abgene, Epson, United Kingdom) methodology.
qPCR primers: CXCL4/CXCL4L1: forward 5′-CTGAAGAAGATGGGGACCTG-3′; reverse 5′-TTCAGCGTGGCTATCAGTTG-3′.
Generation of HEK-293 stable transfectants for CXCL4 and CXCL4L1
Full-length CXCL4 and CXCL4L1 cDNAs were obtained by RT-PCR performed on total mRNA derived from human platelets, cloned in pcDNA 3.1 expression vectors (Invitrogen), sequenced, and transfected into HEK-293 cell line by electroporation. Stably transfected cells were selected using DMEM media containing 300 μg/mL hygromycin.
Furthermore, 3′5′ UTRs containing CXCL4 (GenBank accession no: NM_002619) and CXCL4L1 (GenBank accession no: NM_002620) were obtained from spleen cDNA by PCR amplification using specific primers: CXCL4 primers: 5′-CCGCAGCATGAGCTCCGC-3′ (forward); 5′-TTTAAAATCATAAGGATAACACAAATATCAGAAG-3′ (reverse). CXCL4L1 primers: 5′-ACTGCCTGCAGAACCCCAGC-3′ (forward); 5′-TCTTTGCAGATGTAATGCTTAAGAGCTAC-3′ (reverse).
Purified PCR products were inserted into pCRII-TOPO plasmid and subcloned into pCDNA3.1 hygro(+) after EcoRI digestion for CXCL4L1 or KpnI and EcoRV for CXCL4.
Generation of CXCL4- and CXCL4L1-GFP fusion protein
Full-length CXCL4 and CXCL4L1 cDNAs obtained as described in “Generation of HEK-293 stable transfectants for CXCL4 and CXCL4L1” were cloned in-frame with GFP sequence in the expression vector pEGFP-N3 (Clontech, Mountain View, CA), sequenced, and transfected into HMVECs by electroporation. Cells were cultured on glass slides and 24 and 48 hours after transfection, fixed with paraformaldehyde and incubated 30 minutes with concanavalin-A/Alexa Fluo-660 conjugated (Molecular Probes). Laser confocal microscopy was performed as described in “Immunofluorescence and confocal microscopy.”
Western blot and ELISA
For Western blot analysis, 30 μg HEK-293 cell lysates or 40 μL conditioned media were electrophoresed on 15% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Blotting was performed as previously described.24 The immunoblots were visualized with the enhanced chemiluminescence (ECL plus) Western blotting detection system (Amersham Pharmacia Biotech, Uppsala, Sweden).24
For enzyme-linked immunosorbent assay (ELISA) quantification of CXCL4/CXCL4L1 in supernatants and in lysates of different cell types, cells were plated at a density of 1 × 106 cells/mL. After overnight culture in serum-free media, media were changed and cells cultured for additional 24 hours in serum-free media. At this time conditioned media were collected, cells washed twice with PBS and then lysated with an equal volume of RIPA buffer (50 mM TRIZMA, 0.15 M NaCl, 1% NP-40, 1 mM EDTA, 1 mM EGTA, 2% protease inhibitors cocktail). CXCL4 and CXCL4L1 were measured using the commercial PF4-ELISA kits (Roche, Indianapolis, IN or Zymutest PF4-RK 006A, Hyphen BioMed, Les Ulis, France). Culture medium was used as blank.
For ELISA on HEK-293 transfectants, 5 × 105 cells/mL were cultured for 24 hours in serum-free DMEM. Media were then changed and cells cultured for another 24 hours in 1 mL serum-free DMEM. At this time, conditioned media were collected and cells washed twice with PBS and then lysated. After protein quantification, CXCL4 and CXCL4L1 were measured using the commercial PF4-ELISA. Assays were performed in triplicate and results analyzed using the Softmax Pro4.0 software (Molecular Devices, St Gregoire, France).
For brefeldin A (BFA, Sigma-Aldrich) treatment, cells were cultured at a concentration of 1 × 106 cells/mL for 24 hours in serum-free DMEM. Media were then changed and cells incubated for 6 hours in the presence or absence of 100 to 500 ng/mL BFA in serum-free DMEM. For cycloheximide (CHX; Sigma-Aldrich) treatment, cells were cultured at a concentration of 1 × 106 cells/mL for 24 hours in serum-free DMEM. Media were then changed and cells incubated for 10 hours in presence or absence of 1 to 10 μg/mL CHX in serum-free DMEM. Stimuli with phorbol 12-myristate 13 acetate (PMA; Sigma-Aldrich) were performed on cells cultured at a concentration of 1 × 106 cells/mL in serum-free DMEM for 24 hours. Media were then changed and cells incubated for 6 hours in the presence or absence of 25 to 50 ng/mL PMA in serum-free DMEM. In some experiments, PMA stimulation was performed as described in the presence or absence of PKC inhibitors Gö6976 (1 μM), RO318220 (5 μM), or bisindolylmalheimide (BIM, 5 μM). PKC inhibitors were purchased from Calbiochem (Darmstadt, Germany).
Statistical analysis
Comparison between groups was performed by the Student t test. A P value below .05 was considered to be statistically significant.
Results
Expression of CXCL4 and CXCL4L1 mRNAs and proteins in human cells
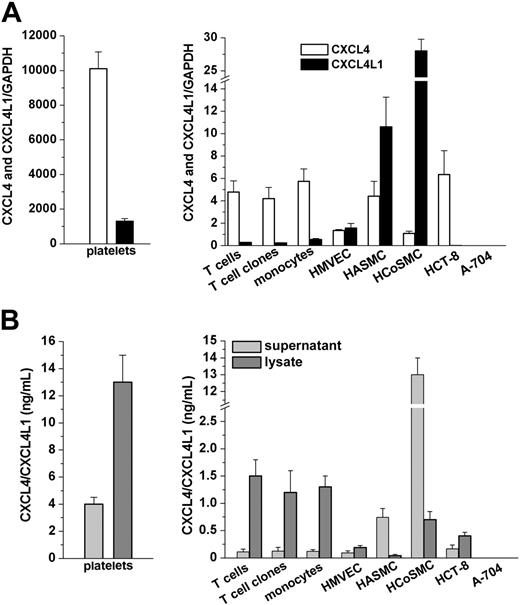
The expression of CXCL4 and CXCL4L1 mRNA was assessed in different cell types, including platelets, cultured T cells, cultured monocytes, and primary cultures of HMVECs, HASMCs, HCoSMCs, HCT-8 and A-704 cell lines (Figure 1A). Given the high similarity between CXCL4 and CXCL4L1 mRNA sequences, we optimized an RT-PCR assay able to quantitate both variants by selecting 2 pairs of primers and 2 probes hybridizing to nonhomolog portions of the 2 mRNAs (Figure S1, available online at the Blood website; see the Supplemental Figures link at the top of the online article). Real-time RT-PCR revealed the presence of CXCL4 and CXCL4L1 mRNAs in all cell types analyzed, unique exceptions being represented by the A-704 cell line (Figure 1A right). CXCL4 was consistently more prevalent than CXCL4L1 in all cell types analyzed, except in HASMCs and in HCoSMCs (Figure 1A). Absence of platelet contamination in freshly purified cell preparations was confirmed by FACS analysis after staining with anti-GPIIb antibody (data not shown) and by RT-PCR using GPIIIa and GPIIb quantitation (Figure S2). Furthermore, CXCL4 and CXCL4L1 expression was demonstrated in 5 different T-cell clones obtained by limiting dilution of T-cell cultures, definitely excluding the possible contamination of our T-cell preparations with platelets.
Detection of CXCL4 and CXCL4L1 mRNA expression and of CXCL4/CXCL4L1 protein secretion in different human cell types. (A) CXCL4 and CXCL4L1 mRNA levels in platelets (left) and in different human cell types (right). GAPDH was used as a housekeeping gene to correct for small differences in cDNA contents. Total mRNA/cDNA (25 ng) was processed in all experiments. Mean values (± SEM) of triplicate determinations of samples obtained from at least 5 independent donors are reported. (B) CXCL4/CXCL4L1 protein levels in lysate and supernatant from platelets (left) and from different human cell types (right), as assessed by ELISA. Cells were cultured for 24 hours in serum-free medium at a concentration of 1 × 106 cells/mL medium as described in “Materials and methods.” Mean values (± SEM) of duplicates determinations of samples obtained from at least 5 independent donors are reported.
Detection of CXCL4 and CXCL4L1 mRNA expression and of CXCL4/CXCL4L1 protein secretion in different human cell types. (A) CXCL4 and CXCL4L1 mRNA levels in platelets (left) and in different human cell types (right). GAPDH was used as a housekeeping gene to correct for small differences in cDNA contents. Total mRNA/cDNA (25 ng) was processed in all experiments. Mean values (± SEM) of triplicate determinations of samples obtained from at least 5 independent donors are reported. (B) CXCL4/CXCL4L1 protein levels in lysate and supernatant from platelets (left) and from different human cell types (right), as assessed by ELISA. Cells were cultured for 24 hours in serum-free medium at a concentration of 1 × 106 cells/mL medium as described in “Materials and methods.” Mean values (± SEM) of duplicates determinations of samples obtained from at least 5 independent donors are reported.
The expression of CXCL4/CXCL4L1 proteins by cell types analyzed with RT-PCR was confirmed by the assessment with a sensitive, commercially available ELISA of their levels in either supernatants or lysates of equal numbers of different cell types (Figure 1B). In all the cell types analyzed the quantity of CXCL4/CXCL4L1 secreted in supernatants was lower than that present in cell lysates, with the exception of HASMCs and HCoSMCs, the only cell types expressing more CXCL4L1 than CXCL4 mRNA (Figure 1B). No detectable amount of CXCL4/CXCL4L1 was observed either in the supernatant or in the lysate of the A-704 cell line (Figure 1B).
Distinct subcellular localization of CXCL4 and CXCL4L1 in HEK-293 and HMVEC transfectants
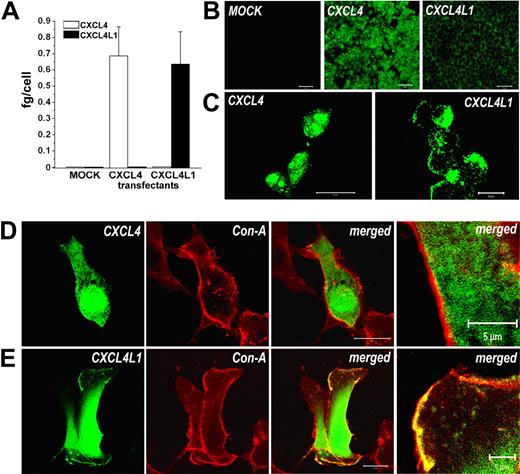
Given the differences in CXCL4 and CXCL4L1 amino acid sequence, particularly at the leader sequence level, we wondered whether the 2 proteins differed in their subcellular localization. To verify this hypothesis, each gene was cloned in an expression vector and stable transfectants were generated by using the HEK-293 EBNA cell line. In all the clones obtained, the expression of CXCL4 and CXCL4L1 mRNA was verified by quantitative RT-PCR (Figure 2A) and 20 clones expressing comparable levels of CXCL4 (n = 10) or CXCL4L1 (n = 10) mRNA were selected and used in all subsequent experiments. The expression of CXCL4 and CXCL4L1 proteins was analyzed by confocal microscopy. No CXCL4/CXCL4L1 immunoreactivity was present in mock-transfected cells (Figure 2B left), whereas a strong cytoplasmic staining was observed in CXCL4 transfectants (Figure 2B-C middle and left, respectively). Surprisingly, in CXCL4L1 transfectants staining was mainly limited to regions adjacent to plasmatic membrane (Figure 2B-C right and right, respectively).
CXCL4 and CXCL4L1 expression by HEK-293 and HMVEC transfectants. (A) CXCL4 and CXCL4L1 mRNA expression by HEK-293 transfectants, as assessed by real-time qRT-PCR. Columns represent mean values (± SEM) of 10 selected clones. (B) Expression of CXCL4/CXCL4L1 in HEK-293 transfectants, as detected by immunofluorescence. Absence of signal in mock transfectants (left), strong cytoplasmic signal in CXCL4 transfectants (green, middle panel), staining in a region adjacent to plasmatic membrane in CXCL4L1 transfectants (green, right). Bar represents 50 μm. (C) High-power magnification of immunofluorescence performed on CXCL4 (left) or CXCL4L1 transfectants (right). Bar represents 20 μm. (D) Confocal microscopy of HMVECs transfected with CXCL4-GFP. GFP fluorescence (green) was localized in the cytoplasm; concanavalin A stains the membrane (red). Merged image (yellow) demonstrates absence of coexpression; bar represents 20 μm. At the extreme part of the figure a high-power magnification of a detail of the merged image is shown; bar represents 5 μm. (E) Confocal microscopy of HMVECs transfected with CXCL4L1-GFP. GFP fluorescence (green) was localized in the cytoplasm and on cytoplasmic membrane, as demonstrated by merged image (yellow; bar represents 20 μm). At the extreme part of the figure a high-power magnification of a detail of the merged image is shown (bar represents 5 μm). Images in panels B and C were acquired using an LSM 510 Meta confocal microscope (Zeiss) equipped with a 20 ×/0.50 NA Plan-Neofluor objective lens (Zeiss). Images in panel D and E were acquired using an LSM 510 Meta laser scanning confocal microscope equipped with a 40×/1.30 NA oil Plan-Neofluor objective lens. An electronic zoom is shown in the extreme panel. LSM 510 Meta confocal microscope software version 3.0 (Zeiss) was used to capture all the images.
CXCL4 and CXCL4L1 expression by HEK-293 and HMVEC transfectants. (A) CXCL4 and CXCL4L1 mRNA expression by HEK-293 transfectants, as assessed by real-time qRT-PCR. Columns represent mean values (± SEM) of 10 selected clones. (B) Expression of CXCL4/CXCL4L1 in HEK-293 transfectants, as detected by immunofluorescence. Absence of signal in mock transfectants (left), strong cytoplasmic signal in CXCL4 transfectants (green, middle panel), staining in a region adjacent to plasmatic membrane in CXCL4L1 transfectants (green, right). Bar represents 50 μm. (C) High-power magnification of immunofluorescence performed on CXCL4 (left) or CXCL4L1 transfectants (right). Bar represents 20 μm. (D) Confocal microscopy of HMVECs transfected with CXCL4-GFP. GFP fluorescence (green) was localized in the cytoplasm; concanavalin A stains the membrane (red). Merged image (yellow) demonstrates absence of coexpression; bar represents 20 μm. At the extreme part of the figure a high-power magnification of a detail of the merged image is shown; bar represents 5 μm. (E) Confocal microscopy of HMVECs transfected with CXCL4L1-GFP. GFP fluorescence (green) was localized in the cytoplasm and on cytoplasmic membrane, as demonstrated by merged image (yellow; bar represents 20 μm). At the extreme part of the figure a high-power magnification of a detail of the merged image is shown (bar represents 5 μm). Images in panels B and C were acquired using an LSM 510 Meta confocal microscope (Zeiss) equipped with a 20 ×/0.50 NA Plan-Neofluor objective lens (Zeiss). Images in panel D and E were acquired using an LSM 510 Meta laser scanning confocal microscope equipped with a 40×/1.30 NA oil Plan-Neofluor objective lens. An electronic zoom is shown in the extreme panel. LSM 510 Meta confocal microscope software version 3.0 (Zeiss) was used to capture all the images.
To confirm that the different intracellular localization of CXCL4 and CXCL4L1 observed in HEK transfectants was not a peculiarity of the HEK-293 cell line, and to further address the cellular distribution of CXCL4 and CXCL4L1 in another cell type, we analyzed CXCL4 and CXCL4L1 expression in cultures of HMVECs after transient transfection of these cells with CXCL4- and CXCL4L1-GFP fusion protein. When transfected alone, GFP yielded a diffuse fluorescence distribution throughout the cell body, consistent with the cytosolic localization of a nonsecretory protein (data not shown). In CXCL4-GFP transfectants, fluorescence was localized within the cytoplasm (Figure 2D), whereas in CXCL4L1-GFP transfectants the fluorescent signal was present not only in the cytoplasm but also on scattered areas of plasmatic membrane, as demonstrated by its colocalization with the membrane marker concanavalin A (Figure 2E).
Secretion pattern of CXCL4 and CXCL4L1 in HEK-293 transfectants
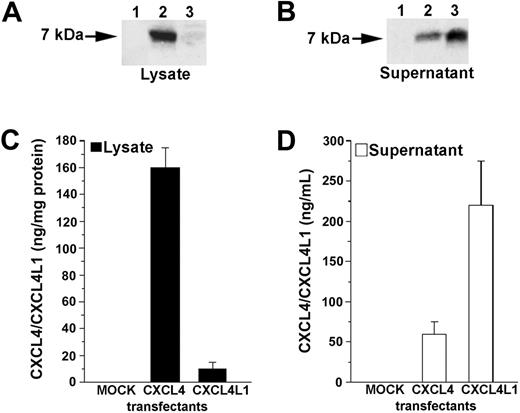
To verify whether CXCL4 and CXCL4L1 were secreted by stable HEK-293 transfectants, Western blot analysis was performed on cell lysates and culture media of CXCL4-, CXCL4L1-, and mock-transfected cells. Cell lysate of mock cells did not contain detectable CXCL4/CXCL4L1 protein. Strong immunoreactivity was found in lysate obtained from CXCL4 transfectant, whereas a very low CXCL4L1 immunoreactivity was detected in the cell lysate of CXCL4L1 transfectant (Figure 3A). No CXCL4/CXCL4L1 protein was detected in supernatant obtained from mock-transfected cells. A low amount of CXCL4 protein was found in the culture medium of CXCL4 transfectant, whereas a strong signal was observed in supernatants of CXCL4L1 transfectants (Figure 3B). Analysis of lysates and supernatants obtained from 2 representative clones expressing comparable amounts of total CXCL4/CXCL4L1 proteins is depicted in Figure 3A-B.
Detection of CXCL4 and CXCL4L1 proteins in lysates and supernatants of HEK-293 transfectants. (A) Western blot analysis of lysate from mock (lane 1), CXCL4 (lane 2), and CXCL4L1 (lane 3) transfectants. Absence of immunoreactivity in mock lysate; strong immunoreactivity in lysate of a CXCL4 transfectant, and absence of protein in the lysate of a CXCL4L1 transfectant. One representative experiment is shown. (B) Western blot analysis of supernatant from mock (lane 1), CXCL4 (lane 2), and CXCL4L1 (lane 3) transfectants. Absence of immunoreactivity in mock supernatant, low immunoreactivity in supernatant of a CXCL4 transfectant, and strong immunoreactivity in the supernatant of a CXCL4L1 transfectant. One representative experiment is shown. (C) CXCL4/CXCL4L1 proteins in lysate from mock, CXCL4, and CXCL4L1 transfectants. Absence of protein in mock lysate, high protein levels in lysate of a CXCL4 transfectant, and absence of protein in the lysate of a CXCL4L1 transfectant, as assessed by ELISA. Cells were cultured for 24 hours in serum-free DMEM at a concentration of 5 × 105 cells/mL medium as described in “Materials and methods.” Columns represent mean values (± SEM) of 4 experiments performed in 2 selected clones. (D) CXCL4/CXCL4L1 proteins in supernatant from mock, CXCL4, and CXCL4L1 transfectants. Absence of protein in mock supernatant, low levels of protein in supernatant of a CXCL4 transfectant, and high levels of protein in the supernatant of a CXCL4L1 transfectant, as assessed by ELISA. Cells were cultured for 24 hours in serum-free DMEM at a concentration of 5 × 105 cells/mL medium as described in “Materials and methods.” Columns represent mean values (± SEM) of 4 experiments performed in 2 selected clones.
Detection of CXCL4 and CXCL4L1 proteins in lysates and supernatants of HEK-293 transfectants. (A) Western blot analysis of lysate from mock (lane 1), CXCL4 (lane 2), and CXCL4L1 (lane 3) transfectants. Absence of immunoreactivity in mock lysate; strong immunoreactivity in lysate of a CXCL4 transfectant, and absence of protein in the lysate of a CXCL4L1 transfectant. One representative experiment is shown. (B) Western blot analysis of supernatant from mock (lane 1), CXCL4 (lane 2), and CXCL4L1 (lane 3) transfectants. Absence of immunoreactivity in mock supernatant, low immunoreactivity in supernatant of a CXCL4 transfectant, and strong immunoreactivity in the supernatant of a CXCL4L1 transfectant. One representative experiment is shown. (C) CXCL4/CXCL4L1 proteins in lysate from mock, CXCL4, and CXCL4L1 transfectants. Absence of protein in mock lysate, high protein levels in lysate of a CXCL4 transfectant, and absence of protein in the lysate of a CXCL4L1 transfectant, as assessed by ELISA. Cells were cultured for 24 hours in serum-free DMEM at a concentration of 5 × 105 cells/mL medium as described in “Materials and methods.” Columns represent mean values (± SEM) of 4 experiments performed in 2 selected clones. (D) CXCL4/CXCL4L1 proteins in supernatant from mock, CXCL4, and CXCL4L1 transfectants. Absence of protein in mock supernatant, low levels of protein in supernatant of a CXCL4 transfectant, and high levels of protein in the supernatant of a CXCL4L1 transfectant, as assessed by ELISA. Cells were cultured for 24 hours in serum-free DMEM at a concentration of 5 × 105 cells/mL medium as described in “Materials and methods.” Columns represent mean values (± SEM) of 4 experiments performed in 2 selected clones.
Western blot analysis was confirmed using an ELISA on both lysates and supernatants of mock and transfected clones (Figure 3C-D). Analysis of lysates and supernatants obtained from 2 representative clones expressing comparable amounts of total CXCL4/CXCL4L1 proteins (CXCL4 transfectant, 225 ± 47 ng/5 × 105 cells; CXCL4L1 transfectant, 248 ± 55 ng/5 × 105 cells), as assessed by ELISA is depicted in Figure 3C-D. However, independently of the total amount of protein synthesized by different clones, an extremely low ratio between secreted and intracellular protein (0.26 ± 0.05, n = 4) was always observed in CXCL4 transfectants, suggesting that CXCL4 protein is mostly retained within the cells. On the contrary, a high ratio between secreted and intracellular protein (45.6 ± 11, n = 4) was always observed in CXCL4L1 transfectants, suggesting that CXCL4L1 is mostly secreted.
The finding that CXCL4/CXCL4L1 detected in the medium was secreted and not released as a consequence of cell lysis was confirmed by carrying out a propidium iodide staining. In each sample, more than 95% of cells were viable as demonstrated by the lack of spontaneous propidium uptake (data not shown).
Regulation of CXCL4 and CXCL4L1 secretion
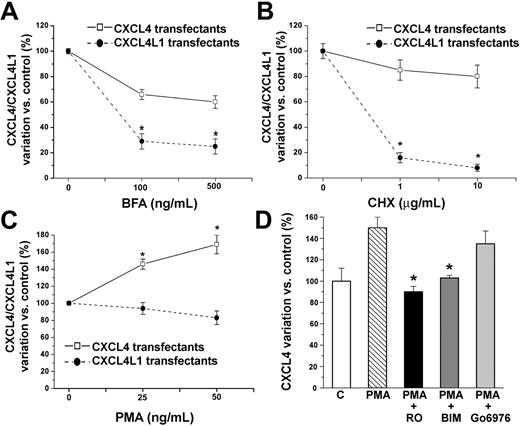
To investigate the regulatory mechanisms of CXCL4 and CXCL4L1 secretion, we performed ELISA on transfectant supernatants after cell treatment with a classical inhibitor of endoplasmic reticulum (ER)/Golgi-dependent protein secretion, such as BFA. Treatment of transfectants with BFA had a small effect on CXCL4 secretion (100% ± 4% control versus 60% ± 20% BFA treated, P < .001, Figure 4A), whereas a strong reduction of protein secretion was observed in BFA-treated CXCL4L1 transfectants (100% ± 4% control versus 25% ± 12% BFA, P < .001, Figure 4A). Similarly, treatment with CHX, an inhibitor of protein synthesis, did not lead to a significant reduction of basal secretion of CXCL4 (100% ± 5% control versus 80% ± 10% CHX, Figure 4B), whereas CXCL4L1 secretion was almost completely inhibited (100% ± 5% control versus 8% ± 3% CHX, P < .001, Figure 4B). These data suggest the presence of a storage compartment for CXCL4 and of a constitutive secretion pathway for CXCL4L1.
Constitutive secretion of CXCL4L1 and PKC-dependent regulated secretion of CXCL4 in HEK transfectants. (A) BFA treatment induced a strong dose-dependent decrease in CXCL4L1 secretion by CXCL4L1 transfectants and low effects in CXCL4 secretion by CXCL4 transfectants. Cells were cultured for 6 hours in serum-free medium at a concentration of 1 × 106 cells/mL medium in presence or absence of 100 to 500 ng/mL BFA. Mean ± SEM of 4 experiments performed in 2 selected clones is shown. *P < .001 versus untreated cells. (B) CHX treatment induced a strong dose-dependent decrease in CXCL4L1 secretion by CXCL4L1 transfectants. No effect was observed on CXCL4 secretion by CXCL4 transfectants. Cells were cultured for 10 hours in serum-free medium at a concentration of 1 × 106 cells/mL medium in presence or absence of 1 to 10 μg/mL CHX. Mean ± SEM of 4 experiments performed in 2 selected clones is shown. *P < .001 versus untreated cells. (C) PMA treatment induced a dose-dependent increase in CXCL4 secretion by CXCL4 transfectants. No effect was observed in CXCL4L1 transfectants. Cells were cultured for 6 hours in serum-free medium at a concentration of 1 × 106 cells/mL medium in the presence or absence of 25 to 50 ng/mL PMA. Mean ± SEM of 4 experiments performed in 2 selected clones is shown. *P < .001 versus untreated cells. (D) Effect of PKC inhibitors on PMA-induced CXCL4 secretion by CXCL4 transfectants. Cells were cultured for 6 hours in serum-free medium at a concentration of 1 × 106 cells/mL medium in presence or absence of 50 ng/mL PMA, and in the presence or absence of different PKC inhibitors. Data represent mean ± SEM of 3 independent experiments performed in duplicate in 2 selected clones. *P < .001 versus PMA-treated cells.
Constitutive secretion of CXCL4L1 and PKC-dependent regulated secretion of CXCL4 in HEK transfectants. (A) BFA treatment induced a strong dose-dependent decrease in CXCL4L1 secretion by CXCL4L1 transfectants and low effects in CXCL4 secretion by CXCL4 transfectants. Cells were cultured for 6 hours in serum-free medium at a concentration of 1 × 106 cells/mL medium in presence or absence of 100 to 500 ng/mL BFA. Mean ± SEM of 4 experiments performed in 2 selected clones is shown. *P < .001 versus untreated cells. (B) CHX treatment induced a strong dose-dependent decrease in CXCL4L1 secretion by CXCL4L1 transfectants. No effect was observed on CXCL4 secretion by CXCL4 transfectants. Cells were cultured for 10 hours in serum-free medium at a concentration of 1 × 106 cells/mL medium in presence or absence of 1 to 10 μg/mL CHX. Mean ± SEM of 4 experiments performed in 2 selected clones is shown. *P < .001 versus untreated cells. (C) PMA treatment induced a dose-dependent increase in CXCL4 secretion by CXCL4 transfectants. No effect was observed in CXCL4L1 transfectants. Cells were cultured for 6 hours in serum-free medium at a concentration of 1 × 106 cells/mL medium in the presence or absence of 25 to 50 ng/mL PMA. Mean ± SEM of 4 experiments performed in 2 selected clones is shown. *P < .001 versus untreated cells. (D) Effect of PKC inhibitors on PMA-induced CXCL4 secretion by CXCL4 transfectants. Cells were cultured for 6 hours in serum-free medium at a concentration of 1 × 106 cells/mL medium in presence or absence of 50 ng/mL PMA, and in the presence or absence of different PKC inhibitors. Data represent mean ± SEM of 3 independent experiments performed in duplicate in 2 selected clones. *P < .001 versus PMA-treated cells.
We also analyzed the ability of CXCL4 and CXCL4L1 transfectants to secrete these proteins in response to stimulants. Activation of PKC by PMA treatment induced CXCL4 release in culture medium (100% ± 4% control versus 169% ± 36% PMA, P < .001, Figure 4C), whereas the same agent had no effect on CXCL4L1 release from HEK-293 transfectants (100% ± 5% control versus 84% ± 21% PMA, Figure 4C).
To better assess the involvement of PKC in PMA-induced CXCL4 secretion, 3 different PKC inhibitors were used. Strong inhibition of PMA-induced CXCL4 secretion was obtained with the broad-spectrum PKC inhibitors RO-31-8220 and BIM (Figure 4D). The inhibitor of the classical group PKCs Gö6976, which is relatively specific for PKCα and PKCβ1, showed lower and no significant inhibition of PMA-induced CXCL4 secretion, suggesting the involvement of nonconventional PKC isozymes in PMA-induced CXCL4 secretion (Figure 4D).
To establish whether the expression of CXCL4 and CXCL4L1 is also regulated at transcriptional level, vectors containing full-length 3′ and 5′ UTR CXCL4 or CXCL4L1 cDNAs were transfected into HEK cells and mRNA expression of stable transfectants was assessed by qPCR after stimulation by 50 ng/mL PMA for 6 hours. CXCL4 and CXCL4L1 transcription was significantly increased after PMA stimulation with 3′5′ UTR containing constructs but not with constructs without 3′5′ UTR (Figure S3).
CXCL4 and CXCL4L1 exhibit distinct intracellular pathways of secretion
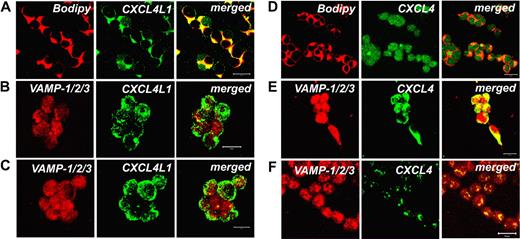
To provide additional evidence that CXCL4 and CXCL4L1 follow distinctly regulated intracellular pathways of secretion, we assessed by confocal microscopy whether in CXCL4- and CXCL4L1-HEK transfectants the 2 chemokines are associated with Golgi or dense-core secretory granules (DCGs), respectively. DCGs, also referred to as large dense-core vesicles, are indeed typical organelles competent for regulated exocytosis, expressed by neurons and neurosecretory cells, but also by a wide variety of other cell types.25 To this end, Bodipy TR C5 ceramide was used to identify the Golgi, whereas a pAb raised against sequences of VAMP-1/2/3 was chosen to identify DCGs. Double-label immunofluorescence performed on CXCL4L1 transfectants demonstrated a strong association between Bodipy and CXCL4L1, indicating that this chemokine consistently localizes within Golgi (Figure 5A). By contrast, no colocalization between CXCL4L1 and the DCG marker VAMP-1/2/3 was observed, a number of vesicles containing CXCL4L1 alone being observed in the cytoplasm or on the surface of the cells (Figure 5B). Treatment of CXCL4L1 transfectants with PMA for 6 hours did not induce any meaningful change of CXCL4L1 content and distribution in CXCL4L1 transfectants (Figure 5C), thus supporting the assumption that CXCL4L1 secretion involves a constitutive pathway, but not DCGs. On the other hand, CXCL4 signal was poorly colocalized with Bodipy in CXCL4 transfectants (Figure 5D), but mostly associated within DCGs, as shown by its strong colocalization with VAMP-1/2/3 (Figure 5E). More importantly, treatment of CXCL4 transfectants with PMA for 6 hours almost completely removed the intracellular CXCL4 staining (Figure 5F), thus clearly demonstrating that CXCL4 exhibits a regulated secretory pathway.
Different intracellular localization of CXCL4 and CXCL4L1 in HEK transfectants. (A) Strong expression of CXCL4L1 (green) in the Golgi of CXCL4L1-HEK transfectants, as shown by costaining with the Golgi marker Bodipy (red). Colocalization is shown in yellow (bar represents 20 μm). (B) Virtually absent colocalization between the DCG marker VAMP-1/2/3 (red) and CXCL4L1 (green) in CXCL4L1-HEK transfectants. Colocalization is shown in yellow (bar represents 20 μm). (C) Absence of any effect of PMA treatment in CXCL4L1-HEK transfectants on CXCL4L1 (green) distribution and localization (bar represents 20 μm). (D) Marginal colocalization of CXCL4 (green) with the Golgi marker Bodipy (red) in CXCL4-HEK transfectants. Colocalization is shown in yellow (bar represents 20 μm). (E) Localization of CXCL4 (green) within DCGs in CXCL4-HEK transfectants, as demonstrated by strong costaining with the DCG marker VAMP-1/2/3. Colocalization is shown in yellow (bar represents 20 μm). (F) Treatment of CXCL4-HEK transfectants with PMA for 6 hours induces degranulation of CXCL4-containing DCGs. Colocalization of CXCL4 (green) within DCGs of CXCL4-HEK transfectants, as stained with VAMP-1/2/3 is shown in yellow (bar represents 20 μm). Images were acquired using an LSM 510 Meta confocal microscope (Zeiss) equipped with a 20×/0.50 Plan-Neofluor objective lens (Zeiss). LSM 510 Meta confocal microscope software version 3.0 (Zeiss) was used to capture all the images.
Different intracellular localization of CXCL4 and CXCL4L1 in HEK transfectants. (A) Strong expression of CXCL4L1 (green) in the Golgi of CXCL4L1-HEK transfectants, as shown by costaining with the Golgi marker Bodipy (red). Colocalization is shown in yellow (bar represents 20 μm). (B) Virtually absent colocalization between the DCG marker VAMP-1/2/3 (red) and CXCL4L1 (green) in CXCL4L1-HEK transfectants. Colocalization is shown in yellow (bar represents 20 μm). (C) Absence of any effect of PMA treatment in CXCL4L1-HEK transfectants on CXCL4L1 (green) distribution and localization (bar represents 20 μm). (D) Marginal colocalization of CXCL4 (green) with the Golgi marker Bodipy (red) in CXCL4-HEK transfectants. Colocalization is shown in yellow (bar represents 20 μm). (E) Localization of CXCL4 (green) within DCGs in CXCL4-HEK transfectants, as demonstrated by strong costaining with the DCG marker VAMP-1/2/3. Colocalization is shown in yellow (bar represents 20 μm). (F) Treatment of CXCL4-HEK transfectants with PMA for 6 hours induces degranulation of CXCL4-containing DCGs. Colocalization of CXCL4 (green) within DCGs of CXCL4-HEK transfectants, as stained with VAMP-1/2/3 is shown in yellow (bar represents 20 μm). Images were acquired using an LSM 510 Meta confocal microscope (Zeiss) equipped with a 20×/0.50 Plan-Neofluor objective lens (Zeiss). LSM 510 Meta confocal microscope software version 3.0 (Zeiss) was used to capture all the images.
Secretion pattern of CXCL4 and CXCL4L1 in T cells and HCoSMCs
To verify the in vivo relevance of our observations, we assessed the mode of CXCL4/CXCL4L1 secretion in cultured T cells, which preferentially expressed CXCL4, and in HCoSMCs, which synthesized almost exclusively CXCL4L1. Treatment of T cells with PMA induced CXCL4 release in culture medium (Figure 6A). To assess the involvement of PKC on PMA-induced CXCL4 secretion by T cells, 3 different PKC inhibitors were used. As already observed in HEK transfectants, Ro318220 and BIM showed strong inhibition of PMA-induced CXCL4 secretion in T cells, whereas Gö6976, which is relatively specific for PKCα and PKCβ1, showed much less inhibition of PMA-induced CXCL4 secretion, suggesting the involvement of nonconventional PKC isozymes in PMA-induced CXCL4 secretion by T cells (Figure 6A). Similar results were also obtained in cultured human monocytes (data not shown). By contrast, PMA treatment of HCoSMCs had no effect on CXCL4/CXCL4L1 release in culture medium (Figure 6B), in agreement with their almost exclusive expression of CXCL4L1. These results are consistent with a regulated, PKC-dependent CXCL4 secretion by T cells, and with a constitutive secretion of CXCL4L1 by HCoSMCs.
Distinct regulation of CXCL4 and CXCL4L1 secretion by PKC activation in T cells and HCoSMCs. (A) Effect of PKC inhibitors on PMA-induced CXCL4 secretion by T cells (CD3+). Cells were cultured for 6 hours in serum-free medium at a concentration of 1 × 106 cells/mL medium in the presence or absence of 50 ng/mL PMA, and in the presence or absence of different PKC inhibitors. Data represent mean ± SEM of 3 independent experiments performed in duplicates. *P < .05 versus PMA-treated cells. (B) Constitutive secretion of CXCL4L1 by HCoSMCs is not further modulated by PMA or PKC inhibitors. Cells were cultured for 6 hours in serum-free medium at a concentration of 1 × 106 cells/mL medium in the presence or absence of 50 ng/mL PMA and in the presence or absence of different PKC inhibitors. Data represent mean ± SEM of 3 independent experiments performed in duplicate.
Distinct regulation of CXCL4 and CXCL4L1 secretion by PKC activation in T cells and HCoSMCs. (A) Effect of PKC inhibitors on PMA-induced CXCL4 secretion by T cells (CD3+). Cells were cultured for 6 hours in serum-free medium at a concentration of 1 × 106 cells/mL medium in the presence or absence of 50 ng/mL PMA, and in the presence or absence of different PKC inhibitors. Data represent mean ± SEM of 3 independent experiments performed in duplicates. *P < .05 versus PMA-treated cells. (B) Constitutive secretion of CXCL4L1 by HCoSMCs is not further modulated by PMA or PKC inhibitors. Cells were cultured for 6 hours in serum-free medium at a concentration of 1 × 106 cells/mL medium in the presence or absence of 50 ng/mL PMA and in the presence or absence of different PKC inhibitors. Data represent mean ± SEM of 3 independent experiments performed in duplicate.
Discussion
CXCL4 is a CXC chemokine exerting pleiotropic effects in coagulation,3 angiogenesis control,6 modulation of the immune system,13 and cancer growth.8 The critical role of CXCL4 in several pathophysiologic conditions is further underlined by the existence in human beings of a highly homologous gene encoding a variant of CXCL4, named as CXCL4L1.18–20 CXCL4L1, compared with CXCL4, exhibits significantly reduced heparin-binding properties and thus a lower sequestration by glycosaminoglycans. Moreover, CXCL4L1 shows a high amino acid divergence in the signal peptide region, suggesting the possible existence of differences in the expression pattern of the 2 chemokines or difference in their secretion mechanism. However, this hypothesis had not been verified before. Indeed, the extremely high amino acid homology between the mature proteins of CXCL4 and CXCL4L1 makes it impossible to obtain antibodies selectively recognizing one or the other variant (L. L., unpublished results, January 2006). Thus, in this study, we first tried to assess the expression of CXCL4 and CXCL4L1 at mRNA levels. Analysis of CXCL4 and CXCL4L1 in different cell types demonstrated that CXCL4 and CXCL4L1 mRNAs were coexpressed in all cell types analyzed, CXCL4 being usually consistently more prevalent than CXCL4L1, with the exception of VSMCs, which were the only cell type showing a predominance of CXCL4L1. In particular, HCoSMCs expressed almost exclusively CXCL4L1.
The 2 main pathways for secretion in a cell are the constitutive and the regulated secretory pathways.26 The regulated secretory pathway is characterized by the concentration of secreted material into electron-dense granules. These granules are stored within cells until induced to release their content by a specific secretagogue following changes in cytoplasmic second messengers, such as PKC, calcium, or cyclic AMP.26 To verify whether CXCL4 and CXCL4L1 had a different mode of secretion, we established stable transfectants selectively expressing CXCL4 or CXCL4L1. Accordingly, colabeling experiments demonstrated that CXCL4 is contained within DCGs and that its release is induced only following PKC activation, whereas basal synthesis of this chemokine is irrelevant, as demonstrated by treatment with CHX, which had no effect on its secretion. The constitutive secretory pathway, on the other hand, is characterized by the continuous fusion of small vesicles with the plasma membrane, with secretion occurring rapidly after protein synthesis. The secreted proteins are not stored within the vesicles, and secretion is closely linked to the level of protein biosynthesis.26 In agreement with this finding, CXCL4L1 was mostly observed in the Golgi apparatus, and continuously synthesized and released by the cells, as demonstrated by the observation that CHX treatment completely abolished its secretion. Because chemokines display their biological functions in an autocrine/paracrine fashion, the existence of 2 nonallelic variants of CXCL4, which exhibit a difference in their mode of secretion, may be of particular relevance for the biology of each cell type within tissues.27,28
T cells, as well as monocytes, which preferentially expressed CXCL4, under basal conditions stored the chemokine at the intracellular level, but they immediately released CXCL4 after PMA stimulation. The main purpose of the regulated secretory pathway is to allow an acute, massive increase in release of the stored material in response to a particular stimulus. Indeed, CXCL4 has powerful immunoregulatory properties if added to T-cell cultures at the time of activation and signaling on TCR. It inhibits the production of Th1 cytokines,13,14 and promotes the production of Th2 cytokines,13 through the interaction with its receptor CXCR3-B.13 Furthermore, CXCL4 favors the expansion of human CD4+CD25+ T-regulatory cells while inhibiting the proliferation of effector T cells.29 Accordingly, the surface expression of CXCR3-B is enhanced on CD4+CD25+ T-regulatory cells primed with CXCL4.30 Tissue resident epithelial cells also expressed almost exclusively CXCL4, consistently with the inhibitory properties of CXCL4 on the growth of resident epithelia.28 The recent description of CXCR3-B expression by different types of tissue epithelium,31,32 is in agreement with this observation. Very recently, indeed, the interaction between CXCL4 and CXCR3-B has been advocated as a basic mechanism for the control of epithelial cell growth to avoid neoplastic transformation.33 Finally, the prevalent expression of CXCL4 in platelets is consistent with its release after activation and degranulation.1,3
Unlike all other cell types examined, VSMCs express almost exclusively CXCL4L1. This is in agreement with the powerful angiostatic effect of CXCL4L1, which is much higher than that displayed by CXCL4.20 Because the constitutive secretory pathway is primarily used for secretion of proteins that are required at a relatively constant level, basal secretion by VSMCs of CXCL4L1 probably represents a physiologic regulatory mechanism to maintain vascular homeostasis and to avoid EC proliferation and aberrant angiogenesis. Particularly high levels of CXCL4L1 were expressed in HCoSMCs. Indeed, both heart size and cardiac function are angiogenesis-dependent, and disruption of angiogenesis in the heart contributes to the progression from adaptive cardiac hypertrophy to heart failure, suggesting a possible role for CXCL4L1 in the regulation of physiologic or pathologic coronary angiogenesis.34
Taken together, the results of this study demonstrate that the existence of 2 nonallelic variants of CXCL4 with such a high homology in human beings is related to the difference in their mode of secretion. The constitutive secretion of CXCL4L1 predominantly by VSMCs, and the regulated secretion of CXCL4 by platelets and the cells of the immune system, suggest that these 2 chemokines display nonredundant roles, and constitute a finely regulated system that distinctly contributes to homeostatic and inflammatory processes.
Authorship
Contribution: P.R., L.L., and A.B. designed research; L.L., R.G., B.M., E.L., C.M., C.S., F.L., F.F., E.R., N.A-C., L.B., G.S.N., and F.A. performed research and analyzed data; P.R., S.R., A.B., E.M., M.S., and L.L. wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Paola Romagnani, Interdepartmental Laboratory of Cellular and Molecular Nephrology, University of Florence, Viale Pieraccini 6, 50139, Firenze, Italy; e-mail: p.romagnani@dfc.unifi.it; and Andreas Bikfalvi, INSERM E113 “Molecular Angiogenesis Laboratory,” Université Bordeaux I, Avenue des Facultés, 33405 Talence, France; e-mail: a.bikfalvi@angio.u-bordeaux1.fr.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
This work was supported by funds from the European Framework Program 6, EU Project contract no. LSHB-CT-2005-518167 “INNOCHEM” (S.R.), by Ministero della Sanità (Programma per la Ricerca Finalizzata 2000), and by the 6th framework program project “STROMA” (no. FP6 LSHC-CT-2003-5032; A.B.).
B.M. is recipient of a FIRC fellowship. R.G. is recipient of PhD fellowship from the EU 6th framework program “STROMA”(no. FP6 LSHC-CT-2003-5032).


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal