Abstract
The JAK2V617F mutation has been shown to occur in the overwhelming majority of patients with polycythemia vera (PV). To study the role of the mutation in the excessive production of differentiated hematopoietic cells in PV, CD19+, CD3+, CD34+, CD33+, and glycophorin A+ cells and granulocytes were isolated from the peripheral blood (PB) of 8 patients with PV and 3 healthy donors mobilized with G-CSF, and the percentage of JAK2V617F mutant allele was determined by quantitative real-time polymerase chain reaction (PCR). The JAK2V617F mutation was present in cells belonging to each of the myeloid lineages and was also present in B and T lymphocytes in a subpopulation of patients with PV. The proportion of hematopoietic cells expressing the JAK2V617F mutation decreased after differentiation of CD34+ cells in vitro in the presence of optimal concentrations of SCF, IL-3, IL-6, and Epo. These data suggest that the JAK2V617F mutation may not provide a proliferative and/or survival advantage for the abnormal PV clone. Although the JAK2V617F mutation plays an important role in the biologic origins of PV, it is likely not the sole event leading to PV.
Introduction
The Philadelphia chromosome negative (Ph–) myeloproliferative disorders (MPDs) include polycythemia vera (PV), essential thrombocythemia (ET), and chronic idiopathic myelofibrosis (CIMF).1-5 These Ph– MPDs are thought to originate at the level of the pluripotent hematopoietic stem cell (HSC).1-5 Recently, there have been several reports identifying a recurrent mutation in the JH2 pseudo kinase domain of the cytoplasmic tyrosine kinase-Janus kinase (JAK2) gene in the hematopoietic cells of patients with Ph– MPDs.6-10 The somatic mutation, a valine-to-phenylalanine substitution at amino acid position 617 of JAK2 (JAK2V617F) has been found in more than 80% of patients with PV, and 50% of patients with ET and CIMF.6-10
JAK2V617F has been shown to be a gain-of-function mutation.6-10 The mutation leads to constitutive tyrosine phosphorylation of JAK2 and phosphorylation of downstream effectors STAT5 and ERK.6-10 Erythroid precursors possessing the mutation are hypersensitive to erythropoietin (Epo).7-10 The formation of Epo-independent colonies, which characterizes PV, has been shown to be associated with hematopoietic cells harboring this mutation.6,7,11 In addition, the mutation is sufficient to produce an erythrocytosis resembling PV in mice that had received transplants of murine bone marrow (BM) cells transduced with a retrovirus encoding JAK2V617F.7
The Ph– MPDs are clonal HSC diseases likely involving both myeloid and lymphoid lineages.1,2,12-27 Using glucose-6-phosphate dehydrogenase isoenzyme analyses, Fialkow et al,13 Adamson et al,14 Fialkow et al,15 Jacobson et al,16 and Fialkow et al17 have shown that the chronic MPDs are biologically interrelated clonal HSC disorders which involve each of the myeloid-cell types (granulocytes, erythrocytes, platelets, monocytes). Using similar assays for cell clonality, some investigators have reported that B18-20 or T lymphocytes21-23 can also be involved in the malignant process in some patients with PV, ET, and CIMF. Some investigators, however, have presented data that indicate that T cells are polyclonal in PV in contrast to the cells belonging to the various myeloid lineages.24,25 Using different assays of clonality, some studies have suggested monoclonality of T lymphocytes in CIMF (based on Ras mutational analysis and fluorescent in situ hybridization analysis).26,27 Using 9p, 10q, and 11q LOH as clonality markers, Kralovics et al28 have, however, shown that a minor proportion of T cells may be generated from the PV clone in some patients with PV. Recently, several groups have reported, in sporadic cases, the absence of the JAK2 mutation in T cells, B cells, and nonhematopoietic tissues, indicating that JAK2 mutation is an acquired event that is restricted to cells belonging to myeloid lineages.6,7,29 Several recent reports have shown that the JAK2V617F mutation induces a proliferative advantage in various immortalized cell lines.7-9 Tefferi et al30 have reported a time-dependent increase of JAK2V617F mutation in the granulocytes of some patients with PV. These results suggest that the JAK2V617F mutation might contribute to the growth and/or survival advantage of cells belonging to the abnormal clone in Ph– MPDs.
Although the currently available data clearly suggest that the JAK2V617F mutation likely participates in the pathogenesis of the Ph– MPDs, the exact role of activated JAK2 signaling in the pathobiology of these MPDs remains unknown. In addition, the location along the hematopoietic hierarchy of the genetic event leading to the mutation remains to be established.
The pluripotent HSC, a rare cell in the BM, is ultimately responsible for the lifelong generation of blood cells.31,32 The differentiation of HSCs into the various hematopoietic lineages is thought to be a stepwise process involving progenitor cells having a gradually more restricted developmental and proliferative potential. The identification of a common lymphoid progenitor cell and a common myeloid progenitor cell in the BM has provided strong supporting evidence for such a stepwise differentiation schema.33-35 As originally defined, a common lymphoid progenitor cell could give rise to T, B, and natural killer (NK) but not myeloid cells, whereas a common myeloid progenitor cell could generate myeloid but not lymphoid cells.33-35 Recently, Hou et al36 have identified a human B cell/myeloid common progenitor cell based on the absence of CXCR4 expression. These progenitors are capable of producing B cells and differentiating into multiple myeloid lineages.36 It is, however, thought that bi-lineage differentiation is infrequent once the HSC differentiates into a specific lymphoid or myeloid progenitor cell.37,38
In this study, we demonstrate that the JAK2V617F mutation is specific to the cells belonging to the various myeloid lineages in the majority of patients with PV, but that it is also present in B lymphocytes or B and T lymphocytes in a subpopulation of patients with PV. In addition, we have demonstrated that the proportion of hematopoietic cells expressing the JAK2V617F mutation decreases after differentiation of PV peripheral-blood (PB) CD34+ cells in vitro in the presence of optimal concentrations of SCF, IL-3, IL-6, and Epo, and that this reduction of JAK2V617F cells is dependent on the presence of Epo. These data suggest that the acquired JAK2 mutation is likely not the sole event leading to PV.
Patients, materials, and methods
Patients and healthy donors
All human tissue samples were obtained after informed consent was provided according to the guidelines of the Institutional Review Board of the University of Illinois College of Medicine. Mobilized PB samples were obtained from healthy donors mobilized with G-CSF (Amgen, Thousand Oaks, CA, at 5 μg/kg/day subcutaneously for 5 days). All patients met the WHO diagnostic criteria for PV and were treated with phlebotomy only.1,3,4 Phlebotomized units of blood (400-600 mL) were obtained from each of the PV patients.
Purification of human PB CD19+, CD3+, CD34+, CD33+, and glycophorin A+ cells and granulocytes
The PB was layered onto Ficoll-Hypaque (1.077 g/mL) (Amersham Biosciences, Piscataway, NJ), and low-density mononuclear cells (MNCs) were separated after centrifugation. Granulocytes were isolated by standard techniques as previously described.39 Populations of CD34+, CD33+, CD19+, CD3+, and glycophorin A+ cells were isolated from the MNCs using a magnetic-activated cell-isolation kit (Miltenyi Biotec, Auburn, CA) according to the manufacturer's instructions. The MNCs were assayed for the percentage of CD34+ cell numbers flow cytometrically as previously described.40 Because patients with PV constitutively mobilize CD34+ cells into the PB to a moderate degree,40 we were able to isolate 2 to 6 × 106 CD34+ cells from a single phlebotomy unit. Magnetically isolated CD34+, CD33+, and glycophorin A+ cells with a purity of 95% or greater were used for subsequent experiments, including genomic DNA (gDNA) and total RNA extractions and in vitro differentiation assays. The magnetically isolated CD19+ and CD3+ cell populations were further stained with PE-conjugated anti–human CD19 and CD3 monoclonal antibodies (mAbs), respectively, and sorted using a FACSVantage (fluorescence activated cell sorter; Becton Dickinson, Mountain View, CA). Using this combination of immunomagnetic cell separation and FACS techniques, each of the CD19+ and CD3+ cell populations had a purity of 99% or greater. These cells were then used for gDNA and total RNA extractions.
PCR sequencing analysis and real-time quantitative PCR assay using the allelic discrimination method
gDNA was extracted from granulocytes, CD19+, CD3+, CD34+, CD33+, and glycophorin A+ cells using Easy-DNA Kit (Invitrogen, Carlsbad, CA). Total RNA was isolated from CD34+ and CD33+ cells using the Micro-to-Midi Total RNA Purification System (Invitrogen). The DNA and RNA concentrations of each sample were measured using a spectrophotometer (SmartSpec Plus; Bio-Rad, Hercules, CA). First-strand complimentary DNAs (cDNAs) were prepared by reverse transcription of total RNAs with random primers using SuperScript III First-Strand Synthesis System (Invitrogen). For amplification of the JAK2 gDNA, the polymerase chain reaction (PCR) primers 5′-GATCTCCATATTCCAGGCTTACACA-3′ and 5′-TATTGTTTGGGCATTGTAACCTTCT-3′, which cover exon 12 of the JAK2 gene and give rise to a PCR product of 520 bp (base pair) were used. For amplification of the JAK2 cDNAcontaining the reported mutation point, the primers used were 5′-AGAAGTAGGAGACTACGGTCAA-3′ and 5′-CACTAAGTTTGATGAAAGGAGGATT-3′, which give rise to a PCR product of 401 bp. PCR was performed with DNApolymerase iTaq (Bio-Rad). The PCR products were purified from agarose gels and sequenced directly using the ABI 3730xl DNA analyzer (Applied Biosystems, Foster City, CA) at the Core DNA Sequencing Facility of the University of Illinois.
gDNA and cDNA from each cell population were also used for the determination of the percentage of JAK2V617F/JAK2total by real-time quantitative kinetic PCR assay using the allelic discrimination method.41 The primers used for gDNA were 5′-TTCTTTGAAGCAGCAAGTATGATGA-3′ and 5′-GGCATTAGAAAGCCTGTAGTTTTAC-3′; the primers used for cDNA were 5′-TTTCTTTGAAGCAGCAAGTATGATG-3′ and 5′-CCAAATTTTACAAACTCCTGAACCA-3′. The probe sequences for both gDNA and cDNA were as follows: allele G probe, [FAM]5′-CGTCTCCACAGACAC-3′[DBH1]; allele T probe, JOE-CGTCTCCACAGAAAC-3′[DBH1]. All PCR primers and probes were obtained from Sigma (St Louis, MO). Real-time PCR amplification and allele detection were performed with an ABI Prism 7900 Sequence Detection System (Applied Biosystems) using the Taqman PCR Core Reagents Kit (Applied Biosystems). A 25-μL reaction was used which contained 300 nM each of the primers, 200 nM of either of the allele-specific probes, and 120 ng gDNA or 5 ng cDNA template. The general amplification conditions included an initial incubation step of 2 minutes at 50°C, an enzyme heat activation step of 10 minutes at 95°C, followed by 45 cycles of 15 seconds at 95°C for denaturation and 1 minute at 59.5°C for annealing and extension.
To generate a standard curve for the percentage of JAK2V617F/JAK2total against ΔCt (CtJAK2V617F – CtJAK2WT), different amounts of gDNA or cDNA from known homozygous samples for the 2 alleles were mixed.41,42 In this way, we created pools for gDNA or cDNA with JAK2V617F allele frequencies of 0%, 1%, 5%, 10%, 25%, 50%, 75%, 90%, 95%, and 100%. All samples were quantified in triplicate for each specific allele, and the mean ΔCt was used to calculate JAK2V617F/JAK2total according to the standard curve using Excel software (Microsoft, Redmond, WA).
In vitro CD34+ cell-differentiation assay
Purified CD34+ cells were incubated in suspension cultures using standard techniques.43 Two milliliters of culture mixture containing 1 × 105 CD34+ cells, Iscove modified Dulbecco medium (IMDM), 20% fetal bovine serum (FBS), 1% bovine serum albumin (BSA), 0.01 mM 2-mercaptoethanol (StemCell Technologies, Vancouver, Canada), and a cytokine cocktail (SCF 100 ng/mL, IL-3 20 ng/mL, and IL-6 100 ng/mL) plus various concentrations of Epo (0 U/mL, 0.025 U/mL, 0.1 U/mL, 0.5 U/mL, or 4 U/mL) were incubated in 24-well tissue plates at 37°C in 5% CO2. All the cytokines were obtained from Amgen. After 6 days of incubation, the cultures were demi-depopulated by the removal of half the culture volume, which was then replaced by newly prepared medium containing the same combination of cytokines. The cultures were continued for 6 additional days, and cells were collected by centrifugation. Total RNA was extracted from the cells harvested at days 6 and 12. Real-time PCR analysis was then performed using methods described in “PCR sequencing analysis and real-time quantitative PCR assay using the allelic discrimination method.” Cytospin preparations of the cells were also prepared and stained with May-Grünwald-Giemsa. A 200 manual cell differential was performed using standard morphologic criteria.
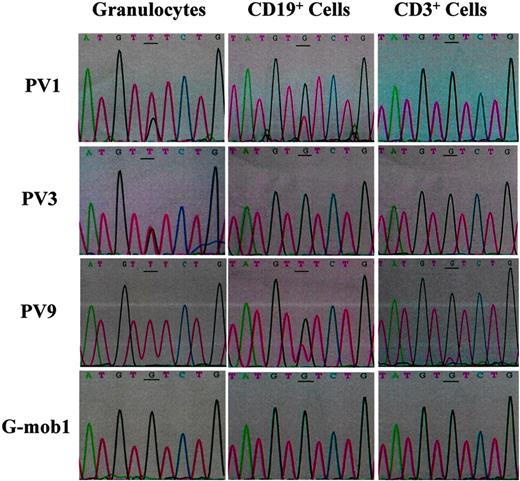
Sequencing of JAK2 PCR products from gDNAs (from 5 directions) of granulocytes, CD19+ and CD3+ cells purified from the PB of PV1, PV3, PV9, and a G-CSF–mobilized healthy volunteer. The mutational point is underlined. Mob indicates G-CSF–mobilized healthy donors.
Sequencing of JAK2 PCR products from gDNAs (from 5 directions) of granulocytes, CD19+ and CD3+ cells purified from the PB of PV1, PV3, PV9, and a G-CSF–mobilized healthy volunteer. The mutational point is underlined. Mob indicates G-CSF–mobilized healthy donors.
Statistical analysis
The data were obtained by examining 8 patients with PV and 3 healthy control subjects. Two-tailed Student t test (Excel software; Microsoft) was used to analyze statistical differences in the percentages of the mutation of different cell populations in PV patients and PV CD34+ cells versus their differentiated progenies. Statistical significance was assumed for P values less than .05.
Results
JAK2V617F mutation is not a myeloid lineage-specific mutation, but is also present in T and B cells in some patients with PV
PV is believed to result from the transformation of a multipotent HSC.1,2,12-27 However, several groups have reported the absence of the JAK2V617F mutation in T cells and B cells of patients with PV, indicating that JAK2V617F mutation is an acquired myeloid lineage–specific mutation.6,7,29 We attempted to determine whether the JAK2 mutation was present in lymphoid cells of patients with PV. gDNA was extracted from highly purified CD19+ B cells and CD3+ T cells (≥ 99%, using the combination of immunomagnetic cell separation and FACS sorting) as well as granulocytes from the PB of 8 patients with PV and 3 G-CSF–mobilized healthy donors, and the JAK2V617F mutational status was analyzed by PCR/direct sequencing. Sequences of the granulocyte gDNA demonstrated that all of the 8 patients with PV had the JAK2V617F mutation, whereas the 3 healthy donors did not contain the mutation (Figure 1; Table 1). The granulocytes of PV9 displayed a homozygous G-T substitution pattern, suggesting that PV9 is homozygous for the mutant allele. The granulocytes of the other 7 patients with PV showed heterozygous G-T substitutions at varying degrees (Figure 1; Table 1). Surprisingly, the sequences of gDNAs from CD19+ B cells of PV1 and PV9 as well as CD3+ T cells of PV9 showed heterozygous G-T substitutions (Figure 1), indicating that the JAK2V617F mutation was also present in these cell populations. Sequencing analysis revealed no G-T substitution in the gDNA from CD19+ or CD3+ cell populations other than PV1 CD19+, PV9 CD19+, and PV9 CD3+ cells, suggesting that these cell populations were not involved in this mutation.
Detection of the JAK2V617F mutation in gDNA of various hematopoietic lineages isolated from the PB of patients with PV and healthy volunteers by direct sequencing
Donors* . | Granulocytes . | CD34+ cells . | CD33+ cells . | Gly-A+ cells . | CD19+ cells . | CD3+ cells . |
|---|---|---|---|---|---|---|
| PV1 | + | + | + | + | + | - |
| PV2 | + | + | + | + | - | - |
| PV3 | + | + | + | + | - | - |
| PV4 | + | + | + | + | - | - |
| PV7 | + | + | + | + | - | - |
| PV8 | + | + | + | + | - | - |
| PV9 | ++ | ++ | ++ | ++ | + | + |
| PV10 | + | + | + | + | - | - |
Donors* . | Granulocytes . | CD34+ cells . | CD33+ cells . | Gly-A+ cells . | CD19+ cells . | CD3+ cells . |
|---|---|---|---|---|---|---|
| PV1 | + | + | + | + | + | - |
| PV2 | + | + | + | + | - | - |
| PV3 | + | + | + | + | - | - |
| PV4 | + | + | + | + | - | - |
| PV7 | + | + | + | + | - | - |
| PV8 | + | + | + | + | - | - |
| PV9 | ++ | ++ | ++ | ++ | + | + |
| PV10 | + | + | + | + | - | - |
The purity of the CD19+ cells and CD3+ cells was 99% or greater. The purity of the other cell types was 95% or greater.
- indicates no detectable G-T substitution in the sequence; +, heterozygous G-T substitution; ++, homozygous G-T substitution; Mob, G-CSF mobilized healthy subject.
Mob1, Mob2, and Mob3 had no detectable G-T substitution in the sequence.
The presence of the JAK2V617F mutation in CD34+ cells, CD33+ cells, and glycophorin A+ cells was also examined similarly. As can be seen in Table 1, the JAK2V617F mutation was present in each of the CD34+, CD33+, and glycophorin A+ cell populations from the 8 patients with PV. Again, there was no detectable mutation in any of the corresponding cell populations isolated from the healthy control subjects (Table 1).
These results indicate that the JAK2V617F mutation is not only a myeloid lineage–specific mutation, but that it is also present in T and B lymphocytes in a subpopulation of patients with PV. The presence of JAK2V617F mutation in cells belonging to both myeloid and lymphoid lineages in PV9 indicates that the mutation likely originates at the level of a cell capable of generating both lymphoid and myeloid cells. In PV1, the JAK2V617F mutation was present in both B cells and cells belonging to the myeloid lineages, but not T cells, indicating the occurrence of the mutation in a common myeloid–B-precursor cell. Such primitive progenitor cells have been described.35 Because the JAK2V617F mutation involves only myeloid lineages in PV2, PV3, PV4, PV7, PV8, and PV10, the mutation in this group likely occurs in a myeloid-specific progenitor cell.
Quantitative assessment of the JAK2V617Fmutation in various hematopoietic lineages
We then examined the same specimens using a real-time quantitative kinetic PCR assay41 by an allelic discrimination method to determine the percentage of JAK2V617F/JAK2total in each cell population. The standard curve for the percentage of JAK2V617F/JAK2total against ΔCt was generated using gDNA mixtures from known homozygous samples for the 2 alleles. The percentage of JAK2V617F/JAK2total in each sample was calculated according to the mean ΔCt and the standard curve.
There was no detectable CtJAK2V617F in any of the cell populations isolated from healthy donors, confirming their JAK2V617F allele frequencies of 0%. The CtJAK2WT was not detectable in the granulocytes and CD33+ cells of PV9, indicating that the JAK2V617F allele frequencies were 100%. As can be seen in Table 2, the percentage of JAK2V617F/JAK2total in the gDNA of PV1 CD19+, PV9 CD19+, and PV9 CD3+ cells were 32.8%, 34.7%, and 7.6%, respectively, confirming the presence of the JAK2V617F mutation in these lymphoid-cell populations as determined by PCR/direct sequencing.
Percentage of JAK2V617F/JAK2total in the gDNA of various hematopoietic lineages isolated from patients with PV and healthy volunteers determined by real-time PCR
Donors* . | Granulocytes, % . | CD34+ cells, % . | CD33+ cells, % . | Gly-A+ cells, % . | CD19+ cells, % . | CD3+ cells, % . |
|---|---|---|---|---|---|---|
| PV1 | 73.0 | 68.9 | 50.2 | 17.8 | 32.8 | 0 |
| PV2 | 8.7 | 12.3 | 6.3 | 1.3 | 0 | 0 |
| PV3 | 64.6 | 67.5 | 34.0 | 25.9 | 0 | 0 |
| PV4 | 21.5 | 33.8 | 20.4 | 27.9 | 0 | 0 |
| PV7 | 37.2 | 38.9 | 33.5 | 48.4 | 0 | 0 |
| PV8 | 49.6 | 46.2 | 24.8 | 43.3 | 0 | 0 |
| PV9 | 100 | 98.2 | 100 | 97.2 | 34.7 | 7.6 |
| PV10 | 64.3 | 48.7 | 51.1 | 46.0 | 0 | 0 |
Donors* . | Granulocytes, % . | CD34+ cells, % . | CD33+ cells, % . | Gly-A+ cells, % . | CD19+ cells, % . | CD3+ cells, % . |
|---|---|---|---|---|---|---|
| PV1 | 73.0 | 68.9 | 50.2 | 17.8 | 32.8 | 0 |
| PV2 | 8.7 | 12.3 | 6.3 | 1.3 | 0 | 0 |
| PV3 | 64.6 | 67.5 | 34.0 | 25.9 | 0 | 0 |
| PV4 | 21.5 | 33.8 | 20.4 | 27.9 | 0 | 0 |
| PV7 | 37.2 | 38.9 | 33.5 | 48.4 | 0 | 0 |
| PV8 | 49.6 | 46.2 | 24.8 | 43.3 | 0 | 0 |
| PV9 | 100 | 98.2 | 100 | 97.2 | 34.7 | 7.6 |
| PV10 | 64.3 | 48.7 | 51.1 | 46.0 | 0 | 0 |
The purity of the CD19+ cells and CD3+ cells was 99% or greater. The purity of the other cell types was 95% or greater. Values are JAK2V617F mutant allele/JAK2 total allele × 100. JAK2V617F/JAK2total was calculated according to the mean ΔCt and the standard curve by real-time quantitative PCR.
The G-CSF—mobilized healthy subjects 1, 2, and 3 had 0% of JAK2V617F/JAK2total in the gDNA of various hematopoietic lineages.
The percentage of JAK2V617F/JAK2total was less than 50% in each of the cell populations of PV2, PV4, PV7, and PV8, including granulocytes, CD34+, CD33+, and glycophorin A+ cells, suggesting that these patients were likely heterozygous for the mutation and mixed with wild-type cells. The percentage of JAK2V617F/JAK2total was greater than 50% in most of the cell populations isolated from PV1, PV3, PV9, and PV10, including granulocytes, CD34+ and CD33+ cells. These findings could be accounted for by these patients being homozygous for the mutation and by a mixture of wild-type cells or heterozygous cells being present in the PB.
When the percentage of JAK2V617F/JAK2total in CD34+ cells was compared with cells belonging to various cell lineages, the percentage of JAK2V617F/JAK2total in the CD34+ cells was significantly greater than that in the CD33+ cells (P < .05) but not granulocytes (P > .05). There was a modest decrease in the mutant allele frequency in the glycophorin A+ cells as compared with the CD34+ cells, which did not reach statistical significance (Table 2). In PV1 and PV9, the percentage of JAK2V617F/JAK2total in CD34+ cells was also greater than that in CD19+ cells and CD3+ cells.
We then attempted to examine whether PV CD34+ cells also contain a higher percentage of the JAK2V617F/JAK2total in mRNA as compared with CD33+ cells. Real-time quantitative PCR was performed, using the cDNA of the CD34+ and CD33+ cells isolated from the 8 patients with PV and 3 healthy donors, and the percentage of JAK2V617F/JAK2total in each of the cDNA was calculated according to the mean ΔCt and a standard curve. Similar to the observation in gDNA, the percentage of JAK2V617F/JAK2total in cDNA of the CD34+ cells was greater than that in CD33+ cells (P < .05) (Table 3).
Percentage of JAK2V617F/JAK2total in the cDNA of CD34+ cells and CD33+ cells isolated from the PB of patients with PV and healthy subjects mobilized with G-CSF
Cells . | PV1 . | PV2 . | PV3 . | PV4 . | PV7 . | PV8 . | PV9 . | PV10 . |
|---|---|---|---|---|---|---|---|---|
| CD34+, % | 85.0 | 34.6 | 98.7 | 71.0 | 71.0 | 76.6 | 100 | 83.9 |
| CD33+, % | 60.9 | 30.7 | 71.4 | 57.1 | 72.9 | 67.9 | 100 | 80.5 |
Cells . | PV1 . | PV2 . | PV3 . | PV4 . | PV7 . | PV8 . | PV9 . | PV10 . |
|---|---|---|---|---|---|---|---|---|
| CD34+, % | 85.0 | 34.6 | 98.7 | 71.0 | 71.0 | 76.6 | 100 | 83.9 |
| CD33+, % | 60.9 | 30.7 | 71.4 | 57.1 | 72.9 | 67.9 | 100 | 80.5 |
The purity of the CD34+ cells and CD33+ cells was 95% or greater. Values are JAK2V617F mutant allele/JAK2total allele × 100 in the cDNA. JAK2V617F/JAK2total was determined according to the mean ΔCt and the standard curve by real-time quantitative PCR.
*G-CSF—mobilized health subjects 1, 2, and 3 had 0% CD34+ and D33+ cells.
Zhao et al10 have recently shown that the genomic DNA from PV MNCs give rise to significantly lower amounts of the mutant PCR products than the corresponding cDNA samples. We also attempted to compare the percentage of JAK2V617F/JAK2total in gDNA and the corresponding cDNA determined by real-time quantitative PCR. In each of the PV CD34+ and CD33+ cell populations, the gDNA contained a significantly smaller percentage of JAK2V617F/JAK2total than the corresponding cDNA (P < .005; Tables 2 and 3). The fact that cells contained a greater percentage of mutant mRNA products than the percentage of the mutant gDNA indicates that the mutant allele might have a transcriptional advantage over the wild-type or that the mutant mRNA is characterized by increased stability. These data also suggest that analysis of cDNA might be a more sensitive tool for the detection of the mutation of JAK2 than the gDNA, as has been suggested by Zhao et al10 previously.
JAK2V617F mutation in the cellular progenies of PV CD34+ cells differentiated in vitro by optimal concentrations of SCF, IL-3, IL-6, and Epo
We have shown that there is a higher percentage of JAK2V617F/JAK2total in the CD34+ cells than CD33+ cells, suggesting that CD34+ cells with the JAK2V617F mutation may not confer a proliferative and/or survival advantage. To test this hypothesis, CD34+ cells isolated from PB of the 8 patients with PV and 2 G-CSF–mobilized healthy donors were cultured in vitro in the presence of optimal concentrations of SCF, IL-3, IL-6, and Epo. Under this culture condition, there was approximately an 8-fold and approximately 25-fold increase in total cell number after 6 and 12 days of culture of both normal and PV CD34+ cells, respectively. The expanded cells were harvested after 6 and 12 days of culture, morphologically analyzed, and the percentage of JAK2V617F/JAK2total in the cDNA of cells generated was determined by real-time quantitative PCR. cDNA was used for this analysis because cDNA is likely a more sensitive target for the detection of the mutation than gDNA. Morphologic analysis of the cells after May-Grünwald-Giemsa staining revealed that the predominant cells in the culture of PV and G-CSF–mobilized PB CD34+ cells were myeloblasts and promyelocytes after 6 days of culture, whereas the predominant cells were myelocytes, metamyelocytes, and normoblasts after 12 days of culture (Table 4).
Morphologic analysis of PB CD34+ cells isolated from patients with PV and healthy G-CSF–mobilized donors cultured in vitro for 6 days and 12 days
. | Myeloblast, % . | Promyeloblast, % . | Myelocyte, % . | Metamyelocyte, % . | Normoblasts, % . |
|---|---|---|---|---|---|
| PV | |||||
| Day 6 | 14.6 ± 4.1 | 61.3 ± 6.2 | 11.2 ± 8.2 | 2.3 ± 1.5 | 4.9 ± 4.1 |
| Day 12 | 2.1 ± 1.7 | 31.0 ± 6.5 | 23.2 ± 10.0 | 18.4 ± 6.3 | 7.6 ± 4.3 |
| Mob | |||||
| Day 6 | 10.9 ± 2.5 | 49.1 ± 5.1 | 35.5 ± 7.7 | 2.0 ± 1.0 | 2.5 ± 3.0 |
| Day 12 | 2.5 ± 1.4 | 26.5 ± 4.9 | 41.3 ± 12.2 | 20.0 ± 6.8 | 9.7 ± 4.6 |
. | Myeloblast, % . | Promyeloblast, % . | Myelocyte, % . | Metamyelocyte, % . | Normoblasts, % . |
|---|---|---|---|---|---|
| PV | |||||
| Day 6 | 14.6 ± 4.1 | 61.3 ± 6.2 | 11.2 ± 8.2 | 2.3 ± 1.5 | 4.9 ± 4.1 |
| Day 12 | 2.1 ± 1.7 | 31.0 ± 6.5 | 23.2 ± 10.0 | 18.4 ± 6.3 | 7.6 ± 4.3 |
| Mob | |||||
| Day 6 | 10.9 ± 2.5 | 49.1 ± 5.1 | 35.5 ± 7.7 | 2.0 ± 1.0 | 2.5 ± 3.0 |
| Day 12 | 2.5 ± 1.4 | 26.5 ± 4.9 | 41.3 ± 12.2 | 20.0 ± 6.8 | 9.7 ± 4.6 |
The purity of the CD34+ cells was 95% or greater. The CD34+ cells were cultured in the presence of SCF, IL-3, IL-6, and Epo at their optimal concentration for 12 days. Cytospin preparations of the cells (day 6 and day 12) were prepared and stained with May-Grünwald-Giemsa. A 100- to 200-cell differential was performed under × 400 magnification using an Olympus BH-2 light microscope. Data are presented as mean ± SD.
Mob indicates G-CSF—mobilized healthy subject.
Compared with the input CD34+ cells, the percentage of JAK2V617F/JAK2total in the cells progressively decreased after 6 and 12 days of culture (P < .01) (Table 5). The exception to this observation was PV9, who was homozygous for the mutant allele. These data indicate that in vitro differentiation of PV CD34+ cells under the optimal conditions favors the development of hematopoietic cells that do not contain the JAK2V617F mutation.
Percentage of JAK2V617F mutation in the cDNA of CD34+ progenitor cells and their differentiated cellular progenies
. | . | Following culture . | . | |
|---|---|---|---|---|
| Donors* . | CD34+ cells before culture, % . | MNCs at day 6, % . | MNCs at day 12, % . | |
| PV1 | 85.0 | 73.7 | 66.9 | |
| PV2 | 34.6 | 10.5 | 5.1 | |
| PV3 | 98.7 | 85.7 | 77.9 | |
| PV4 | 71.0 | 28.3 | 12.1 | |
| PV7 | 71.0 | 39.9 | 9.8 | |
| PV8 | 76.6 | 74.7 | 53.1 | |
| PV9 | 100 | 100 | 100 | |
| PV10 | 83.9 | 70.8 | 43.5 | |
. | . | Following culture . | . | |
|---|---|---|---|---|
| Donors* . | CD34+ cells before culture, % . | MNCs at day 6, % . | MNCs at day 12, % . | |
| PV1 | 85.0 | 73.7 | 66.9 | |
| PV2 | 34.6 | 10.5 | 5.1 | |
| PV3 | 98.7 | 85.7 | 77.9 | |
| PV4 | 71.0 | 28.3 | 12.1 | |
| PV7 | 71.0 | 39.9 | 9.8 | |
| PV8 | 76.6 | 74.7 | 53.1 | |
| PV9 | 100 | 100 | 100 | |
| PV10 | 83.9 | 70.8 | 43.5 | |
The purity of the CD34+ cells was 95% or greater. The CD34+ cells were cultured in the presence of SCF, IL-3, IL-6, and Epo at their optimal concentration for 6 and 12 days. Each value represents the JAK2V617F mutant allele/JAK2 total allele × 100 in the cDNA. JAK2V617F/JAK2total was calculated according to the mean ΔCt and the standard curve by real-time quantitative PCR.
G-CSF—mobilized healthy subjects 1 and 2 had 0% JAK2V617F mutation.
JAK2V617F mutation in the cellular progenies of PV CD34+ cells cultured in vitro in the presence of SCF, IL-3, IL-6, and various concentrations of Epo
PV erythroid progenitor cells possessing the JAK2 mutation are hypersensitive to Epo.7-10 The formation of Epo-independent colonies, which characterizes PV, has been shown to be associated with hematopoietic cells harboring this mutation.6,7,11 One might predict that the percentage of JAK2V617F/JAK2total in the cells differentiated in vitro from PV CD34+ cells would vary when different concentrations of Epo are added to cultures.
To test this hypothesis, PB CD34+ cells isolated from 4 different patients with PV and a G-CSF–mobilized healthy donor were cultured in vitro in the presence or absence of various concentrations of Epo (0.0 U/mL, 0.025 U/mL, 0.1 U/mL, 0.5 U/mL, or 4 U/mL) with or without optimal concentrations of SCF, IL-3, and IL-6. In the presence of optimal concentrations of SCF, IL-3, and IL-6 but various Epo concentrations (0.0 U/mL, 0.025 U/mL, 0.1 U/mL, 0.5 U/mL, and 4 U/mL) there was an approximately 15-fold, approximately 18-fold, approximately 20-fold, approximately 25-fold, and approximately 25-fold increase, respectively, in total cell number after 12 days of culture of both normal and PV CD34+ cells. The expanded cells were harvested after 12 days of culture and morphologically analyzed, and the percentage of JAK2V617F/JAK2total in the cDNA of cells was determined by real-time quantitative PCR. Morphologic analysis of the cells after May-Grünwald-Giemsa staining revealed that with higher concentration of Epo, progressively higher percentages of erythroblasts and normoblasts were observed in the cultures of both PV and G-CSF–mobilized PB CD34+ cells (data not shown).
As can be seen in Table 6, the percentage of JAK2V617F/JAK2total in the cells generated from PV CD34+ cells in the presence of SCF, IL-3, and IL-6 but in the absence of Epo was only slightly lower than that of the input CD34+ cells. The percentage of JAK2V617F/JAK2total progressively decreased when increasing concentrations of Epo were added, and the percentage of JAK2V617F/JAK2total plateaued at a concentration of 0.5 U/mL Epo (Table 6).
Percentage of JAK2V617F mutation in the cDNA of CD34+ progenitor cells and their differentiated cellular progenies generated by SCF, IL-6, IL-3, and various dose of Epo
. | . | Following 12 days of culture with SCF ± IL-6 ± IL-3 plus different concentrations of Epo . | . | . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Donors* . | CD34+ cells before culture, % . | 0 U/mL, % . | 0.025 U/mL, % . | 0.1 U/mL, % . | 0.5 U/mL, % . | 4 U/mL, % . | ||||
| PV2 | 34.6 | 32.4 | 29.5 | 17.0 | 4.9 | 5.1 | ||||
| PV4 | 71.0 | 49.3 | 35.6 | 19.1 | 9.8 | 12.1 | ||||
| PV7 | 71.0 | 65.9 | 40.1 | 18.9 | 10.7 | 9.8 | ||||
| PV8 | 76.6 | 71.4 | 70.1 | 57.9 | 46.4 | 53.1 | ||||
. | . | Following 12 days of culture with SCF ± IL-6 ± IL-3 plus different concentrations of Epo . | . | . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Donors* . | CD34+ cells before culture, % . | 0 U/mL, % . | 0.025 U/mL, % . | 0.1 U/mL, % . | 0.5 U/mL, % . | 4 U/mL, % . | ||||
| PV2 | 34.6 | 32.4 | 29.5 | 17.0 | 4.9 | 5.1 | ||||
| PV4 | 71.0 | 49.3 | 35.6 | 19.1 | 9.8 | 12.1 | ||||
| PV7 | 71.0 | 65.9 | 40.1 | 18.9 | 10.7 | 9.8 | ||||
| PV8 | 76.6 | 71.4 | 70.1 | 57.9 | 46.4 | 53.1 | ||||
The purity of the CD34+ cells was ≥95%. The CD34+ cells were cultured in the presence of SCF, IL-3, IL-6 and different concentrations of Epo for 12 days. Each value represents the JAK2V617F mutant allele/JAK2 total allele × 100 in the cDNA. JAK2V617F/JAK2total was calculated according to the mean ΔCt and the standard curve by real time quantitative PCR.
The G-CSF—mobilized healthy subject had 0% JAK2V617F mutation.
PV PB CD34+ cells were also similarly cultured in the presence and absence of various concentrations of Epo alone. The percentage of JAK2V617F/JAK2total in the cells generated in the absence of Epo was slightly higher as compared with the input CD34+ cells. The percentage of JAK2V617F/JAK2total in the cells generated in the presence of optimal doses of Epo was, however, dramatically decreased (Table S1, available on the Blood website; see the Supplemental Table link at the top of the online article).
These data indicate that the addition of an optimal concentration of Epo in vitro to PV CD34+ cells is required for the development of hematopoietic cells that do not contain the JAK2V617F mutation.
Discussion
The pluripotent HSC is a rare cell present in the BM and less frequently in normal PB, which is responsible for the lifelong generation of blood cells.31,32 The differentiation of HSCs into various hematopoietic lineages is thought to be a stepwise process via the generation of progenitor cells having a gradually more restricted developmental and proliferative potential. The identification of a common lymphoid progenitor cell and a common myeloid progenitor cell has provided strong supporting evidence for such a stepwise differentiation schema.33-35 As originally defined, a common lymphoid progenitor cell would give rise to T, B, and NK but not myeloid cells, whereas a common myeloid progenitor cell could generate myeloid but not lymphoid cells.33-35 Recently, Hou et al36 have identified a human B cell/myeloid common progenitor cell based on the absence of CXCR4 expression. In this study, we have documented the JAK2V617F mutation is present in the T and B lymphocytes of a patient with PV, indicating the JAK2V617F mutation occurs at the multipotent HSC level in a subpopulation of patients with PV. We also showed that the JAK2V617F mutation was present in both B cells and cells belonging to the myeloid lineages, but not T cells in a PV patient, indicating the occurrence of the mutation in a common myeloid–B-lymphocyte precursor cell in a subpopulation of patients with PV. The JAK2V617F mutation involves only myeloid lineages in the rest of the 6 patients with PV, suggesting the mutation likely occurs in myeloid-specific progenitor cells in the majority of patients with PV. Jamieson et al44 recently reported that the JAK2V617F mutation can be detected in a CD34+CD38–CD90+Lin– subpopulation of hematopoietic cells isolated from 6 of 6 patients with PV. Our findings and those of Jamieson et al44 indicate that at least in some patients, the JAK2V617F mutation originates at the level of an HSC. It remains possible, however, that the JAK2 mutation might impair differentiation of stem cells into cells belonging to lymphoid lineages accounting for 75% of patients in our series not having B- or T-cell involvement.
Lasho et al29 have reported the absence of the JAK2V617F mutation in both B and T lymphocytes in one patient each with PV, ET, and CIMF who displayed homozygous JAK2V617F in their granulocytes. Baxter et al6 and James et al7 have also reported the absence of detectable JAK2V617F mutation in T cells from some patients with MPDs. The incidence of the involvement of the JAK2V617F mutation in T cells of patients with PV might be limited (1 of 8 patients with PV in this study). Studies of larger numbers of patients will be required to determine its true incidence. The incidence of the involvement of the JAK2V617F mutation in B cells might be more common than T cells in PV. By analysis of clonal cytogenetic markers in different cell types, Reeder et al27 have shown that the percentage of abnormal nuclei was variable in both lymphocyte populations but always higher in B lymphocytes as compared with T lymphocytes in CIMF. In this study, we found 2 of 8 patients with PV with the involvement of the JAK2V617F mutation in B cells.
The Ph– MPDs are clonal HSC diseases likely involving both myeloid and lymphoid lineages.1,2,12-27 Using X-chromosome–linked polymorphism analysis, numerous reports have shown a monoclonal pattern of the myeloid lineages (mainly the granulocyte fraction) in patients with PV, ET, and CIMF.13-17,24,25 Using similar clonality assays, there are several reports demonstrating that B18-20 and T lymphocytes21-23 can be involved by the malignant clone in at least some patients with PV, ET, and CIMF. Studies based on Ras mutational analyses and fluorescent in situ hybridization analysis have also suggested monoclonality of T lymphocytes in CIMF.26,27 In addition, using 9p, 10q, and 11q LOH as clonality markers, Kralovics et al28 have shown that a minor proportion of T cells may be generated from the PV clone in some patients with PV.
The JAK2V617F mutation has been shown to result in constitutive tyrosine phosphorylation and its downstream effectors STAT5 and ERK in various cell lines.7-10 Erythroid progenitor cells carrying the mutation were able to proliferate in the absence of Epo, a characteristic of PV.6,7,11 The mutation was sufficient to produce an erythrocytosis resembling PV in mice after transplantation with murine BM cells transduced with a retrovirus containing JAK2V617F.7 Although the currently available data clearly demonstrate that the JAK2V617F mutation participates in the pathogenesis of the Ph– MPDs, the exact role for activated JAK2 signaling in the pathogenesis of these Ph– MPDs remains uncertain at this time.
Tefferi et al30 have reported a time-dependent increase of JAK2V617F mutation in the granulocytes in some patients with PV. Several groups have shown the JAK2V617F mutation induced a proliferative advantage in cell lines, suggesting the mutation might contribute to the growth and/or survival advantages of the abnormal clone in Ph– MPDs.7-9 However, James et al7 have shown that transduction with the wild-type allele neutralized JAK2V617F-mediated growth factor independence by Ba/F3 cell lines and cotransfection studies with both mutant and wild-type allele suggested that the latter might function as a negative-dominant allele.
In this study, we showed that in most of the patients with PV, there is a higher percentage of JAK2V617F/JAK2total in the PB CD34+ cells than in the more-differentiated CD33+ cells. It would, however, be important to compare the percentage of JAK2V617F/JAK2total in the BM CD34+ cells with CD34+ cells circulating in the PB. We still cannot exclude the possibility that CD34+ cells bearing the JAK2V617F mutation have the increased ability to circulate into the PB. In this study, we have also shown that there is a decreased percentage of JAK2V617F/JAK2total in the differentiated cell progenies generated in vitro from PV PB CD34+ cells in the presence of optimal concentration of SCF, IL-3, IL-6, and Epo. Note here that the cytokine concentrations used in these cultures were optimal to promote cell proliferation. The concentration of Epo used might lead to the activation of both wild-type and mutant JAK2. It is possible that these optimal conditions neutralized or even eliminated the competitive advantage of JAK2V617F hematopoietic progenitor cells. Indeed, when in vitro cultures were performed in the absence of exogenous Epo for 12 days, cells containing the JAK2 mutation predominanted, whereas the addition of increasing concentrations of Epo reduced the numbers of such cells harboring the mutation. Because serum Epo levels have been shown to be subnormal in patients with PV,1,45 this differentiation assay might represent the events occurring in vivo in patients with PV bearing the JAK2V617F mutation. Prchal et al46 have also recently observed similar data with regard to the JAK2 mutational status of erythroid cells generated in vitro from PV erythroid progenitor cells. Their data indicate that in vitro expansion of PV progenitor cells also favors expansion of erythroid precursor cells that lack the JAK2V617F mutation. In contrast to the previous studies using cell lines,7-9 these data suggest that JAK2V617F mutation does not provide a proliferative and/or survival advantage in vitro under the culture conditions used.
These studies demonstrate the importance of the role of the JAK2V617F mutation in the biologic origins of PV. The exact mechanism by which this mutation leads to the generation of excess differentiated hematopoietic cells in patients with PV will, however, require further study.
Prepublished online as Blood First Edition Paper, June 6, 2006; DOI 10.1182/blood-2006-04-017392.
Supported by the Department of Defense (grant MP048007) (M.X.) and a research grant from the Myeloproliferative Disorder Foundation (M.X.).
T.I. and M.X. conceived and performed the studies. R.H. followed the patients, obtained the patient samples, and contributed to the composition of the final manuscript. E.B. obtained institutional review board approval for obtaining the patient and healthy volunteer samples and performed the cell-purification procedures. M.X. acquired and analyzed the data and wrote the manuscript.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank the patients and healthy volunteers for their agreement to participate in this study.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal