Abstract
CD4+CD25+FOXP3+ regulatory T cells (Treg's) play an important role in the maintenance of immune tolerance. The mechanisms controlling the induction and maintenance of Treg's in humans need to be defined. We find that human myeloid dendritic cells (DCs) are superior to other antigen presenting cells for the maintenance of FOXP3+ Treg's in culture. Coculture of DCs with autologous T cells leads to an increase in both the number of Treg's, as well as the expression of FOXP3 protein per cell both in healthy donors and myeloma patients. DC-mediated expansion of FOXP3high Treg's is enhanced by endogenous but not exogenous interleukin-2 (IL-2), and DC-T-cell contact, including the CD80/CD86 membrane costimulatory molecules. DCs also stimulate the formation of Treg's from CD25- T cells. The efficacy of induction of Treg's by DCs depends on the nature of the DC maturation stimulus, with inflammatory cytokine-treated DCs (Cyt-DCs) being the most effective Treg inducers. DC-induced Treg's from both healthy donors and patients with myeloma are functional and effectively suppress T-cell responses. A single injection of cytokine-matured DCs led to rapid enhancement of FOXP3+ Treg's in vivo in 3 of 3 myeloma patients. These data reveal a role for DCs in increasing the number of functional FOXP3high Treg's in humans.
Introduction
Regulatory T cells (Treg's) maintain immunologic self-tolerance and also suppress immune responses to tumors, transplants, and infectious agents.1 Current evidence points to the existence of several types of CD4+ Treg's.1,2 Antigen-induced Treg's typically regulate immunity via the production of cytokines such as interleukin-10 (IL-10) or transforming growth factor-β (TGF-β), while naturally occurring or thymus-produced CD4+CD25+ Treg's require cell-contact-dependent mechanisms.2,3 CD1d-restricted natural killer (NK) T cells also can exert regulatory roles.4 A major advance has been the discovery of the transcription factor FOXP3 as a marker and critical controller of suppressor T-cell function.5,6 The importance of this factor in immune regulation is best illustrated by the presence of FOXP3 mutations in humans with X-linked syndrome of immune dysregulation, polyendocrinopathy, and enteropathy (IPEX) as well as in scurfy mice.7-9 These patients and mice lack CD4+CD25+ FOXP3+ regulatory T cells and suffer from the early onset of severe autoimmune and chronic inflammatory disease. The ability of Treg cells to regulate immunity has prompted attempts to stimulate Treg's to treat autoimmunity10-12 or to dampen Treg's to increase immunity to pathogens13 and tumors.14-17
Most prior studies to activate CD4+CD25+ Treg's in humans have used T-cell receptor (TCR) stimulation via soluble or immobilized anti-CD3, along with anti-CD28 and IL-2.18 Recent studies have also shown the ability of human dendritic cells (DCs) to induce FOXP3 expression in human CD4+ T cells.19 However, the evaluation of FOXP3 in these studies was based on analysis of bulk mRNA or total proteins in cell lysates. This has limited a quantitative evaluation of antigen-presenting cell (APC) requirements for the expansion and kinetics of activation of human Treg's. The recent availability of specific antibodies to FOXP3 has permitted evaluation of the expression of this factor in T cells at a single-cell level.20 Here we use these antibodies to examine the activation, maintenance, and expansion of CD4+CD25+ FOXP3+ Treg's by DCs, primarily human monocyte-derived DCs (Mo-DCs).
DCs are APCs specialized to initiate and regulate immunity.21 The potency of DCs at inducing immunity has led to attempts to enhance antitumor immunity by injection of antigen-loaded DCs.22 The ability of DCs to induce immunity is strongly linked to their activation or maturation status. Therefore, DCs are generally differentiated ex vivo prior to adoptive transfer in most current trials. The most common approach in current DC vaccines is to culture DCs in the presence of a cocktail of inflammatory cytokines.23 This leads to a uniform population of mature DCs with enhanced expression of costimulatory molecules. However, while optimization of DC maturation protocols have largely focused on the ability of human DCs to expand effector or protective T cells, their ability to concurrently induce Treg's in vivo has received less attention. Here we will show that human DCs are also specialized for the expansion and maintenance of Tregs in blood-cell cultures from healthy donors and patients with cancer, and that the most active type of DCs are those induced by exposure to inflammatory cytokines (Cyt-DCs). Rapid expansion of Treg's by Cyt-DCs was also evident in vivo within 1 week after DC injection in all 3 patients with myeloma tested.
Patients, materials, and methods
Healthy donors and patients with myeloma
Peripheral blood was obtained from healthy donors and patients with myeloma, following informed consent approved by Rockefeller University Institutional Review Board. Buffy coats purchased from New York Blood Center were also used as a source of mononuclear cells from healthy donors.
Generation of Mo-DCs
Peripheral blood mononuclear cells (PBMCs) were isolated from peripheral blood via density gradient centrifugation using Ficoll-Hypaque (Amersham Pharmacia Biotech, Uppsala, Sweden). CD14+ cells were selected with CD14 microbeads (Miltenyi Biotec, Auburn, CA) and used to generate DCs as described.24 The CD14- fraction was either cultured in RPMI-1640 with l-glutamine (Mediatech, Herndon, VA) supplemented with 5% pooled human serum (Labquip, Niagara Falls, NY) or frozen and used as the source of T cells in DC/T-cell cocultures. DCs were generated by culturing CD14+ cells in RPMI-1640 medium with l-glutamine supplemented with 1% single-donor plasma, gentamicin (20 μg/mL; BioWittaker, Walkersville, MD), and 0.01 M HEPES (Cambrex, Walkersville, MD). Granulocyte-macrophage colony-stimulating factor (GM-CSF, 20 ng/mL; Immunex, Seattle, WA) and IL-4 (20 ng/mL; R&D Systems, Minneapolis, MN) were added to the culture on days 0, 2, and 4.
Isolation of BDCA-1+ DCs, BDCA-2+ DCs, and CD19+ cells
Myeloid and plasmacytoid DCs were isolated using the BDCA-1 and BDCA-2 isolation kits, respectively (Miltenyi Biotec). Plasmacytoid DCs (BDCA-2+) were isolated first, and the negative fraction was then subjected to isolation of both B cells (CD19+) and myeloid DCs (BDCA-1+).
Maturation of DCs
DCs were either used without maturation or were differentiated overnight with an inflammatory cytokine mixture; IL-1β (10 ng/mL), IL-6 (1000 U/mL), tumor necrosis factor-α (TNF-α; 10 ng/mL) (all from R&D Systems, Minneapolis, MN), and prostaglandin E2 (1 μg/mL; Sigma, St Louis, MO).23 DCs were also matured with the 2B6 antibody (Ab) (10 μg/mL; MacroGenics, Rockville, MD),24 which blocks the inhibitory Fcγ receptor (FcγRIIb). LPS (20 ng/mL; Sigma), poly I:C (25 μg/mL; InvivoGen, San Diego, CA), and a CD40 ligand (CD40L) transfected cell line (a generous gift from Dr J. Banchereau, Baylor Institute for Immunology Research, Dallas, TX) were also used as DC maturation stimuli.
Purification of T cells and isolation of CD25- cells
CD3+ T cells were obtained from the CD14- fraction by negative selection using Pan T cell Isolation Kit II (Miltenyi Biotec). The purity of the cell populations was verified via flow cytometry. CD25- T cells were obtained from the purified CD3+ T cells using fluorescence-activated cell-sorting (FACS) to gate on the CD25- population. For some experiments, CD25- fractions were obtained by depleting the CD25+ cells with CD25 beads (Miltenyi Biotec). Cell sorting was used in all experiments where the generation of FOXP3+ cells from the CD25- fraction was examined.
DC-T-cell cocultures
After DC maturation (24 hours), DCs and T cells (either bulk CD3+ cells or CD25- T cells) were cocultured at a 1:10 ratio at 106 T cells/mL in RPMI-1640 supplemented with 5% pooled human serum. IL-2 (0-200 U/mL) was added to some cultures.
Blocking experiments
Cytokine-matured DCs were treated with anti-CD80 and anti-CD86 (1-5 μg/mL each; R&D Systems) or mouse IgG1 (2-10 μg/mL; BD Biosciences, San Diego, CA) for 45 minutes prior to coculture with T cells. Monoclonal anti-IL-2 antibody (1 μg/mL; R&D Systems) or mouse IgG1 isotype control was added at the time of DC-T-cell coculture to block the effects of IL-2.
Flow cytometry for FOXP3 detection
FOXP3 expression in T cells was assessed at various time points using the APC anti-human FOXP3 Staining Kit (e-Biosciences, San Diego, CA). Rat IgG2a APCs (BD Biosciences) were used as isotype control. Samples were also simultaneously stained with CD25 PE (Miltenyi Biotec), CD3 FITC, CD4 FITC, CD4 PE, and CD8 PE (BD Biosciences). For some initial experiments, another anti-FOXP3 Ab was used (a generous gift from Dr Bridget Fox, University of Oxford, United Kingdom).
T-cell proliferation assay
CFSE-labeled T cells (0.3 μM CFSE; Molecular Probes, Eugene, OR) were cultured alone or in the presence of cytokine-matured DCs in RPMI-1640 with l-glutamine supplemented with 5% pooled human serum in 6-well plates. Flow cytometry was performed on day 5 or 6 of coculture to assess FOXP3 expression among proliferating cells.
Suppression assay
Cytokine-matured DCs (Cyt-DCs) were used as stimulators for CFSE-labeled allogeneic T-cell responders. CD25+ or CD25- cells were tested as suppressors and were from the same donor as the stimulating DCs. T cells stimulated with DCs were harvested at approximately day 7 and sorted into CD25+ high cells and CD25- cells. The stimulators and responder cells were cultured in 96-well round-bottom plates at a ratio of 1:10 and suppressors (CD25+ or CD25- cells) were added at a ratio of 1:3, 1:10, or 1:30. Proliferation of the CFSE-labeled cells was assessed at days 3 to 4 of coculture by flow cytometry.
DC-mediated expansion of Treg's in vivo in patients with advanced cancer
We recently carried out a clinical trial of cytokine-matured Mo-DCs in patients with advanced cancer. All patients received 3 injections of 5 × 106 Mo-DCs matured using an inflammatory cytokine cocktail.25 The first injection involved unpulsed DCs as a control, followed at monthly intervals by 2 injections of DCs loaded with NK T-cell ligand, α-galactosyl ceramide (α-GalCer). In the current study, induction of Treg's was evaluated only after the first (unpulsed) DC injection. PBMCs from 4 patients with adequate numbers of cryopreserved PBMCs from preinjection, and days 1, 4, 7, and 28 after DC injection were thawed together and analyzed for the presence of CD4+CD25+ FOXP3+ T cells by flow cytometry.
Statistical analysis
Data from 2 groups were compared using the Student t test, and significance set at a P value less than .05.
Results
Myeloid DCs from blood are superior APCs for maintaining human CD4+CD25+ FOXP3+ Treg's
Most prior studies to activate CD4+CD25+ Treg's in humans have used TCR stimulation via soluble or immobilized anti-CD3, along with anti-CD28 and IL-2.26 To identify APCs active in the stimulation of human Treg's, we purified from blood populations of CD19+ B cells, CD14+ monocytes, BDCA1+ myeloid DCs, or BDCA2+ plasmacytoid DCs. These APCs were cultured with autologous purified CD3+ T cells and the frequency of CD4+FOXP3+ cells was monitored 7 days later. Culture of T cells alone without exogenous IL-2 led to a decline in the proportion of FOXP3+ T cells. In contrast, the frequency of FOXP3+ T cells was maintained and/or enhanced in cocultures with monocytes or myeloid DCs, and the amount of FOXP3 protein per cell was enhanced as well. Myeloid DCs were superior to other APCs, including monocytes, for the induction of both the number of FOXP3+ Treg's (Figure 1A-B), as well as expression of FOXP3 protein per cell (Figure 1C). Thus, DCs are able to expand the number of Treg's in human blood cultures and increase the level of the FOXP3 transcription factor that is critical for Treg function.
Comparison of different cell populations for the induction of CD4+CD25+ FOXP3+ Treg's. (A) Purified CD3+ T cells were cultured alone or with the indicated populations: myeloid DCs (Myel DCs), plasmacytoid DCs (PDCs), monocytes (Monos) and B cells. After 7 days of culture, the frequency of CD4+ FOXP3+ Treg's was assessed by FACS and compared with the frequency of the CD4+ FOXP3+ Treg's at day 0. Data show a summary of 2 experiments. *P < .05 for comparisons with myeloid DCs. (B) The graph depicts fold changes in numbers of CD4+ FOXP3+ Treg's at day 0 compared with numbers after culture either alone or with myeloid DCs (Myel DCs), plasmacytoid DCs (PDCs), monocytes (Monos), or B cells. *P < .05 for comparisons with myeloid DCs. (C) The amount of FOXP3 protein per CD4+ FOXP3+ cell was examined by determining the geometric mean fluorescence of the FOXP3 expression by FACS at day 7. *P < .05 for comparisons with myeloid DCs. Data shown represent means ± SD.
Comparison of different cell populations for the induction of CD4+CD25+ FOXP3+ Treg's. (A) Purified CD3+ T cells were cultured alone or with the indicated populations: myeloid DCs (Myel DCs), plasmacytoid DCs (PDCs), monocytes (Monos) and B cells. After 7 days of culture, the frequency of CD4+ FOXP3+ Treg's was assessed by FACS and compared with the frequency of the CD4+ FOXP3+ Treg's at day 0. Data show a summary of 2 experiments. *P < .05 for comparisons with myeloid DCs. (B) The graph depicts fold changes in numbers of CD4+ FOXP3+ Treg's at day 0 compared with numbers after culture either alone or with myeloid DCs (Myel DCs), plasmacytoid DCs (PDCs), monocytes (Monos), or B cells. *P < .05 for comparisons with myeloid DCs. (C) The amount of FOXP3 protein per CD4+ FOXP3+ cell was examined by determining the geometric mean fluorescence of the FOXP3 expression by FACS at day 7. *P < .05 for comparisons with myeloid DCs. Data shown represent means ± SD.
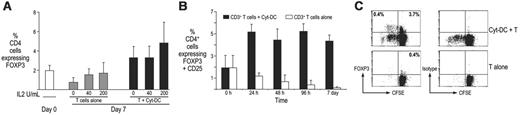
Human Mo-DCs expand FOXP3+ CD4+ T cells in culture
Mo-DCs are an abundant source of DCs and as such, are under evaluation for clinical immunotherapy. To test whether such DCs also induce Treg's, we cultured bulk CD3+ T cells either alone or with Mo-DCs for 7 days (Cyt-DCs), in the absence or presence of graded doses of exogenous IL-2 at 0 to 200 U/mL (Figure 2). The Cyt-DCs used in this experiment were matured using a commonly used cocktail of inflammatory cytokines (TNFα, IL-1β, IL-6) plus PGE2 for 24 hours prior to DC:T cell coculture. In cultures without Cyt-DCs, the frequency of FOXP3+ cells as well as the expression of FOXP3 protein per cell were lower than in the starting population, but could be sustained when IL-2 was added to the cultures (Figure 2A, left). In contrast, there was some expansion of CD4+ FOXP3+ Treg's in cocultures with Cyt-DCs, even when no exogenous IL-2 was added (Figure 2A). The amount of FOXP3 protein expressed per cell as detected by flow cytometry was also significantly lower in T cells cultured alone, compared with Cyt-DC-T cell cocultures (data not shown). As in other studies, most of the FOXP3+ cells were CD4+, although a minor subpopulation of CD8+ FOXP3+ cells were also present at baseline and expanded with DCs (data not shown). The increase in FOXP3+ T cells in these cocultures was rapid, detectable within 24 hours, and maintained over time (Figure 2B). To test if cell proliferation was contributing to the numbers of Treg's, the starting population of T cells was labeled with CFSE prior to culture with Cyt-DCs. Analysis of CFSE dilution showed that some of the FOXP3+ cells underwent proliferation in response to stimulation with Cyt-DCs (Figure 2C). Thus, Cyt-DCs expand and maintain the numbers of CD4+CD25+ FOXP3+ Treg's in culture.
Expansion of CD4+ CD25+ FOXP3+ T cells from CD4-CD25- T cells
CD25 was clearly expressed in parallel with FOXP3 in our DC-T-cell cocultures, so we tested whether Cyt-DCs could induce FOXP3 expression from starting populations of CD25- T cells, where FOXP3 expression was minimal (Figure 3A, left panel). Coculture of CD25- T cells with cytokine matured DCs led to the induction of both CD25 and FOXP3 in a fraction of the CD4+ T cells. The expression of both CD25 as well as FOXP3 increased continuously over the 1-week culture (Figure 3B). Hence, Cyt-DCs are able to stimulate a fraction of CD25- T cells to express both CD25 as well as FOXP3, without the need for exogenous IL-2.
Induction of Treg's by DCs is dependent on cell-cell contact, CD80/CD86 interactions, and IL-2
To examine whether DC-mediated expansion of Treg's requires cell-cell contact between DCs and T cells, we cocultured these cells with or without transwell inserts. The induction of FOXP3+ T cells was reduced in cultures with transwells, indicating the need for cell-cell contact (Figure 4A). Costimulation via B7 has been shown to be critical for homeostasis of CD25+ Treg's in mice.27 When we cocultured T cells with Cyt-DCs either alone or in the presence of anti-CD80/CD86 antibodies, the presence of anti-CD80/CD86 antibodies reduced the induction of FOXP3+ T cells (Figure 4B). IL-2 is critical for the maintenance of Treg's in vivo.28 To test the need for IL-2 in our cultures, we cocultured T cells with Cyt-DCs either alone or in the presence of anti-IL-2 antibodies. The presence of anti-IL-2 monoclonal Ab (mAb) also reduced the number of FOXP3+ T cells (Figure 4B). Therefore, DC-mediated induction of Treg's depends on cell-cell contact, which in part includes CD80/CD86-mediated interactions, and IL-2 produced in these cocultures.
Expansion of CD4+CD25+ FOXP3+ Treg cells by monocyte-derived cytokine-matured DCs. (A) CD3 purified T cells were cultured alone or with monocyte-derived Cyt-DCs in the absence or presence of graded doses of IL-2 (0-200 U/mL). After 7 days, the numbers of CD4+ FOXP3+ Treg's were monitored by flow cytometry. Data (mean ± SD) show a summary of 5 experiments. P < .05 for comparisons with Cyt-DCs. (B) T cells were cultured either alone or with Cyt-DCs, and CD4+ FOXP3+ T cells were enumerated by FACS on days 0, 1, 2, 4, and 7. Data (mean ± SD) show a summary of 2 experiments. (C) T cells were labeled with CFSE and cultured alone or with Cyt-DCs, and proliferation monitored by flow cytometry on day 5. The figure represents 1 of 4 similar experiments.
Expansion of CD4+CD25+ FOXP3+ Treg cells by monocyte-derived cytokine-matured DCs. (A) CD3 purified T cells were cultured alone or with monocyte-derived Cyt-DCs in the absence or presence of graded doses of IL-2 (0-200 U/mL). After 7 days, the numbers of CD4+ FOXP3+ Treg's were monitored by flow cytometry. Data (mean ± SD) show a summary of 5 experiments. P < .05 for comparisons with Cyt-DCs. (B) T cells were cultured either alone or with Cyt-DCs, and CD4+ FOXP3+ T cells were enumerated by FACS on days 0, 1, 2, 4, and 7. Data (mean ± SD) show a summary of 2 experiments. (C) T cells were labeled with CFSE and cultured alone or with Cyt-DCs, and proliferation monitored by flow cytometry on day 5. The figure represents 1 of 4 similar experiments.
Induction of FOXP3+ T cells in CD4+CD25- T cells by DCs. (A) CD25- T cells were isolated by flow sorting and cultured alone (T alone) or with Cyt-DCs. After 7 days of culture, CD4+CD25+ FOXP3+ T cells were monitored by flow cytometry. One of 4 similar experiments. (B) CD25- T cells were cultured alone or with Cyt-DCs, and expression of FOXP3 protein was monitored by flow cytometry on days 0, 1, 4, and 7 of culture. The graph shows a summary of 2 experiments (mean ± SD).
Induction of FOXP3+ T cells in CD4+CD25- T cells by DCs. (A) CD25- T cells were isolated by flow sorting and cultured alone (T alone) or with Cyt-DCs. After 7 days of culture, CD4+CD25+ FOXP3+ T cells were monitored by flow cytometry. One of 4 similar experiments. (B) CD25- T cells were cultured alone or with Cyt-DCs, and expression of FOXP3 protein was monitored by flow cytometry on days 0, 1, 4, and 7 of culture. The graph shows a summary of 2 experiments (mean ± SD).
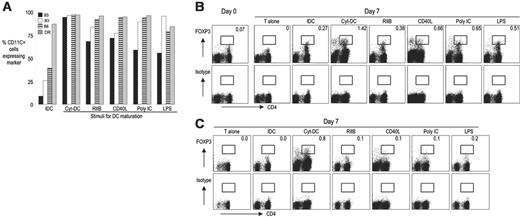
The nature of the DC maturation stimulus impacts on the expansion of Treg's
The ability of DCs to stimulate immunity is linked to their maturation status, which influences both the quantity and quality of the T-cell response. Human DCs undergo some aspects of maturation, particularly an up-regulation of costimulatory molecules like CD86, in response to a number of different stimuli, including inflammatory cytokines, ligands for Toll-like receptors, and selective blockade of inhibitory FcγR.24 These stimuli, however, have distinct effects on DC phenotype and their functional properties.21 To test whether the nature of maturation stimulus has an impact on DC-mediated expansion of Treg's, we cultured bulk CD3+ T cells and CD25- T cells alone or with immature DCs, or DCs matured for 24 hours using inflammatory cytokines (Cyt-DC; IL-1β, IL-6, TNF-α, and PGE2), Toll-like receptor ligands (LPS, poly I:C), CD40L-transfected cells, or 2B6 anti-FcγRIIB antibody. DC maturation was monitored by examining the up-regulation of CD83, CD80, CD86, and human leukocyte antigen (HLA)-DR (Figure 5A). As seen in previous experiments, FOXP3 expression decreased when T cells were cultured alone, while the addition of all forms of Mo-DCs, increased the frequency of FOXP3+ T cells as well as the amount of FOXP3 protein per cell (P < .005). Of the maturation stimuli tested, Cyt-DCs led to the greatest induction of FOXP3+ T cells (Figure 5b). This is particularly evident when the induction of FOXP3high cells (Figure 5B; see box) is compared. Notably, in addition to CD4+ FOXP3+ cells, a small but distinct population of CD4- FOXP3+ cells is also induced in these cultures. These cells express CD8 and are therefore likely CD8+ Treg's. The superiority of Cyt-DCs for the induction of Treg's was also evident in the induction of CD25+ FOXP3+ Treg's from CD25- cells (Figure 5C). Thus, the nature of the DC maturation stimulus has a major impact on the ability of DCs to induce Treg's.
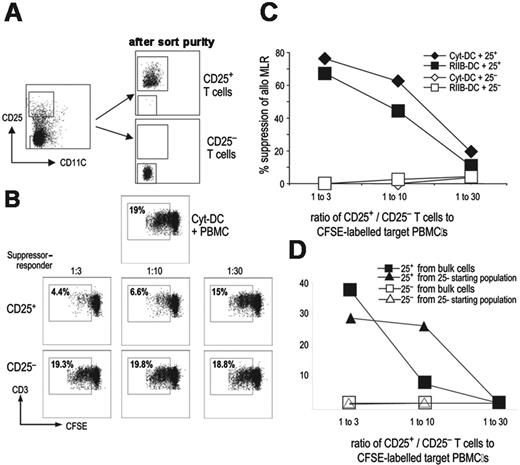
DC-induced Treg's are functional and can suppress MLR induced by mature DCs
To test the suppressive function of human Treg's, we used an assay involving reduction of T-cell proliferation in the allogeneic mixed leukocyte reaction (MLR). DCs are potent stimulators of the MLR. To test the regulatory properties of DC induced Treg's, we chose to use the more demanding criterion of suppression of an MLR driven by mature DCs. DCs were cocultured with autologous T cells, and on day 7 of culture, CD25high or CD25- cells (used as controls) were isolated by FACS (Figure 6A). These cells were then added as suppressors (suppressor-target ratio, 1:3-1:30) to an allogeneic MLR driven by mature DCs, with the DCs derived from the same donor as had been used to generate the Treg's. CD25high but not the CD25- cells were able to inhibit T-cell proliferation (median percent suppression, 50%; Figure 6B). When we added the Treg's to an MLR stimulated by DCs from the original autologous donor versus a different or third-party donor, the Treg's more actively suppressed autologous DCs (data not shown). Therefore, Treg's induced by DCs are functional and able to suppress T-cell responses. Although Cyt-DCs induced larger numbers of Treg's than DCs matured in other ways (eg, blockade of the inhibitory Fc receptor with 2B6 mAb), the suppressive properties of the elicited Treg's were comparable (Figure 6C). As discussed, DCs are also efficient at the induction of Treg's from CD25- precursors. To directly evaluate the suppression mediated by such induced Treg's, CD25+ cells from cocultures of DCs and purified CD25- T cells were sorted and tested for their suppressive properties. These induced Treg's were also effective at suppression of the MLR (Figure 6D). Thus, DC-expanded Treg's have suppressive function in vitro.
Generation of FOXP3+ cells by cytokine matured DCs is contact, CD80/CD86, and IL-2 dependent. (A) T cells were cultured with Cyt-DCs in the presence or absence of transwell inserts. Data (mean ± SD) shown in the left panel are CD4+FOXP3+ cells expressed as a percentage of control cultures with no transwells. *P < .05. The representative FACS plots on the right are from 1 of the 3 experiments summarized in the panel on the left. (B) T cells were cultured with Cyt-DCs treated with anti-CD80 and anti-CD86 antibodies (1-5 μg/mL each), anti-IL-2 antibody (1-5 μg/mL), or isotype control. Data (mean ± SD) shown in the panel on the left are CD4+FOXP3+ cells expressed as a percentage of control cultures with isotype mAb-treated DCs.*P < .05. The representative FACS plots on the right are from 1 of the 3 experiments summarized in the panel on the left. The numbers in the FACS plots represent percentage of cells in that quadrant.
Generation of FOXP3+ cells by cytokine matured DCs is contact, CD80/CD86, and IL-2 dependent. (A) T cells were cultured with Cyt-DCs in the presence or absence of transwell inserts. Data (mean ± SD) shown in the left panel are CD4+FOXP3+ cells expressed as a percentage of control cultures with no transwells. *P < .05. The representative FACS plots on the right are from 1 of the 3 experiments summarized in the panel on the left. (B) T cells were cultured with Cyt-DCs treated with anti-CD80 and anti-CD86 antibodies (1-5 μg/mL each), anti-IL-2 antibody (1-5 μg/mL), or isotype control. Data (mean ± SD) shown in the panel on the left are CD4+FOXP3+ cells expressed as a percentage of control cultures with isotype mAb-treated DCs.*P < .05. The representative FACS plots on the right are from 1 of the 3 experiments summarized in the panel on the left. The numbers in the FACS plots represent percentage of cells in that quadrant.
Effect of DC maturation stimulus on expansion of Treg's. (A) Maturation status of immature DCs (IDC) or DCs matured with inflammatory cytokines (Cyt-DC), FcγRIIB blockade (RIIB), CD40 ligand (CD40L), poly I:C, or LPS was monitored by examining the surface expression of CD83, CD80, CD86, and HLA-DR. The figure represents 1 of 3 similar experiments. (B) T cells were cultured either alone (T alone), or with immature DCs (IDC), or DCs matured with inflammatory cytokines (Cyt-DC), FcγRIIB blockade (RIIB), CD40 ligand matured DCs (CD40L), or the Toll-like receptor ligands poly I:C, or LPS. After 7 days of culture, the presence of FOXP3+ T cells was monitored by flow cytometry. The figure represents 1 of 5 similar experiments. The numbers represent the percentage of FOXP3high cells in the gated area. (C) As in panel B, different types of mature DCs were tested for the ability to induce Treg's in the CD4+ CD25- population. The plots represent 1 of 3 similar experiments.
Effect of DC maturation stimulus on expansion of Treg's. (A) Maturation status of immature DCs (IDC) or DCs matured with inflammatory cytokines (Cyt-DC), FcγRIIB blockade (RIIB), CD40 ligand (CD40L), poly I:C, or LPS was monitored by examining the surface expression of CD83, CD80, CD86, and HLA-DR. The figure represents 1 of 3 similar experiments. (B) T cells were cultured either alone (T alone), or with immature DCs (IDC), or DCs matured with inflammatory cytokines (Cyt-DC), FcγRIIB blockade (RIIB), CD40 ligand matured DCs (CD40L), or the Toll-like receptor ligands poly I:C, or LPS. After 7 days of culture, the presence of FOXP3+ T cells was monitored by flow cytometry. The figure represents 1 of 5 similar experiments. The numbers represent the percentage of FOXP3high cells in the gated area. (C) As in panel B, different types of mature DCs were tested for the ability to induce Treg's in the CD4+ CD25- population. The plots represent 1 of 3 similar experiments.
Suppressive function of DC-induced Treg's. (A) T cells were cultured with DCs for 7 days, and the cocultures were labeled with CD25 PE and CD11c FITC. Cell sorting was used to obtain CD25high and CD25- fractions of CD11c- cells. The plot shows the postsort purity of the fractions used as suppressors in the MLR. (B) Suppression of the allogeneic MLR by Treg's induced by mature DCs. Mature DCs (from the same donor as used to generate the CD25+ FOXP3+ T cells) were cultured with CFSE labeled allogeneic responder T cells at a ratio of 1:10. CD25+ suppressors (middle panel) and CD25- cells (bottom panel) obtained via cell sorting were added to the cultures at suppressor-responder ratios of 1:3 to 1:30. Three to 4 days later, flow cytometry was performed to determine the proliferation in the cultures. Top panel shows control MLR without any cells added. (C) FOXP3+ Treg's were generated by culturing T cells with either Cyt-DCs or FcγRIIB blockade-matured DCs (RIIB-DC). Seven days later, DC-expanded T cells were subjected to cell-sorting as in panel A, and CD25+ and CD25- populations were obtained and used as suppressors. The MLR was set up with mature DCs (stimulators) and CFSE-labeled allogeneic T cells as responders as in panel B. The sorted CD25+ and CD25- T cells were added to compare the suppressive ability of the Treg's generated by DCs matured by 2 different maturation stimuli. (D) Bulk CD3+ T cells and sorted CD3+ CD25- T cells were cocultured with Cyt-DCs. After 7 days of coculture, T cells from both conditions were flow sorted to obtain CD25high as well as CD25- T cells as described in panel A. The CD25+ and CD25- cells were used as suppressors in an MLR. The MLR was set up with mature DCs and CFSE-labeled allogeneic T cells as responders as in panel B, and the CD25+ and CD25- populations were added to compare the suppressive ability of the Treg's generated from either bulk T cells or CD25- T cells (de novo Treg's).
Suppressive function of DC-induced Treg's. (A) T cells were cultured with DCs for 7 days, and the cocultures were labeled with CD25 PE and CD11c FITC. Cell sorting was used to obtain CD25high and CD25- fractions of CD11c- cells. The plot shows the postsort purity of the fractions used as suppressors in the MLR. (B) Suppression of the allogeneic MLR by Treg's induced by mature DCs. Mature DCs (from the same donor as used to generate the CD25+ FOXP3+ T cells) were cultured with CFSE labeled allogeneic responder T cells at a ratio of 1:10. CD25+ suppressors (middle panel) and CD25- cells (bottom panel) obtained via cell sorting were added to the cultures at suppressor-responder ratios of 1:3 to 1:30. Three to 4 days later, flow cytometry was performed to determine the proliferation in the cultures. Top panel shows control MLR without any cells added. (C) FOXP3+ Treg's were generated by culturing T cells with either Cyt-DCs or FcγRIIB blockade-matured DCs (RIIB-DC). Seven days later, DC-expanded T cells were subjected to cell-sorting as in panel A, and CD25+ and CD25- populations were obtained and used as suppressors. The MLR was set up with mature DCs (stimulators) and CFSE-labeled allogeneic T cells as responders as in panel B. The sorted CD25+ and CD25- T cells were added to compare the suppressive ability of the Treg's generated by DCs matured by 2 different maturation stimuli. (D) Bulk CD3+ T cells and sorted CD3+ CD25- T cells were cocultured with Cyt-DCs. After 7 days of coculture, T cells from both conditions were flow sorted to obtain CD25high as well as CD25- T cells as described in panel A. The CD25+ and CD25- cells were used as suppressors in an MLR. The MLR was set up with mature DCs and CFSE-labeled allogeneic T cells as responders as in panel B, and the CD25+ and CD25- populations were added to compare the suppressive ability of the Treg's generated from either bulk T cells or CD25- T cells (de novo Treg's).
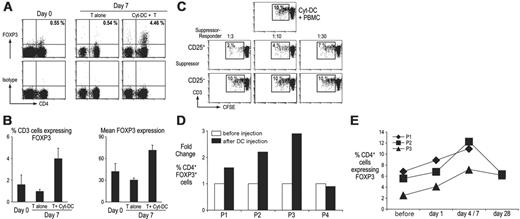
Cytokine DCs expand functional FOXP3high Treg's from patients with myeloma
To test whether Cyt-DCs lead to an increase in Treg's in blood cells from cancer patients, we monitored the expansion of FOXP3+ Treg's in cocultures of Cyt-DCs and bulk T cells from 3 patients with myeloma (Figure 7A-B). As with healthy donors, DCs significantly expanded the frequency of FOXP3+ T cells, as well as the level of FOXP3high cells in all 3 patients tested. The Treg's induced in these cultures were functional and able to efficiently suppress the MLR (Figure 7C). Therefore, Cyt-DCs are also efficient at expansion of functional FOXP3+ T cells in patients with myeloma.
Cytokine DCs expand regulatory T cells in vivo
The most common approach used for maturation of Mo-DCs for clinical immunotherapy has been an inflammatory cytokine cocktail.22 Recently, we carried out a clinical trial of cytokine-matured Mo-DCs in patients with advanced cancer.25 All patients received an initial injection of unpulsed cytokine cocktail-matured DCs. To examine the effect of DC injection on the induction of Treg's in vivo, we analyzed the number of FOXP3+ Treg's in these patients before and after the first DC vaccine in 3 patients with myeloma (P1-P3) and 1 patient with advanced renal cell cancer (P4). Injection of the DC vaccine was associated with an increase in FOXP3+ Treg's in 3 of 4 patients tested (Figure 7D-E). There was no evidence for clinical deterioration or decline in virus specific immune responses after this DC injection.25 DC-mediated expansion of Treg's is rapid and associated with a near doubling of circulating Treg's as early as 7 days after DC injection. Thus, a single injection of Cyt-DCs can lead to an increase in CD4+CD25+ FOXP3+ Treg's in patients with cancer.
Expansion of FOXP3+ Treg's by DCs from patients with myeloma. (A) CD14- cells were used as the source of T cells and either cultured alone or with autologous cytokine-matured DCs. Seven days later, FOXP3+ CD4+ T-cell numbers were examined by flow cytometry and compared with those seen in fresh blood at the time of blood draw. Numbers represent percentage of cells in that quadrant. (B) Results show mean (±SD) FOXP3+ CD4+ T-cell expansions from 3 different patients. Left panel shows expansion of CD4+ FOXP3+ T cells at the time of blood draw (day 0), and after 7 days of culturing T cells alone (T alone) and with cytokine matured DCs (T + Cyt-DCs). The right panel shows the geometric mean fluorescence of the FOXP3 expression in CD4+ FOXP3+ T cells before and after culture. (C) CD25+ and CD25- T cells were sorted after 7 days of culture with cytokine-matured DCs as described in Figure 5. An MLR was set up using patient DCs as stimulators and CFSE-labeled allogeneic T cells as responder cells at a 1:10 ratio. Patient CD25+ and CD25- cells were added as suppressors at a ratio of 1:3 to 1:30. Four days later, flow cytometry was performed to determine the proliferation of the CFSE-labeled responder cells. Figure represents 1 of 2 similar experiments. (D) Cytokine-matured DCs expand FOXP3+ CD4+ T cells in vivo. Flow cytometry was performed to examine FOXP3 expression in T cells before and after injection of cytokine-matured DCs in 4 patients. (E) The kinetics of FOXP3 expansion was examined in 3 patients who showed an increase in FOXP3+ CD4+ T cells. T cells were obtained before injection, 1 to 7 days after, and 28 days after injection of cytokine-matured DCs.
Expansion of FOXP3+ Treg's by DCs from patients with myeloma. (A) CD14- cells were used as the source of T cells and either cultured alone or with autologous cytokine-matured DCs. Seven days later, FOXP3+ CD4+ T-cell numbers were examined by flow cytometry and compared with those seen in fresh blood at the time of blood draw. Numbers represent percentage of cells in that quadrant. (B) Results show mean (±SD) FOXP3+ CD4+ T-cell expansions from 3 different patients. Left panel shows expansion of CD4+ FOXP3+ T cells at the time of blood draw (day 0), and after 7 days of culturing T cells alone (T alone) and with cytokine matured DCs (T + Cyt-DCs). The right panel shows the geometric mean fluorescence of the FOXP3 expression in CD4+ FOXP3+ T cells before and after culture. (C) CD25+ and CD25- T cells were sorted after 7 days of culture with cytokine-matured DCs as described in Figure 5. An MLR was set up using patient DCs as stimulators and CFSE-labeled allogeneic T cells as responder cells at a 1:10 ratio. Patient CD25+ and CD25- cells were added as suppressors at a ratio of 1:3 to 1:30. Four days later, flow cytometry was performed to determine the proliferation of the CFSE-labeled responder cells. Figure represents 1 of 2 similar experiments. (D) Cytokine-matured DCs expand FOXP3+ CD4+ T cells in vivo. Flow cytometry was performed to examine FOXP3 expression in T cells before and after injection of cytokine-matured DCs in 4 patients. (E) The kinetics of FOXP3 expansion was examined in 3 patients who showed an increase in FOXP3+ CD4+ T cells. T cells were obtained before injection, 1 to 7 days after, and 28 days after injection of cytokine-matured DCs.
Discussion
By tracking FOXP3+ cells at the single-cell level, our data demonstrate that human DCs effectively expand CD4+CD25+ FOXP3+ T cells in culture and in vivo. Culture of purified human T cells alone results in a decrease in the number of FOXP3+ cells as well as the amount of FOXP3 protein per cell. In the presence of exogenous IL-2, the starting frequency of Treg's is sustained, but the levels of FOXP3 protein remain low. In contrast, when DCs are added to these cultures, the Treg's respond in several ways: (1) the frequency of Treg's increases, generally to more than 5% of the culture when cytokine-matured DCs are used; (2) the amount of FOXP3 protein per cell increases; (3) FOXP3high CD4+CD25+ T cells develop from CD25- precursors; and (4) some of the FOXP3+ cells are able to proliferate. In addition to sustaining expression of CD25 and FOXP3, the expanded Treg's are functionally active and suppress DC stimulation in the allogeneic MLR.
Prior studies have shown that the injection of LPS-matured DCs into mice leads to an expansion of adoptively transferred CD4+CD25+ Treg's.29 The targeting of antigens to DCs in the steady state leads to the induction of tolerance by both deletion of effectors as well as induction of Treg's. It is of interest that stimulation of human T cells by immature DCs can also lead to induction of another type of Treg, called Tr1, comprising IL-10-producing cells that lack FOXP3.30,31 Tr1 cells exert their suppression through the release of IL-10, whereas FOXP3+ Treg's require cell-cell contact.32 The effect of DC maturation also varies with the 2 types of Treg. An immature form of DC seems essential for the induction of Tr1, whereas cytokine matured DCs are more active than immature DCs in expanding FOXP3+ Treg's.30,31 Together, these studies support complementary roles for the induction of different types of regulatory T cells, Tr1 and CD4+CD25+ FOXP3+ T cells by DCs in different states of maturation. The finding that DCs matured via inflammatory cytokines are the most efficient at inducing Treg's is of interest, as excess production of inflammatory cytokines would represent an overactive immune stimulus. Further research will need to relate the nature of the DC maturation stimulus to the ability of human DCs to stimulate Treg's in vivo, especially in the context of vaccine biology, as the goal in many vaccines is to minimize the concurrent induction of Treg's.
These data also have clear implications for clinical DC-mediated immunotherapy. Mo-DCs are currently being investigated for immunotherapy of cancer and pathogens. Interestingly, nearly all of the DC vaccine studies to date have used inflammatory cytokines to activate DCs ex vivo. Here we show that such DCs are also able to efficiently expand Treg's in vitro and in vivo, including in patients with cancer. Therefore, although DCs matured using inflammatory cytokines are effective at inducing effector T cells, they are also likely to induce FOXP3+ Treg's. Vaccine-mediated induction of FOXP3+ Treg's may have been an underappreciated effect in current trials of human DC vaccination. The nature of the DC maturation stimulus may be an important variable determining the balance between the induction of effector-versus-FOXP3+ regulatory T cells. For example, alternate DC activation approaches like FcγRIIb blockade might lead to less induction of Treg's, while maintaining the ability to activate effector T cells. However, as shown here, the induction of Treg's takes place with these other maturation stimuli as well, supporting the need to combine DC therapy with approaches to selectively remove Treg's or dampen their function.16 Unfortunately the current use of CD25-based methods for targeting Treg's remain a challenge, because the CD25 marker is also expressed by FOXP3- effector cells. Furthermore, as shown here, DCs are also efficient at inducing FOXP3+ Treg's from CD25- cells in humans. Therefore, the depletion of CD25+ T cells prior to DC vaccination is not likely to be sufficient for removal of all FOXP3+ Treg's.
The ability of DCs to efficiently expand Treg's also has clear implications for the generation of Treg's for therapeutic purposes. The protocols described here using purely autologous systems and human sera can be readily extended to clinical grade conditions to generate Treg's for adoptive transfer in autoimmunity and transplantation. To our knowledge, the only other approach shown to expand FOXP3+ Treg's in vivo in humans involves the administration of very high doses of IL-2 in patients with melanoma.33 This approach is, however, associated with considerable toxicity. An important advantage of the use of DCs is that these cells are efficient in antigen capture, processing and presentation. This allows DCs to be used to generate antigen-specific Treg's, which are known to be much more effective regulators of immunity in vivo.10,34
Several studies have shown an increase in FOXP3+ Treg's in patients with cancer.1,35 Another aspect of several cancers, including myeloma, is chronic inflammation with an increase in inflammatory cytokines in the tumor bed. For example, increased levels of several inflammatory cytokines have been observed in myeloma patients.36 Therefore, the cytokine milieu of the tumor bed may account for the observed induction of Treg's in patients with myeloma. Our data also extend the recent findings of Beyer et al,37 showing that Treg's in myeloma are functional, and suggest that their expansion should be monitored in the context of antitumor vaccination in myeloma.37,38 Optimizing the balance of vaccine-induced effector-versus-regulatory T cells may be critical to effective immunotherapy against cancer or viral infections.39
Prepublished online as Blood First Edition Paper, June 8, 2006; DOI 10.1182/blood-2006-03-011353.
Supported in part by funds from the National Institutes of Health (AI054375 [K.M.D.], PO1 AI51573 [R.M.S.], CA106802 and CA109465 [M.V.D.], and MO1-RR00102 to The Rockefeller University General Clinical Research Center [RU GCRC]), the Dana Foundation, the American Society of Clinical Oncology Career Development award (K.M.D.), Damon Runyon Eli Lilly Clinical Investigator Award (M.V.D.), and the Montreal General Hospital Foundation (D.K.B.).
K.M.D. designed, performed, and coordinated research, analyzed data, and wrote the paper; D.K.B. performed research, analyzed data, and wrote the paper; M.V.D. designed research, analyzed data, and wrote the paper; E.M. performed research; and R.M.S. designed research, analyzed data, and wrote the paper.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors would like to thank Rockefeller flow cytometry core and Klara Velinzon for help with flow sorting, and Judy Adams for her help with graphics.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal