Abstract
Environmentally exposed epithelial Langerhans cells (LCs) encounter diverse innate stress signals, which lead to the activation of complex intracellular signaling cascades. Among these, p38 MAPK is consistently phosphorylated. For which aspects of LC activation triggering of p38 signaling is sufficient remains to be elucidated. We show that conditional induction of a dominant active form of MAPK kinase 6 (d.a.MKK6), a direct upstream kinase of p38, in LCs efficiently induces the up-regulation of costimulatory molecules and enhances their T-cell stimulatory capacity. These immediate effects showed no or only a minor requirement for classical NF-κB signaling. Concomitant with LC activation, d.a.MKK6 induced the alternative NF-κB member RelB, whose nuclear localization marks mature DCs. Specific inhibition of nuclear RelB during d.a.MKK6-induced LC activation further enhanced their maturation state. This observation was validated using the p38 activator anisomycin, thus suggesting a novel LC intrinsic control mechanism regulated by RelB.
Introduction
Immature dendritic cells (DCs) such as epidermal/mucosal Langerhans cells (LCs) reside in peripheral tissues. Upon activation they migrate from peripheral sites to lymphoid organs and thereby acquire potent T-cell stimulatory capacity, a process referred to as DC maturation.1,2 Various triggers are capable of inducing DC maturation. Pathogen recognition receptors (PRRs) such as Toll-like receptors (TLRs) induce pathogen-associated molecular pattern (PAMP)–dependent DC activation. Other stimuli are PAMP independent. These include inflammatory cytokines, environmental danger stimuli (eg, UV light and haptens), or dying cells.2,3 Additionally, adhesion-dependent microenvironmental signals seem to counteract DC maturation, because epidermal shear stress itself triggers LC maturation.4 Loss-of-function studies revealed that the classical NF-κB pathway and the p38 MAPK cascade are fundamentally involved in or required for DC maturation.5-9
Two NF-κB signaling pathways are known. The classical NF-κB pathway involves inhibitors of κB (IκBs), which upon phosphorylation and subsequent degradation release bound classical NF-κB dimers (p65/p50 or c-rel/p50).10 Inhibition of the classical NF-κB pathway in human monocyte-derived DCs (mo-DCs) (eg, by adenoviral delivery of an IκBα superrepressor [IκBα-SR] mutant) results in impaired DC maturation.6-8 In the alternative (noncanonical) NF-κB cascade, the p100 (NF-κB2) molecule retains the alternative NF-κB member RelB in the cytoplasm. Upon phosphorylation by the IκB kinase 1 (IKK1), p100 is degraded to p52, RelB is released, and RelB/p52 dimers translocate to the nucleus.10 Nuclear localization of RelB is considered a marker for mature DCs, because diverse maturation stimuli induce RelB translocation to the nuclei of DCs in vitro.11 Similarly, in vivo nuclear RelB marks mature DCs in lymphoid organs and inflammatory sites such as rheumatoid arthritis lesions.12,13 RelB is known to regulate DC development. RelB-deficient mice selectively lack myeloid-related CD8α− DCs but not LCs.14 Similarly, differentiation of human CD11b+ interstitial-type DCs (intDCs) but not LCs from progenitor cells is inhibited by expressing a truncated p100 molecule that specifically captures RelB in the cytoplasm.15 RelB deficiency was also shown to impair murine DC antigen presentation function in vivo.16 Furthermore, DCs from p100-deficient mice, which have enhanced RelB activity but also lack p52, showed enhanced DC maturation.17 However, expression of dominant negative NF-κB–inducing kinase (NIK), an inducer of IKK1-mediated p100 processing, had no effect on human mo-DC maturation.18 Therefore, while a critical function of RelB in DC subset development is well established, its function in DC maturation remains unclear.
p38 mitogen-activated protein kinase (p38 MAPK) is an evolutionary highly conserved stress response pathway in eukaryotic cells. The p38 is activated by the upstream MAPK kinases MKK6 or MKK3 and is then translocated to the nucleus where it in turn phosphorylates respective target molecules.19 Various studies proved that p38 is functionally involved in DC maturation, because addition of specific p38 inhibitors during UV-B irradiation,20 hapten stimulation,21 or TLR4 ligation6 of mo-DCs resulted in impaired DC activation. Whether the specific activation of p38 MAPK is a trigger for DC maturation is not known.
In this study we investigated the selective triggering of one defined signaling pathway in LCs. Addressing this question required a new experimental approach, specifically, a system for conditional activation of genes in physically unmanipulated LCs. We found that conditional induction of a dominant active version of MKK6 (d.a.MKK6) in immature LCs strongly induces up-regulation of costimulatory molecules (UCM) and turns LCs into potent T-cell stimulators. Concomitant with these effects, d.a.MKK6-expression induced RelB. Our conditional activation system offered the opportunity to specifically investigate the role of nuclear RelB during the LC maturation process. This revealed that RelB failed to promote but rather counteracted LC activation by signals that specifically trigger p38.
Materials and methods
Cytokines and reagents
Human stem-cell factor (SCF), thrombopoietin (TPO), and tumor necrosis factor alpha (TNFα) were purchased from PeproTech (London, United Kingdom); transforming growth factor beta 1 (TGF-β1) was purchased from R&D Systems (Wiesbaden, Germany); fms-related tyrosine kinase 3 ligand (FL, Flt3L) was obtained from Amgen (Seattle, WA); granulocyte-macrophage colony-stimulating factor (GM-CSF) was kindly provided by Novartis Research Institute (Vienna, Austria); CD40 ligand (CD40L) was kindly provided by Amgen (Seattle, WA); LPS, doxycycline (DOX), and anisomycin were purchased from Sigma-Aldrich (Vienna, Austria); SB230580 (SB) was puchased from Calbiochem (San Diego, CA).
Isolation of cord-blood CD34+ cells
Cord-blood samples from healthy donors were collected during healthy full-term deliveries. Approval was obtained from the Medical University of Vienna institutional review board for these studies. Informed consent was provided in accordance with the Declaration of Helsinki. Cord-blood mononuclear cells (MNCs) were isolated by density gradient centrifugation using Lymphoprep (Axis-Shield, Oslo, Norway). CD34+ cells were isolated from MNCs by MACS Direct CD34 Progenitor Cell Isolation Kit (Miltenyi Biotec, Bergisch Gladbach, Germany) according to the manufacturer's instructions. The purity of the isolated CD34+ cells was more than 95%.
Retroviral vectors and gene transduction
Complementary DNAs of MKK6b(DE) (d.a. MKK6b containing S>D and T>E mutations obtained from J. Han, La Jolla, CA), human RelB (obtained from R. Thomas, Brisbane, Australia), p100ΔN (amino acids 407 to 900 of p100, obtained from C. V. Paya, Rochester, MN), and IκBα-SR and Moloney murine leukemia virus (MMLV) ecotropic receptor (obtained from R. de Martin, Vienna, Austria) were subcloned into the MMLV–based retroviral vector pBMN (obtained from G. P. Nolan, Stanford, CA) upstream of an internal ribosome entry site (IRES) followed by either GFP or murine CD8α (mCD8α). The packaging cell lines Phoenix E (ecotropic retrovirus) and Phoenix-Gag-Pol (Ph-GP; amphotropic virus) were transfected by calcium phosphate precipitation. For amphotropic virus, Ph-GP cells were cotransfected with the gibbon ape leukemia virus envelope protein (obtained from D. B. Kohn, Los Angeles, CA). Infection of target cells was done as previously described.15 Briefly, target cells were plated on RetroNectin (Takara Bio, Shiga, Japan)–coated nontissue culture plates coated with virus (3 to 5 hours) in specific growth medium. Infections were repeated 2 to 3 times at intervals of 12 to 24 hours. CD34+ cells were infected in expansion mix (SCF, FL, TPO; each 50 ng/mL). Double and triple gene transduction was performed by cotransfection of Phoenix cells.
Fluorescence activated cell sorter (FACS)–detectable tetracycline-inducible gene-expression system
The created tetracycline-inducible system (Tet-ON system) consists of 2 vectors. The tetracycline activator (rtTA2s-M2, TA, excised from pUHDrtTA2s-M2 provided by H. Boujard, Heidelberg, Germany)22 was subcloned into the pBMN vector upstream of IRES-mCD8α. The resultant vector was designated TA-mCD8α. The tet-response vector is a self-inactivating vector encoding a mutated 3′ LTR, which upon infection leads to an inactivated 5′ LTR. This enables expression of a “gene of interest” from a tet-response element followed by either IRES-GFP (HR-GFP, obtained from F. Rossi, University of British Columbia, Vancouver)23 or IRES-nerve growth factor receptor (HR-NGFR, constructed by replacing IRES-GFP of the HR-GFP vector by IRES-NGFR excised from pGC-IRES-NGFR, obtained from A. Iwama, Boston, MA). Complementary DNAs of interest were subcloned into the tet-response vectors. CD34+ cells were first infected with TA-mCD8α and then with the tet-response vector. Gene expression was induced by 1 to 2 μg/mL DOX.
In vitro culture of CD34+ cord-blood stem cells
LCs were generated from CD34+ cells (2 × 104 to 7 × 104/mL per well) in 24-well tissue culture plates (Nunc, Rochester, NY) in serumfree X-VIVO 15 medium (BioWhittaker, Walkersville, MD) supplemented with GlutaMAX (2.5 mM; Gibco/Invitrogen, Carlsbad, CA), penicillin/streptomycin (125 U/mL each), GM-CSF (100 ng/mL), SCF (20 ng/mL), FL (50 ng/mL), TNFα (2.5 ng/mL), and TGF-β1 (0.5 ng/mL).24 LC maturation was induced by 500 ng/mL CD40L, 1 μg/mL LPS, or 40 ng/mL anisomycin for 48 hours. For FACS analysis, LC clusters were disrupted immediately before staining.
Cell lines
U937T cells (obtained from G. Grosveld, Memphis, TN) were transduced with pBMN–eco receptor–IRES–mCD8α and sorted for mCD8αhigh cells by FACS for ecotropic virus–infectible cells. U937T NF-κB reporter cells were generated by transducing U937T cells with a self-inactivating retroviral vector (obtained from G. P. Nolan) containing a 5 × NF-κB–GFP reporter cassette (obtained from R. de Martin), followed by single-cell cloning. Control vector or d.a.MKK6-inducible U937 Tet-ON cells were generated by cotransducing U937 cells (purchased from American Type Culture Collection [ATCC], Manassas, VA) with TA-mCD8α either together with HR-NGFR or d.a.MKK6-NGFR followed by single-cell cloning for obtaining optimal DOX-inducible clones. All cell lines were maintained in RPMI plus 10% FCS medium.
The photomicrograph in Figure 1A was made using a Nikon TMS inverted microscope equipped with a 10×/0.25 Ph1 DL objective lens and a Coolpix 995 digital camera (Nikon, Tokyo, Japan). Adobe Photoshop 7.0 software (Adobe Systems, San Jose, CA) was used for image processing.
Flow cytometry
Flow cytometry analysis was performed as previously described.15 Murine monoclonal antibodies (mAbs) of the following specificities were used: FITC-conjugated anti-CD1a (BD Pharmingen, Heidelberg, Germany); phycoerythrin (PE)–conjugated anti-CD86, MHC II, NGFR, CD40 (BD Pharmingen), CD11b (BD Biosciences, Palo Alto, CA), CD80, CD83, mCD8α (Caltag Laboratories, Hamburg, Germany), and Langerin (Immunotech, Marseille, France); biotinylated mAbs anti-CD86, CD80, NGFR, and CD11b (BD Pharmingen) combined with streptavidin (SA)–PerCP or SA-APC (BD Pharmingen); PerCP-conjugated anti-mCD8α (BD Pharmingen); and allophycocyanin (APC)–conjugated anti-CD1a (BD Pharmingen) and anti-CD14 and anti-CD83 (Caltag Laboratories). Isotype control mAbs were provided by O. Majdic (Vienna, Austria). For FACS sorting we used the BD FACSAria flow cytometer.
Western blot analysis
Whole-cell lysates were prepared by resuspending cell pellets in an appropriate volume of lysis buffer (20 mM Tris [pH 7.5], 150 mM NaCl, 2.5 mM EDTA, 1% Triton X-100) supplemented with protease inhibitors (protease inhibitor cocktail set III; Calbiochem), NaF (50 mM), and Na3VO4 (1 mM). After 15 minutes on ice, extracts were centrifuged (10 minutes, 4°C, 18 000g rpm). Nuclear and cytoplasmic extracts were prepared using the nuclear extract kit from Active Motif (Carlsbad, CA) according to the manufacturer's instructions. Proteins resolved by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) were transferred to a polyvinylidene difluoride membrane (Immobilon-P; Millipore, Billerica, MA). Membranes were probed with anti-MKK6 (Stressgen Biotechnologies, San Diego, CA), anti–phosphorylated or anti–total p38 MAPK, anti–phosphorylated or anti–total JNK (Cell Signaling Technology, Beverly, MA), anti–RelB sc-226 (Santa Cruz Biotechnology, Santa Cruz, CA), anti–p65 sc-372, antiactin (A-2066; Sigma-Aldrich, Vienna, Austria), anti–PCNA sc-56 antibodies followed by horseradish peroxide–conjugated goat anti–rabbit or goat anti–mouse IgG (H+L) antibodies (Pierce Biotechnology, Rockford, IL). Detection was performed with the chemiluminescent substrate SuperSignal WestPico or WestDura (Pierce Biotechnology).
Mixed-leukocyte reaction (MLR)
Cells were γ-irradiated (20 Gy), and graded numbers of these stimulator cells were cocultured with a constant number of 1 × 105 purified allogeneic T cells in RPMI medium containing 10% FCS using U-bottom, 96-well tissue culture plates (Nalge Europe, Brussels, Belgium). Cultures were pulsed at day 6 for 18 hours with 1 μCi (0.037 MBq)–per-well methyl-3H thymidine (Amersham Biosciences, Little Chalfont, United Kingdom). Incorporated radioactivity was measured using a 1450 microbeta plate reader (Wallac-Trilux Instrument; Life Science, Vienna, Austria). Results are presented as the mean ± SD obtained from triplicate cultures.
Cytokine measurement
Cells were seeded (1 × 105/100 μL) in 96-well plates, and supernatants were collected 48 hours later. Quantification of IL-1β, IL-6, IL-8, TNFα, IL-12 p70, and IL-10 in culture supernatants was performed using the Cytometric Bead Array (CBA) Flex system (Becton Dickinson, Heidelberg, Germany) downscaling according to the manufacturer and analyzing acquired data with BD CBA software. The Luminex system was used alternatively.
Statistical analysis
Statistical analysis was performed using the paired, 2-tailed Student t test; P values less than < .05 were considered significant.
Results
Conditional activation of genes in immature Langerhans cells
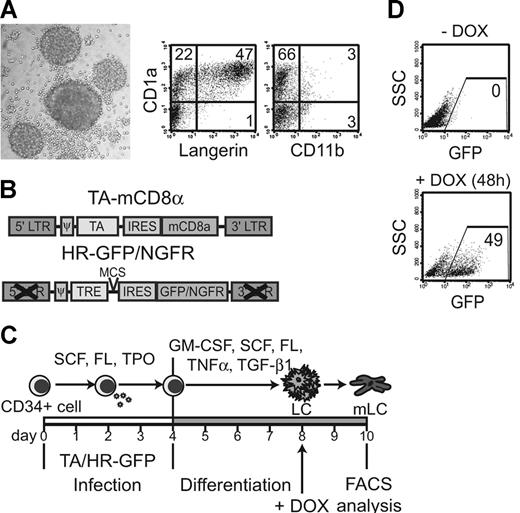
To investigate selective p38 MAPK triggering in immature human LCs, we chose to express a dominant active version of the common upstream activator of all 4 p38 MAPK isoforms, the MAPK kinase 6b (d.a.MKK6).25,26 Expression of d.a.MKK6 is known as a tool for selective p38 MAPK activation.27 We used an in vitro system to generate postmitotic immature LCs from human CD34+ hematopoietic progenitor cells (HPCs) in serumfree medium in the presence of 5 cytokines including TGF-β1.24 These immature LCs form typical round clusters via E-cadherin adhesions and are CD1a+Langerin+ (Figure 1A) In contrast to interstitial-type DCs, they lack CD11b expression (Figure 1A).15,28-30 Simple physical manipulation leading to LC cluster disruption induces LC activation.28 To strictly avoid this unspecific activation, we established an inducible expression system, thus enabling gene induction in physically unmanipulated LCs. In this inducible expression system, CD34+ HPCs are transduced with 2 bicistronic retroviral vectors (Figure 1B) under expansion conditions (SCF, FL, TPO; Figure 1C). Cells are then replated in specific LC differentiation conditions,24 and gene expression is turned on by the addition of DOX later during culture (ie, after cells have already acquired immature LC characteristics) (Figure 1C). Two response vectors were used encoding either GFP or NGFR as marker molecule. Upon DOX addition, a substantial percentage of generated LCs expressed GFP, whereas control cells (–DOX) remained GFP negative (Figure 1D). Importantly, this Tet-ON system showed undetectable background levels of marker molecule expression without DOX (Figure 1D).
Tet-inducible system for postmitotic LCs. (A) Immature CD34+-cell–derived LC clusters were generated under LC-promoting conditions and analyzed by FACS for CD1a, Langerin, and CD11b expression (numbers depict percentages). Photomicrograph shows typical round LC clusters. (B) Schematic representation of bicistronic retroviral vectors. The first (TA-mCD8α) encodes the tet-activator (TA) upstream of IRES-mCD8α; the second is a self-inactivating vector that encodes a tet-response element (TRE) followed by either IRES-GFP or IRES-NGFR (HR-GFP/NGFR). (C) CD34+ cells are sequentially infected with the constructs shown in panel B and cultured in a 2-step differentiation model as indicated. Gene expression is turned on by DOX (2 μg/mL) in immature LCs on day 8 and analyzed 24 hours or 48 hours later. (D) Representative FACS diagrams show day-10 LCs grown with or without DOX. Induced gene expression is marked by GFP expression.
Tet-inducible system for postmitotic LCs. (A) Immature CD34+-cell–derived LC clusters were generated under LC-promoting conditions and analyzed by FACS for CD1a, Langerin, and CD11b expression (numbers depict percentages). Photomicrograph shows typical round LC clusters. (B) Schematic representation of bicistronic retroviral vectors. The first (TA-mCD8α) encodes the tet-activator (TA) upstream of IRES-mCD8α; the second is a self-inactivating vector that encodes a tet-response element (TRE) followed by either IRES-GFP or IRES-NGFR (HR-GFP/NGFR). (C) CD34+ cells are sequentially infected with the constructs shown in panel B and cultured in a 2-step differentiation model as indicated. Gene expression is turned on by DOX (2 μg/mL) in immature LCs on day 8 and analyzed 24 hours or 48 hours later. (D) Representative FACS diagrams show day-10 LCs grown with or without DOX. Induced gene expression is marked by GFP expression.
Conditional d.a.MKK6 expression in primary LCs induces UCM and enhances T-cell stimulation
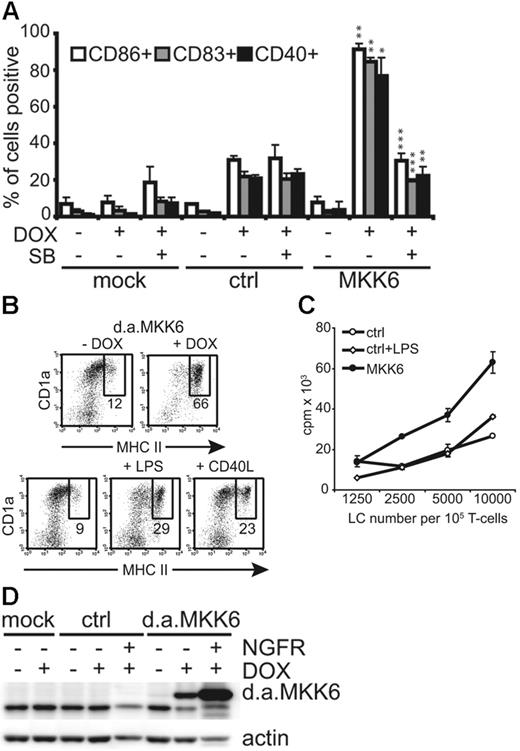
CD1a+ LCs induced to express d.a.MKK6-NGFR were analyzed after 48 hours for various DC maturation marker molecules. Gated CD1a+NGFR+ cells expressing d.a.MKK6 showed a mature DC phenotype as indicated by the up-regulation of CD86, CD83, and CD40. Conversely, control vector–transduced LCs remained immature (Figure 2A) The d.a.MKK6-induced UCM could be inhibited by the addition of the specific p38 MAPK inhibitor SB203580, thus confirming p38 signal requirement for this effect (Figure 2A). DOX-induced d.a.MKK6 expression also resulted in a higher percentage of major histocompatibility complex (MHC) IIhi LCs as compared with LCs grown in the absence of DOX or LCs stimulated with LPS or CD40L (Figure 2B). To investigate whether d.a.MKK6-matured LCs were also functionally activated, we performed an allogeneic mixed-leukocyte reaction (allo-MLR). MLR was initiated with sorted CD1a+NGFR+ LCs expressing d.a.MKK6 or control vector. Expression of d.a.MKK6 clearly enhanced the T-cell stimulatory capacity of LCs relative to control transduced cells (Figure 2C). However, LPS stimulation, although leading to p38 phosphorylation (data not shown), did not increase the T-cell stimulatory capacity of LCs. This reflects the rather moderate up-regulation of MHC II observed upon LPS administration (Figure 2B). Western blot analysis of sorted NGFR+CD1a+ LCs confirmed the ectopic expression of d.a.MKK6 (Figure 2D). Low d.a.MKK6 expression could also be detected in the sorted NGFR− population due to the presence of cells expressing low NGFR levels (Figure 2D and data not shown). The up-regulation of CD86, CD83, and CD40 seen with gated MKK6-NGFR− LCs was most likely due to low levels of d.a.MKK6 expression. However, this effect was minor compared with the strong UCM observed with MKK6-NGFR+ cells, thus arguing against the possibility that this effect is solely due to extrinsic factors (eg, cytokines) (data not shown).
Induction of d.a.MKK6 in immature LCs results in UCM and enhanced T-cell stimulatory capacity. (A) Bars represent percentages (mean ± SD of 3 independent experiments) of gated CD1a+NGFR− LCs (mock infected or grown without DOX) or CD1a+NGFR+ LCs (d.a.MKK6 or control transduced grown with DOX) expressing CD86, CD83, and CD40. The effect of the specific p38 MAPK inhibitor SB230580 (15 μM/mL) was determined. P values (*P < .05, **P < .01, ***P < .001) were determined for control (ctrl) + DOX versus MKK6 + DOX or MKK6 + DOX versus MKK6 + DOX/SB. (B) Representative dot plots show CD1a versus MHC II expression of TA/d.a.MKK6-NGFR–transduced day 10 LCs grown with or without 2 μg/mL DOX (upper panel) compared with mock-infected LCs stimulated with either 1 μg/mL LPS or 500 ng/mL CD40L for 48 hours (lower panel). DOX-stimulated cells were gated for NGFR expression. (C) Allo-T-cell–stimulatory capacity of sorted CD1a+NGFR+ LCs expressing either control vector (ctrl) or d.a.MKK6 (MKK6) was determined. In parallel, control-transduced LCs were stimulated with 1 μg/mL LPS. One representative of 4 experiments is shown. (D) Total-cell lysates of CD1a+ LCs (mock, ctrl, or d.a.MKK6 infected) stimulated with or without DOX were analyzed by Western blot for MKK6 or actin expression. DOX-stimulated cultures were separated into CD1a+NGFR+ and CD1a+NGFR− LCs by FACS sorting prior to preparing cell lysates (1 representative of 3 independent experiments). For panels A and C, results are presented as the mean ± SD obtained from triplicate cultures.
Induction of d.a.MKK6 in immature LCs results in UCM and enhanced T-cell stimulatory capacity. (A) Bars represent percentages (mean ± SD of 3 independent experiments) of gated CD1a+NGFR− LCs (mock infected or grown without DOX) or CD1a+NGFR+ LCs (d.a.MKK6 or control transduced grown with DOX) expressing CD86, CD83, and CD40. The effect of the specific p38 MAPK inhibitor SB230580 (15 μM/mL) was determined. P values (*P < .05, **P < .01, ***P < .001) were determined for control (ctrl) + DOX versus MKK6 + DOX or MKK6 + DOX versus MKK6 + DOX/SB. (B) Representative dot plots show CD1a versus MHC II expression of TA/d.a.MKK6-NGFR–transduced day 10 LCs grown with or without 2 μg/mL DOX (upper panel) compared with mock-infected LCs stimulated with either 1 μg/mL LPS or 500 ng/mL CD40L for 48 hours (lower panel). DOX-stimulated cells were gated for NGFR expression. (C) Allo-T-cell–stimulatory capacity of sorted CD1a+NGFR+ LCs expressing either control vector (ctrl) or d.a.MKK6 (MKK6) was determined. In parallel, control-transduced LCs were stimulated with 1 μg/mL LPS. One representative of 4 experiments is shown. (D) Total-cell lysates of CD1a+ LCs (mock, ctrl, or d.a.MKK6 infected) stimulated with or without DOX were analyzed by Western blot for MKK6 or actin expression. DOX-stimulated cultures were separated into CD1a+NGFR+ and CD1a+NGFR− LCs by FACS sorting prior to preparing cell lysates (1 representative of 3 independent experiments). For panels A and C, results are presented as the mean ± SD obtained from triplicate cultures.
d.a.MKK6-matured LCs show selective production of cytokines
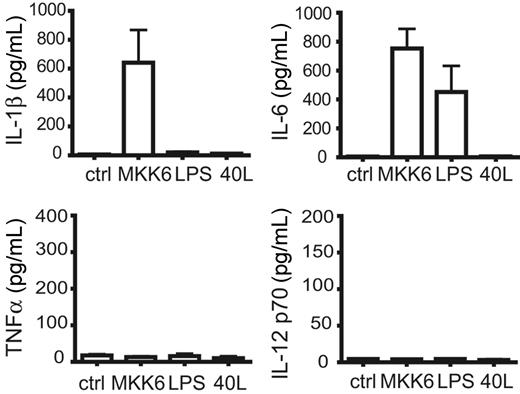
LCs in vivo synthesize the early-acting proinflammatory cytokines IL-1β and, to a lesser extent, IL-6 but not TNFα.31 In all experiments induction of d.a.MKK6 in LCs led to the production of high amounts of IL-1β, an effect not observed upon LPS or CD40L stimulation (Figure 3) Furthermore, d.a.MKK6 expression stimulated LCs to produce IL-6, which was also observed by LPS addition. IL-8 secretion was also enhanced by d.a.MKK6 (data not shown) whereas IL-12 p70 and TNFα were never produced (Figure 3). IL-10 was not detected under all conditions tested (data not shown). In line with previous observations32 we also observed that IL-1β addition potentiated the effect of CD40L and IFNγ on IL-12 p70 production by LCs (data not shown).
d.a.MKK6 selectively induces IL-1β and IL-6 production of CD34+-cell–derived LCs. CD34+ cells transduced with TA and d.a.MKK6-NGFR (or control) were differentiated to LCs. On day 8, gene expression was induced by DOX addition (2 μg/mL), and sorted CD1a+NGFR+ cells were replated for cytokine production in GM-CSF (100 ng/mL), FL (50 ng/mL), SCF (20 ng/mL), and DOX (2 μg/mL). For comparison, control-transduced CD1a+ LCs were in parallel stimulated with 2 μg/mL LPS or 500 ng/mL CD40L. IL-6, IL-1β, TNFα, and IL-12 p70 amounts (mean ± SEM of 3 independent experiments) were determined after 48 hours.
d.a.MKK6 selectively induces IL-1β and IL-6 production of CD34+-cell–derived LCs. CD34+ cells transduced with TA and d.a.MKK6-NGFR (or control) were differentiated to LCs. On day 8, gene expression was induced by DOX addition (2 μg/mL), and sorted CD1a+NGFR+ cells were replated for cytokine production in GM-CSF (100 ng/mL), FL (50 ng/mL), SCF (20 ng/mL), and DOX (2 μg/mL). For comparison, control-transduced CD1a+ LCs were in parallel stimulated with 2 μg/mL LPS or 500 ng/mL CD40L. IL-6, IL-1β, TNFα, and IL-12 p70 amounts (mean ± SEM of 3 independent experiments) were determined after 48 hours.
d.a.MKK6 strongly induces RelB in LCs, but conditional RelB expression alone is not sufficient for LC activation
Nuclear expression of the nonclassical NF-κB member RelB is considered a “hallmark” phenotypic characteristic of mature DCs.33 Conditional expression of d.a.MKK6 in immature LCs strongly induced RelB expression (Figure 4A) To investigate whether RelB overexpression in immature LCs is sufficient to activate them, we inserted RelB in a tet-response construct followed by IRES-NGFR and evaluated conditional RelB expression in primary CD34+-cell–derived LCs as depicted in Figure 1C. RelB overexpression upon DOX addition was confirmed by Western blot analysis (Figure 4B). Phenotypic analysis of gated NGFR+ cells revealed that enforced expression of RelB in primary immature LCs fails to induce up-regulation of CD86, CD83, or CD40. In comparison, side-by-side analysis of DOX-induced d.a.MKK6 expression showed a strong UCM induction (Figure 4B).
Conditional expression of RelB in LCs does not induce maturation. (A) Representative Western blot analysis for RelB. CD1a+NGFR+ LCs induced to express d.a.MKK6-NGFR or control vector in the presence of DOX for 48 hours were sorted, and total-cell lysates were prepared. As control, lysates of CD1a+ LCs grown in the absence of DOX were used. Actin detection was the loading control. (B) (Left) CD1a+NGFR+ LCs induced to express RelB-NGFR were sorted, and RelB expression was analyzed by Western blot. As control, lysates of CD1a+ RelB-transduced LCs grown in the absence of DOX or of CD1a+ mock-infected LCs were used. Actin detection was the loading control. (Right) Control vector (ctrl), RelB-NGFR, or d.a.MKK6-NGFR was induced in immature LCs by DOX (2 μg/mL), and gated NGFR+ cells were analyzed for CD86, CD83, and CD40 by FACS after 48 hours. Bars represent the mean percentages ± SD of 3 independent experiments. P values (**P < .01, ***P < .001) were determined for control versus MKK6 or control versus RelB.
Conditional expression of RelB in LCs does not induce maturation. (A) Representative Western blot analysis for RelB. CD1a+NGFR+ LCs induced to express d.a.MKK6-NGFR or control vector in the presence of DOX for 48 hours were sorted, and total-cell lysates were prepared. As control, lysates of CD1a+ LCs grown in the absence of DOX were used. Actin detection was the loading control. (B) (Left) CD1a+NGFR+ LCs induced to express RelB-NGFR were sorted, and RelB expression was analyzed by Western blot. As control, lysates of CD1a+ RelB-transduced LCs grown in the absence of DOX or of CD1a+ mock-infected LCs were used. Actin detection was the loading control. (Right) Control vector (ctrl), RelB-NGFR, or d.a.MKK6-NGFR was induced in immature LCs by DOX (2 μg/mL), and gated NGFR+ cells were analyzed for CD86, CD83, and CD40 by FACS after 48 hours. Bars represent the mean percentages ± SD of 3 independent experiments. P values (**P < .01, ***P < .001) were determined for control versus MKK6 or control versus RelB.
d.a.MKK6-induced UCM is independent of concomitantly induced weak classical NF-κB activation
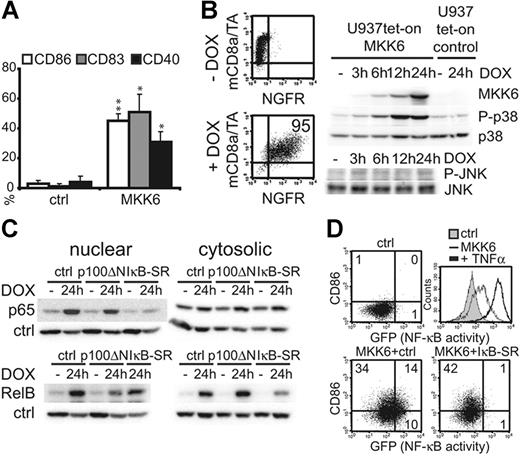
Due to cell-number limitations of primary LCs, we used the human myelomonocytic U937 cell line for biochemical analysis of signaling processes downstream of d.a.MKK6 (ie, for subcellular fractionation experiments). Although these homogeneous model cells differ in many aspects from primary LCs, they showed UCM induction (Figure 5A) and enhanced T-cell stimulatory capacity (data not shown) in response to d.a.MKK6, as similarly observed for LCs. To obtain optimal inducible U937 clones, we cotransduced the cells with the TA and tet-response vectors (Figure 1B). Upon DOX addition, the d.a.MKK6-inducible cell line acquired the marker molecule NGFR (Figure 5B). Expression of d.a.MKK6 started 3 to 6 hours after DOX administration and thereafter increased within 24 hours (Figure 5B). As expected, p38 MAPK phosphorylation paralleled the induced d.a.MKK6 expression leading to sustained p38 activation (Figure 5B). The specificity of MKK6 for p38 MAPK was confirmed, because a related MAPK, c-Jun n-terminal kinase (JNK), was not phosphorylated by d.a.MKK6 (Figure 5B).19 However, d.a.MKK6 induction also resulted in nuclear translocation of the NF-κB member p65, which was inhibited by coexpression of an IκBα-SR mutant (IκBα-SR)34 known to capture p65 and p50 but not RelB in the cytoplasm (Figure 5C, upper left panel). As seen with primary LCs, d.a.MKK6 elevated overall RelB expression, which was diminished by the coexpression of the IκBα-SR (Figure 5C, lower right panel), consistent with previous observations that the classical NF-κB pathway controls RelB transcription.35 Furthermore, d.a.MKK6 led to nuclear translocation of RelB. A truncated p100 molecule (p100ΔN), containing the ankyrin-rich RelB binding domain only, was described to capture RelB but not other NF-κB members in the cytoplasm.15,36 Coexpression of d.a.MKK6 together with p100ΔN revealed that p100ΔN decreases d.a.MKK6-induced nuclear RelB and concomitantly increases cytoplasmic RelB (Figure 5C, lower panels). To analyze whether d.a.MKK6-induced UCM was dependent on NF-κB activation, we coinfected a U937 NF-κB–GFP reporter cell line with d.a.MKK6 alone or together with the IκBα-SR. Using this cell line, UCM and NF-κB activation could be correlated at the single-cell level. Analysis of GFP induction (NF-κB activity) versus surface marker expression revealed that some cells up-regulate CD86 without NF-κB activation while others showed both. Therefore, d.a.MKK6-induced UCM and d.a.MKK6-induced NF-κB activation are not interdependent. In line with this, IκBα-SR abrogated d.a.MKK6-induced GFP induction but not CD86 expression (Figure 5D, lower right panel). This NF-κB independency was also observed for d.a.MKK6-induced CD80 up-regulation (data not shown). The d.a.MKK6-induced NF-κB activation was previously shown in myoblasts.37 However, in our system, NF-κB activation resulting from d.a.MKK6 was only minor compared with the high GFP levels induced by TNFα stimulation (Figure 5D, histogram overlay).
Classical NF-κB signaling is dispensable for d.a.MKK6-induced UCM. (A) U937Te cells were infected with either empty pBMN-IRES-GFP vector or pBMN-d.a.MKK6-IRES-GFP. GFP+ cells were analyzed by FACS for the expression of CD86, CD83, and CD40. Bars represent mean percentages ± SD of 3 independent experiments (*P < .05, **P < .01). (B) U937 cells were cotransduced with TA-mCD8α and HR-NGFR or d.a.MKK6-NGFR, and optimal inducible clones were generated. FACS diagrams show NGFR versus mCD8α expression of a d.a.MKK6-inducible U937 clone grown with or without DOX for 48 hours. Representative Western blot analysis of cell lysates from d.a.MKK6- or control vector–inducible U937 cells treated with DOX (1 μg/mL) for the indicated time points. MKK6, phosphorylated or total p38, and phosphorylated or total JNK were detected. One representative of 4 independent experiments is shown. (C) Representative Western blot analysis of nuclear and cytosolic extracts from a d.a.MKK6-inducible U937 clone transduced with either pBMN-IRES-GFP, pBMN-IκB-SR-IRES-GFP, or pBMN-p100ΔN-IRES-GFP grown with or without 2 μg/mL DOX (24 hours). GFP+ cells were FACS sorted prior to preparation. Extracts were probed with anti-p65, anti-RelB, antiactin (cytosolic control), and anti-PCNA (nuclear control) antibodies. (D) Representative FACS blots of a U937T NF-κB–GFP reporter cell line coinfected with empty vector controls or d.a.MKK6-NGFR together with pBMN-IRES-mCD8α or pBMN-IκB-SR-IRES-mCD8α. NGFR+ mCD8α+ cells were analyzed for CD86 versus GFP. Histogram overlay shows GFP induction (NF-κB activity) in response to TNFα stimulation (2.5 ng/mL, 48 hours) of control transduced cells (bold black line) compared with cells grown in the absence of TNFα (full gray line) or cells transduced with d.a.MKK6 (thin black line). One representative of 3 independent experiments is shown.
Classical NF-κB signaling is dispensable for d.a.MKK6-induced UCM. (A) U937Te cells were infected with either empty pBMN-IRES-GFP vector or pBMN-d.a.MKK6-IRES-GFP. GFP+ cells were analyzed by FACS for the expression of CD86, CD83, and CD40. Bars represent mean percentages ± SD of 3 independent experiments (*P < .05, **P < .01). (B) U937 cells were cotransduced with TA-mCD8α and HR-NGFR or d.a.MKK6-NGFR, and optimal inducible clones were generated. FACS diagrams show NGFR versus mCD8α expression of a d.a.MKK6-inducible U937 clone grown with or without DOX for 48 hours. Representative Western blot analysis of cell lysates from d.a.MKK6- or control vector–inducible U937 cells treated with DOX (1 μg/mL) for the indicated time points. MKK6, phosphorylated or total p38, and phosphorylated or total JNK were detected. One representative of 4 independent experiments is shown. (C) Representative Western blot analysis of nuclear and cytosolic extracts from a d.a.MKK6-inducible U937 clone transduced with either pBMN-IRES-GFP, pBMN-IκB-SR-IRES-GFP, or pBMN-p100ΔN-IRES-GFP grown with or without 2 μg/mL DOX (24 hours). GFP+ cells were FACS sorted prior to preparation. Extracts were probed with anti-p65, anti-RelB, antiactin (cytosolic control), and anti-PCNA (nuclear control) antibodies. (D) Representative FACS blots of a U937T NF-κB–GFP reporter cell line coinfected with empty vector controls or d.a.MKK6-NGFR together with pBMN-IRES-mCD8α or pBMN-IκB-SR-IRES-mCD8α. NGFR+ mCD8α+ cells were analyzed for CD86 versus GFP. Histogram overlay shows GFP induction (NF-κB activity) in response to TNFα stimulation (2.5 ng/mL, 48 hours) of control transduced cells (bold black line) compared with cells grown in the absence of TNFα (full gray line) or cells transduced with d.a.MKK6 (thin black line). One representative of 3 independent experiments is shown.
Inhibition of nuclear RelB enhances d.a.MKK6-induced LC maturation
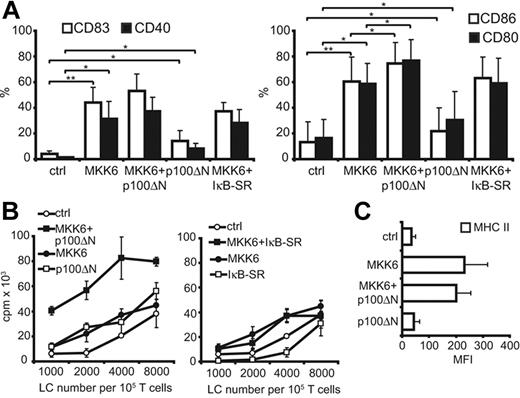
Because conditional expression of RelB in LCs was not sufficient to induce LC activation, we next focused our investigation on the inverse effect (ie, inhibition of nuclear shuttling of RelB by p100ΔN during d.a.MKK6-induced LC activation). The d.a.MKK6-NGFR was induced alone or was coinduced together with p100ΔN-GFP in primary immature LCs by DOX addition. NGFR+GFP+ cells were gated and analyzed for the up-regulation of various DC maturation marker molecules. Coinduced p100ΔN affected neither the up-regulation of CD83 nor CD40 (Figure 6A, left panel). Notably, however, p100ΔN led to a significant enhancement of d.a.MKK6-induced CD86 and CD80 expression (Figure 6A, right panel; Table 1) Increased expression of these costimulatory molecules was paralleled by the enhancement of d.a.MKK6-induced T-cell stimulatory capacity in response to induced p100ΔN expression (Figure 6B, left panel). This effect was not mediated by prolonged survival of the MKK6/p100ΔN-cotransduced LCs as confirmed by 7-AAD analysis (data not shown). Furthermore, the enhanced d.a.MKK6-induced T-cell stimulation by nuclear RelB inhibition was not accompanied by enhanced surface expression of MHC II (Figure 6C). The p100ΔN induction alone slightly enhanced costimulatory molecule expression (Figure 6A). However, the effect of p100ΔN expression alone on T-cell proliferation was not consistently observed in all experiments (Figure 6B, left panel). Inhibiting nuclear RelB substantially differed from inhibiting the classical NF-κB pathway. Whereas p100ΔN increased d.a.MKK6-induced CD86 and CD80 expression, coinduction of IκBα-SR had no effect on these 2 costimulatory molecules (Figure 6A, right panel). Furthermore, the inhibition of the classical NF-κB pathway did not increase d.a.MKK6-enhanced T-cell stimulation (Figure 6B, right panel) but rather promoted enhanced cell death of d.a.MKK6 expressing LCs (data not shown).
Diminished nuclear RelB during d.a.MKK6-induced maturation leads to enhanced DC activation. TA-transduced CD34+ cells were cotransduced with control vectors (ctrl), d.a.MKK6-NGFR + ctrl (MKK6), d.a.MKK6-NGFR + p100ΔN-GFP, ctrl + p100ΔN-GFP (p100ΔN), or d.a.MKK6-NGFR + IκB-SR-GFP. Genes were coinduced in day 8 LCs by DOX (2 μg/mL) for 48 hours. (A) GFP+NGFR+ cells were gated for CD1a expression and analyzed by FACS for the expression of CD86, CD80, CD83, and CD40. Bar diagrams depict mean percentages ± SD of 4 independent experiments (paired Student t test was performed; horizontal bars cluster the statistical comparisons, *P < .05, **P < .01). (B) CD1a+GFP+NGFR+ cells were sorted and subjected to an allo-MLR. Diagrams represent 1 representative experiment. (C) GFP+NGFR+ cells were gated for CD1a expression and analyzed by FACS for MHC II expression. Bar diagram depicts mean percentages ± SD of 4 independent experiments.
Diminished nuclear RelB during d.a.MKK6-induced maturation leads to enhanced DC activation. TA-transduced CD34+ cells were cotransduced with control vectors (ctrl), d.a.MKK6-NGFR + ctrl (MKK6), d.a.MKK6-NGFR + p100ΔN-GFP, ctrl + p100ΔN-GFP (p100ΔN), or d.a.MKK6-NGFR + IκB-SR-GFP. Genes were coinduced in day 8 LCs by DOX (2 μg/mL) for 48 hours. (A) GFP+NGFR+ cells were gated for CD1a expression and analyzed by FACS for the expression of CD86, CD80, CD83, and CD40. Bar diagrams depict mean percentages ± SD of 4 independent experiments (paired Student t test was performed; horizontal bars cluster the statistical comparisons, *P < .05, **P < .01). (B) CD1a+GFP+NGFR+ cells were sorted and subjected to an allo-MLR. Diagrams represent 1 representative experiment. (C) GFP+NGFR+ cells were gated for CD1a expression and analyzed by FACS for MHC II expression. Bar diagram depicts mean percentages ± SD of 4 independent experiments.
Significant enhancement of d.a.MKK6-induced CD86 and CD80 expression by p100ΔN
| Experiment* . | Ctrl . | MKK6 . | MKK6 + p100ΔN . | P‡ . | |
|---|---|---|---|---|---|
| Ctrl vs MKK6 . | MKK6 vs MKK6 + p100ΔN . | ||||
| % CD86†; | .006 | .033 | |||
| 1 | 36 | 83 | 90 | ||
| 2 | 5 | 70 | 85 | ||
| 3 | 1 | 43 | 54 | ||
| 4 | 11 | 45 | 70 | ||
| % CD80† | .026 | .014 | |||
| 1 | 27 | 71 | 88 | ||
| 2 | 5 | 72 | 88 | ||
| 3 | 2 | 41 | 54 | ||
| 4 | 31 | 49 | 78 | ||
| Experiment* . | Ctrl . | MKK6 . | MKK6 + p100ΔN . | P‡ . | |
|---|---|---|---|---|---|
| Ctrl vs MKK6 . | MKK6 vs MKK6 + p100ΔN . | ||||
| % CD86†; | .006 | .033 | |||
| 1 | 36 | 83 | 90 | ||
| 2 | 5 | 70 | 85 | ||
| 3 | 1 | 43 | 54 | ||
| 4 | 11 | 45 | 70 | ||
| % CD80† | .026 | .014 | |||
| 1 | 27 | 71 | 88 | ||
| 2 | 5 | 72 | 88 | ||
| 3 | 2 | 41 | 54 | ||
| 4 | 31 | 49 | 78 | ||
CD34+ cells from 4 individual cord-blood donor samples (experiments 1 to 4).
Percentages of gated CD1a+GFP+NGFR+ LCs transduced with control vectors (ctrl), d.a.MKK6 + ctrl (MKK6), or d.a.MKK6 + p100ΔN positive for CD86 or CD80 expression.
The paired t test was performed for statistical analysis of CD86 or CD80 expression.
Negative regulatory role of RelB in LC activation induced by physiologic innate stress signals
To determine whether the role of RelB as counterregulator in d.a.MKK6-induced LC activation is of physiologic relevance, we inhibited nuclear RelB during diverse LC activation stimuli. The potentiated T-cell stimulation of activated LCs expressing p100ΔN could also clearly be observed when ribotoxic stress by anisomycin instead of d.a.MKK6 was induced (Figure 7A). Anisomycin is a commonly used strong p38 MAPK activator38 leading to p38 phosphorylation in primary LCs (Figure 7B). However, NF-κB was not activated by anisomycin, because no GFP induction could be observed in U937 NF-κB–GFP reporter cells (Figure 7B). Moreover, nuclear RelB inhibition in LCs matured with the hapten DNFB similarly resulted in an increase in T-cell stimulatory capacity, although this effect was not as strong and consistent as observed with d.a.MKK6 or anisomycin (data not shown). Neither LPS nor LPS and IL-1β administration could enhance the T-cell stimulatory capacity of LCs (Figure 7C). More importantly, T-cell proliferation could not be amplified by nuclear RelB inhibition during these 2 stimuli (Figure 7C) or by CD40 ligation (data not shown), although both LPS and CD40L induced translocation of RelB to the nucleus of primary LCs (Figure 7D).
Anisomycin-induced T-cell stimulatory capacity of LCs is also enhanced by nuclear RelB inhibition. (A) (Left) Empty vector controls (ctrl), d.a.MKK6-NGFR + HR-GFP (MKK6), HR-NGFR + p100ΔN-GFP (p100ΔN), or d.a.MKK6-NGFR + p100ΔN-GFP (MKK6+p100ΔN) were coinduced in immature LCs by DOX addition. CD1a+NGFR+GFP+ LCs were sorted, and an allo-MLR was performed. (Right) HR-GFP control vector (ctrl) or p100ΔN-GFP (p100ΔN) was induced by DOX either in the presence or absence of 40 ng/mL anisomycin. CD1a+GFP+ LCs were sorted and subjected to an allo-MLR. Diagrams represent 1 representative experiment. (B) Representative Western blot analysis for phosphorylated or total p38 MAPK of total-cell lysates from immature LCs stimulated with 40 ng/mL anisomycin for the indicated time points. The representative histogram overlay shows GFP induction (NF-κB activity) analyzed by FACS. U937 NF-κB–GFP reporter cells were left untreated or stimulated with 40 ng/mL anisomycin or 2.5 ng/mL TNFα for 24 hours. (C) Control vector (ctrl) or p100ΔN-GFP (p100ΔN) was induced in LCs either in the absence or presence of 1 μg/mL LPS or LPS + 20 ng/mL IL-1β. CD1a+GFP+ LCs were sorted, and allo-MLRs were performed. In parallel, CD1a+GFP+NGFR+ LCs induced to coexpress control vectors (ctrl), d.a.MKK6 + control (MKK6), MKK6 + p100ΔN, or p100ΔN + control (p100ΔN) were subjected to an allo-MLR. (D) Representative Western blot analysis of nuclear and cytosolic extracts from day 8 primary immature LCs either left unstimulated (n.s.), stimulated with 1 μg/mL LPS, or stimulated with 500 ng/mL CD40L for 48 hours. Extracts were probed with anti-RelB and antiactin antibodies.
Anisomycin-induced T-cell stimulatory capacity of LCs is also enhanced by nuclear RelB inhibition. (A) (Left) Empty vector controls (ctrl), d.a.MKK6-NGFR + HR-GFP (MKK6), HR-NGFR + p100ΔN-GFP (p100ΔN), or d.a.MKK6-NGFR + p100ΔN-GFP (MKK6+p100ΔN) were coinduced in immature LCs by DOX addition. CD1a+NGFR+GFP+ LCs were sorted, and an allo-MLR was performed. (Right) HR-GFP control vector (ctrl) or p100ΔN-GFP (p100ΔN) was induced by DOX either in the presence or absence of 40 ng/mL anisomycin. CD1a+GFP+ LCs were sorted and subjected to an allo-MLR. Diagrams represent 1 representative experiment. (B) Representative Western blot analysis for phosphorylated or total p38 MAPK of total-cell lysates from immature LCs stimulated with 40 ng/mL anisomycin for the indicated time points. The representative histogram overlay shows GFP induction (NF-κB activity) analyzed by FACS. U937 NF-κB–GFP reporter cells were left untreated or stimulated with 40 ng/mL anisomycin or 2.5 ng/mL TNFα for 24 hours. (C) Control vector (ctrl) or p100ΔN-GFP (p100ΔN) was induced in LCs either in the absence or presence of 1 μg/mL LPS or LPS + 20 ng/mL IL-1β. CD1a+GFP+ LCs were sorted, and allo-MLRs were performed. In parallel, CD1a+GFP+NGFR+ LCs induced to coexpress control vectors (ctrl), d.a.MKK6 + control (MKK6), MKK6 + p100ΔN, or p100ΔN + control (p100ΔN) were subjected to an allo-MLR. (D) Representative Western blot analysis of nuclear and cytosolic extracts from day 8 primary immature LCs either left unstimulated (n.s.), stimulated with 1 μg/mL LPS, or stimulated with 500 ng/mL CD40L for 48 hours. Extracts were probed with anti-RelB and antiactin antibodies.
Discussion
NF-κB and p38 are involved in or required for DC maturation. However, it remained unknown for which aspects of LC activation the p38 stress response pathway is responsible. For addressing this question, we developed a system allowing us to conditionally activate candidate signaling proteins in physically unmanipulated immature human LCs.
We demonstrated that p38 MAPK activation by conditional expression of d.a.MKK6 induces UCM and potent T-cell stimulatory capacity of immature LCs. Furthermore, d.a.MKK6 induced the secretion of IL-1β and IL-6, whereas other cytokines, such as TNFα or IL-12 p70, were not produced. Moreover, d.a.MKK6 induced RelB. Preventing nuclear translocation of RelB by coinduced p100ΔN in LCs showed that RelB counterregulates rather than mediates d.a.MKK6-induced immunostimulation and UCM. This negative regulatory role of RelB was confirmed in ribotoxic stress-induced LC activation. Therefore, our data show that (1) MKK6 clearly induces LC activation and that (2) upon DC maturation stimuli that preferentially activate p38, RelB rather counteracts than promotes LC activation.
The herein applied Tet-ON system will be a useful tool to investigate controlled induction of gene expression in human and murine primary cells of different lineages. The advantages of this system are the undetectable background levels of gene expression in the absence of DOX and the use of FACS-assayable marker molecules. Additionally, the gene of interest is synthesized de novo upon DOX addition, thereby also allowing tightly controlled expression of cytosolic regulatory proteins.
DOX-induced d.a.MKK6 expression resulted in the up-regulation of DC costimulatory molecules without a requirement for classical NF-κB signaling. Until now all studies dealing with the role of p38 during DC activation used pharmacologic inhibitors. We rationalized that the selective triggering of p38 in LCs would provide novel and complementary information on its involvement in DC maturation. Because various signals activate p38 (eg, UV light, haptens, TLR ligation), our data suggest that triggering the MKK6/p38 pathway is a general mechanism of LC activation.
LPS has little effect on UCM induction of primary LCs. One reason may be the strength and kinetics of p38 activation, because p38 phosphorylation is only transiently induced by LPS (data not shown). In general, LCs are thought to be poor responders to LPS. This might at least partially relate to the fact that the LC phenotype in vivo and in vitro is highly dependent on TGF-β1.39,40 TGF-β1 was shown to inhibit LC activation in response to noncognate stimuli such as LPS, TNFα, and IL-1β.41 Additionally, it has been demonstrated that immature human LCs generated in vitro in the presence of TGF-β1 or human LCs freshly isolated from epidermis express either no42,43 or intermediate44 levels of TLR4. Interestingly, we show in our experiments that d.a.MKK6-induced UCM is not inhibited by IκB-SR. It will be interesting to analyze whether constitutive active TGF-β1 signaling might render LCs (but not other DC subtypes) refractory to signaling via NF-κB–dependent pathways.
Our demonstration that d.a.MKK6-induced LC activation is independent of the classical NF-κB pathway is in line with studies showing that p38 but not NF-κB inhibitors abrogate DC maturation under certain activation conditions (such as mo-DC maturation by haptens).21 Furthermore, this concept might generally apply also to PAMP-dependent signals because triggering of TLR4 via a ROS-dependent activation of the MAPKKK ASK1, one of several upstream kinases of MKK6, is exclusively dependent on p38 activation but independent of NF-κB.45
A key finding of our study is that inhibition of nuclear shuttling of RelB in LCs further enhances their T-cell stimulatory capacity upon d.a.MKK6 induction. RelB is translocated to the nuclei of DCs by diverse DC activation signals.11,33 However, its function in DC maturation remained unclear. We inhibited nuclear RelB directly during the maturation process of LCs by inducing p100ΔN, thus ruling out RelB effects on DC development. These experiments revealed that inhibition of nuclear RelB amplifies the T-cell stimulatory capacity of d.a.MKK6-matured LCs and also of LCs activated by anisomycin. Anisomycin, a commonly used strong p38 MAPK activator,38 has previously been used to mimic the ribotoxic stress response, which can result from viral infection of DCs.46 Anisomycin-induced p38 activation is thought to be reactive oxygen species (ROS) dependent.47 Interestingly, anisomycin does not activate classical NF-κB (Figure 7C), thus supporting our concept of NF-κB–independent DC activation. The effect of p100ΔN expression on T-cell stimulation was not observed during LPS- or CD40L-induced LC activation. Although all of these diverse DC activation stimuli lead to p38 activation, they differ in the coactivation of other signaling events (eg, classical NF-κB activation or ROS production). Notably, RelB was recently shown to play a transcriptional role in the activation of genes involved in the protection against oxidative stress.48 Future studies should evaluate what intracellular events define whether RelB counterregulates LC activation or not and whether changes in autocrine cytokine production play a role.
LCs undergo spontaneous maturation in vitro in serumfree medium even in the presence of TGF-β1. We observed that p100ΔN alone slightly but consistently enhanced UCM by LCs. This suggests that RelB counterregulates spontaneous maturation.
Supporting our data, evidence has been presented that RelB rather represents a negative regulator of immune responses. First, RelB-containing dimers gradually replace p65-containing dimers during mo-DC maturation. This dimer exchange was correlated with transcriptional down-regulation of certain proinflammatory genes.49 Second, in vivo observations in dominant negative IKKα transgenic mice support a negative regulatory function of RelB in macrophages during inflammation.50 Furthermore, RelB-deficient fibroblasts show enhanced production of proinflammatory mediators.51 We propose that under certain LC activation conditions the regulatory role of nuclear RelB might be to counteract prolonged LC activation. Interestingly, such a scenario might be compatible with the observation that RelB-deficient mice develop T-cell–dependent skin lesions similar to human atopic dermatitis.52 However, alternative interpretations of our data exist. It must be considered that d.a.MKK6 induces excessive RelB, and this might be counterregulated by p100ΔN to levels that might promote LC maturation. Further studies on controlled nuclear RelB inhibition during LC activation in vivo are required to elucidate this RelB-dependent control mechanism.
We found that d.a.MKK6 strongly induces IL-1β production by LCs, an effect not observed upon LPS or CD40L stimulation. This observation might be due to sustained p38 MAPK activation resulting from d.a.MKK6, which is in contrast to a transient p38 activation obtained by LPS (data not shown). This is reminiscent of a recent study demonstrating that IL-1β production only occurs upon synergistic activation of at least 2 different TLRs, possibly due to sustained signaling resulting from TLR synergism.53 In line with our observations, the authors found no IL-1β production with LPS alone.53 The secretion of IL-1β is a tightly regulated process. Activation of the proinflammatory caspase-1 via the inflammasome leads to the processing of pro–IL-1β, finally resulting in the secretion of IL-1β.54 How d.a.MKK6 leads to the activation of caspase-1 and to IL-1β secretion remains to be investigated. Additionally, up-regulation of pro–IL-1β in the cell was previously shown to depend on NF-κB or p38 MAPK activity.55,56 Furthermore, it also must be taken into account that d.a.MKK6-induced IL-1β might further enhance d.a.MKK6 effects, because IL-1β stimulation of LCs itself resulted in minor UCM (data not shown).
In conclusion, our data identified that d.a.MKK6 expression strongly induces LC activation. We propose that d.a.MKK6/p38 activation is a general trigger of DC activation. This together with our observations that MKK6/p38-induced LC activation occurs independently of classical NF-κB, and that RelB plays a previously unrecognized negative regulatory role upon specific LC activation stimuli, may provide novel information for therapeutic strategies to enhance or diminish DC-based immune responses.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Contribution: A.J. designed and performed research and wrote the paper; B.P., S.T., L.X.H., B.H., P.M.R., and F.G. contributed reagents and analytic tools; and H.S. designed research and wrote the paper.
Acknowledgments
This work was supported by grants START-Y156 and SFB-F2304 from the Austrian Science Fund (Fonds zur Förderung der wissenschaftlichen Forschung [FWF]) and grant 10294 from the Austrian National Bank. We thank W. Ellmeier, J. Stockl, J.-F. Fortin, and G. Zlabinger for critically reading the manuscript and for helpful discussions. Furthermore, we thank all the collaborating nurses and doctors from the obstetric departments at Lainz Hospital and Kaiser Franz Josef Hospital, Vienna, for providing cord-blood samples.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal