Abstract
Graft-versus-host disease (GVHD) after allogeneic hematopoietic cell transplantation (HCT) is associated with considerable morbidity and mortality, particularly in patients who do not respond to primary therapy, which usually consists of glucocorticoids (steroids). Approaches to therapy of acute GVHD refractory to “standard” doses of steroids have ranged from increasing the dose of steroids to the addition of polyclonal or monoclonal antibodies, the use of immunotoxins, additional immunosuppressive/chemotherapeutic interventions, phototherapy, and other means. While many pilot studies have yielded encouraging response rates, in most of these studies long-term survival was not improved in comparison with that seen with the use of steroids alone. A major reason for failure has been the high rate of infections, including invasive fungal, bacterial, and viral infections. It is difficult to conduct controlled prospective trials in the setting of steroid-refractory GVHD, and a custom-tailored therapy dependent upon the time after HCT, specific organ manifestations of GVHD, and severity is appropriate. All patients being treated for GVHD should also receive intensive prophylaxis against infectious complications.
Introduction
Graft-versus-host disease (GVHD) is the most frequent complication after allogeneic hematopoietic cell transplantation (HCT). First described as “secondary disease” in mice1 the syndrome was shown to be triggered by immunocompetent donor cells.2,3 As soon as the clinical basis for human HCT was established, it was apparent that GVHD would be a formidable problem even with transplantation of marrow cells from sibling donors who were identical with the patient for the antigens of the major histocompatibility complex (MHC), termed HLA (human leukocyte antigen) in humans
The development of acute GVHD is dependent upon various risk factors, which affect the manifestations of the disease and, possibly, the response to first-line therapy. Furthermore, treatment responses may be incomplete or mixed, rendering the assessment of refractory acute GVHD difficult. It appears justified, therefore, to provide a brief background description of the pathophysiology and classification of GVHD and outline up-front therapeutic strategies, which often overlap with what we consider therapy for refractory GVHD.
Pathophysiology and risk factors
Understanding the pathophysiology of GVHD is a prerequisite to designing effective prophylactic and therapeutic strategies. A 3-step process best reflects the current view of the development of GVHD (reviewed in Ferrara et al4 ). In this model, total body irradiation (TBI) or other cytotoxic modalities used to prepare patients for HCT result in tissue damage and the release of inflammatory cytokines into the circulation. In this milieu, transplanted donor T lymphocytes (and other cellular compartments) are activated. Studies in mice have shown that host antigen-presenting cells, in particular dendritic cells, are essential,5 and the cytokines released by tissue damage up-regulate MHC gene products on those cells, which also present minor histocompatibility antigens (miHAs) to donor T cells. Activated T cells express interferon γ (IFN-γ), interleukin-2 (IL-2), and tumor necrosis factor α (TNFα) among others, leading to T-cell expansion, with the overall response depending upon polarization to a Th1 (IL-2, TNFα, etc) versus a Th2 (IL-10, IL-4, etc) pattern.4 These events are followed by the generation of cytotoxic and inflammatory cytokines, cytotoxic effector cells (using Fas- and perforin-mediated mechanisms), large granular lymphocytes (LGLs), and nitric oxide. Interactions of innate (LGL/natural killer [NK] cells) and adaptive (alloreactive T lymphocytes) immune responses lead to organ damage. Additional complexity has been added by the recent description of NKT cells, and regulatory T cells (Tregs) and the inclusion of chemokines.6 Finally, there is evidence that B cells can contribute to the development of GVHD, predominantly in its chronic form,7 particularly in male patients who received transplants from female donors. Conversely, host B cells may attenuate GVHD by secreting IL-10.8
The major risk factors for the development of GVHD are histoincompatibility between donor and patient, older patient (and possibly donor) age, greater intensity of the transplant conditioning regimen, the use of peripheral blood progenitor cells rather than marrow as a source of stem cells (certainly for chronic GVHD), and donor/recipient sex mismatch, especially with allosensitized female donors.9–11 Recent data indicate that acute GVHD is more likely to occur if donor T-cell chimerism is established rapidly after transplantation.12
Incidence of acute GVHD
The incidence of acute GVHD in patients who receive donor cells from which T lymphocytes have not been depleted in vitro is in the range of 10% to 80%, dependent upon the risk factors present, and despite of in vivo prophylaxis.13 The time of onset of GVHD with conventional transplant strategies has typically been 2 to 3 weeks after HCT. With the use of reduced-intensity conditioning regimens, however, acute GVHD may occur later in the course (see “Classification of GVHD”). It has also been speculated that the incidence of GVHD would be lower with low-intensity conditioning regimens, although the reported frequency was only minimally reduced; however, the overall severity grade of acute GVHD may be lower than observed with conventional conditioning,14 consistent with the established concept that regimen intensity correlates with GVHD severity.15 At the same time, a recent report suggests that the complications associated with GVHD are similar in patients conditioned with reduced intensity and with conventional regimens.16 About one third of patients with acute GVHD respond to initial therapy, leaving some 10% to 50% of all patients who underwent transplantation in need for secondary treatment.17
Classification of GVHD
The major target organs of acute GVHD are skin, liver, and intestinal tract, although other organs can be involved. Glucksberg et al proposed the first classification scheme for acute GVHD18 ; the acute form was distinguished from a chronic form by the time of onset (less or more than 100 days after HCT).
However, this distinction between acute and chronic GVHD is no longer tenable. Observations in patients who underwent transplantation after reduced-intensity conditioning regimens or in patients receiving donor lymphocyte infusions at various time intervals after HCT indicate that patients may present with a clinical picture of acute GVHD several months after HCT, while GVHD with characteristics of the “chronic” form can occur as early as 50 or 60 days after transplantation.14,19 The recent National Institutes of Health (NIH) Consensus Conference proposed to recognize 2 categories of GVHD as follows: (1) acute GVHD (absence of features consistent with chronic GVHD), comprising (a) classic acute GVHD (before day 100) and (b) persistent, recurrent, or late acute GVHD (after day 100, often upon withdrawal of immunosuppression); (2) chronic GVHD, comprising (a) classic chronic GVHD (no signs of acute GVHD), and (b) an overlap syndrome, in which features of both acute and chronic GVHD are present.20 This classification is pertinent to the present discussion. For example, patients with persistent or recurrent late acute GVHD may not require prolonged therapy as traditionally given for the treatment of chronic GVHD. At the same time, patients with an overlap syndrome should be treated as for chronic GVHD, but may require a shorter treatment interval of more intensive immunosuppression to control acute inflammatory manifestations. (This classification does not consider GVHD following blood transfusions.)
Therapy
A common approach has been to treat GVHD with agents that were successful in the prevention or treatment of rejection in solid organ transplantation and to use the same agents for prophylaxis for GVHD if therapeutic results were promising. This deserves to be stressed for several reasons: (1) Considering our understanding of the pathophysiology of GVHD, preventing the initiation (prophylaxis) of an adverse alloresponse is one thing, interrupting an ongoing cascade of signals (therapy) is another. (2) Even assuming that the mechanism of action of a given drug would be appropriate for its use as a therapeutic agent, if a patient develops GVHD while receiving that agent (prophylactically), it is unlikely that it would be effective when given (again) therapeutically. (3) While alloreactivity is responsible for both organ graft rejection and GVHD, HCT is associated with GVHD as well as a potential for graft rejection, and to prevent graft rejection, a graft-facilitating effect of donor T cells is desirable. (4) Dependent upon the time after HCT when GVHD develops, the milieu in regard to cytokine profile, cell composition, and tissue damage differs, and these differences are likely to affect the responses to therapeutic interventions.
Primary therapy and its impact
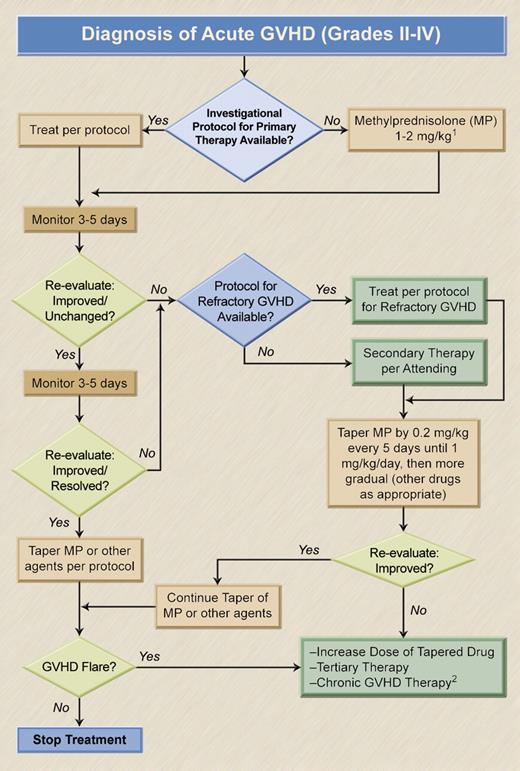
A general algorithm for the management of acute GVHD is given in Figure 1. Preferred treatment options and indications are summarized in Table 1. The response to primary therapy is of central importance as responses correlate with survival. Glucocorticosteroids (steroids) are the mainstay of acute GVHD therapy.13 Steroid-induced lysis of lymphocytes during interphase and anti-inflammatory properties of steroids may lead to prompt improvement, including patients with hyperacute presentation of GVHD. Complete responses occur in 25% to 40% of patients, and clinically relevant improvement, defined as regression of skin rash or decrease in the volume of diarrhea and the extent of liver function abnormalities21 in 40% to 50% of patients with grades II to IV acute GVHD. However, the likelihood of response decreases with increasing severity of the disease.22,23
Management of acute GVHD. 1Patients with grade IIa GVHD may respond to MP, 1 mg/kg, possibly combined with beclomethasone, 1-2 mg 4 times a day, and budesonide, 3 mg twice a day. In patients with grades IIb to IV GVHD, the starting dose of MP should probably be 2 mg/kg (with or without the addition of beclomethasone and budesonide). (Grade IIa is defined as skin rash of < 50% body surface area, not progressing rapidly within the first day; bilirubin < 3 mg/dL; < 20 mL nonhemorrhagic diarrhea/kg per day, without abdominal cramping. Grades IIb-IV include all cases outside the parameters defined for grade IIa.) 2The decision will likely depend upon the timing of the GVHD flare and the clinical presentation of the disease.20 Illustration by A. Y. Chen and H. Crawford.
Management of acute GVHD. 1Patients with grade IIa GVHD may respond to MP, 1 mg/kg, possibly combined with beclomethasone, 1-2 mg 4 times a day, and budesonide, 3 mg twice a day. In patients with grades IIb to IV GVHD, the starting dose of MP should probably be 2 mg/kg (with or without the addition of beclomethasone and budesonide). (Grade IIa is defined as skin rash of < 50% body surface area, not progressing rapidly within the first day; bilirubin < 3 mg/dL; < 20 mL nonhemorrhagic diarrhea/kg per day, without abdominal cramping. Grades IIb-IV include all cases outside the parameters defined for grade IIa.) 2The decision will likely depend upon the timing of the GVHD flare and the clinical presentation of the disease.20 Illustration by A. Y. Chen and H. Crawford.
Treatment of steroid-refractory acute GVHD
| Organ with predominant GVHD manifestation . | Secondary therapy . |
|---|---|
| Skin | ATG (thymoglobulin, ATGAM); denileukin diftitox; monoclonal antibodies (anti-CD25, anti-CD3, anti-CD52); phototherapy (PUVA, ECP); chemotherapy (MMF, calcineurin inhibitors, pentostatin, sirolimus) |
| Liver | ATG; denileukin diftitox; monoclonal antibodies; chemotherapy (sirolimus, pentostatin, calcineurin inhibitors); phototherapy |
| Intestinal tract | “Nonabsorbable” steroids (beclomethasone, budesonide); ATG; TNFα blockade (infliximab, etanercept); chemotherapy (sirolimus, pentostatin, calcineurin inhibitors); mesenchymal “stem” cells; octreotide |
| Organ with predominant GVHD manifestation . | Secondary therapy . |
|---|---|
| Skin | ATG (thymoglobulin, ATGAM); denileukin diftitox; monoclonal antibodies (anti-CD25, anti-CD3, anti-CD52); phototherapy (PUVA, ECP); chemotherapy (MMF, calcineurin inhibitors, pentostatin, sirolimus) |
| Liver | ATG; denileukin diftitox; monoclonal antibodies; chemotherapy (sirolimus, pentostatin, calcineurin inhibitors); phototherapy |
| Intestinal tract | “Nonabsorbable” steroids (beclomethasone, budesonide); ATG; TNFα blockade (infliximab, etanercept); chemotherapy (sirolimus, pentostatin, calcineurin inhibitors); mesenchymal “stem” cells; octreotide |
A typical steroid regimen for primary therapy of GVHD may consist of methylprednisolone (MP), 2 mg/kg per day for 7 or 14 days, followed by gradual dose reduction if the patient responds. A prospective, randomized study comparing 2 mg/kg per day of MP to 10 mg/kg per day failed to show any advantage of the higher dose.24 The same investigators treated 211 patients with grades I to IV GVHD with MP, 2 mg/kg per day, and they were able to taper MP in 150 patients (71%) beginning after 5 days of therapy, whereas 61 patients (29%) required continuation of therapy.25 These “nonresponders” were randomized to receive MP at an increased dose of 5 mg/kg per day for 10 days, either alone or combined with thymoglobulin (6.25 mg/kg over 10 days), and 26% of patients achieved complete responses. There was no significant difference in the response rates between the 2 secondary therapies. Of importance, nonrelapse mortality was 27% for the day-5 responders compared with 49% for nonresponders (P = .009). Based on similar experience at our center, we generally do not increase the dose of MP above 2 mg/kg in nonresponding patients but, rather, add another agent to the treatment regimen.
Major drawbacks of systemic steroid therapy are acute and delayed complications, in particular, the increased risk of infections, osteopenia, and aseptic necrosis. Major efforts have, therefore, been directed at regimens that would reduce systemic steroid use. An additional consideration was that with such an approach higher response rates might be achieved and fewer patients would have to be considered steroid refractory. A randomized trial in 60 patients with intestinal GVHD compared oral MP, at 1 mg/kg per day, plus placebo to MP plus beclomethasone. Treatment responses by day 10 were 55% and 71%, respectively, and the durable responses by day 30 were 41% and 71% for the 2 groups (P = .02).26,27 In a follow-up study with similar design28 in 129 patients with gastrointestinal GVHD, MP was tapered at day 10 if GVHD had improved, and the study drug (beclomethasone/placebo) was continued. The day-200 survival was 92% for patients on beclomethasone and 76% for placebo-treated patients (P = .01). Patients with recurrent or continuous GVHD had greater steroid exposure and a higher risk of death. There was a 2% incremental increase in the risk of death for every 1.0-mg/kg increase in cumulative MP exposure relative to the lowest dose (P = .045).
These trials suggest that for patients with certain GVHD manifestations, systemic steroid exposure can be reduced, both in regard to daily doses and the duration of therapy, and secondary therapy may not be required. However, not all attempts at sparing steroids by combination therapy have resulted in improved outcome.29,30
Secondary therapy (steroid-refractory GVHD)
Timely assessment of nonresponse to primary therapy.
An important question is what constitutes refractoriness and, therefore, when should secondary therapy be initiated. The dynamics of treatment responses differ between target organs and may differ considerably between patients. While erythematous skin rashes may show improvement within 24 hours, it is unlikely that significant improvement would have occurred by that time in hepatic or intestinal dysfunction. If the manifestations of GVHD in any organ worsen over 3 days of treatment or if the skin does not improve by 5 days (particularly if other organ manifestations are present), it is unlikely that a response will be achieved in a timely fashion, and secondary therapy should be considered.
As discussed, however, there is no complete agreement as to what the optimum dose of steroids should be. Thus, a physician may decide to increase the dose of MP to 5 mg/kg per day if a response is not observed at 2 mg/kg. Is such a patient steroid refractory? While some published data suggest that the response rate of GVHD may increase with increasing doses of MP, there is no data to show that long-term survival is improved.31 Thus, I would consider a patient whose GVHD does not improve on 2 mg/kg per day of MP as being steroid refractory and would institute secondary therapy.
As we can usually not exclude the possibility that some benefit, even though unsatisfactory, was derived from primary therapy, the next question is whether the agent used for initial treatment should be continued. Most physicians, including myself, will continue steroids despite the well-known side effects. In patients with prominent intestinal manifestation of GVHD, the addition of beclomethasone (4 × 2 mg/d) and budesonide (2 × 3 mg/d) is reasonable and may allow to reduce the doses of systemically administered steroids. Preliminary data also suggest that the use of lithium to stimulate the Wnt pathway (as proposed by G. Huls) may facilitate re-epithelialization of the intestine (unpublished observations, 2006).
The choice of secondary therapy.
Table 1 provides a summary of available agents and their preferred use for secondary therapy of GVHD.
Antibodies, polyclonal or monoclonal, are the most widely used secondary agents. There is considerable experience with antithymocyte globulin (ATG), which has been in use for more than 3 decades.32 ATG may be associated with acute febrile reactions, hypotension, thrombocytopenia, and, in rare instances, even anaphylactic reactions. In heavily pretreated patients ATG may lead to the development of posttransplantation lymphoproliferative disorders (usually Epstein-Barr virus [EBV]+), a complication that it has in common with monoclonal anti–T-cell antibodies.33 However, some 20% to 50% of patients will improve on ATG, and responses of skin GVHD have been observed in 60% to 75% of patients. Acute toxicity is generally controlled by premedication with steroids, antihistamines, and meperidine. If needed, supplemental intravenous fluid can be administered. If EBV reactivation occurs, treatment with anti-CD20 monoclonal antibody (rituximab, 375 mg/m2) is usually therapeutic.34 Numerous ATG preparations are available. One regimen uses ATGAM (equine ATG) at 30 mg/kg per day every other day for 6 doses or 15 mg/kg per day for 12 doses. The rabbit preparation thymoglobulin is generally given at one tenth the dose of ATGAM.35–37 A recently described intriguing effect of thymoglobulin, in addition to its broad anti–T-cell effect, is the induction of CD4+CD25+ regulatory T cells,38 which may play a role in establishing tolerance. Early institution of ATG appears to be associated with improved survival.39
A broad array of monoclonal antibodies in murine or humanized form with pan-T or T-subset reactivity has been used as secondary therapy of GVHD. Responses, sometimes sustained, have been observed with anti-CD2, anti-CD3, anti-CD5, and other antibodies.40–42 In a dose-escalation trial with the anti-CD147 antibody, ABX-CBL, approximately half of the patients with steroid-refractory acute GVHD improved, and survival was superior to that observed in a historical comparison group treated with horse ATG (ATGAM).43 The ABX-CBL antibody was originally generated against cytotoxic T lymphocytes and was found to be effective in treating organ graft rejection and refractory GVHD.44 Based on the encouraging pilot data, and despite a frequently observed side effect of myalgia, a phase 3 trial was conducted comparing ABX-CBL with ATG (ATGAM) in 92 patients with an end point of day-180 survival. ABX-CBL was given intravenously at 0.1 mg/kg per day for 14 consecutive days; dependent upon treatment responses, “maintenance” for up to 3 cycles (2 infusions per week for 2 weeks) was allowed.45 Disappointingly, the response rates did not differ between the 2 arms, and day-180 survival was nonsignificantly better in the ATG arm.
Good responses were also obtained with the humanized antibody, HuM291 (visilizumab), directed at the invariant CD3 epsilon chain of the T-cell receptor.34 Among 15 patients with steroid-refractory GVHD, 7 achieved complete and 8 partial responses. Sustained responses were achieved with a single dose of antibody (3 mg/m2). A follow-up study enrolled 44 patients, 86% of whom had grades III to IV acute GVHD. The overall response rate was 32% (complete responses 14%) at 42 days, and survival at 6 months was 32%.46 In both trials, 40% to 50% of patients experienced a rise in plasma titers of EBV DNA, which was controlled with administration of rituximab (375 mg/m2). Neither visilizumab nor ABX-CBL is currently available.
The anti-CD52 antibody, alemtuzumab, long in use for T-cell depletion, has also been administered for the treatment of acute and chronic GVHD. As the antibody is reactive against T and B lymphocytes, the risk of EBV-related lymphoproliferative disorders is low.47,48 However, dependent upon the doses used, the risk of infections, in particular of viral etiology, appears to be high.49
A particular target for monoclonal antibody therapy is the interleukin-2 receptor alpha subunit (CD25), prominently expressed on activated T lymphocytes. With murine antibodies such as B-B10, response rates of 60% to 70% were reported. Subsequently, several “humanized” antibodies with the same specificity were developed, and response rates in the range of 50% were observed. Based on encouraging data on the efficacy of daclizumab in steroid-refractory acute GVHD, a randomized, multicenter trial tested the efficacy of the combination of daclizumab and steroids, compared with steroids alone as frontline therapy. While the response rates were comparable (53% vs 51%), overall results were disappointing, with only 29% of patients surviving with combination treatment, compared with 60% given steroids alone (P = .002).50 In addition to emphasizing the need for controlled trials, these observations underscore what we discussed: the timing of therapy, the combinations used, and the sequence of drug administration all may impact on treatment outcome.
One strategy to enhance the efficacy of monoclonal antibodies or biologic ligands has been to link them to a toxin (immunotoxin) that would be delivered to the appropriate target cells. Denileukin diftitox, which links the first 386 amino acids of the diphtheria toxin to amino acids 1 to 133 of IL-2, has received considerable attention. Following binding to CD25, the catalytic A-fragment of the toxin is translocated to the cytoplasm, where it interferes with cellular protein synthesis leading to cell death. Basic studies with this approach suggest that only one molecule of toxin needs to enter the cell in order to be cytotoxic. The initial clinical study by Ho et al51 tested 3 dose schedules, finally settling on a regimen of 9 μg/kg on days 1, 3, 5, 15, 17, and 19. Complete remissions occurred in 33%, and 38% of patients improved by at least one severity grade for an overall response rate of 71%. Premedication similar to that used for monoclonal or polyclonal antibodies was required. Hepatic function abnormalities were common, while severe organ toxicity was infrequent. Best responses were observed in patients with skin and intestinal GVHD. The authors emphasized that 10 patients had previously been treated unsuccessfully with daclizumab. Among 12 patients who ultimately had complete responses, 7 (58%) were surviving, compared with only 1 of 12 patients who did not achieve a response (P < .001). From that study, it appeared that the reduction of CD3+ T lymphocytes within the first week of treatment correlated significantly with the eventual achievement of a complete response (P = .03). Unfortunately, the overall survival of the cohort included in that study remained disappointingly low. Nevertheless, denileukin diftitox is 1 of 4 arms in a currently ongoing randomized phase 2 trial for primary therapy of acute GVHD. While the compound is available for clinical use, toxicity is not negligible, and recommendations for broader use in the clinic should await the results of ongoing studies.
The use of the anti-TNFα monoclonal antibody, infliximab, is based on a rationale similar to that for blockade of the interleukin-1 receptor or the use of soluble IL-1 receptor. The effects of TNFα, which is present at high levels in patients with GVHD, are pleiotropic. While TNFα by itself can induce apoptosis, it also triggers expression of adhesion molecules, histocompatibility antigens, and various cytokines with inflammatory function, including IL-1, IL-6, and interferon β. Past trials showed that anti-TNFα antibodies improved GVHD manifestations; however, GVHD flares upon discontinuation of therapy were frequent.52 Pilot observations with infliximab, a chimeric antibody composed of human constant and murine variable regions, suggested that 10 mg/kg on a weekly basis for 4 doses was particularly effective in patients with gastrointestinal GVHD. Such a pattern may not be surprising as murine studies have shown that TNFα plays a predominant role in GVHD manifestations in the intestinal tract.53 A retrospective analysis by Couriel et al in 21 patients with steroid-refractory acute GVHD showed a response rate of 60% with infliximab, with most responses being complete.54 The rate of infection, in particular with fungal organisms, was high (hazard ratio, 13.6). Overall survival was 38%; however, all surviving patients developed chronic GVHD. Nevertheless, because of the encouraging initial response rate a phase 3 study was conducted using infliximab as up-front treatment in combination with MP in comparison with MP given alone. The response rates in the 2 arms were 66% and 63%, and no long-term benefit of the antibody was observed. Overall, these data suggest that blockade of TNFα can improve GVHD symptomatology, but a complete arrest of the GVHD process is apparently not achieved.
In parallel to the various trials with monoclonal antibodies and immunotoxins, old and new chemotherapeutic/immunomodulatory drugs have been used in the treatment of refractory GVHD. Mycophenolate mofetil (MMF) is the morpholino ethylester of mycophenolic acid (MPA) and possesses immunosuppressive as well as antibacterial, antifungal, and antiviral activities. The active compound, MPA, inhibits inosin monophosphate dehydrogenase, thereby interfering with the synthesis of guanosine triphosphate. As lymphocytes do not have a salvage pathway, they are dependent upon neosynthesis, and are, therefore, preferentially affected by treatment with MMF. As MMF is given orally, its efficacy depends upon absorption, which may be impaired if the intestinal tract is involved by GVHD. In addition, MMF can cause intestinal toxicity. Peak levels of MPA are achieved within 2 hours of an oral dose, and due to enterohepatic recirculation, a second peak occurs at 6 to 12 hours.17 Some studies have shown poor correlations between the dose of MMF administered and the MPA levels reached. However, it has been recommended that the area under the curve for MPA should be in the range of 30 to 60 μg per hour per milliliter and the trough level in the range of 1 to 3.5 mg/L. Plasma concentrations in patients after HCT are lower than observed in solid organ transplantation, presumably due to impairment of hepatic and intestinal function and altered enterohepatic circulation. While the administration of MMF every 8 hours instead of twice a day resulted in satisfactory plasma levels, it is not clear whether this approach has a beneficial effect on GHVD prevention.55 Patients who also receive tacrolimus tend to have higher trough levels of MPA than patients not on tacrolimus. Clinical phase 2 studies in patients with acute GVHD (primary therapy) showed an overall improvement in 72% of patients, predominantly in the skin. In steroid-refractory grades II to IV acute GHVD, we observed at our center a 42% response rate in 19 patients treated with 1 g MMF twice a day.17 In 4 patients, the drug needed to be stopped because of neutropenia or nausea; 2 other patients had GVHD progression on treatment. Survival at 2 years was 16%. Thus, again, there was a small proportion of patients who appeared to derive long-term benefit, but it was not clear whether this strategy was superior to any other approach that has been tested, including continuous administration of steroids only.
Yet another drug we have used for the treatment of refractory GVHD is pentostatin.56,57 Pentostatin is a nucleoside analog, and its administration results in effects similar to those observed with adenosine deaminase deficiency. The resulting accumulation of 2′ deoxyadenosine 5′ triphosphate leads to cell death, predominantly in T cells and NK cells. Pentostatin also reduces the generation of TNFα. It can induce prolonged lymphopenia. In a phase 1 study, which enrolled 24 patients with steroid refractory GVHD, pentostatin was given intravenously at doses of 1, 2, 3, or 4 mg/m2 per day for 3 days. The dose was reduced in patients with impaired renal function. Aside from lymphopenia, the drug was well tolerated. There was a suggestion that infections with adenovirus, human herpes virus type 6, and cytomegalovirus (CMV) were more frequent than otherwise expected. A dose of 1.5 mg/m2 per day was established as the maximum tolerated dose of pentostatin. Among 22 evaluable patients, 14 (64%) achieved complete remission; the response rate was highest in the skin (81%), followed by the gut (79%), and the liver (55%). Of note, 6 patients who had responded to treatment and subsequently flared responded to retreatment with pentostatin. However, only 26% of patients were surviving at approximately 1 year.
Sirolimus (rapamycin) has also been tested in the treatment of refractory acute GVHD.58,59 In a phase 1 to 2 trial, we treated 21 patients (all with grades III or IV GVHD) with sirolimus, at a loading dose of 15 mg/m2 orally on day 1, followed by 5 mg/m2 per day for 13 days or 5 or 4 mg/m2 per day for 14 days without a loading dose.59 In 10 patients, the drug was discontinued because of a lack of improvement of GVHD, myelosuppression, or the occurrence of seizures in 2 patients. Myelosuppression was the most frequent side effect. Five patients had evidence of hemolytic uremic syndrome, a complication observed particularly when the drug was combined with calcineurin inhibitors. However, 12 patients showed improvement of GVHD, 5 with complete and 7 with partial responses, for a response rate of 57%. Six of 12 responding patients but only 1 nonresponding patient were surviving more than 400 days after transplantation. These results in a very high-risk patient population were quite encouraging. Further recommendations will have to be based on the results from ongoing studies. Careful monitoring of blood levels and dose adjustments may be helpful in reducing toxicity.
Tacrolimus or cyclosporine may be useful therapeutically in patients who have not been exposed to those agents as part of their prophylaxis. A switch from cyclosporine to tacrolimus in our hands was useful only in patients in whom the switch occurred because of neurotoxicity.60
Phototherapy pursues a different strategy. Based on initial observations in patients with cutaneous T-cell lymphoma or various dermatologic disorders, 8-methoxypsoralen is given to patients orally before external exposure to ultraviolet irradiation in the UVA range (320-400 nm) to sensitize circulating T lymphocytes to UV energy. The dose of methoxypsoralen ranges from 10 mg for patients weighing less than 30 kg to 50 mg for patients weighing more than 80 kg, and is given about 90 minutes before UV exposure. A starting dose of UVA may be 0.25 J (2 sessions on consecutive days, to be repeated every 2 weeks with escalation of exposure in steps of 0.25 J as tolerated). This approach is particularly effective in patients with acute cutaneous GVHD, and has been useful as a steroid-sparing measure.61
A more effective strategy may be the use of extracorporeal photopheresis (ECP). Here, patient blood (approximately 5 × 109 leukocytes) is exposed extracorporeally to 8-methoxypsoralen followed by UVA irradiation before being returned to the patient.62,63 ECP results in apoptosis in all leukocyte subsets within 24 to 48 hours. Apoptotic cells are taken up by antigen-presenting cells. There is evidence that this process leads to suppression of T-cell reactivity, impaired cytokine release, and the induction of regulatory T cells.64,65 This approach has been shown to have more systemic benefits than the external application of PUVA. Greinix et al conducted a phase 2 study in 38 patients with steroid-refractory or steroid-dependent acute GVHD.62 ECP was given on 2 consecutive days at weekly intervals until maximal response. Maximum responses were seen after 1 to 13 (median 4) cycles. Steroids could be discontinued after a median of 55 days (range, 17-284 days) without flares of acute GVHD. The complete response rate was 86% with grade II, 55% with grade III, and 30% with grade IV acute GVHD. Complete resolution of GVHD occurred in 82% of cases with skin, and 61% with liver and gut GVHD. Transplant-related mortality at 4 years was 14% in patients who achieved complete remissions, and 73% in patients who did not (P < .001). At a median follow-up of almost 4 years, 22 (79%) of 28 surviving patients had no evidence of GVHD. A Kaplan-Meier estimate of survival at 4 years was 59% for patients with complete remissions, compared with 11% for the remaining patients. Of interest, these figures are virtually identical to the 50% to 58% survival among patients responding to denileukin diftitox or sirolimus, compared with 8% to 11% survivors among nonresponders to the same agents. One wonders whether this is sheer coincidence or whether the numbers reflect some biologic risk factors.
In my opinion, physico-chemical approaches are useful, particularly with GVHD of the skin. They allow for steroid sparing, and may, indeed, improve survival in the hands of experienced investigators. However, as with other strategies, it is difficult to predict which patients will respond.
Finally, several studies in recent years have investigated the potential usefulness of mesenchymal stem cells in tolerance induction and therapy of GVHD. A report by Le Blanc et al66 suggested that even HLA-haploidentical mesenchymal cells are a potent tool for the treatment of GVHD. Ongoing trials should generate more comprehensive data on the usefulness of such an approach in the management of GVHD.
Other considerations
Infections are the major (nonrelapse) cause of death in patients receiving therapy for GVHD, and all patients should receive systematic infection prophylaxis. This should include Pneumocystis jirovecii prophylaxis with trimethoprim-sulfamethoxazole (or dapsone), and prevention of herpes virus reactivation with acyclovir. Patients who develop chronic GVHD should also receive penicillin as prophylaxis against infections with encapsulated organisms such as pneumococcus. While on GVHD therapy, patients need to be monitored for viral reactivation, in particular, CMV and EBV, and if there is evidence of activation, treatment with ganciclovir or rituximab, respectively, should be given. Patients also need to be monitored for yeast or mold infections (skin, mucosa, or systemic) by surveillance cultures and determination of galactomannan levels, and appropriate treatment with topical fluconazole, or systemic voriconazole or a caspofungin should be initiated.
The usefulness of prophylactic intravenous immunoglobulin is controversial; however, patients whose IgG levels fall below 4 g/L (400 mg/dL) may benefit.
Patients with intestinal GVHD may benefit at least from short-term “gut rest”; whether prolonged withholding of oral intake is beneficial is not clear. Also, steroid administration has a potent catabolic effect. While enteral nutrition with a bland diet may be preferable and maintain a certain level of bowel function, this may not be possible in patients with severe GVHD and large volumes of diarrhea, and parenteral nutritional support should be provided. Octreotide may reduce the amount of diarrhea.
Finally, patients with severe GVHD are often physically incapacitated, and prolonged therapy with steroids may result in muscle weakness and wasting. It is important, therefore, to make every effort possible to keep patients mobile and possibly involve a physical therapist to develop an individual exercise program.
Summary
The prognosis of patients who develop acute GVHD after HCT and do not respond to primary therapy is poor. Numerous strategies to treat these patients with second-line therapy have been undertaken, aiming at inactivation of alloreactive donor T lymphocytes or NK cells, host antigen-presenting cells, cytokines, or cytokine receptors, or at tissue repair in the recipient. Overall, results have been disappointing. Only very few controlled studies for steroid-refractory GVHD have been conducted, and it has been difficult, if not impossible, to identify patients who have not responded to steroids who are likely to respond to another agent. The consequence has been a somewhat indiscriminate use of whatever agent is available or what the physician's preference might be. There is agreement that efforts must be aimed at reducing steroid exposure and protecting the patient against infectious complications. In my opinion, a more custom-tailored approach to a given patient is desirable. In patients with predominant GVHD of the skin that is not fully responding to steroids, the use of phototherapy is an attractive and possibly the least toxic strategy at the present time. The liver and intestinal tract are slower to respond, and the clinician is tempted to add various agents if responses do not become quickly apparent. Despite all concerns about toxicity, polyclonal antibodies (ie, ATG) remain a useful tool to treat visceral GVHD, although precautions must be taken, particularly in regard to fungal infections and EBV reactivation, by monitoring galactomannan levels and EBV DNA copy numbers in peripheral blood. For predominant gastrointestinal involvement by GVHD, the use of oral nonabsorbable steroids holds promise, possibly in combination with TNFα blockade, while carefully monitoring for evidence of infections.
Authorship
Conflict-of-interest disclosure: The author declares no competing financial interests.
Correspondence: H. Joachim Deeg, Fred Hutchinson Cancer Research Center, 1100 Fairview Ave N, D1-100, PO Box 19024, Seattle, WA 98109-1024; e-mail: jdeeg@fhcrc.org.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported in part by NIH grants HL36444, CA18029, and HL082941 (Bethesda, MD).
I thank Drs Mary Flowers and Paul Carpenter for their thoughtful comments and Helen Crawford and Bonnie Larson for help with article preparation. Dr Carpenter provided an initial concept of the algorithm in Figure 1.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal