Abstract
The deregulation of inflammatory response during sepsis seems to reflect the overproduction of mediators, which suppress leukocyte functions. We investigated the intracellular mechanisms underlying the inability of neutrophils from severe septic patients to migrate toward chemoattractants. Patients with sepsis (52) and 15 volunteers were prospectively enrolled. Patients presented increased circulating levels of tumor necrosis factor-α, interferon-γ, interleukin (IL)–8, and IL-10. Patients showed reduced neutrophil chemotaxis to formyl-methionyl-leucyl-phenylalanine (FMLP), leukotriene B4 (LTB4) or IL-8. No difference in the transcription or expression of the IL-8 receptor, CXCR1, was detected in neutrophils from controls and patients. However, septic neutrophils failed to increase tyrosine phosphorylation and actin polymerization in response to IL-8 or LTB4. In contrast, septic neutrophils, similar to controls, showed phagocytic activity that induced actin polymerization and augmented phosphotyrosine content. Treatment of control neutrophils with cytokines and lipopolysaccharide (LPS) to mimic endogenous septic environment inhibited actin polymerization and tyrosine phosphorylation in response to IL-8 or LTB4. High expression of G protein–coupled receptor kinase 2 (GRK2) and GRK5 was detected in septic neutrophils and control cells treated with cytokines plus LPS. Data suggest that endogenous mediators produced during sepsis might continually activate circulating neutrophils, leading to GRK activation, which may induce neutrophil desensitization to chemoattractants.
Introduction
Sepsis is a complex clinical syndrome resulting from a damaging host response to infection.1 In the United States, more than 700 000 patients per year develop sepsis, with mortality rates reported to vary between 30% and 70%, despite the best available supportive care.2
Polymorphonuclear neutrophils (PMNs) play the first line in the host defense against microorganisms, being recruited to the inflammatory sites by chemoattractants such as leukotriene B4 (LTB4) and chemokines.3,4 Once emigrated, these leukocytes are able to phagocytose and to generate large amounts of reactive oxygen and nitrogen species, such as hydrogen peroxide and nitric oxide, which are crucial products for the microbicidal activity of these cells.5,6 As neutrophils appear to play a crucial role in the control of the infectious process, one can hypothesize that a deficient migratory ability of neutrophils may aggravate infections. Indeed, impairment of neutrophil migration has been reported in leukemia,7 diabetes,8 and AIDS,9 diseases associated with high susceptibility to infection. Furthermore, previous studies from our group showed that failure of neutrophil migration is observed in severe sepsis induced by cecal ligation and puncture and Staphylococcus aureus administration.10,11 In these lethal models, failure of neutrophil migration to the site of infection was accompanied by increased numbers of bacteria in the peritoneal fluid and blood. Conversely, in sublethal infection in which massive neutrophil migration was observed, bacterial infection was restricted to the peritoneal cavity, and the animals exhibited a low mortality rate.10,11 More recently, we have also reported that blood neutrophils obtained from patients with sepsis failed to respond in vitro to the chemotactic stimuli FMLP and LTB4. This unresponsiveness was directly associated to a poor prognosis.12
Evidence from literature suggests that the high levels of circulating cytokines/chemokines observed in severe sepsis may mediate the impairment of neutrophil migration, in addition to being involved in the deleterious physiopathologic findings of the disease, such as coagulation disorders, cardiovascular collapse, and organ failure.13 The intravenous administration of tumor necrosis factor-α (TNF-α) or interleukin-8 (IL-8) inhibited neutrophil migration to mouse peritoneal cavity and anti–TNF-α antibody partially prevented the inhibition of neutrophil migration in endotoxemia model.14 However, the molecular mechanisms involved in the reduced ability of neutrophils to migrate during sepsis were not completely clarified.
Independent of their chemical nature, most chemoattractants exert their action via binding to specific G protein–coupled receptors (GPCRs) controlling complex cascades of signaling events. Among these, activation of the tyrosine kinase pathway is a key event to mediate actin filament assembly, a fundamental step for neutrophil responses, including cell locomotion and phagocytosis.15 However, desensitization of GPCR family receptors is an important determinant of the intensity and duration of agonist stimulation. G protein–coupled receptor kinases (GRKs), specific kinases interacting with GPCR protein, induce receptor phosphorylation and thereby signal GPCR desensitization in the continuing presence of chemoattractants.16 Therefore, an increased expression of GRKs could augment chemotactic receptor desensitization and in turn reduce neutrophil migratory response.17
In the present study, we hypothesized that the impairment of neutrophil migration observed in sepsis could result from signal receptor desensitization mediated by continuous and excessive chemotactic receptor activation. To address this question, we investigated the intracellular mechanisms underlying the inability of neutrophils from severe septic patients to migrate toward IL-8 and LTB4. We have demonstrated that the systemic septic environment can overstimulate circulating neutrophils, therefore inducing GRK2 and GRK5 expression and receptor desensitization. GRKs, probably leading to GPCR phosphorylation, might impair chemoattractant-induced tyrosine kinase activity and the subsequent rearrangement of the actin network, thus compromising the ability of neutrophils from patients with sepsis to migrate.
Patients, materials, and methods
Patients
The prospectively enrolled patients admitted and treated for sepsis in the Intensive Care Unit of the Department of Surgery, Faculty of Medicine of Ribeirão Preto, University of São Paulo, Brazil, from June 2001 to June 2003. All patients presented clinical and/or laboratory variables that fulfilled the criteria for sepsis.18 Healthy male and female volunteers served as controls. The study was approved by the Human Subjects Institutional Committee of the Faculty of Medicine of Ribeirão Preto, University of São Paulo (HCRP 4989/99), and written informed consent was obtained from patients (or their caretakers) and volunteers. Patients were excluded for the following reasons: older than 75 years or younger than 15 years; mean arterial pressure less than 50 mm Hg; bradycardia (heart rate < 50 bpm) or tachycardia (heart rate > 125 bpm); intervention with high doses of vasopressor agents (dopamine > 7.5 μg/kg/min, dobutamine > 10 μg/kg/min); oliguria (urine output < 50 mL/h); irreversible circulatory shock; and when informed consent could not be obtained. All patients enrolled were evaluated according to the Acute Physiological and Chronic Health Evaluation (APACHE) II score.
Neutrophil isolation
Blood neutrophils from patients with sepsis or healthy volunteers were isolated by Percoll gradient19 and suspended in RPMI medium (97% of viable cells, as assessed by trypan blue exclusion). In some experiments, neutrophils isolated from healthy subjects were pretreated (2 hours, 37°C) with IL-1β (2 ng/mL) plus interferon-γ (IFN-γ; 10 ng/mL) and lipopolysaccharide (LPS; 10 μg/mL), and further incubated (1 hour, 37°C) with IL-8 (10–9 M) or LTB4 (10–8 M).
Neutrophil migration assay
Chemotaxis was assayed in 48-well microchemotaxis chambers (Neuro Probe, Gaithersburg, MD) using 5-μm PVP-free polycarbonate filter.19 Neutrophils (106 cells/mL in RPMI-0.01% bovine serum albumin [BSA]) were allowed to migrate toward FMLP (10–7 M), LTB4 (10–8 M), or IL-8 (10–9 M), or medium alone (random migration; 37°C, 5% CO2). After 1 hour, filters were removed, fixed, and stained, and neutrophils that migrated through the membrane were counted under a light microscope on at least 5 randomly selected fields.19 Each sample was assayed in triplicate. Results are expressed as number of neutrophils per field.
Phagocytosis assay
The ability of septic or healthy neutrophils to phagocytose (1 hour, 37°C, 5% CO2) human plasma-opsonized zymosan (10 particles/cell) was assayed,20 in the presence or not of cytochalasin B (CyB; 15 μg/mL) or genistein (80 μM), and expressed as the phagocytic index (% of phagocytic cells × number of interiorized particles). The contents of F-actin and phosphotyrosine of phagocyting cells were analyzed by fluorescence microscopy.
Cytokine assay
Plasma circulating levels of TNF-α, IFN-γ, IL-8, and IL-10 were determined by double-ligand enzyme-linked immunosorbent assay (ELISA).12 The results are expressed as picograms of cytokine per milliliter of serum.
RNA extraction and RT-PCR analysis
Total RNA extracted from neutrophils by Trizol (Gibco-BRL, Carlsbad, CA) was reverse-transcripted using Superscript II reverse transcriptase (RT; Gibco-BRL).21 The primers for CXCR1 amplification were sense (5′) 5′ CAG ATC CAC AGA TGT GGG AT-3′; and antisense, (3′) 5′ AGC AGC CAA GAC AAA CAA ACT-3′ amplifying 468 bp. For β-actin: sense, (5′) 5′-GGC GAC GAG GCC CAG A-3′; and antisense, (3′)5′-CGA TTT CCC GCT CGG C-3′ amplifying 463 bp. The reverse transcription product was amplified, and the polymerase chain reaction (PCR) products were separated in 1.5% agarose gel and identified by ethidium bromide.21,22 Gel Pro-Analyzer 3.1 software (Media Cybernetics, Silver Spring, MD) was used for densitometric analysis. Results are shown as units of β-actin mRNA.
CXCR1 expression
Quantification of CXCR1 expression surface antigens was performed in flow cytometry using PE-conjugated antibodies anti-CXCR1 (R&D Systems, Minneapolis, MN) or anti-γ1 and γ2 (BD Pharmingen, San Diego, CA) using a FACSCalibur flow cytometer and the CellQuest software (Becton Dickinson, San Jose, CA).23
Fluorescence microscopy for F-actin, phosphotyrosine or GRK
The contents of F-actin, GRK2, GRK5, and tyrosine-phosphorylated residues were determined in neutrophils stimulated with IL-8, LTB4, or zymosan by fluorescence microscopy. After treatment, neutrophil slides were prepared by cytospin centrifuge, and F-actin was stained with TRITC-labeled phalloidin (Sigma, St Louis, MO).19 Immmunocytochemistry for phosphotyrosine or GRKs was developed using biotin conjugated-primary antibodies (anti-pTyr, anti-GRK2, or anti-GRK5, 1:50; Santa Cruz Biotechnology, Santa Cruz, CA) and streptavidin-conjugated FITC (1:50; Caltag, Burlingame, CA).24 Microscopic analysis of fluorescent images was done using an epifluorescence microscope (Olympus BX40-F4; Tokyo, Japan) equipped with appropriate filters for FITC or TRITC, and using 100×/1.30 NA oil-immersion objectives. Image capturing was performed with a cooled charged-coupled device CoolSNAP camera (Photometrics, Tucson, AR). All images were captured using identical camera settings: time of exposure, brightness, contrast and sharpness, and an appropriated white balance set according to the fluorescence filter and acquired and analyzed by Image-Pro Plus 4.0 (Media Cybernetics). The mean fluorescence density was determined from a linear measurement of individual cells' fluorescence. All cells of at least 5 randomly chosen fields of each slide, performed in duplicate, were analyzed from at least 10 individual experiments. Results are shown as the mean ± SD of the mean fluorescence densities of each field subtracted from the mean density of the area measured as background for each individual slide. Figures show representative gray images taken by Adobe Photoshop software (Adobe Systems, San Jose, CA).
Immunoblot for GRK
GRK2 and GRK5 expression in septic neutrophils or in cells treated with cytokines plus LPS and further stimulated with IL-8 or LTB4 were determined by immunoblots19,24 with polyclonal anti-GRK2 or anti-GRK5 antibody (Ab; 1:500; Santa Cruz Biotechnology). Immunoreactive proteins were visualized on the PVDF (Hybond-P; Amersham Biosciences. Arlington Heights, IL) by 3,3′-diaminobenzidine, and band densitometry was quantified by Scion Image Software (Scion, Frederick, MD).
Statistical analysis
Data were analyzed by Prism 3 software (GraphPad Software, San Diego, CA). The Kolmogorov-Smirnov test was used to verify data distribution. Results are expressed as mean ± SD, except for cytokine levels, expressed as medians and 25th and 75th percentiles. Unpaired t test and Mann-Whitney were used for comparing means or medians between 2 groups, respectively. Analysis of variance (ANOVA) followed by Bonferroni was used for multiple comparisons for unpaired data. A P value of less than .05 was taken as statistically significant.
Results
Clinical data
Patients with sepsis (52) from different sources were enrolled in this study. The main diagnoses included pneumonia in 17 (35.4%) patients, intra-abdominal infection in 6 (12.5%) patients, and trauma in 4 (8.3%) patients. The remaining 21 (43.7%) patients consisted of diverse causes and miscellaneous (Table 1). Characteristics of the patients concerning age, sex, APACHE II score, and bacteriological data are summarized in Table 1. Controls consisted of 15 healthy volunteers, 7 (46.7%) men and 8 (53.3%) women, with a mean age of 30.9 ± 7.4 years (range, 23-47 years).
Characteristics of patients with sepsis
. | Data . |
|---|---|
| No. patients | 52 |
| Average age, y (range) | 52.1 (17-89) |
| Male/female ratio, % | 51.9/48.1 |
| APACHE II score, mean ± SD | 26.66 ± 7.68 |
| Microorganism isolates, no. patients | |
| Gram-negative rods | 5 |
| Gram-positive rods | 1 |
| Gram-positive coccus | 5 |
| Levenings | 3 |
| Miscellaneous | 12 |
| Negative culture | 24 |
| Not determined | 2 |
. | Data . |
|---|---|
| No. patients | 52 |
| Average age, y (range) | 52.1 (17-89) |
| Male/female ratio, % | 51.9/48.1 |
| APACHE II score, mean ± SD | 26.66 ± 7.68 |
| Microorganism isolates, no. patients | |
| Gram-negative rods | 5 |
| Gram-positive rods | 1 |
| Gram-positive coccus | 5 |
| Levenings | 3 |
| Miscellaneous | 12 |
| Negative culture | 24 |
| Not determined | 2 |
Cytokine concentrations
Cytokine levels were determined in serum samples of patients with sepsis and controls. The median time between the onset of sepsis and blood collection was 3 days (range, 1-5 days). Table 2 shows that median serum levels of TNF-α, IFN-γ, IL-8, and IL-10 were significantly increased in patients with sepsis compared with controls.
Concentrations of cytokines detected in sera from controls and patients with sepsis
. | TNF-α . | . | IFN-γ . | . | IL-8 . | . | IL-10 . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
. | No. individuals . | Median concentration, pg/mL (25th-75th percentiles) . | No. individuals . | Median concentration, pg/mL (25th-75th percentiles) . | No. individuals . | Median concentration, pg/mL (25th-75th percentiles) . | No. individuals . | Median concentration, pg/mL (25th-75th percentiles) . | ||||
| Controls | 9 | 0 (0-71.7) | 5 | 170.8 (0-283.6) | 9 | 0 (0-18.4) | 9 | 155.5 (29.1-233.8) | ||||
| Patients | 9 | 202.4 (147.1-334.6)* | 26 | 528.1 (176-815)* | 12 | 155.7 (122.8-257.5)* | 12 | 343.2 (256-465.9)† | ||||
. | TNF-α . | . | IFN-γ . | . | IL-8 . | . | IL-10 . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
. | No. individuals . | Median concentration, pg/mL (25th-75th percentiles) . | No. individuals . | Median concentration, pg/mL (25th-75th percentiles) . | No. individuals . | Median concentration, pg/mL (25th-75th percentiles) . | No. individuals . | Median concentration, pg/mL (25th-75th percentiles) . | ||||
| Controls | 9 | 0 (0-71.7) | 5 | 170.8 (0-283.6) | 9 | 0 (0-18.4) | 9 | 155.5 (29.1-233.8) | ||||
| Patients | 9 | 202.4 (147.1-334.6)* | 26 | 528.1 (176-815)* | 12 | 155.7 (122.8-257.5)* | 12 | 343.2 (256-465.9)† | ||||
Data are medians of the levels of cytokines detected in serum samples obtained 1 to 5 days after the onset of sepsis.
P < .05 compared with respective controls (Mann-Whitney Utest).
P < .01 compared with respective controls (Mann-Whitney Utest).
Neutrophil chemotaxis
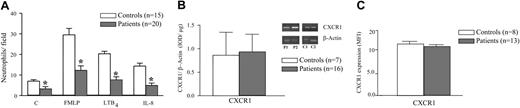
The ability of neutrophils obtained from 20 patients with sepsis (mean age ± SD, 56.3 ± 17.2 years; range, 26-83 years) and 15 healthy donors to migrate in vitro toward the chemoattractants was assayed. Figure 1A shows that even random migration was found to be reduced in patients. Moreover, migration of neutrophils toward all chemoattractants tested was significantly inhibited (> 50%) in patients with sepsis when compared with controls. Interestingly, although controls and patients differed in age distribution, when patients with sepsis (n = 6, mean age ± SD, 39.2 ± 7.6 years; range, 23-47 years) were analyzed within the same range of controls, impaired chemotactic response of septic neutrophils was either observed versus control cells (P < .05, unpaired t test; asterisk in Figure 1A), respectively, for RPMI (3.5 ± 3.1 vs 7.2 ± 2.9 neutrophils/field), FMLP (13.8 ± 9.4 vs 30.5 ± 12.1 neutrophils/field), LTB4 (9.5 ± 8.8 vs 21.2 ± 5.2 neutrophils/field), and IL-8 (7.2 ± 6.0 vs 14.3 ± 5.3 neutrophils/field).
IL-8 receptor expression
To evaluate whether inhibition of chemotactic response of septic neutrophils was related to alterations in chemoattractant receptor expression, CXCR1 mRNA and protein levels were determined by RT-PCR and flow cytometry, respectively. As seen in Figure 1B there was no significant difference in CXCR1 mRNA expression in neutrophils from controls or patients with sepsis. In agreement, no difference between the median fluorescence intensity for CXCR1 was detected between groups (Figure 1C), indicating that cell membrane CXCR1 expression was similar in neutrophils from controls and patients with sepsis.
Actin polymerization and phosphotyrosine content
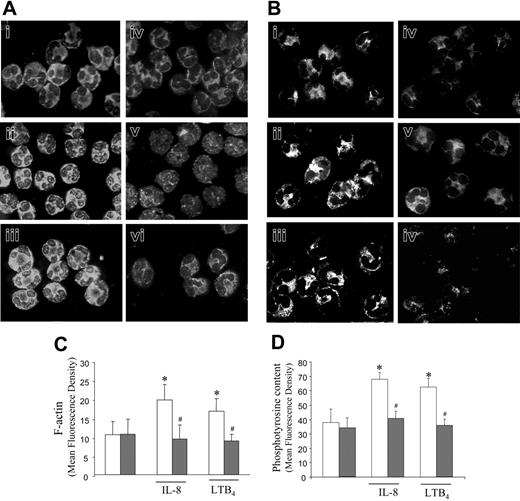
During migration, concurrent changes in actin cytoskeleton enable cells to move toward the stimulus. In response to external stimuli, there is a dynamic remodeling of the cytoskeleton, which is involved with changes in cell shape and motility and signal transduction.25,26 We investigated the alterations in actin cytoskeleton dynamic and the pattern of tyrosine kinase activation in neutrophils from patients with sepsis and controls after stimulation with IL-8 or LTB4. Figure 2 shows that under basal conditions (nonstimulation) no significant difference in F-actin content was observed in neutrophils from both controls (Figure 2Ai) and septic patients (Figure 2Aiv). However, while treatment of control cells with IL-8 (Figure 2Aii) or LTB4 (Figure 2Aiii) increased actin polymerization leading to an increase in cell fluorescence, no alteration in actin assembly was observed in neutrophils from patients with sepsis treated with IL-8 (Figure 2Av) or LTB4 (Figure 2Avi). A similar pattern of results was observed by immunocytochemistry for phosphotyrosine in both groups. There was no difference in the fluorescence intensity for phosphotyrosine in cells from controls (Figure 2Bi) or patients with sepsis (Figure 2Biv) incubated with medium alone. However, while the addition of IL-8 or LTB4 to cultures significantly increased the phosphotyrosine content in control cells (Figure 2Bii and 2Biii, respectively), neutrophils from patients with sepsis were not able to respond to IL-8 (Figure 2Bv) or LTB4 (Figure 2Bvi). Differences in the intensity of fluorescence among all treatments are demonstrated in Figure 2C and 2D that clearly show the inability of septic neutrophils in signaling with an increase in actin polymerization (Figure 2C) and tyrosine phosphorylation (Figure 2D) after chemoattractant stimulation.
Chemotactic activity and CXCR1 receptor expression on neutrophils. (A) Chemotactic response of septic neutrophils (▦, n = 20) or control cells (□, n = 15) toward FMLP (10–7 M), LTB4 (10–8 M), or IL-8 (10–9 M), or medium alone (C indicates controls) in a microchemotaxis chamber. Data are means ± SD. *P < .01 compared with respective control. (B) IL-8 receptor gene expression. Expression of CXCR1 mRNA in neutrophils from controls (□, n = 7) and patients with sepsis (▦, n = 16) evaluated by RT-PCR as described in “Patients, materials, and methods.” Results are shown as units of β-actin mRNA. Insert shows 2 representative experiments showing CXCR1 and β-actin mRNA expression in neutrophils from controls (C1, C2) and patients with sepsis (P1, P2). (C) Flow cytometric analysis of CXCR1 receptor expression on neutrophils from controls (□, n = 8) and patients with sepsis (▦, n = 13). Results are shown as median fluorescence intensity (MFI). Error bars indicate SD.
Chemotactic activity and CXCR1 receptor expression on neutrophils. (A) Chemotactic response of septic neutrophils (▦, n = 20) or control cells (□, n = 15) toward FMLP (10–7 M), LTB4 (10–8 M), or IL-8 (10–9 M), or medium alone (C indicates controls) in a microchemotaxis chamber. Data are means ± SD. *P < .01 compared with respective control. (B) IL-8 receptor gene expression. Expression of CXCR1 mRNA in neutrophils from controls (□, n = 7) and patients with sepsis (▦, n = 16) evaluated by RT-PCR as described in “Patients, materials, and methods.” Results are shown as units of β-actin mRNA. Insert shows 2 representative experiments showing CXCR1 and β-actin mRNA expression in neutrophils from controls (C1, C2) and patients with sepsis (P1, P2). (C) Flow cytometric analysis of CXCR1 receptor expression on neutrophils from controls (□, n = 8) and patients with sepsis (▦, n = 13). Results are shown as median fluorescence intensity (MFI). Error bars indicate SD.
Effect of phagocytic activity of septic neutrophils on actin polymerization and phosphotyrosine content
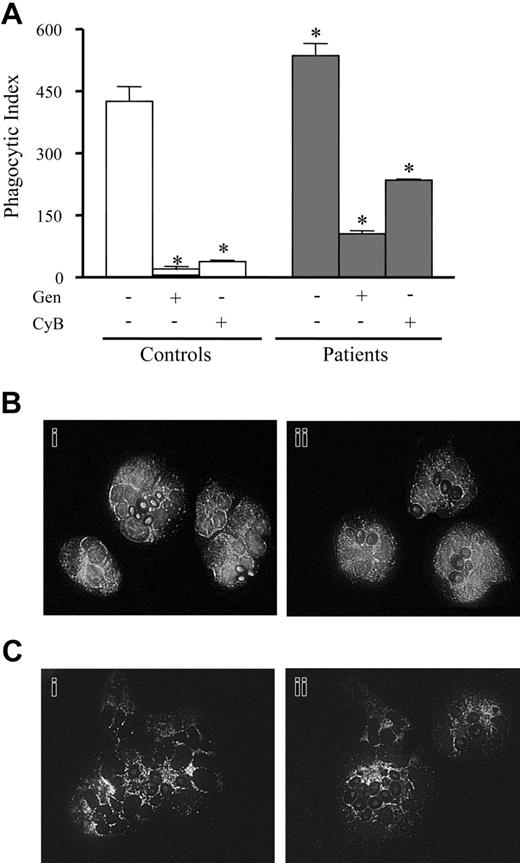
Phagocytosis is absolutely dependent on actin cytoskeleton integrity and can modulate tyrosine kinase activation in leukocytes. The ability to phagocytose opsonized zymosan and the modulation of this process by tyrosine kinase was evaluated in septic neutrophils. As shown in Figure 3A, neutrophils from septic patients (gray bars) presented greater phagocytic activity (P < .05) compared with control neutrophils (open bars). Incubation with either CyB (15 μg/mL), a disruptor of microfilament organization, or genistein (80 μM), an inhibitor of tyrosine kinases, reduced phagocytosis of opsonized targets in controls and septic cells (Figure 3A). Cytochemistry analysis of cells under phagocytic activity for F-actin (Figure 3B) or phosphotyrosine (Figure 3C) showed that neutrophils from both groups, control (Figure 3Bi, 3Ci) and septic (Figure 3Bii, 3Cii) were able to respond to opsonized zymosan stimulation, increasing acting polymerization (Figure 3B) and phosphotyrosine content (Figure 3C).
IL-8 and LTB4 did not increase actin polymerization and protein tyrosine phosphorylation from septic neutrophils. The contents of F-actin (A, C) and phosphotyrosine (B, D) were analyzed by cytofluorescence in neutrophils from volunteers (i-iii; □) or patients with sepsis (iv-vi; ▦), treated with medium alone (i, iv), IL-8 (10–9 M; ii-iii), or LTB4 (10–8 M; iv-v). Actin filaments were stained with TRITC-phalloidin (A), and tyrosine-phosphorylated proteins were immunolabeled with FITC-conjugated antiphosphotyrosine Ab (B). Panels show images representative of at least 5 independent experiments. In addition, cells were imaged (×1000) as described, and the fluorescence intensity of F-actin (C) or phosphotyrosine (D) was quantified. Data are means ± SD from 5 independent experiments. *P < .05 compared with cells incubated with medium alone (ANOVA followed by Bonferroni); #P < .05 compared with control neutrophils (unpaired t test).
IL-8 and LTB4 did not increase actin polymerization and protein tyrosine phosphorylation from septic neutrophils. The contents of F-actin (A, C) and phosphotyrosine (B, D) were analyzed by cytofluorescence in neutrophils from volunteers (i-iii; □) or patients with sepsis (iv-vi; ▦), treated with medium alone (i, iv), IL-8 (10–9 M; ii-iii), or LTB4 (10–8 M; iv-v). Actin filaments were stained with TRITC-phalloidin (A), and tyrosine-phosphorylated proteins were immunolabeled with FITC-conjugated antiphosphotyrosine Ab (B). Panels show images representative of at least 5 independent experiments. In addition, cells were imaged (×1000) as described, and the fluorescence intensity of F-actin (C) or phosphotyrosine (D) was quantified. Data are means ± SD from 5 independent experiments. *P < .05 compared with cells incubated with medium alone (ANOVA followed by Bonferroni); #P < .05 compared with control neutrophils (unpaired t test).
Effect of cytokine-LPS treatment on LTB4- or IL-8–induced actin assembly and tyrosine phosphorylation
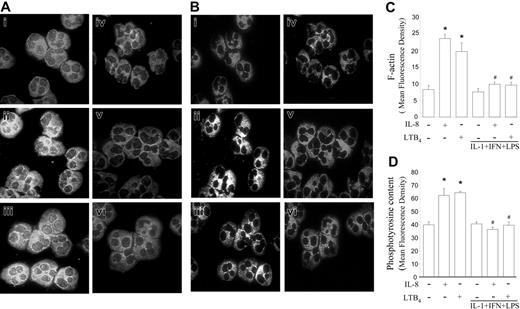
Bacterial products and cytokine serum levels are elevated in sepsis, and, for this, we have investigated with fluorescence microscopy whether pretreatment of neutrophils from healthy volunteers with cytokines and LPS would interfere with actin assembling (Figure 4A) and tyrosine phosphorylation (Figure 4B) in response to chemoattractants. Basal levels of F-actin (Figure 4Ai) and phosphotyrosine (Figure 4Bi) in blood neutrophils from controls increased significantly after stimulation with IL-8 (Figure 4Aii and 4Bii) or with LTB4 (Figure 4Aiii and 4Biii). In contrast, pretreatment of neutrophils with cytokines (IL-1β+ IFN-γ) plus LPS (Figure 4Aiv and 4Biv) impaired actin polymerization (Figure 4A) and tyrosine phosphorylation (Figure 4B) induced by IL-8 (Figure 4Av and 4Bv) or LTB4 (Figure 4Avi and 4Bvi). The quantification of cell fluorescence, as described in “Patients, materials, and methods,” is shown in Figure 4C and 4D. Treatment with cytokines and LPS was not able to induce, per se, significant changes in actin cytoskeleton or in phosphotyrosine content when compared with nontreated control cells, but prevented the increase in actin polymerization (Figure 4C) and tyrosine phosphorylation (Figure 4D). Accordingly, pretreatment with LPS and cytokines also inhibited the chemotactic response of neutrophils to IL-8 (Nontreated cells, 29.3 ± 3.5; LPS + Ctk, 13.4 ± 2.3; n = 5; P < .05).
Effect of phagocytosis on actin polymerization and protein tyrosine phosphorylation in septic neutrophils. (A) Phagocytic index of neutrophils from controls (□) or patients with sepsis (▦), treated (+) or not (–) with CyB (15 μg/mL) or with genistein (Gen; 80 μM). Data are means ± SD. *P < .05 compared with controls (ANOVA followed by Bonferroni). Fluorescence microscopy (magnification: × 1000) for F-actin (B) and phoshotyrosine (C) of neutrophils from controls (i; □) and patients with sepsis (ii; ▦), after phagocytosis of opsonized zymosan, performed as described in “Patients, materials, and methods.”
Effect of phagocytosis on actin polymerization and protein tyrosine phosphorylation in septic neutrophils. (A) Phagocytic index of neutrophils from controls (□) or patients with sepsis (▦), treated (+) or not (–) with CyB (15 μg/mL) or with genistein (Gen; 80 μM). Data are means ± SD. *P < .05 compared with controls (ANOVA followed by Bonferroni). Fluorescence microscopy (magnification: × 1000) for F-actin (B) and phoshotyrosine (C) of neutrophils from controls (i; □) and patients with sepsis (ii; ▦), after phagocytosis of opsonized zymosan, performed as described in “Patients, materials, and methods.”
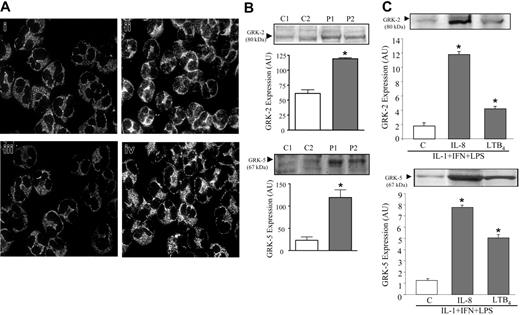
GRK2 and GRK5 expression
GRKs have been described as key modulators of GPCR desensitization. The IL-8 receptors, CXCRs, and the LTB4 receptor, BLT1, can be phosphorylated by GRKs, resulting in receptor desensitization.16,17 Alterations in GRK2 and GRK5 expression on neutrophils from patients with sepsis were analyzed by immunocytochemistry or immunoblotting (Figure 5A). Compared with control cells (Figure 5Ai and 5Aiii), a significant increase in fluorescence was clearly seen in septic neutrophils, indicating high expression of GRK2 (Figure 5Aii) and GRK5 (Figure 5Aiv). Confirming these results, an increase in GRK2 and GRK5 expression was also observed on the immunoblottings of septic neutrophils (Figure 5B). Moreover, in vitro treatment of healthy neutrophils with LPS + IFN-γ+ IL-1β+ IL-8 significantly induced increase in either GRK2 or GRK5 (Figure 5C) expression. Stimulation with LTB4 also increased GRK2 and GRK5 expression in neutrophils (Figure 5C), although with a lower magnitude than IL-8.
Discussion
Arising from the body's response to infection, sepsis is a life-threatening and commonly lethal disorder, accompanied by an inability to regulate the inflammatory responses. The impairment of neutrophil migration toward the inflammatory focus limits the appropriate clearance of microorganisms in the peripheral tissue, and has been ascribed as a harmful feature during severe sepsis.10,11 In animal models, the reduction of neutrophil migration to infection sites accompanied by lethality have been directly associated with increased circulating levels of cytokines, chemokines, and nitric oxide.10,11 Furthermore, the unresponsiveness of blood neutrophils from septic patients to chemotactic mediators in vitro27,28 has been directly correlated to a poor prognosis.12
Confirming the severity of sepsis scored as APACHE II, patients with sepsis enrolled in this study presented a significant increase in circulating proand anti-inflammatory cytokines, TNF-α, IFN-γ, IL-8 (CXCL8), and IL-10, accompanied by a high mortality rate. Supporting previous data,12 we are showing that limited chemotactic ability of septic neutrophils occurs for structurally different chemoattractants, including bacterial products such as FMLP, chemokines, IL-8 (CXCL8), and also lipid mediators, such as LTB4. The failure of septic patient neutrophils to mount a chemotactic response could not be ascribed to a deficiency in the transcription or expression of chemotactic receptors since CXCR1, an IL-8 (CXCL8) receptor in humans,29 was similarly expressed in neutrophils obtained from controls and patients with sepsis. The chemokine receptors CXCR1 and CXCR2 are central for many PMN functions.29 Although some reports have shown that in vitro treatment with cytokines and/or bacterial products could down-regulate CXCR1 and CXCR2 expression on human neutrophils,23,30 studies with patients with sepsis, confirming our data, showed that expression of CXCR1, the high-affinity receptor for IL-8, were not altered in sepsis.31,32
Most neutrophil chemoattractants recognize membrane GPCR on the leukocytes and, as a result of receptor activation by the chemoattractant, a complex sequence of signaling events are triggered, inducing profound alterations in cytoskeleton dynamics and activating signaling pathway, allowing neutrophils to develop motile, adhesive, phagocytic, and antimicrobial responses.33
In the classical view of signaling initiated by activation of GPCR by chemoattractants, the Gβγ complex activates phospholipase Cβ isoforms that, ultimately, results in calcium mobilization and activation of protein kinase C (PKC) that mediates the activation of NADPH oxidase complex, regulating the respiratory burst, phagocytosis, and bacterial killing in neutrophils.34 In addition, downstream to G proteins, other intracellular signals are triggered, including phosphatidylinositol 3-kinase (PI3K) and mitogen-activated protein kinase (MAPK) pathways, Src family of tyrosine kinases, Rho family of small guanosine triphosphate–binding proteins, and phosphatases that affects many aspects of neutrophil functioning, particularly chemotaxis and survival.35 Activation of these pathways by chemoattractants leads to protein phosphorylation, especially on tyrosine residues of several adapter proteins, which amplifies the signal transduction and priming cells to respond to adhesive interactions via integrins.15 Signaling for motility finds its climax with the polymerization of F-actin, which results in lamella formation and overall rearrangement of the cellular cytoskeleton and cell crawling.33
Effects of LTB4 and IL-8 on actin-assembling and protein tyrosine phosphorylation of neutrophils treated in vitro with cytokines and LPS. Neutrophils from healthy individuals were incubated with medium alone (i-iii) or with cytokines + LPS (iv-vi) and further stimulated with IL-8 (10–9 M; ii, v) or LTB4 (10–8 M; iii, vi), as described in “Patients, materials, and methods.” F-actin, stained with TRITC-phalloidin (A, C; left panels), and tyrosine-phosphorylated proteins, immunolabeled with FITC-conjugated antiphosphotyrosine Ab (B, D; right panels), were analyzed by fluorescence microscopy (magnification: × 1000) and quantified as described. Data are means ± SD from 10 independent experiments. *P < .05 compared with control neutrophils; #P < .05 compared with cells incubated with medium alone (ANOVA followed by Bonferroni).
Effects of LTB4 and IL-8 on actin-assembling and protein tyrosine phosphorylation of neutrophils treated in vitro with cytokines and LPS. Neutrophils from healthy individuals were incubated with medium alone (i-iii) or with cytokines + LPS (iv-vi) and further stimulated with IL-8 (10–9 M; ii, v) or LTB4 (10–8 M; iii, vi), as described in “Patients, materials, and methods.” F-actin, stained with TRITC-phalloidin (A, C; left panels), and tyrosine-phosphorylated proteins, immunolabeled with FITC-conjugated antiphosphotyrosine Ab (B, D; right panels), were analyzed by fluorescence microscopy (magnification: × 1000) and quantified as described. Data are means ± SD from 10 independent experiments. *P < .05 compared with control neutrophils; #P < .05 compared with cells incubated with medium alone (ANOVA followed by Bonferroni).
Increase of GRK2 and GRK5 expression in septic neutrophils. (A) Neutrophils from controls (i, iii) or patients with sepsis (ii, iv) were immunostained for GRK2 (i-ii) and GRK5 (iii-iv). The panels show a representative experiment out of at least 7 independent experiments. (B) Immunoblotting for GRK2 (top panel) and GRK5 (bottom panel) in neutrophils from patients with sepsis (▦) or healthy volunteers (□). The blots, obtained from 2 representative experiments, show GRK2 and GRK5 expression in neutrophils from controls (C1, C2) and patients with sepsis (P1, P2). (C) Immunoblotting for GRK2 (top) and GRK5 (bottom) in neutrophils from healthy volunteers incubated with cytokines and LPS, plus IL-8 (10–9 M) or LTB4 (10–8 M), as described in “Patients, materials, and methods.” For immunoblotting, the densitometry of each band, expressing the content of GRK2 and GRK5, was analyzed and expressed in arbitrary units (AU). Data show means ± SD of at least 6 experiments. *P < .05 compared with nontreated cells (ANOVA followed by Bonferroni).
Increase of GRK2 and GRK5 expression in septic neutrophils. (A) Neutrophils from controls (i, iii) or patients with sepsis (ii, iv) were immunostained for GRK2 (i-ii) and GRK5 (iii-iv). The panels show a representative experiment out of at least 7 independent experiments. (B) Immunoblotting for GRK2 (top panel) and GRK5 (bottom panel) in neutrophils from patients with sepsis (▦) or healthy volunteers (□). The blots, obtained from 2 representative experiments, show GRK2 and GRK5 expression in neutrophils from controls (C1, C2) and patients with sepsis (P1, P2). (C) Immunoblotting for GRK2 (top) and GRK5 (bottom) in neutrophils from healthy volunteers incubated with cytokines and LPS, plus IL-8 (10–9 M) or LTB4 (10–8 M), as described in “Patients, materials, and methods.” For immunoblotting, the densitometry of each band, expressing the content of GRK2 and GRK5, was analyzed and expressed in arbitrary units (AU). Data show means ± SD of at least 6 experiments. *P < .05 compared with nontreated cells (ANOVA followed by Bonferroni).
Our data show that rather than a down-regulation of receptor expression on septic neutrophils, a failure of receptor signaling in response to chemoattractants assigned for impairing the neutrophil migration. We have demonstrated that neutrophils from patients with sepsis present a significant reduction in the basal phosphotyrosine protein content and failed to display increase in tyrosine kinase activity and alterations in actin cytoskeleton dynamics in response to IL-8 (CXCL8) or LTB4. Moreover, the inability of septic neutrophils to increase actin polymerization and tyrosine kinase activity seems to be restricted to the incapacity of chemoattractants, such as IL-8 or LTB4, to trigger the signal transduction through their respective receptors. Neutrophils from patients with sepsis showed a high and cytochalasin b–sensitive phagocytic activity against opsonized particulated stimuli that induced increase and rapid redistribution of F-actin and augment in phosphotyrosine content, indicating that these cells present functional cytoskeleton and signaling pathways that are able to respond to other stimuli than chemoattractants.
Aiming to reproduce in part the endogenous septic environment, human neutrophils obtained from healthy individuals were treated with cytokines and LPS and further stimulated with chemoattractants. Interestingly, this in vitro treatment also inhibited chemotactic response, actin polymerization, and the increase of phosphotyrosine content in response to IL-8 or LTB4 stimulation, suggesting that endogenous mediators produced during sepsis might activate circulating neutrophils, impairing these cells to respond to further chemoattractant stimulation.
In similarity with other GPCRs, chemotactic receptor activation by agonists can lead to receptor desensitization, a phenomenon modulated by GRKs, which phosphorylates GPCR and often results in a rapid attenuation of receptor responsiveness.15,35 Desensitization of migratory responses is an important determinant to regulate neutrophil influx in pathologic conditions. Continuous and excessive chemokine receptor activation can induce an increase in the expression of GRKs, which phosphorylate GPCRs to signal receptor desensitization.16 Prolonged LTB4 stimulation was reported to desensitize human neutrophils for chemotaxis and calcium influx, through the phosphorylation, by GRK6, of the LTB4 receptor.36 Macrophage inflammatory protein-2 was shown to induce, in vitro, expression of GRK2 and GRK5 in blood neutrophils.17 Studies on CXCR1 and CXCR2 functionality showed that high concentrations of IL-8 (CXCL8) decrease migratory responses.37 This effect seems to be related to GRK2-induced phosphorylation of CXCR1, desensitizing this receptor and probably allowing its internalization.38
Since CXCR1 expression was maintained on neutrophils during severe sepsis, it was conceivable to assume that desensitization of chemoattractant receptors may account for the reduced chemotactic responses. Supporting this idea, we demonstrate that neutrophils from patients with sepsis show a significant increase in GRK2 and GRK5 expression. Furthermore, treatment of control neutrophils with cytokines plus LPS and further stimulation with IL-8 (CXCL8) or LTB4 also up-regulated GRK2 and GRK5, indicating a key role for those mediators in neutrophil desensitization.
In summary, we present evidence that the systemic septic environment can highly activate circulating neutrophils, inducing GRK expression and desensitization of migratory activity. Increase in the GRK expression is associated with impairment of chemoattractant-induced tyrosine kinase activity and subsequent rearrangement of actin network, hence compromising the ability of neutrophils from patients with sepsis to migrate toward the infectious focus. The shift in chemotaxis from activation to desensitization provides a delicate interplay between many cellular factors that tightly regulate the neutrophil migration in sepsis.
Prepublished online as Blood First Edition Paper, July 18, 2006; DOI 10.1182/blood-2006-05-024638.
Supported by grants from the Fundacão de Amparo à Pesquisa do Estado de São Paulo (FAPESP), Conselho Nacional de Degenvolvimento Científico e Tecnológico (CNPq), CAPES, and Fundacão de Amparo à Pesquisa do Estado do Rio Janeiro (FAPERJ) (Brazil).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Fabiola L. Mestriner and Giuliana Bertozi Francisco (University of São Paulo) and Marcos B. Batista (Universidade do Estado do Rio de Janeiro) for technical assistance.
The authors have no conflicting financial interests.
Dr Arraes' current affiliation is the Department of Clinical Analysis, State University of Maringá, Maringá, Paraná, Brazil.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal