Abstract
Juvenile myelomonocytic leukemia (JMML) is a rare clonal myeloproliferative disorder. Although allogeneic stem cell transplantation can induce long-term remissions, relapse rates remain high and innovative approaches are needed. Since donor lymphocyte infusions have clinical activity in JMML, T-cell-mediated immunotherapy could provide a nonredundant treatment approach to compliment current therapies. γ-Globin, an oncofetal protein overexpressed by clonogenic JMML cells, may serve as a target of an antitumor immune response. We predicted 5 γ-globin-derived peptides as potential human leukocyte antigen (HLA)-A2 restricted cytotoxic T lymphocyte (CTL) epitopes and showed that 4 (g031, g071, g105, and g106) bind A2 molecules in vitro. Using an artificial antigen-presenting cell (aAPC) that can process both the N- and C-termini of endogenously expressed proteins, we biochemically confirmed that g105 is naturally processed and presented by cell surface A2. Furthermore, g105-specific CD8+ CTLs generated from A2-positive healthy donors were able to specifically cytolyze γ-globin+, but not γ-globin- JMML cells in an A2-restricted manner. These results suggest that this aAPC-based approach enables the biochemical identification of CD8+ T-cell epitopes that are processed and presented by intact cells, and that CTL immunotherapy of JMML could be directed against the γ-globin-derived epitope g105.
Introduction
Results from recent clinical studies have renewed the hope that immunotherapeutic strategies can be effective in the treatment of patients with cancer.1-4 Although immune responses can be detected in clinical trials of tumor antigen-based vaccines and adoptive therapy, additional progress is needed to increase the magnitude and duration of these responses for most patients.5 Significant clinical responses in heavily pretreated patients with metastatic melanoma have recently been observed following the transfer of highly avid antitumor lymphocytes.6,7 Since this approach is not feasible for all patients or diseases, new techniques must be developed to reliably generate high-quality antitumor T cells that can target antigens endogenously expressed by tumors.
It is crucial to identify the appropriate targets in order to generate effective antitumor immune responses. Antigens that are expressed or overexpressed by tumor cells but not by normal tissues should be favored to reduce the risk of toxicity. Moreover, whether a particular antigenic epitope is processed and presented by the target cell in the context of human leukocyte antigen (HLA) is vital, since only then can it serve as a genuine target of an antitumor immune response. Recently, we reported the generation of an artificial antigen-presenting cell (aAPC) that expresses HLA-A2, CD80, and CD83 and is able to support the priming and prolonged expansion of A2-restricted peptide-specific CD8+ cytotoxic T cells.8 We also showed that our aAPC can be engineered to present, via its transduced A2 molecule, any antigenic peptide of choice using a specially designed fusion protein that requires only N-terminal processing.9 Given the versatility of this aAPC system, we next sought to address whether our aAPC could process whole proteins and present particular immunogenic peptides.
Juvenile myelomonocytic leukemia (JMML) is a rare clonal myeloproliferative disorder of early childhood arising from pluripotent stem cells.10-13 Patients with JMML respond poorly to most standard chemotherapy regimens and, although allogeneic stem cell transplantation can be curative, relapse rates are unacceptably high.14,15 Since a graft-versus-leukemia effect is demonstrated by the efficacy of donor lymphocyte infusions, it is possible that JMML can be treated with T-cell-mediated immunotherapy.16-18 Identification of appropriate antigenic targets would be mandatory for the successful immune-based therapy of JMML. One promising candidate tumor antigen, γ-globin, is an oncofetal protein that is expressed by clonogenic JMML cells but is not necessary for normal erythropoiesis in children or adults.19
We demonstrate herein that our aAPC can process both the N- and C-termini of A2-restricted peptides in a proteasome-dependent way. Furthermore, A2-restricted peptides can be biochemically identified in acid-stripped peptide pools derived from the cell surface of aAPCs. Using this aAPC-based strategy we demonstrated that the γ-globin-derived peptide, g105, is naturally processed and presented. Furthermore, we show that γ-globin-specific cytotoxic T lymphocytes (CTLs) can be generated, and that these CTLs were able to recognize primary JMML cells in an HLA-restricted manner. These findings suggest that γ-globin may serve as a genuine JMML-associated antigen and demonstrate the versatility of our aAPC as a tool for identifying relevant antigenic epitopes.
Materials and methods
cDNA
Partial influenza virus MP1 cDNA was produced by overlapping polymerase chain reaction (PCR) according to the published sequence. Full-length human telomerase reverse transcriptase (hTERT) cDNA was a gift from Dr Ishikawa (Kyoto University, Japan). Full-length γ-globin cDNA was cloned from a human fetal liver cDNA library as described elsewhere.20 Partial MP1, partial hTERT, and full-length γ-globin cDNAs were fused to the C-terminus of enhanced green fluorescent protein (EGFP) cDNA (Clontech, Palo Alto, CA) in frame using standard molecular biology techniques to generate EGFP-MP1, EGFP-hTERT, and EGFP-γ-globin, respectively. All the constructs were verified by DNA sequencing.
Peptides
Synthetic peptides described in Table 1 and Pol (476ILKEPVHGV484) of HIV-pol, TAX (11LLFGYPVYV19) of HTLV1-TAX, and I540 (540ILAKFLHWL548) and R572 (572RLFFYRKSV580) of hTERT were purchased from New England Peptides (Fitchburg, MA).
Predicted γ-globin-derived peptides can bind HLA-A2
Peptide . | Sequence . | Protein . | Fluorescence index* . |
|---|---|---|---|
| g018 | KVNVEDAGGE | γ-globin | 0 |
| g031 | RLLVVYPWT | γ-globin | 0.8 |
| g071 | SLGDAIKHL | γ-globin | 0.7 |
| g105 | KLLGNVLVTV | γ-globin | 2.4 |
| g106 | LLGNVLVTV | γ-globin | 3.0 |
| F271 | FLWGPRALV | MAGE-3 | 1.9 |
| G58 | GILGFVFTL | Flu MP1 | 1.0 |
| A98-ID | AHTKDGFNF | Idiotype | 0 |
Peptide . | Sequence . | Protein . | Fluorescence index* . |
|---|---|---|---|
| g018 | KVNVEDAGGE | γ-globin | 0 |
| g031 | RLLVVYPWT | γ-globin | 0.8 |
| g071 | SLGDAIKHL | γ-globin | 0.7 |
| g105 | KLLGNVLVTV | γ-globin | 2.4 |
| g106 | LLGNVLVTV | γ-globin | 3.0 |
| F271 | FLWGPRALV | MAGE-3 | 1.9 |
| G58 | GILGFVFTL | Flu MP1 | 1.0 |
| A98-ID | AHTKDGFNF | Idiotype | 0 |
Calculated arbitrary units by T2 binding
Cells
aAPCs were generated by transducing K562 cells (American Type Culture Collection [ATCC], Manassas, VA) with HLA-A*0201, CD80, and CD83 as described previously.8 Using a retrovirus vector, pMX, and a packaging cell line, 293GPG, aAPCs were further transduced with either EGFP-MP1, EGFP-hTERT, or EGFP-γ-globin fusion cDNA to produce aAPC/MP1, aAPC/hTERT, or aAPC/γ-globin, respectively. EGFP-positive cells were isolated by flow cytometry-guided sorting. The expression of transduced molecules was stable even after 2 months in culture.
The human T-cell leukemia line Jurkat was obtained from ATCC and cultured in RPMI 1640 supplemented with 10% FCS. Primary JMML cells, JMML1 (46, XY; hemoglobin F [HbF], 55%) and JMML2 (45, XY,-7; HbF, 1%) were isolated from HLA-A2-positive patients with JMML after obtaining written informed consent from their parents. The diagnosis of JMML was based on internationally accepted criteria.12 JMML tumor cells were purified from spontaneous colonies in methylcellulose-based medium (StemCell Technologies, Vancouver, BC, Canada) and cultured in RPMI 1640 supplemented with 10% human AB serum and 10 ng/mL granulocyte-macrophage colony-stimulating factor (GM-CSF; Immunex, Thousand Oaks, CA) for a short period of time. GM-CSF receptor (GM-CSFR) alpha chain expression by JMML cells was confirmed by flow cytometry analysis using specific monoclonal antibody (mAb; BD Pharmingen, San Diego, CA). Peripheral blood samples from normal donors and patients with JMML were collected in compliance with protocols approved by the Institutional Review Boards of the Dana-Farber Cancer Institute (DFCI) and University of Nagoya, respectively.
Flow cytometry analysis
γ-Globin expression was examined by intracellular staining with anti-γ-globin mAb (Caltag, Burlingame, CA) according to the manufacturer's instructions.
HLA-A*0201-binding assay
Candidate peptides were identified using publicly available algorithms (http://bimas.dcrt.nih.gov:80/cgi-bin/molbio/ken_parker_comboform). The HLA-A*0201-binding assay of synthetic peptides was performed as previously reported.20 Briefly, T2 cells were pulsed with 50 μg/mL of peptide and 5 μg/mL of β2-microglobulin (Calbiochem, La Jolla, CA) for 18 hours at room temperature. HLA-A*0201 expression was then measured by flow cytometry using anti-A2 mAb (BB7.2) and the PE-conjugated F(ab′)2 fragment of goat anti-mouse IgG secondary antibody (SouthernBiotech, Birmingham, AL).
Chromium release assay
The chromium (Cr) release assay was performed as previously reported.20 Briefly, peptide pulsed target cells were labeled by 51Cr and cytotoxicity assays were performed for the stated time periods. Where indicated, target cells were preincubated with anti-A2 mAb (BB7.2) or isotype control (mIgG2b) for 30 minutes at 37°C prior to the addition of CTLs. Data were calculated as mean percentage of specific cytolysis ± SD, subtracting spontaneous chromium release.
Peptide elution and sequencing
Cell-surface HLA-A2-bound peptides were acid stripped from aAPC transfectants and separated by reverse-phase high-performance liquid chromatography (HPLC) on a C18 column (Source 5RPC St 4.6/150; Amersham Pharmacia Biotech, Piscataway, NJ) as described.9 The peptides were eluted with an acetonitrile gradient (2% to 100%). The specific HPLC fractions corresponding to a reference peptide were collected, lyophilized, and resuspended in 0.1% TFA. Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS) analysis was performed on a Voyager Biospectrometry Workstation (Applied Biosystems, Foster City, CA) in the positive delayed-reflector mode. All mass spectra were collected with acquisition range 0.8 to 3 kDa following the same instrumental parameters. All mass spectra were acquired by 100 shots evenly distributed on each spot. External calibration of each sample plate and preparation was performed using the corresponding adjacent well. The sequence of the peptide was confirmed using electrospray ionization (ESI) ion trap liquid chromatography tandem mass spectrometry (LC/MS/MS; Finnigan LCQDeca; Thermo Electron Corporation, Waltham, MA) by the DFCI protein sequencing core facility.
ELISPOT analysis
Enzyme-linked immunospot (ELISPOT) analysis for interferon-γ (IFN-γ) secretion was performed as previously described.8 Where indicated, aAPC derivatives were treated with 10 μM lactacystin or beta-lactone (Calbiochem) for 4 hours and then peptide-stripped with citrate buffer. Stripped cells were further treated with 10 μM of a proteasome inhibitor for 10 hours and then used as stimulators in the presence of the inhibitor. Control cells were similarly treated with MeSO4 (Sigma, St Louis, MO).
Production of HLA-A2 peptide-specific CD8+ T cells
γ-Globin-derived peptide-specific cytotoxic CD8+ T cells were generated as described previously.8 Briefly, purified HLA-A2-positive CD8+ lymphocytes were plated at 2 × 106 cells/well in RPMI 1640 supplemented with 10% human AB serum. aAPCs were pulsed with synthetic peptide for 6 hours at room temperature. aAPCs were then irradiated at 200 Gy, washed, and added to the CD8+ responder cells at a responder-stimulator ratio of 20:1. T-cell cultures were repeatedly stimulated on a weekly basis and supplemented with 10 IU/mL IL-2 (Chrion, Emeryville, CA) and 10 ng/mL IL-15 (Peprotech, Rocky Hill, NJ) between the stimulations.
Structure and expression of transduced genes. (A) Primary structure of EGFP-MP1 is shown. The position of the A2-restricted antigenic peptide, MP158-66, is shown in the black box. (B) Expression of transduced EGFP-MP1 (open curve) was analyzed by flow cytometric analysis using the FL1 channel. Untransduced aAPCs were used as a control (filled curve).
Structure and expression of transduced genes. (A) Primary structure of EGFP-MP1 is shown. The position of the A2-restricted antigenic peptide, MP158-66, is shown in the black box. (B) Expression of transduced EGFP-MP1 (open curve) was analyzed by flow cytometric analysis using the FL1 channel. Untransduced aAPCs were used as a control (filled curve).
Results
Generation of aAPCs transduced with partial MP1 gene containing the A2-restricted MP158-66 peptide sequence
K562 is a human erythroleukemic cell line, which lacks the expression of any HLA class I or class II molecules on the cell surface,21 but abundantly expresses the adhesion molecules CD54 (ICAM-1) and CD58 (LFA-3). aAPCs were generated by retrovirally transfecting K562 with HLA-A2, CD80, and CD83, and has been shown to prime and expand antigen-specific CD8+ T cells from HLA-A2-positive donors.8 Furthermore, aAPCs can endogenously process and present A2-restricted peptides fused to the C-terminus of EGFP or puromycin N-acetyltransferase in a proteasome-dependent way, suggesting that aAPCs can properly process the N-terminus of A2 restricted peptides.9 Since the C-terminus of class I-restricted peptides is widely known to be generated solely by proteasome machinery,22-24 we postulated that aAPCs can endogenously process both N- and C-termini of HLA-A2-restricted peptides. To test this, we generated aAPC/MP1 by transducing aAPCs with the EGFP-MP1 fusion gene, which contains the A2-restricted MP158-66 antigenic peptide sequence flanked by upstream and downstream MP1 sequences (Figure 1A). The expression of transduced EGFP-MP1 fusion gene by aAPC/MP1 was confirmed by flowcytometric analysis, and EGFP-positive cells were collected by flow cytometry-guided sorting (Figure 1B). Control aAPC expressing the EGFP moiety alone was established in a similar way.
Proteasome-dependent processing of the N- and C-termini of MP158-66 by aAPCs
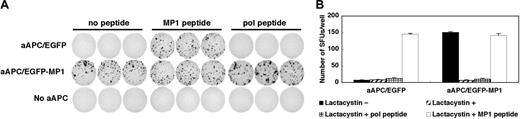
We first examined whether MP158-66 peptide is naturally processed and presented by HLA-A2 molecules on the surface of aAPC/MP1. MP158-66 peptide-specific CD8+ T-cell lines were stimulated by either aAPC/EGFP or aAPC/MP1, and an IFN-γ ELISPOT was performed. aAPC/MP1 induced IFN-γ secretion to the same extent as aAPC/EGFP pulsed with MP158-66 peptide, while aAPC/EGFP did not induce any IFN-γ secretion (Figure 2A). IFN-γ secretion was identical whether or not the stimulating aAPC/MP1 was pulsed with MP158-66 or an irrelevant HIV-pol peptide. These results suggest that aAPC/MP1 are able to naturally process and present MP158-66 peptides derived from the transduced partial MP1 gene, and that the antigenic density of the MP158-66 peptide in A2 groove is sufficient to induce effector function of MP158-66-specific CD8+ T cells.
Next, we confirmed that the proteasome machinery participates in the processing of MP158-66 peptide. aAPCs were pretreated with the proteasome inhibitor lactacystine and used as stimulators in an IFN-γ ELISPOT. As shown in Figure 2B, endogenous processing and subsequent presentation of MP158-66 peptides were completely abrogated by treating aAPC/MP1 with lactacystin, suggesting that successful processing of transduced MP1 is predominantly under the control of the proteasome using the conditions examined. The fact that the exogenous addition of MP158-66 but not the HIV-pol peptide successfully restored the induction of IFN-γ secretion suggests that the expression of A2 molecules itself was not substantially affected by lactacystin. Another proteasome inhibitor beta-lactone also inhibited the MP158-66 peptide processing to the same extent (data not shown). Considering that proteasome subunits are expressed at the protein level as shown previously,9 these results suggest that aAPCs can process both the N-and C-terminus of the EGFP-MP1 fusion protein in a proteasome-dependent manner.
aAPCs can process both the N- and C-termini of I540 and R572 peptides derived from a partial TERT gene
hTERT, the catalytic subunit of telomerase, is very closely associated with telomerase activity and has been considered a candidate tumor-associated antigen.25-27 Although at least 2 A2-restricted peptides (I540 and R572) derived from hTERT have been suggested, it remains controversial whether these peptides are naturally processed and presented by primary tumor cells.28-30 We biochemically examined the presence of I540 and R572 in the HLA-A2 groove on the surface of parental aAPCs, which constitutively express hTERT. aAPCs (2 × 108) were washed and then acid-treated to elute cell-surface peptides. Flow-through peptide fractions were collected following filtration through a membrane with a 5-kDa cut-off. They were further separated by reverse-phase HPLC, and the peptide fractions corresponding to synthetic I540 and R572 peptides were collected and subjected to mass spectrometry. Neither I540 nor R572 was detected by mass spectrometry under the conditions tested (data not shown).
aAPC/MP1 can naturally process and present MP1 peptide via A2 on the cell surface in a proteasome-dependent way. (A-B) MP158-66-specific IFN-γ ELISPOT analysis. (A) MP158-66-specific CD8+ T cells were stimulated with aAPC/EGFP, aAPC/MP1, or without any APCs in the presence or absence of indicated peptides. (B) APCs were pretreated with lactacystin and acid to inhibit the processing and presentation of endogenous peptides. Then, IFN-γ ELISPOT was performed in the presence or absence of lactacystin. The results are expressed as mean ± SD.
aAPC/MP1 can naturally process and present MP1 peptide via A2 on the cell surface in a proteasome-dependent way. (A-B) MP158-66-specific IFN-γ ELISPOT analysis. (A) MP158-66-specific CD8+ T cells were stimulated with aAPC/EGFP, aAPC/MP1, or without any APCs in the presence or absence of indicated peptides. (B) APCs were pretreated with lactacystin and acid to inhibit the processing and presentation of endogenous peptides. Then, IFN-γ ELISPOT was performed in the presence or absence of lactacystin. The results are expressed as mean ± SD.
HPLC separation and mass spectrometric analysis of I540 and R572 peptides from the cell surface of aAPC/hTERT. (A) Primary structure of EGFP-hTERT is shown. (B) Expression of transduced EGFPTERT (open curve) was analyzed by flow cytometric analysis using the FL1 channel. Untransduced aAPCs were used as a control (filled curve). (C) Cell-surface peptides from aAPC/hTERT were acid stripped and separated by reverse-phase HPLC as described in “Materials and methods.” Peptide fractions were collected, subjected to mass spectral analysis, and fractions corresponding to those of synthetic I540 and R572 peptides were identified.
HPLC separation and mass spectrometric analysis of I540 and R572 peptides from the cell surface of aAPC/hTERT. (A) Primary structure of EGFP-hTERT is shown. (B) Expression of transduced EGFPTERT (open curve) was analyzed by flow cytometric analysis using the FL1 channel. Untransduced aAPCs were used as a control (filled curve). (C) Cell-surface peptides from aAPC/hTERT were acid stripped and separated by reverse-phase HPLC as described in “Materials and methods.” Peptide fractions were collected, subjected to mass spectral analysis, and fractions corresponding to those of synthetic I540 and R572 peptides were identified.
We then established aAPC/hTERT cells, which were engineered to overexpress partial hTERT encompassing I540 and R572 (Figure 3A-B). We isolated aAPC/hTERT surface-bound peptides by acid elution and reverse-phase HPLC, and then collected peptide fractions corresponding to synthetic I540 and R572 peptides that were subjected to mass spectrometry. We successfully isolated both I540 and R572 peptides in the fraction nos. 42 and 38, respectively (Figure 3C). Since HLA-A2 is the only HLA expressed by aAPCs, these results strongly indicate that aAPC/hTERT, which expresses a partial hTERT protein, is able to process and present I540 and R572 in the groove of surface A2 molecules. Taken all together, these results suggest that the aAPC-based strategy described may allow us to biochemically identify A2-restricted peptides derived from an ectopically overexpressed protein.
γ-Globin, a candidate tumor antigen of JMML, contains predicted antigenic epitopes that bind to HLA-A2
JMML is a rare clonal myeloproliferative disorder.10-13 Although allogeneic stem cell transplantation can induce long-term remissions, relapse rates remain high and novel modalities are needed.14,15 Since donor lymphocyte infusion after relapse is efficacious in JMML,16-18 antigen-directed T-cell-mediated immunotherapy might have clinical activity. One candidate tumor-associated antigen for the immune-based therapy of JMML is γ-globin, which is expressed at high levels in most patients with JMML.19 Most clonogenic JMML cells constitutively express this fetal protein, which is not necessary for the normal erythropoesis of children and adults. To determine whether γ-globin can serve as an immune target in JMML, we sought to determine whether γ-globin is naturally processed and presented by the HLA complex. Using conventional bioinformatic techniques,31 we predicted 5 γ-globin-derived peptides (g018, g031, g071, g105, and g106) that could theoretically bind to the HLA-A2 molecule (Table 1). In order to confirm the in vitro binding of these candidate peptides, we performed T2-binding assays using synthetic peptides. As shown in Table 1, except for g018, the remaining 4 peptides were able to bind to A2 molecules. Interestingly, the binding of 2 peptides, g105 and g106, was stronger than that of MAGE3 and MP158-66 peptides. These results suggest that γ-globin-derived peptides can bind HLA-A2 molecules at least in vitro.
Identification of γ-globin-derived peptides on the cell surface of aAPC/γ-globin. (A) Cell-surface peptides from aAPC/γ-globin were acid stripped and separated by reverse-phase HPLC as described in “Materials and methods.” Peptide fractions were collected, subjected to mass spectral analysis, and fractions corresponding to those of synthetic g031 and g105 peptides were identified. (B) The fraction corresponding to the g105 peptide was subjected to sequencing.
Identification of γ-globin-derived peptides on the cell surface of aAPC/γ-globin. (A) Cell-surface peptides from aAPC/γ-globin were acid stripped and separated by reverse-phase HPLC as described in “Materials and methods.” Peptide fractions were collected, subjected to mass spectral analysis, and fractions corresponding to those of synthetic g031 and g105 peptides were identified. (B) The fraction corresponding to the g105 peptide was subjected to sequencing.
A γ-globin-derived peptide is naturally processed and presented by aAPCs transduced with full-length γ-globin gene
Since these results do not indicate which predicted epitopes derived from γ-globin are endogenously processed and presented by tumor cells in vivo, we next used a biochemical strategy to address the question. We first established aAPC/γ-globin by introducing full-length γ-globin cDNA fused to EGFP. As described, A2-bound peptides were eluted from the cell surface of aAPC/γ-globin and were separated by reverse-phase HPLC. Peptide fractions corresponding to 4 synthetic γ-globin derived peptides (g031, g071, g105, and g106) were collected and subjected to mass spectrometry. As shown in Figure 4A, g031 and g105 were detected in the fraction of nos. 39 and 37, respectively. Neither the g071 nor the g106 peptide was detectable under the conditions studied (data not shown). Additional separation of fraction no. 37 was performed, and peptide sequencing completely matched g105 (Table 2). Since HLA-A2 is the only HLA expressed by aAPCs, these results strongly suggest that γ-globin-derived g105 peptide is processed and presented in the groove of the A2 molecule on the surface of aAPC/γ-globin.
Amino acid sequence of the g105 peptide
Seq . | No. . | b . | y . |
|---|---|---|---|
| K | 1 | 129.1 | 1055.7 |
| L | 2 | 242.2 | 927.6 |
| L | 3 | 355.3 | 814.5 |
| G | 4 | 412.3 | 701.4 |
| N | 5 | 526.3 | 644.4 |
| V | 6 | 625.4 | 530.4 |
| L | 7 | 738.5 | 431.3 |
| V | 8 | 837.6 | 318.2 |
| T | 9 | 938.6 | 219.1 |
| V | 10 | 1037.7 | 118.1 |
Seq . | No. . | b . | y . |
|---|---|---|---|
| K | 1 | 129.1 | 1055.7 |
| L | 2 | 242.2 | 927.6 |
| L | 3 | 355.3 | 814.5 |
| G | 4 | 412.3 | 701.4 |
| N | 5 | 526.3 | 644.4 |
| V | 6 | 625.4 | 530.4 |
| L | 7 | 738.5 | 431.3 |
| V | 8 | 837.6 | 318.2 |
| T | 9 | 938.6 | 219.1 |
| V | 10 | 1037.7 | 118.1 |
CTLs specific for γ-globin-derived g105 peptide can recognize primary JMML cells
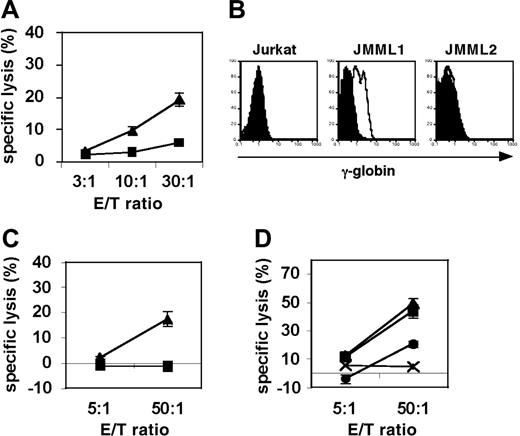
To test whether g105-specific CTLs can target primary JMML cells, we generated g105-specific CD8+ CTLs from A2-positive healthy donors using synthetic peptide. HLA-A2-positive CD8+ T cells from healthy donors were stimulated with g105-peptide-pulsed aAPCs at weekly intervals, and cultures were supplemented with IL-2 and IL-15 twice a week. γ-Globin-derived g105 peptide-specific CTLs were able to specifically cytolyze T2 cells pulsed with g105 peptide but not T2 cells pulsed with the control peptide TAX (Figure 5A). In order to investigate whether g105 is processed and presented by primary JMML tumor cells, we isolated A2+/γ-globin+ JMML1 cells and A2+/γ-globin- JMML2 from HLA-A2-positive patients with JMML. Intracellular staining of γ-globin using specific mAb-detected γ-globin expression in JMML1 but not JMML2 or Jurkat cells (Figure 5B). Using these primary JMML tumor cells as targets, we performed cytotoxicity assays using g105 CTLs. Importantly, A2+/γ-globin+ JMML1 cells but not A2+/γ-globin- JMML2 cells were killed by g105-specific CTLs (Figure 5C). The specific cytotoxicity was blocked by anti-A2 mAb but not isotype control, suggesting that the observed cytotoxicity was A2 restricted (Figure 5D). These results indicate that g105 is naturally processed and presented by primary JMML cells via HLA-A2.
g105-specific CTLs can kill γ-globin-positive JMML cells in an HLA-A2-restricted way. (A) g105 peptide-specific CTLs were generated by repeated stimulations of purified CD8+ T cells from A2-positive healthy donors with g105 peptide-pulsed aAPCs. Between stimulations, IL-2 and IL-15 were added to the cultures every 3 days. Radiolabeled T2 cells were pulsed with g105 peptide (▴) or control peptide, TAX (▪), and used for a 4-hour cytotoxicity assay. (B) Intracellular flow cytometric analysis of γ-globin expression in A2-positive patient-derived JMML1 and JMML2 cells, and the human T-cell leukemic cell line Jurkat. Histograms represent isotype (filled curves) and γ-globin-specific staining (open curves). (C) A2-positive γ-globin-positive JMML1 cells (▴) and A2-positive γ-globin-negative JMML2 cells (▪) were subjected to 8-hour cytotoxicity assays. (D) A2-positive γ-globin-positive JMML1 cells were subjected 20-hour cytotoxicity assays in the absence (▴) or presence of anti-A2 mAb (•) or isotype control (▪). A2-positive γ-globin-negative JMML2 cells (×) were also included as a target. (A, C-D) Results are expressed as means ± SD.
g105-specific CTLs can kill γ-globin-positive JMML cells in an HLA-A2-restricted way. (A) g105 peptide-specific CTLs were generated by repeated stimulations of purified CD8+ T cells from A2-positive healthy donors with g105 peptide-pulsed aAPCs. Between stimulations, IL-2 and IL-15 were added to the cultures every 3 days. Radiolabeled T2 cells were pulsed with g105 peptide (▴) or control peptide, TAX (▪), and used for a 4-hour cytotoxicity assay. (B) Intracellular flow cytometric analysis of γ-globin expression in A2-positive patient-derived JMML1 and JMML2 cells, and the human T-cell leukemic cell line Jurkat. Histograms represent isotype (filled curves) and γ-globin-specific staining (open curves). (C) A2-positive γ-globin-positive JMML1 cells (▴) and A2-positive γ-globin-negative JMML2 cells (▪) were subjected to 8-hour cytotoxicity assays. (D) A2-positive γ-globin-positive JMML1 cells were subjected 20-hour cytotoxicity assays in the absence (▴) or presence of anti-A2 mAb (•) or isotype control (▪). A2-positive γ-globin-negative JMML2 cells (×) were also included as a target. (A, C-D) Results are expressed as means ± SD.
Discussion
γ-Globin is an oncofetal protein, which is not essential for normal erythropoiesis in children and adults. Although a normal self-protein, γ-globin is immunogenic and has been shown to induce IgG autoantibodies in some patients with aplastic anemia and chronic myelogenous leukemia20 (data not shown). Intriguingly, globin genes in general appear to be immunogenic since B-cell responses to both α- and β-globin genes also have been reported.20 It is well known that clonogenic JMML cells express γ-globin, and patients with JMML have elevated levels of γ-globin.19 Therefore, we have hypothesized that γ-globin might serve as a tumor-associated antigen for JMML, and we studied potential HLA-A2-restricted epitopes that could be targets of CD8+ T cells. We have successfully shown that g105 peptide was eluted from the surface A2 molecules on aAPC/γ-globin, and CTLs directed against this peptide were able to recognize and cytolyze JMML cells from A2-positive patients, suggesting that g105 does exist in the A2 groove of JMML tumor cells.
Accumulating evidence suggests that JMML is a clonal disease that involves myeloid cells, monocytes, erythrocytes, platelets, and B and T lymphocytes.32-36 JMML therefore appears to originate from multilineage hematopoietic stem cells, although the JMML “cancer stem cell” and its expression of antigens such as γ-globin has not been directly examined. Very recently, we have developed a novel murine xenotransplant model of JMML using nonobese diabetic/severe combined immunodeficiency (NOD/SCID)/γcnull mice.36 Unlike conventional SCID or NOD/SCID mice, NOD/SCID/γcnull mice lack natural killer (NK) cell activity as well as T and B lymphocytes, which results in an increased incidence of graft rejection. Although not possible in the current study due to the lack of available T-cell clones, infusion of g105-specific CTL clones into NOD/SCID/γcnull given xenotransplants of JMML may help to determine whether g105 is a bona fide tumor “rejection” antigen. Such experiments would address the critically important issue of whether JMML stem cells express γ-globin.
Our data support that γ-globin-derived g105 is naturally processed and presented by JMML tumor cells. Furthermore, the affinity of the g105 peptide for A2 molecules is high and can induce T-cell responses ex vivo. However, these results do not address whether g105 is an immunodominant antigenic peptide in vivo. Determining whether g105-specific CD8+ T cells are present in patients with JMML patients or whether vaccination with g105 induces specific immune responses could indicate the immunodominance of the γ-globin-derived g105 peptide. Once this is demonstrated, study of the clinical utility of immunotherapy directed against the γ-globin-derived g105 peptide in A2-positive patients may be warranted.
We have immunologically and biochemically shown that our aAPC can naturally process both N- and C-termini of candidate antigens and present HLA-A2-restricted epitopes on the cell surface. We have demonstrated the ability of aAPCs to process and present the influenza virus peptide MP158-66 following the introduction of an EGFP-MP1 fusion gene. This process is proteasome dependent, as demonstrated by the inhibition of peptide presentation on the aAPC cell surface by treatment with proteasome inhibitors. Using a relatively simple and direct method of acid stripping, column filtration, and HPLC separation followed by mass spectrometry analysis,37 we were able to confirm biochemically the presence of the hTERT-derived peptides I540 and R572 on the cell surface of aAPCs transduced with an EGFP-fusion gene encoding partial hTERT. However, using the same experimental conditions, we were not able to isolate either of these peptides on the cell surface of parental aAPCs or aAPCs engineered to overexpress full-length hTERT (data not shown). In agreement with previously published work, these results suggest that the density of the hTERT-derived I540 peptide may be too low or absent from the A2 groove on the cells studied. Bulk CTLs or CTLs purified by A2/I540 multimer, but not CTL clones specific for I540, have been able to recognize hTERT-positive tumor cells.29,38 Some have suggested that the generation of hTERT-specific CTL clones with high avidity may not be feasible.39 Our system may enable us to identify other hTERT-derived peptides that are naturally processed and presented and can serve as the target of CTL responses. It is important to note that peptides should be eluted from cells expressing the full-length hTERT gene rather than an ectopically expressed partial gene, since the latter may process and present cryptic hTERT-derived peptides that are not normally generated by the cancer cells.
The robust nature of this approach was demonstrated by the identification of a γ-globin-derived candidate peptide that is processed and presented on the surface of intact cells. We suggest that g105 is a genuine potential immunotherapy target since anti-γ-globin CTLs generated against this epitope can recognize and kill HLA-A2-positive JMML cells that express γ-globin. This approach can potentially be applied to other HLA alleles. Since the parental cell line, K562, is easily transfected, HLA-A2 can be substituted with any HLA class I subtype. In fact, aAPCs expressing HLA-A*0101, HLA-A*2402, or HLA-A*2601 have already been generated, and the analysis of their ability to process and present endogenous peptide is ongoing. We are also investigating whether this approach is applicable to HLA class II-restricted peptides using K562 transduced with HLA-DR, HLA-DM, and the invariant chain. If non-A2-restricted peptides are similarly processed and presented by our aAPCs, the process of determining which candidate antigenic epitopes are processed and presented will be greatly facilitated.
Prepublished online as Blood First Edition Paper, June 15, 2006; DOI 10.1182/blood-2006-04-017566.
Supported by National Institutes of Health grants HL54785-08 (N.H.), CA87720 (M.O.B.), and CA92625-04 (L.M.N.). S.A. was funded by the Dr Mildred Scheel Stiftung der Deutschen Krebshilfe, and L.M.N. was supported by a grant from the Cancer Research Institute.
N.H. and M.O.B. designed and performed research, analyzed data, and wrote the manuscript; Z.X., A.B., A.P.M., and S.A. performed research; S.K. collected clinical samples; and L.M.N. designed research and integrated the whole work.
N.H. and M.O.B. contributed equally to this paper.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank F. Ishikawa for hTERT cDNA, J. Daley and S. Lazo-Kallanian for excellent technical help, and W. N. Haining for critical review of the manuscript.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal