Abstract
CD38 expression of tumor cells has been identified as an important prognostic factor in B-cell chronic lymphocytic leukemia (B-CLL). Although CD38 is involved in effector functions of T cells, the prognostic value of CD38+ T cells has not yet been addressed in B-CLL. In the present study, CD38-expression levels in B-CLL cells and T cells from 204 patients were analyzed by flow cytometry and correlated with clinical and molecular risk parameters. CD38 expression significantly differed in the neoplastic clone from patients with low versus advanced stage, irrespective of the sex of patients. In contrast, CD38 expression was generally higher in T cells from female compared with male patients but only increased in male patients in a stage-dependent manner. In male patients, combined analysis of CD38 in T cells and B-CLL cells identified 4 subgroups with significantly different treatment-free survival. Multivariate analysis including Rai stage and molecular risk parameters of the neoplastic clone identified CD38-expression levels in T cells as an independent prognostic factor in male patients. Combined analysis of CD38 in B-CLL and T cells is superior in predicting outcome of male B-CLL patients than either parameter alone. Further studies are needed to elucidate the underlying mechanisms of the sex-specific role of CD38+ T cells in B-CLL.
Introduction
B-cell chronic lymphocytic leukemia (B-CLL) is a neoplastic disease of slowly proliferating CD5+ B cells that develops in the older population. Its clinical behavior is variable, with some patients having an indolent course without any therapy and others a rapidly progressing one probably requiring aggressive treatment. As an underlying mechanism of differential disease activity, genomic aberrations1 and distinct expression levels of signaling molecules influencing survival as well as proliferation of B-CLL cells have been postulated. Besides the B-cell receptor itself2,3 and Zap-70, a tyrosine kinase involved in B-cell receptor (BCR) signaling,4 CD38, initially described as a mediator of leukocyte adhesion to endothelium,5 was identified as a further candidate, since it induces both survival and proliferation signals in B-CLL cells6 and its expression and signaling capacity correlate with both progression and response to therapy.7 Its clinical importance as negative prognostic marker was established by analysis of CD38 expression in large patient cohorts.7-10
While the study of malignant cells provides many insights into tumor biology, it has also become clear that transformation and progression of tumors is not an independent process but is controlled by their interactions with the tumor microenvironment (for a recent review see Joyce11 ). Supporting factors in the microenvironment include stromal and vascular cells as well as infiltrating immune cells. Dependency on such supportive signals should also be relevant in B-CLL, in which close cooperation with other immune cells can be expected from normal behavior of B cells. In the present study, we focused on T cells and their expression of CD38 for the following reasons. (1) CD38 has been identified as an important signaling molecule of T cells.12 (2) The signaling capacity of T cells has been shown to be dysregulated in B-CLL patients (for a review see Scrivener et al13 ). Such an aberrant immune control might favor a more aggressive outgrowth of the neoplastic B-cell clone and negatively impact on the clinical outcome of patients. (3) T cells might even deliver antiapoptotic and/or proliferative signals to the neoplastic clone when coming into intimate contact with each other in lymphoid compartments like bone marrow or lymph nodes.14 We therefore raised the question of whether aberrant expression of CD38 in T cells might also be of prognostic relevance in B-CLL.
Patients, materials, and methods
Patients and samples
The study was approved by the local ethics committees (those of the Medical University Innsbruck and Provincial Government of Salzburg, Austria) and conducted according to the Declaration of Helsinki. After providing informed patient consent, 204 consecutive, unselected B-CLL patients seen at the Department of Hematology and Oncology, University Hospitals Innsbruck and Salzburg, Austria, between March 2000 and November 2005 were included in this retrospective study. Tumor cells from peripheral blood were collected in heparinized tubes during routine examinations. B-CLL was defined by clinical criteria as well as by cellular morphology and the coexpression of CD19, CD5, and CD23 in lymphocytes simultaneously displaying restriction of light-chain rearrangement. Staging was performed according to modified Rai classification. Progression was defined as by Cheson et al.15 Treatment-free survival was defined by the period between first diagnosis and first CLL-specific treatment. No deaths (CLL-related or unrelated) occurred during this time. Median observation time of clinical course of patients was 50 months, ranging from 2 to 330 months. Characteristics of the patients studied are presented in Table 1. In addition, peripheral-blood samples from 33 healthy age-matched controls were analyzed for CD38-expression levels in T cells.
Patient characteristics
Parameters . | No. of patients (%) . |
|---|---|
| Total no. | 204 (100) |
| Sex | |
| Male | 116 (57) |
| Female | 88 (43) |
| Age, y | |
| Median | 68 |
| Range | 43-89 |
| Rai stage | |
| Low, 0 | 63 (31) |
| Intermediate, 1-2 | 98 (48) |
| High, 3-4 | 34 (17) |
| Unknown | 9 (4) |
| Disease activity | |
| Stable | 101 (50) |
| Progressive | 68 (33) |
| Unknown | 35 (17) |
| Previous therapy | |
| Chemonaive | 138 (68) |
| Treatment ended more than 4 wks before | 57 (28) |
| Under treatment | 9 (4) |
| Molecular risk parameters | |
| Zap-70+ B-CLL cells, at least 20% | 90 (44) |
| Unmutated Ig VH gene* | 48 (37) |
| Unfavorable genetic aberration(s), del17p, 11q, 12+* | 42 (30) |
Parameters . | No. of patients (%) . |
|---|---|
| Total no. | 204 (100) |
| Sex | |
| Male | 116 (57) |
| Female | 88 (43) |
| Age, y | |
| Median | 68 |
| Range | 43-89 |
| Rai stage | |
| Low, 0 | 63 (31) |
| Intermediate, 1-2 | 98 (48) |
| High, 3-4 | 34 (17) |
| Unknown | 9 (4) |
| Disease activity | |
| Stable | 101 (50) |
| Progressive | 68 (33) |
| Unknown | 35 (17) |
| Previous therapy | |
| Chemonaive | 138 (68) |
| Treatment ended more than 4 wks before | 57 (28) |
| Under treatment | 9 (4) |
| Molecular risk parameters | |
| Zap-70+ B-CLL cells, at least 20% | 90 (44) |
| Unmutated Ig VH gene* | 48 (37) |
| Unfavorable genetic aberration(s), del17p, 11q, 12+* | 42 (30) |
The indicated risk parameters were available for a subgroup of patients: Ig VH mutational status (n = 130) and genomic aberrations (n = 140). Statistical analyses for differences in tumor load, clinical stage, sex, disease activity, and treatment-free survival revealed no significant differences between subgroups and the total patients' cohort (data not shown), ruling out that results are biased by patient selection.
Antibodies and reagents
For characterization of lymphocytic subsets, the following fluorochrome-labeled murine antibodies were used: anti–human CD19, CD5, CD38, CD4, CD8, CD3, CD56, CD62L, and CD45RA (all from Beckman Coulter, Fullerton, CA); and FITC-labeled anti–human Zap-70 (clone 2F3.2; Upstate, Biomedica, Vienna, Austria).
Flow cytometric analysis
Fresh blood samples were incubated with a mixture of fluorescence-labeled anti-CD19, anti-CD5, and anti-CD38 monoclonal antibodies (mabs) for 15 minutes followed by lysis of red blood cells with fluorescence-activated cell sorter (FACS) lysing solution (Becton Dickinson, Heidelberg, Germany). At least 5000 cells were analyzed using the FC500 flow cytometer and RXP1.0 software (Beckman Coulter). As gating strategy, CD19+CD5+ cells (B-CLL) and CD19–CD5+ cells (T cells) were analyzed for CD38 expression. Despite the decrease in relative numbers of T cells in patients with B-CLL, we were able to analyze at least 5000 T cells per sample. Representative plots of CD38-expression profiles from 2 patients' samples are presented in Figure 1. Further in-depth characterization of the CD19–CD5+ cell pool concerning cellular subtype and maturation stage was performed by staining T cells with antibodies to CD4, CD8, CD45RA, and CD62L.16
Analysis of Zap-70 expression was performed using a modified protocol from Crespo et al.17 Briefly, blood samples were incubated with phycoerythrin-conjugated antibodies to CD3 and CD56 and with phycoerythrin–cyanin 7–conjugated antibodies to CD19 for 15 minutes. Red cells were lysed with FACS lysing solution (Becton Dickinson). Cells were spun down by centrifugation at 200g, fixed, and permeabilized using the Fix & Perm Kit (An der Grub, Kaumberg, Austria). To the permeabilization solution, 1 μg/sample of directly FITC-labeled anti–Zap-70 mab was added, and cells were incubated for 15 minutes. Cells were then washed with phosphate-buffered saline (PBS) and analyzed immediately by flow cytometry.
Genetic analysis
For detection of genomic aberrations (ie, del17p, del11q, del13q, trisomy 12), interphase fluorescence in situ hybridization (FISH) analysis using the commercially available multicolor probe set (ABBOTT/VYSIS, Vienna, Austria) was performed according to manufacturer's instructions.
Ig heavy chain variable region (VH) mutational analysis was performed according to Fais et al.18 Five hundred nanograms of total RNA was reversely transcribed to cDNA. To determine the VH gene family used by the B-CLL cells, 1 μL cDNA was amplified using all possible combinations of VH leader and heavy chain constant region (CH) primers in 21 separate reactions. The reactions were carried out in volumes of 20 μL using a Biometra T3000 thermocycler (Biometra, Göttingen, Germany) under the following conditions: denaturation at 94°C for 45 seconds, annealing at 62°C for 30 seconds, and extension at 72°C for 45 seconds. After 35 cycles, a final extension step of 10 minutes was included. Polymerase chain reaction (PCR) products were sequenced by an outsource sequencing service (MWG Biotech AG, Martinsried, Germany). Sequences were aligned to the V genes in the IMGT database sequence directory19 and best matches were analyzed for their degree of homology. A cutoff of at least 98% homology to germ line sequences was used for the detection of unmutated Ig VH genes.20
CD38-expression profiles of B-CLL cells (CD19+CD5+) and T cells (CD19–CD5+) as detected by 3-color flow cytometry. Fresh blood samples were incubated with a mixture of fluorescence-labeled anti-CD19, anti-CD5, and anti-CD38 mabs for 15 minutes followed by lysis of red blood cells with a FACS lysing solution. CD19+CD5+ cells (B-CLL) and CD19–CD5+ cells (T cells) were analyzed for CD38 expression. Representative results from a patient with CD38 low risk (< 30% CD38+ B-CLL cells, < 50% CD38+ T cells, left row) and CD38 high risk (≥ 30% CD38+ B-CLL cells, ≥ 50% CD38+ T cells, right row) are presented.
CD38-expression profiles of B-CLL cells (CD19+CD5+) and T cells (CD19–CD5+) as detected by 3-color flow cytometry. Fresh blood samples were incubated with a mixture of fluorescence-labeled anti-CD19, anti-CD5, and anti-CD38 mabs for 15 minutes followed by lysis of red blood cells with a FACS lysing solution. CD19+CD5+ cells (B-CLL) and CD19–CD5+ cells (T cells) were analyzed for CD38 expression. Representative results from a patient with CD38 low risk (< 30% CD38+ B-CLL cells, < 50% CD38+ T cells, left row) and CD38 high risk (≥ 30% CD38+ B-CLL cells, ≥ 50% CD38+ T cells, right row) are presented.
Peripheral-blood samples from a subset of B-CLL patients were characterized for genomic aberrations (n = 140) and Ig VH mutational status (n = 130). Distribution of molecular risk parameters within the patients' cohort is depicted in Table 1.
Statistical analysis
All statistical analyses were performed using StatView 5.1 (Abacus Concepts, Berkeley, CA). The cutoff values for definition of risk groups were determined by receiver operating characteristic (ROC) analysis and subsequent Youden index calculation.21 Using this approach, we established a cutoff between 28% and 32% CD38– B-CLL cells to best distinguish between 2 groups of B-CLL patients with a different clinical outcome. This is well in the range reported by others.7,8 We therefore decided to use a cutoff of at least 30%. For CD38 expression of T cells, a cutoff of at least 50% CD38– T cells gave the highest possible Youden index. Analysis of Zap-70 using a directly labeled mab (see “Antibodies and reagents”) is expected to yield lower mean fluorescence intensities (MFIs) than a 2-step staining procedure with primary and secondary mabs.17 However, it did not change the ratio between MFI of B-CLL cells and internal controls (T/natural killer [NK] cells) in our study. Youden index analysis revealed the same cutoff of at least 20% Zap-70+ B-CLL cells for best discrimination between risk groups as determined by others.17
Significance of differences in CD38-expression levels depending on Rai stage was determined using a factorial analysis of variance (ANOVA) unpaired t test. Comparisons of treatment-free intervals of patients were performed using the Kaplan-Meier method and significance was determined using the log-rank test. Correlation of CD38 expression of T cells with other risk parameters was tested using a chi-square test. Multivariate analysis to determine the interdependency of CD38-expression profile of T cells and other clinical or molecular risk parameters was carried out by Cox proportional hazards regression.
Results
Correlation of CD38 expression in B-CLL and T cells with sex and clinical stage
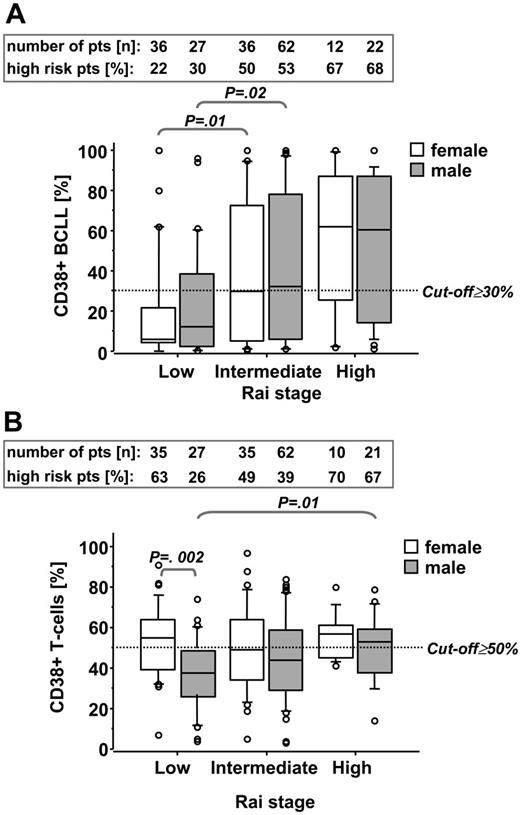
We first quantified relative numbers of CD38+ B-CLL cells (CD19+CD5+) and T cells (CD19–CD5+) in individual B-CLL patients by flow cytometry (Figure 1). The latter population was confirmed as being T cells by their CD3 positivity (data not shown). Since activation status of T cells might interact with the course of disease in B-CLL, we raised the question whether, besides increased expression of CD38 in B-CLL cells, stage-dependent differences in CD38 expression were also detectable in the T-cell population. In addition, since CD38 expression has been shown to be regulated by sex hormones,22 we separately analyzed CD38-expression levels in cells from female and male patients. We could confirm a stage-associated CD38 expression of B-CLL cells also reported by others,8 which was sex neutral (Figure 2A). In contrast, at low Rai stage the percentage (Figure 2B) as well as the absolute numbers of CD38+ T cells (P = .01; data not shown) were higher in female compared with male patients but only increased in male patients in a stage-dependent manner (Figure 2B).
Sex- and stage-related differences of CD38-expression levels in B-CLL and T cells. CD38-expression levels from B-CLL cells (A) and T cells (B) from female and male patients at low, intermediate, and high Rai stage were analyzed. Numbers in the boxes on top of the graphs represent numbers of patients in the outlined subgroups (top line) and percentages of high-risk patients (characterized by ≥ 30% CD38+ B-CLL cells or ≥ 50% CD38+ T cells, respectively) within these subgroups (bottom line). Data are presented in box-and-whisker format: the 25th and 75th percentile form the box, with the mean marked as a line; the 10th and 80th percentile form the whiskers; and all observations <10th percentile or >90th percentile are presented as circles.
Sex- and stage-related differences of CD38-expression levels in B-CLL and T cells. CD38-expression levels from B-CLL cells (A) and T cells (B) from female and male patients at low, intermediate, and high Rai stage were analyzed. Numbers in the boxes on top of the graphs represent numbers of patients in the outlined subgroups (top line) and percentages of high-risk patients (characterized by ≥ 30% CD38+ B-CLL cells or ≥ 50% CD38+ T cells, respectively) within these subgroups (bottom line). Data are presented in box-and-whisker format: the 25th and 75th percentile form the box, with the mean marked as a line; the 10th and 80th percentile form the whiskers; and all observations <10th percentile or >90th percentile are presented as circles.
CD38 expression in B-CLL cells and T cells is differentially regulated but comparably stable over time
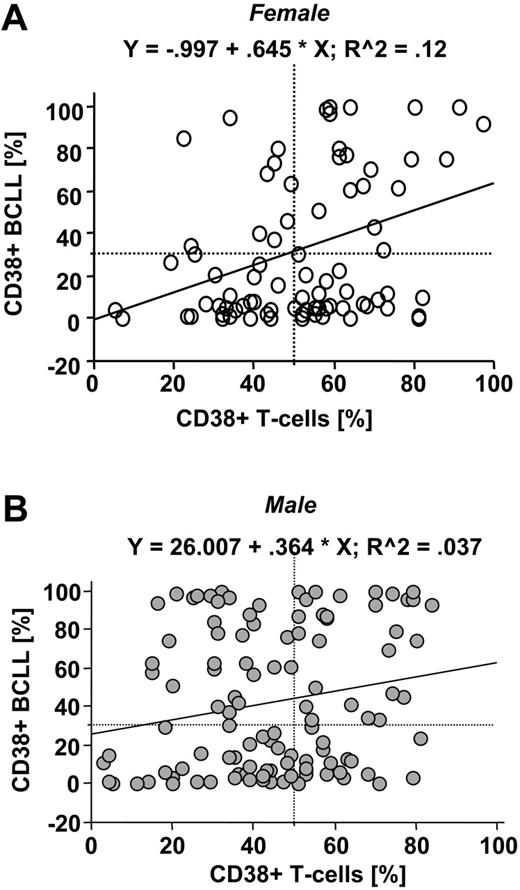
We next addressed whether CD38 expression of T cells correlates with that of B-CLL cells and whether expression levels in both cell populations are stable over time. CD38-expression levels from T cells showed only a very low degree of correlation with the expression levels in B-CLL cells both in female (Figure 3A) and in male patients (Figure 3B), suggesting different regulation by factors yet to be identified. In a subgroup of 112 consecutive patients, analysis of CD38 expression was repeated over time (the numbers of sequential analyses ranging from 2 to 10). In the majority of patients we found stable expression of CD38 in B-CLL cells as well as T cells. Major changes of expression levels in B-CLL cells were found in 11 (9%) of 112 patients and in T cells in 12 (11%) of 112 patients, which led to a change in risk classification (data not shown). These changes were not associated with infectious episodes (data not shown) nor were they induced by therapy, since none of these patients had received treatment between measurements. Interestingly, patients with changes in CD38 expression in B-CLL cells were not the same patients with changes in CD38 expression in T cells, again suggesting differential regulation of CD38 in both subsets.
CD38-expression levels are differently regulated in B-CLL cells and T cells. CD38-expression levels in B-CLL cells were plotted against those detected in T cells. Analysis from female (n = 84; A) and male patients (n = 115; B) revealed a very low degree of correlation as indicated by the correlation coefficient presented on top of the graphs. The fitted regression lines are plotted as solid lines. The dotted lines mark the cutoff values for definition of high-risk groups (ie, expression of CD38 in at least 30% of B-CLL cells and 50% of T cells).
CD38-expression levels are differently regulated in B-CLL cells and T cells. CD38-expression levels in B-CLL cells were plotted against those detected in T cells. Analysis from female (n = 84; A) and male patients (n = 115; B) revealed a very low degree of correlation as indicated by the correlation coefficient presented on top of the graphs. The fitted regression lines are plotted as solid lines. The dotted lines mark the cutoff values for definition of high-risk groups (ie, expression of CD38 in at least 30% of B-CLL cells and 50% of T cells).
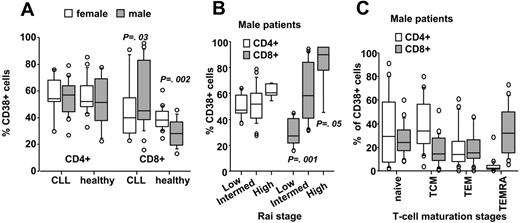
In a subgroup of 64 consecutive patients, we analyzed whether CD38-expression levels were different within CD4+ and CD8+ T-cell subsets. CD38 expression did not significantly differ between CD4+ and CD8+ T cells (Figure 4A). However, there were significantly higher relative numbers of CD38+ cells in the CD8+ T-cell population of male compared with female patients. This was specific for B-CLL, since the opposite, namely higher, percentages of CD38+ cells in females compared with males were detected in the peripheral CD8+ T-cell compartment from healthy age-matched controls (Figure 4A). Comparable to the observation made for the total CD5+ T-cell fraction, CD38-expression levels significantly increased in CD8+ T cells in a stage-dependent manner only in male (Figure 4B) and not in female patients (data not shown). Subset analysis of the T-cell compartment from male patients (n = 23) revealed that the most mature effector memory RA+ T cells (TEMRAs)16 contributed mainly to the CD8+ CD38+ T-cell compartment, whereas naive and central memory T cells (TCMs) were found above all in the CD4+ CD38+ T-cell pool (Figure 4C).
Correlation of sex and stage with CD38-expression levels in CD4+ and CD8+ T cells. CD38-expression levels were significantly different in CD8+ T cells from male (n = 39) compared with female patients (n = 25), whereas the opposite, higher expression of CD38 in peripheral CD8+ T cells from healthy age-matched females (n = 13) compared with males (n = 20) was observed (A). (B) It was also in the CD8+ T-cell subset where a stage-dependent increase in CD38-expression levels was observed in male B-CLL patients. (C) Distribution of naive, central memory (TCM), effector memory (TEM), or effector memory RA+ (TEMRA) cells within the CD4+ and CD8+ pool within the CD38+ T-cell compartment of male patients (n = 23). Data are presented in box-and-whisker format: the 25th and 75th percentile form the box, with the median marked as a line, the 10th and 90th percentiles as whiskers, and all observations <10th percentile and >90th percentile as circles.
Correlation of sex and stage with CD38-expression levels in CD4+ and CD8+ T cells. CD38-expression levels were significantly different in CD8+ T cells from male (n = 39) compared with female patients (n = 25), whereas the opposite, higher expression of CD38 in peripheral CD8+ T cells from healthy age-matched females (n = 13) compared with males (n = 20) was observed (A). (B) It was also in the CD8+ T-cell subset where a stage-dependent increase in CD38-expression levels was observed in male B-CLL patients. (C) Distribution of naive, central memory (TCM), effector memory (TEM), or effector memory RA+ (TEMRA) cells within the CD4+ and CD8+ pool within the CD38+ T-cell compartment of male patients (n = 23). Data are presented in box-and-whisker format: the 25th and 75th percentile form the box, with the median marked as a line, the 10th and 90th percentiles as whiskers, and all observations <10th percentile and >90th percentile as circles.
Combined analysis of CD38 of B-CLL and T cells for risk assessment in B-CLL
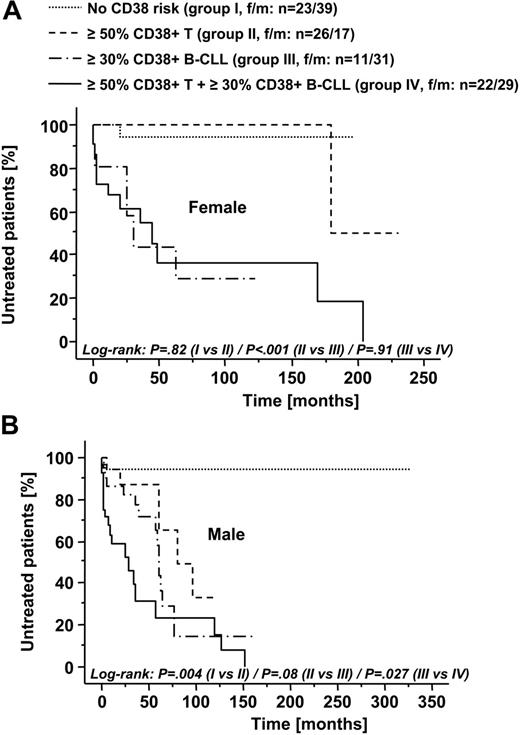
Since we observed an association of CD38-expression levels of both neoplastic cells and T cells from male patients with higher Rai stages, we next evaluated whether they represent independent risk parameters, and, if so, whether combined analysis of CD38 expression of B-CLL cells and T cells would more precisely identify high-risk patients than either parameter alone. Using a cutoff of at least 30% for CD38 expression in B-CLL cells and a cutoff of at least 50% for CD38 expression in T cells (see “Statistical analysis”), we classified both female and male patients into 4 risk groups characterized as no CD38 risk (group I with < 50% CD38+ T cells and < 30% CD38+ B-CLL cells), at least 50% CD38+ T cells (group II), at least 30% CD38+ B-CLL (group III), and at least 30% CD38+ B-CLL and at least 50% CD38+ T cells (group IV). Mean age did not significantly differ between these groups (data not shown). As presented in a Kaplan-Meier plot in Figure 5A, in female patients CD38-expression profile of B-CLL cells but not of T cells negatively interacted with treatment-free survival. In male patients, however, CD38-expression profile of T cells alone identified patients with a more aggressive course of disease. Furthermore, we observed a continuous decrease in treatment-free survival in male patients from group I to group IV (Figure 5B).
CD38 expression of T cells is an independent prognostic factor for male B-CLL patients
The more aggressive course of disease in male patients with at least 50% CD38+ T cells might also be linked to a more unfavorable risk profile of tumor cells within this group. We therefore evaluated whether tumor cells from these patients differed in Zap-70 expression, Ig VH mutational status, or genomic aberrations. As presented in Table 2, the tumor-cell population of both groups of patients had a comparable molecular risk profile. There was even a borderline underrepresentation of unfavorable Zap-70+ B-CLL in the group of male patients with at least 50% CD38+ T cells (Table 2), making it unlikely that the negative prognostic impact of CD38 expression in T cells was just a selection bias resulting from the accumulation of patients with other unfavorable prognostic factors in this population.
Correlation of CD38-expression profile of T cells with Zap-70, Ig VH mutational status, and genomic aberrations in male patients
Parameter . | No CD38 risk, no. patients* . | At least 50% CD38+ T cells, no patients . | P . |
|---|---|---|---|
| Zap-70 | .05 | ||
| Less than 20% positive B-CLL cells | 26 | 15 | |
| At least 20% positive B-CLL cells | 12 | 1 | |
| Homology to Ig VH germ line | .63 | ||
| Less than 98% homology | 21 | 13 | |
| At least 98% homology | 5 | 2 | |
| Genomic aberrations | .59 | ||
| Absent or 13q | 26 | 12 | |
| del11q, del17p, 12+ | 4 | 1 |
Parameter . | No CD38 risk, no. patients* . | At least 50% CD38+ T cells, no patients . | P . |
|---|---|---|---|
| Zap-70 | .05 | ||
| Less than 20% positive B-CLL cells | 26 | 15 | |
| At least 20% positive B-CLL cells | 12 | 1 | |
| Homology to Ig VH germ line | .63 | ||
| Less than 98% homology | 21 | 13 | |
| At least 98% homology | 5 | 2 | |
| Genomic aberrations | .59 | ||
| Absent or 13q | 26 | 12 | |
| del11q, del17p, 12+ | 4 | 1 |
The definition of “no CD38 risk” refers to patients with less than 50% CD38+ T cells and less than 30% CD38+ B-CLL cells.
Univariate analysis revealed that CD38 positivity of at least 30% of B-CLL cells and at least 50% of T cells as well as Ig VH mutational status, genomic aberrations, Zap-70 expression, and clinical stage significantly influenced time from diagnosis to treatment in male patients (Table 3). Multivariate analysis identified CD38 expression in B-CLL cells and CD38 expression in T cells as independent prognostic factors for treatment-free survival (Table 3). Both parameters remained significant when all other risk factors that predicted for treatment-free survival in the univariate analysis (ie, Ig VH mutational status, genomic aberrations, Zap-70 expression, and Rai stage) were included in the model. Of clinical importance, Zap-70, genomic aberrations, and Rai stage became insignificant in the multivariate analysis (Table 3).
Cox regression analysis of treatment-free survival of male B-CLL patients
. | Univariate . | . | Multivariate . | . | ||
|---|---|---|---|---|---|---|
| Parameter . | Relative risk ratio (95% CI) . | P . | Relative risk ratio (95% CI) . | P . | ||
| CD38+ B-CLL cells 30% or greater | 6.6 (3.0-14.4) | < .001 | 6.1 (1.6-23.8) | .009 | ||
| CD38+ T cells 50% or greater | 3.1 (1.6-5.9) | < .001 | 4.0 (1.3-12.5) | .01 | ||
| Ig VH mutational status | 3.1 (1.3-7.2) | < .001 | 3.3 (.96-11.3) | .05 | ||
| High-risk genomic aberrations, 11q-, 17p-, 12+ | 3.0 (1.5-6.1) | .003 | .5 (.15-1.7) | .28 | ||
| Zap-70+ B-CLL cells 20% or greater | 1.8 (1.0-3.4) | .05 | 1.1 (.42-3.3) | .76 | ||
| Rai stage | ||||||
| Intermediate, 1-2 | 9.0 (1.2-67.4) | .03 | 1.8 (2.1-15.1) | .59 | ||
| High, 3-4 | 24.1 (3.2-181.5) | .002 | 1.7 (.18-16.7) | .63 | ||
. | Univariate . | . | Multivariate . | . | ||
|---|---|---|---|---|---|---|
| Parameter . | Relative risk ratio (95% CI) . | P . | Relative risk ratio (95% CI) . | P . | ||
| CD38+ B-CLL cells 30% or greater | 6.6 (3.0-14.4) | < .001 | 6.1 (1.6-23.8) | .009 | ||
| CD38+ T cells 50% or greater | 3.1 (1.6-5.9) | < .001 | 4.0 (1.3-12.5) | .01 | ||
| Ig VH mutational status | 3.1 (1.3-7.2) | < .001 | 3.3 (.96-11.3) | .05 | ||
| High-risk genomic aberrations, 11q-, 17p-, 12+ | 3.0 (1.5-6.1) | .003 | .5 (.15-1.7) | .28 | ||
| Zap-70+ B-CLL cells 20% or greater | 1.8 (1.0-3.4) | .05 | 1.1 (.42-3.3) | .76 | ||
| Rai stage | ||||||
| Intermediate, 1-2 | 9.0 (1.2-67.4) | .03 | 1.8 (2.1-15.1) | .59 | ||
| High, 3-4 | 24.1 (3.2-181.5) | .002 | 1.7 (.18-16.7) | .63 | ||
The analysis included 75 male patients (for whom all risk parameters including high-risk genomic aberrations and Ig VH mutational status were available). The median follow-up for this cohort was 59 months (range, 2-327 months).
CD38-expression profiles of B-CLL cells and T cells significantly correlate with time from diagnosis to first treatment in male patients with B-CLL. Female (A) and male (B) patients were stratified into 4 risk groups according to the expression levels of CD38 in B-CLL cells and T cells (group I: no CD38 risk, ie, CD38 expression in < 50% of T cells and < 30% of B-CLL cells; group II: ≥ 50% CD38+ T cells; group III: ≥ 30% CD38+ B-CLL cells; group IV: ≥ 50% CD38+ T cells and ≥ 30% CD38+ B-CLL cells). Kaplan-Meier analysis of treatment-free survival revealed CD38+ B-CLL cells as a prognostic factor both in female and male patients. In contrast, the presence of at least 50% CD38+ T cells predicted for significantly shorter treatment-free survival in male but not in female patients. Numbers of patients in the respective subgroups are indicated. P values for comparison of risk groups using the log-rank test are presented.
CD38-expression profiles of B-CLL cells and T cells significantly correlate with time from diagnosis to first treatment in male patients with B-CLL. Female (A) and male (B) patients were stratified into 4 risk groups according to the expression levels of CD38 in B-CLL cells and T cells (group I: no CD38 risk, ie, CD38 expression in < 50% of T cells and < 30% of B-CLL cells; group II: ≥ 50% CD38+ T cells; group III: ≥ 30% CD38+ B-CLL cells; group IV: ≥ 50% CD38+ T cells and ≥ 30% CD38+ B-CLL cells). Kaplan-Meier analysis of treatment-free survival revealed CD38+ B-CLL cells as a prognostic factor both in female and male patients. In contrast, the presence of at least 50% CD38+ T cells predicted for significantly shorter treatment-free survival in male but not in female patients. Numbers of patients in the respective subgroups are indicated. P values for comparison of risk groups using the log-rank test are presented.
Kaplan-Meier analysis also revealed a trend to shorter overall survival in male patients with at least 50% CD38+ T cells compared with male patients with less than 50% CD38+ T cells (log-rank, P = .08; data not shown). Significance level might not be reached because of the relatively high proportion of censored cases in this analysis.
Discussion
In the present study we analyzed CD38 expression in B-CLL cells and CD5+ T cells of B-CLL patients. We confirmed CD38 expression of B-CLL cells to be a stable, independent marker for a more aggressive course of disease. The prognostic value of CD38 expression of B-CLL cells was independent of the sex of the patients. For the first time, we also identified expression of CD38 in T cells as an independent risk marker in male but not in female patients. The reasons for these sex differences in the role of CD38+ T cells for disease progression are unknown at present. A comparable complex interaction between sex and Ig VH mutational status has been described previously23 but its contribution to the inferior prognosis of male B-CLL patients remains unresolved at present. An obvious explanation for sex differences would be the modulation of immune functions by sex hormones. In thymocytes and mature T cells, expression of both estrogen and androgen receptors has been described,24-26 and hormone stimulation significantly interacts with T-cell development and function.27 Intriguingly, estrogen has been demonstrated to up-regulate CD38 expression and functions in the myometrium.22 In line with this is our observation of higher expression levels of CD38 in CD5+ T cells from female compared with male patients (Figure 2B). This sex-specific difference in CD38 expression could also be observed when CD8+ T cells from age-matched healthy individuals (n = 33) were analyzed (Figure 4A). However, the opposite, higher CD38 expression in males versus females was observed in the CD8+ T-cell subset from B-CLL patients (Figure 4A), implying that factors other than sex also interact with CD38 expression in T cells from B-CLL patients.
Our observation that CD38+ T cells contribute to disease progression raises the question about the underlying molecular mechanisms. In general, CD38 can be detected in peripheral naive mature T cells; however, upon activation and conversion from naive to memory status, CD38-expression level constantly decreases.28 Our analysis of the CD4 T-cell pool (Figure 4C) corroborates this previous finding. However, a different regulation has to be assumed for CD8+ T cells in which CD38 expression was detected mainly in cells at their most mature stage (Figure 4C). CD38, though itself lacking intracellular signaling activity, has been shown to be critical for T-cell functions such as T-cell receptor (TCR)–dependent activation, cytokine production, proliferation, cytolytic activity,29 and, finally, activation-induced cell death.30 Since the cytokines produced by CD38+ T cells such as interleukin-2, interleukin-4, and interferon-γ29,31 have all been shown to support survival of B-CLL cells in vitro,32-34 enhanced cytokine production by CD8+ T cells might significantly contribute to disease progression of B-CLL. Interactions of T cells with clinical course in B-CLL is not an unexpected finding, since alterations within the T-cell compartment that contribute to progressive disease have already been reported previously. Among these changes, aberrant expression of chemokines,14 cytokines,35,36 and costimulatory and adhesion molecules have been described, and a major part of these changes seems to be directly triggered by the tumor clone itself.37 Besides changes within the conventional CD4+ and CD8+ T-cell pool, increased numbers of regulatory T cells have also been reported and associated with the reduced proliferative capacity of T cells in B-CLL.38 Despite the established role of regulatory T cells in inhibiting T-cell proliferation, their role in tumor progression remains to be determined in B-CLL.38 The results from our study argue against the contribution of CD4+ regulatory T cells to the CD38+ T-cell pool, since sex- and stage-dependent differences were exclusively observed in the CD8+ T-cell subset (Figure 4). However, further investigations are certainly required to determine whether CD38+ T cells are also controlled by regulatory T cells, as demonstrated for T cells in general from both healthy individuals and tumor patients. It is also clear from our observation of a sex-specific role of CD38+ T cells in B-CLL (Figure 5) that an even more complex interaction between T cells and tumor cells than anticipated might exist.
For risk assessment of B-CLL patients, the Rai and Binet staging systems were developed 30 years ago. These standard methods, however, cannot completely predict the course of disease in an individual patient at diagnosis. Genomic aberrations,1,39 absence of somatic hypermutation in the immunoglobulin heavy chain (Ig VH) region of the leukemic clone,7,18,40 and CD38 positivity of B-CLL cells7-10 have recently been identified as independent prognostic parameters. The suitability of a molecular marker for risk assessment also depends on its stability and applicability in clinical laboratory practice. Analysis of CD38 in both B-CLL cells and T cells of male patients meets these criteria. (1) It can be assessed in a single measurement using the gating strategy presented in Figure 1. (2) Although CD38 expression was reported to change upon activation in T cells,28 it remained stable over time in T cells from the majority (∼90%) of patients (data not shown). CD38 expression in B-CLL cells has been demonstrated to be modulated by interferons,41 which might also explain variations in individual patients over time.8 Serum levels of interferons should increase during infectious episodes, which might accumulate in patients with more advanced disease. However, we failed to observe a correlation between changes in CD38 expression of B-CLL or T cells and infectious episodes (Figure 3; data not shown), suggesting regulation by factors different from interferons. Our observation of changes in CD38-expression levels in T cells over time in 10% of patients cells might represent a caveat for the use of these parameters for risk assessment in B-CLL. However, the same frequency of changes was observed for CD38-expression levels in B-CLL cells in our study and by others,8 suggesting similar values as prognostic factors.
From our study, it can be concluded that combined analysis of CD38 in B-CLL cells and T cells for identification of risk groups in male B-CLL might be superior to the use of either parameter alone. For clinical risk evaluation in B-CLL, it is unlikely that one single parameter (being CD38-expression levels of T cells or others) will be enough for accurate prediction of clinical outcome, considering the high complexity of this disease. However, by adding novel independent risk parameters such as CD38 expression in T cells (which indeed can be assessed easily in clinical routine) to established ones, as we propose from the results of our study, the predictive power of molecular profiling should significantly increase. The clinical value of CD38 expression in T cells is also underlined by the fact that multivariate analysis revealed CD38 expression of B-CLL cells and T cells and Ig VH mutational status as independent parameters, whereas important risk factors such as Zap-70, genomic aberrations, and Rai stage became insignificant in this model (Table 3). The prognostic power of CD38 expression in T cells certainly has to be confirmed in prospective studies. The negative impact of CD38+ T cells on the course of disease might also become an important issue in situations when autologous T cells are expanded and/or stimulated for immune therapies and underlines the need for further work on the biologic background of these sex-specific interactions.
Prepublished online as Blood First Edition Paper, July 6, 2006; DOI 10.1182/blood-2006-03-010553.
Supported by grants of the Austrian Science Foundation (I.T., P16153; R.G., SFB021), the Tyrolean Cancer Aid, the Austrian Cancer Aid, the Province of Salzburg (R.G. and I.T.), and the Austrian National Bank (A.E., grant no. 10990). C.H. was supported by the Verein fuer Klinische Malignom-und Zytokinforschung, Salzburg, Austria.
Presented in part in abstract form at the 47th annual meeting of The American Society of Hematology, Atlanta, GA, December 10, 2005.42
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We are indebted to Ms Rajam Csordas-Iyer for critical reading and editorial assistance.
The authors indicate no potential conflicts of interest.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal