Abstract
The 67-kDa laminin receptor (67LR) is a nonintegrin cell-surface receptor with high affinity for laminin, which plays a key role in tumor invasion and metastasis. We investigated the role of 67LR in granulocyte colony-stimulating factor (G-CSF)–induced mobilization of CD34+ hematopoietic stem cells (HSCs) from 35 healthy donors. G-CSF–mobilized HSCs, including CD34+/CD38– cells, showed increased 67LR expression as compared with unstimulated marrow HSCs; noteworthy, also, is the fact that the level of 67LR expression in G-CSF–mobilized HSCs correlated significantly with mobilization efficiency. During G-CSF–induced HSC mobilization, the expression of laminin receptors switched from α6 integrins, which mediated laminin-dependent adhesion of steady-state human marrow HSCs, to 67LR, responsible for G-CSF–mobilized HSC adhesion and migration toward laminin. In vitro G-CSF treatment, alone or combined with exposure to marrow-derived endothelial cells, induced 67LR up-regulation in marrow HSCs; moreover, anti-67LR antibodies significantly inhibited transendothelial migration of G-CSF–stimulated marrow HSCs. Finally, G-CSF–induced mobilization in mice was associated with 67LR up-regulation both in circulating and marrow CD34+ cells, and anti-67LR antibodies significantly reduced HSC mobilization, providing the first in vivo evidence for 67LR involvement in stem-cell egress from bone marrow after G-CSF administration. In conclusion, 67LR up-regulation in G-CSF–mobilized HSCs correlates with their successful mobilization and reflects its increase in marrow HSCs, which contributes to the egress from bone marrow by mediating laminin-dependent cell adhesion and transendothelial migration.
Introduction
Mobilized hematopoietic stem cells (HSCs), obtained by granulocyte colony-stimulating factor (G-CSF) administration, are rapidly replacing traditional bone marrow (BM) harvesting as a source of stem cells for transplantation purposes.1-3
HSCs express several adhesion molecules that play an important role in their retention within the BM microenvironment4-8 Adhesion molecules, such as β1 and β2 integrins (ie, VLA-4, VLA-5, and LFA-1), l-selectin, and the stromal derived factor 1 (SDF-1) chemokine and its receptor CXCR4, are well recognized key players in mobilization and homing of HSCs.9-14 HSC release from BM also involves the activation and secretion of several proteolytic enzymes.15 However, how adhesion molecules, chemokines, and proteases act and crosstalk is only partially understood.
The 67-kDa laminin receptor (67LR) is a nonintegrin cell-surface receptor with high affinity for laminin, which plays a key role in tumor invasion and metastasis.16 67LR expression is increased in neoplastic cells as compared with their normal counterparts and directly correlates with enhanced invasive and metastatic potential.17 Indeed, 67LR overexpression promotes tumor-cell migration and adhesion18,19 and increases extracellular matrix degradation by up-regulating the expression and activity of proteolytic enzymes.20,21 Thus, 67LR overexpression is considered a molecular marker of metastatic aggressiveness in cancers of many tissues.22,23
67LR derives from a 37-kDa cytosolic precursor (37LRP)24,25 and binds laminin through different binding domains.26,27 Laminin conformation changes on binding 67LR, thus interacting more efficiently with integrins28 and becoming more sensitive to the action of proteolytic enzymes.20 67LR is coexpressed and can physically interact with the α6-integrin chain.29
67LR expression regulates adhesion of human T lymphocytes to laminin30 and their in vitro and in vivo migration31 ; 67LR also mediates acute myeloid leukemia-cell adhesion to laminin,32 as well as in vitro and in vivo migration of lymphoma and myeloma cells.31,33 In addition, various laminin isoforms have shown mitogenic and adhesive properties for hematopoietic progenitor cells.34,35
All together, these observations suggest possible 67LR involvement in the process of HSC mobilization. Therefore, we investigated whether 67LR could play a role in the regulation of HSC mobilization induced by G-CSF.
Materials and methods
Reagents
Horseradish peroxidase-conjugated anti–rabbit IgG was from Bio-Rad (Richmond, CO); FITC-labeled goat anti–rabbit IgG was from Jackson ImmunoResearch Labs (West Grove, PA). Protease inhibitors, Ficoll-Hypaque (specific gravity 1077), bovine serum albumin (BSA), and hydrocortisone sodium hemisuccinate were from Sigma Chemical (St Louis, MO). Human placental laminin (LM) was from Chemicon (Temecula, CA). The enhanced chemiluminescence (ECL) detection kit was from Amersham International (Amersham, United Kingdom) and polyvinylidene fluoride (PVDF) filters were from Millipore (Windsor, MA). RPMI 1640 medium, Medium 199, FN-coated culture flasks, heat-inactivated fetal calf serum (FCS), Lipofectamine, and Geneticin were from Life Technologies (Gaithersburg, MD). The 96-well microtiter plates and Transwell plates were from Costar (Cambridge, MA). Chemotaxis PVPF filters were purchased from Corning (Corning, NY). The stromal-derived factor 1α (SDF-1α) was purchased from PeproTech (London, United Kingdom). Human specific fluorescein isothiocyanate (FITC)–, peridinin chlorophyll (PerCP)–, and phycoerythrin (PE)–labeled monoclonal antibodies (mAbs) were purchased from Becton Dickinson (Mountain View, CA). Methylcellulose supplemented with a specific mouse cytokine cocktail was from StemCell Technologies (Vancouver, BC, Canada). Interleukin 3 (IL-3), G-CSF, granulocyte-macrophage colony-stimulating factor (GM-CSF), stem-cell factor (SCF), and erythropoietin (EPO) were from Amgen (Thousand Oaks, CA). Recombinant human G-CSF (rhG-CSF, lenograstim) was purchased from Italfarmaco (Milan, Italy). 67LR cDNA was cloned into the pcDNA3 vector and the resulting plasmid was named 67LR-pcDNA3. The rabbit anti-67LR polyclonal antibody ab711, recognizing both human and rodent 67LR, was from Abcam (Cambridge, United Kingdom); MPRLs, a cocktail of 8 anti-67LR mAbs,36 and the monoclonal anti-67LR antibody MLuC5 were kindly provided by Dr S. Menard and Dr E. Tagliabue (National Cancer Institute, Milan, Italy). Goat polyclonal anti–α6-integrin subunit antibody, anti–phospho-ERKs, and anti-ERK2 polyclonal antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA). The enzyme-linked immunosorbent assay (ELISA) kit for quantitative determination of laminin in human sera was from Takara (Otsu, Shiga, Japan).
Sample collection
Heparinized blood samples were obtained after written informed consent was obtained (according to the procedures outlined by the ethics committee of our institution) before, during, and after the mobilizing procedure, from 35 healthy adults (19 men and 16 women, 20-55 years old). The donors received glycosylated rhG-CSF administered subcutaneously, at 10 μg/kg/d, in 2 divided doses, for 5 days, to mobilize and collect CD34+ cells. Heparinized BM specimens were obtained by aspiration from the posterior iliac crest from healthy young donors (8 men and 7 women, 25-45 years old).
CD34+ HSC separation
Peripheral-blood (PB) and BM mononuclear cells MNCs were isolated by Ficoll-Hypaque centrifugation. CD34+ cells were highly purified by MiniMacs high-gradient magnetic separation columns (Miltenyi Biotec, Auburn, CA). CD34+ cells reached 90% purity by 2 sequential selections through the magnetic cell separator. Purity of the positively selected CD34+ cells was evaluated by flow cytometry. Purified CD34+ cells were resuspended in RPMI medium supplemented with 5% FCS for in vitro cultures.
For CD34+/CD38– enrichment, PBMNCs (5 × 107/mL) were incubated for 30 minutes in ice with a mixture of anti–glycophorin A, -CD3, -CD2, -CD14, -CD16, -CD19, -CD24, -CD56, and -CD66b tetrameric antibody complexes (StemCell Technologies). After 3 washings, cells were incubated for 30 minutes with 60 μL/mL magnetic colloidal iron/dextran particles, and finally processed through the StemSep device for depletion of targeted cells (StemCell Technologies). At the end of the procedure, CD34+ cell recovery was 60% to 80% and purity was in the range of 70% to 95%.
Flow cytometry analysis
Enumeration and immunophenotyping of CD34+ cells were performed by 2- and 3-color flow cytometry, respectively, in which CD34+ cells were identified by a CD45-gating method.37 Briefly, whole blood containing approximately 1 × 106 cells was incubated for 20 minutes at 4°C with the following directly conjugated mAbs: 20 μL of both PerCP-labeled anti-CD45 antibody and PE-conjugated anti-CD34 antibody. The sample was treated with red blood cell lysis buffer (Becton Dickinson) and the cells were washed with PBS containing 1% human serum albumin and 0.1% sodium azide. After treatment with 2% formaldehyde cell fixation buffer (Becton Dickinson) for 10 minutes at 37°C, the cells were washed and stained first with 1 μL anti-67LR polyclonal antibody ab711 for 30 minutes at 4°C and then with a FITC-conjugated antirabbit antibody for 30 minutes at 4°C. The cells were analyzed immediately on a FACScan flow cytometer (Becton Dickinson). At least 5 to 10 × 105 total events were acquired in each sample using CellQuest software (Becton Dickinson). A mononuclear gate was created, based on CD45 expression and side light scatter; the total number of CD34+ cells was calculated on the basis of the relative percentage of CD34+ cells in the total number of nucleated cells. The expression of 67LR on enriched CD34+/CD38– cells was assessed by triple staining using an anti-CD34–PerCP, an anti-CD38–PE, and the polyclonal anti-67LR antibody ab711 detected by a FITC-conjugated antirabbit antibody. Equivalent gating on isotype-matched negative controls was used for background subtraction in all assays.
Cell cultures
CD34+ KG1 cells38 were grown in RPMI supplemented with 5% heat-inactivated FCS, 300 μg/mL glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin. Cells (5 × 106) were transfected with 10 μg 67LR-pcDNA3 or control vector pcDNA3 and 60 μL Lipofectamine for 5 hours at 37°C (5% CO2). Transfected cells were selected by Geneticin at 0.4 mg/mL; the resulting clones were pooled and cultured in the presence of 0.2 mg/mL Geneticin.
Clonogenic human progenitors were measured in methylcellulose, as previously described.39 Clonogenic mouse progenitors were also grown in methylcellulose in the presence of a recombinant mouse growth factor cocktail.
The human BM-derived endothelial-cell line HBMEC was a gift from Dr C. Ellen van der Schoot (Amsterdam, The Netherlands). Cells were cultured in FN-coated culture flasks in Medium 199 supplemented with 10% pooled, heat-inactivated human serum, 10% FCS, 1 ng/mL basic fibroblast factor (Boehringer-Mannheim, Mannheim, Germany), 5 U/mL heparin, 300 μg/mL glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin. In all experiments, HBMEC monolayers were pretreated with IL-1β for 4 hours (PeproTech, Rocky Hill, NJ).
Mobilization of mouse CD34+ cells
Male Balb/c mice aged 8 to 9 weeks were purchased from Charles River Laboratory (Lecco, Italy). All the experiments were approved by the animal care committee of University Federico II, Naples, Italy. Mice received intraperitoneally a daily dose of 300 μg/kg G-CSF for 4 days. Some mice also received intraperitoneal injections of the neutralizing anti-67LR antibody MLuC5 (100 μg in 200 μL saline) on days 3 and 4, immediately after G-CSF treatment. Four hours after the last injection of G-CSF, mice were humanely killed, BM and PB were harvested and analyzed by 2-color flow cytometric analysis, using a mouse-specific PE-conjugated anti-CD34 antibody (Becton Dickinson), and the anti-67LR polyclonal antibody ab711 was revealed by a FITC-conjugated antirabbit secondary antibody, as described (see “Flow cytometry analysis”).
Western blot
CD34+ KG1 cells, PBMNCs, and purified PB or BM CD34+ cells were lysed in 1% Triton X-100/PBS. Then 100 μg protein was electrophoresed on a 12% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE), under reducing conditions, and transferred onto a PVDF membrane. The membrane was blocked and probed with 1 μg/mL anti-67LR polyclonal antibody ab711. Finally, washed filters were incubated with horseradish peroxidase–conjugated antirabbit antibodies and detected by ECL. For MAPK activation, KG1 cells, highly purified BM CD34+ cells, and highly purified G-CSF–mobilized PB CD34+ cells were plated in 35-mm wells previously coated overnight at 4°C with LM (100 μg/well) or heat-denatured BSA, as a negative control. Cells were allowed to adhere for 16 hours at 37°C, lysed, and subjected to Western blot with anti-phospho-ERKs and anti–ERK-2 polyclonal antibodies, as described.
Adhesion assay
The 96-well microtiter plates were coated overnight at 4°C with LM (5 μg/well) or heat-denatured BSA, as a negative control. Plates were incubated for 16 hours at 37°C with 1 × 105 CD34+ KG1 cells, BM CD34+ cells, or G-CSF–mobilized PB CD34+ cells in 100 μL RPMI medium supplemented with 5% FCS. Attached cells were fixed with 3% paraformaldehyde, permeabilized by 2% methanol, and stained with 0.5% crystal violet in 20% methanol. The stain was eluted with a solution of 0.1 M sodium citrate, pH 4.2, in 50% ethanol, and the absorbance measured at 540 nm. In some experiments, cells were preincubated with 1 μg anti-67LR polyclonal antibody ab711 or with nonimmune rabbit immunoglobulins, as a negative control, and then plated on LM. All experiments were performed in triplicate. Results are reported as a percentage of control; 100% values represent cell adhesion to heat-inactivated BSA.
Cell migration assay
Cell migration assays were performed in Boyden chambers, using uncoated 5-μm pore size PVPF polycarbonate filters. CD34+ KG1 (2 × 105) cells or G-CSF–mobilized PB CD34+ cells were plated in the upper chamber in serum-free medium; 25 μg/mL LM or serum-free medium was added in the lower chamber. Cells were allowed to migrate for 90 minutes at 37°C, 5% CO2. Cells on the lower surface of the filter were then fixed in ethanol, stained with hematoxylin, and counted at × 200 magnification (10 random fields/filter). In some experiments, cells were preincubated for 1 hour at 37°C with 1 μg/mL anti-67LR polyclonal antibody ab711 or with nonimmune rabbit immunoglobulins and then plated. All experiments were performed in triplicate.
Transendothelial migration assay
Migration assays were performed on FN-coated filters in Transwell plates of 6.5 mm diameter with 5-μm pore filters. Endothelial cells were plated at 2 to 3 × 104 cells/Transwell to obtain confluent endothelial monolayers. Monolayers of endothelial cells were pretreated for 4 hours with IL-1β and washed with assay medium (DMEM with 0.25% BSA). Then, 1 × 104 G-CSF–stimulated BM CD34+ cells were added to the upper compartment in 0.1 mL assay medium; 0.6 mL assay medium, with or without SDF-1 (100 ng/mL), was added to the lower compartment. After 4 hours of incubation at 37°C, 5% CO2, cells migrating into the lower compartment as well as nonmigrating cells into the upper compartment were collected and analyzed for their colony-forming cell (CFC) content. In blocking experiments, G-CSF–stimulated BM CD34+ cells were preincubated for 30 minutes at 37°C with 1 μg/mL anti-67LR polyclonal antibody ab711 or with nonimmune rabbit immunoglobulin.
Statistical analysis
Results of in vivo and in vitro studies were expressed as a mean ± SEM or SD, as required. Differences between groups were evaluated using the Student t test. Correlation between variables was assessed using the Pearson linear regression.
Results
67LR expression in human G-CSF–mobilized CD34+ PB stem cells
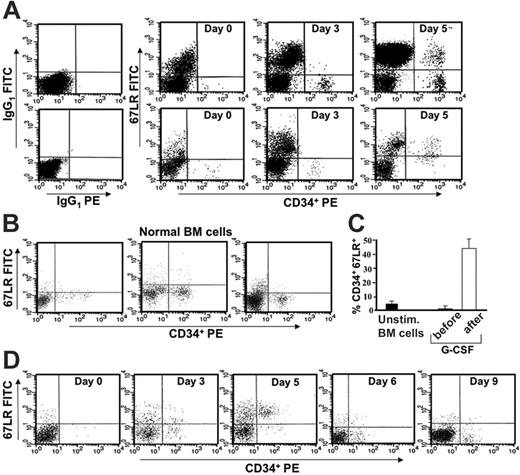
We first investigated whether there was a modulation of 67LR expression in circulating CD34+ cells of healthy subjects following G-CSF stimulation. PBMNCs were obtained from 35 healthy donors before G-CSF mobilization (day 0) and at various time points during G-CSF administration (days 3-5). Phenotypic analysis of 67LR-expressing CD34+ cells was determined by 3-color flow cytometry on a mononuclear gate of CD45+ cells. G-CSF administration increased 67LR expression in circulating CD34+ cells; by contrast, unstimulated BM CD34+ cells showed very low levels of 67LR (Figure 1A-B). The mean percentage ± SEM of 67LR+ unstimulated BM CD34+ cells from 15 healthy subjects was 5.1% ± 1.1% (range, 1%-13%; Figure 1B). Using as cutoff a percentage of 67LR+ circulating CD34+ cells higher than 20%, 67LR expression was increased in 31 of 35 donors. The mean percentage ± SEM of 67LR+ circulating CD34+ cells was 1.86% ± 0.2% (range, 0.5%-7%) before G-CSF administration and 46.3% ± 4.1% (range, 23%-86%) on the day of cell harvesting (day 4 or 5 of G-CSF administration; P < .001; Figure 1C). G-CSF withdrawal was associated with a rapid reduction of 67LR expression on CD34+ cells in all G-CSF–treated donors (Figure 1D).
In vivo G-CSF administration increases 67LR expression in circulating CD34+ cells compared with normal unstimulated marrow CD34+ cells. (A) 67LR expression in circulating CD34+ cells during G-CSF–induced stem-cell mobilization in 2 representative donors (nos. 1 and 20 in Figure 2A-B). Immunophenotyping of 67LR expressing CD34+ cells was performed by 3-color flow cytometry on mononuclear cells gated for side light scatter (SSC-H) and CD45+, with an anti-67LR polyclonal antibody detected by a FITC-conjugated antirabbit antibody. PBMNCs were collected before (day 0) or at various time points (day 3 and 5) during G-CSF administration. (B) Immunophenotyping of 67LR-expressing CD34+ cells on BMMNCs from 3 representative healthy subjects. (C) Percentage of 67LR expressing CD34+ cells in BMMNCs from 15 healthy subjects and in PBMNCs collected from 35 healthy donors before G-CSF administration (day 0, ▪) or at the time of cell harvesting (day 5, □). (D) Immunophenotyping of 67LR expressing CD34+ cells performed by 3-color flow cytometry on mononuclear cells gated for side light scatter (SSC-H) and CD45+, with an anti-67LR polyclonal antibody on PBMNCs collected before (day 0), during (days 3 and 5) G-CSF administration, and at G-CSF withdrawal (days 6 and 9). Increased 67LR expression in circulating CD34+ cells is strictly dependent on G-CSF administration.
In vivo G-CSF administration increases 67LR expression in circulating CD34+ cells compared with normal unstimulated marrow CD34+ cells. (A) 67LR expression in circulating CD34+ cells during G-CSF–induced stem-cell mobilization in 2 representative donors (nos. 1 and 20 in Figure 2A-B). Immunophenotyping of 67LR expressing CD34+ cells was performed by 3-color flow cytometry on mononuclear cells gated for side light scatter (SSC-H) and CD45+, with an anti-67LR polyclonal antibody detected by a FITC-conjugated antirabbit antibody. PBMNCs were collected before (day 0) or at various time points (day 3 and 5) during G-CSF administration. (B) Immunophenotyping of 67LR-expressing CD34+ cells on BMMNCs from 3 representative healthy subjects. (C) Percentage of 67LR expressing CD34+ cells in BMMNCs from 15 healthy subjects and in PBMNCs collected from 35 healthy donors before G-CSF administration (day 0, ▪) or at the time of cell harvesting (day 5, □). (D) Immunophenotyping of 67LR expressing CD34+ cells performed by 3-color flow cytometry on mononuclear cells gated for side light scatter (SSC-H) and CD45+, with an anti-67LR polyclonal antibody on PBMNCs collected before (day 0), during (days 3 and 5) G-CSF administration, and at G-CSF withdrawal (days 6 and 9). Increased 67LR expression in circulating CD34+ cells is strictly dependent on G-CSF administration.
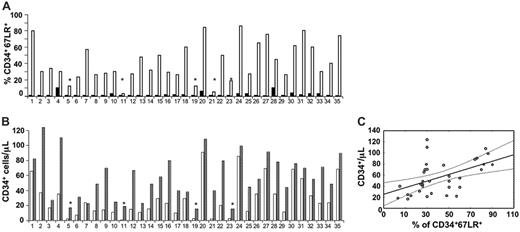
Increased 67LR expression in circulating CD34+ cells after G-CSF administration is significantly correlated with mobilization efficiency. (A) Percentage of 67LR-expressing CD34+ cells in PBMNCs collectd from 35 healthy donors before G-CSF administration (day 0, ▪) or at the time of cell harvesting (day 5, □). Mean percentages ± SEM of 67LR+ circulating CD34+ cells were 2.36% ± 0.4% before G-CSF administration and 42.4% ± 4.1% on the day of cell harvesting (P < .001). (B) 67LR expressing CD34+ cells/μLin PBMNCs collected from 35 healthy donors at the time of cell harvesting (day 5 of G-CSF administration, □) and the corresponding peak value of CD34+ cells/μl (▤). Mean ± SEM of 67LR+ circulating CD34+ cells was 0.11 ± 0.02/μL before G-CSF administration and 29.6 ± 4.2/μL on the day of cell harvesting (P < .001). (A, B) Four of 5 poorly mobilizing donors (*) did not show 67LR increase in circulating CD34+ cells. (C) Pearson linear regression analysis between percentages of 67LR-expressing PB CD34+ cells/μL after G-CSF administration and PB CD34+ cell peak values on the day of collection (r = 0.5, P = .002). Percentages of 67LR+ circulating CD34+ cells are significantly correlated with the degree of mobilization.
Increased 67LR expression in circulating CD34+ cells after G-CSF administration is significantly correlated with mobilization efficiency. (A) Percentage of 67LR-expressing CD34+ cells in PBMNCs collectd from 35 healthy donors before G-CSF administration (day 0, ▪) or at the time of cell harvesting (day 5, □). Mean percentages ± SEM of 67LR+ circulating CD34+ cells were 2.36% ± 0.4% before G-CSF administration and 42.4% ± 4.1% on the day of cell harvesting (P < .001). (B) 67LR expressing CD34+ cells/μLin PBMNCs collected from 35 healthy donors at the time of cell harvesting (day 5 of G-CSF administration, □) and the corresponding peak value of CD34+ cells/μl (▤). Mean ± SEM of 67LR+ circulating CD34+ cells was 0.11 ± 0.02/μL before G-CSF administration and 29.6 ± 4.2/μL on the day of cell harvesting (P < .001). (A, B) Four of 5 poorly mobilizing donors (*) did not show 67LR increase in circulating CD34+ cells. (C) Pearson linear regression analysis between percentages of 67LR-expressing PB CD34+ cells/μL after G-CSF administration and PB CD34+ cell peak values on the day of collection (r = 0.5, P = .002). Percentages of 67LR+ circulating CD34+ cells are significantly correlated with the degree of mobilization.
Noteworthy, 4 of 5 donors not showing 67LR increase on circulating CD34+ cells after G-CSF treatment mobilized poorly (Figure 2A-B: donors 5, 11, 19, and 23); indeed, they obtained a peak of fewer than 20 CD34+ cells/μL and did not achieve the target CD34+ cell yield of 2 × 106 CD34+ cells/kg or more in one apheresis procedure after 5 days of G-CSF administration. Accordingly, linear regression analysis showed that both numbers and percentages of 67LR+ circulating CD34+ cells after G-CSF administration directly correlated with CD34+ cell peak values on the day of collection (r = 0.7, P = .001 and r = 0.5, P = .002, respectively; Figure 2C).
67LR expression was also investigated by Western blot analysis with an anti-67LR polyclonal antibody on highly purified G-CSF–mobilized CD34+ cells and on PBMNCs from the corresponding donors, collected before and at various time points during G-CSF stimulation. Western blot analysis showed increased 67LR expression in PBMNCs during G-CSF treatment and confirmed that G-CSF–mobilized CD34+ cells expressed high levels of 67LR at the time of cell harvesting by apheresis, compared with unstimulated BM HSCs (Figure 3A).
Phenotypic analysis of 67LR expression in enriched CD34+/CD38– cells,40 as determined by flow cytometry on PBMNCs from 5 G-CSF–treated donors, showed that the mean ± SEM of 67LR-expressing CD34+/CD38– cells at day 5 of G-CSF stimulation was 43% ± 3% (range, 36%-60%), whereas it was 7.5% ± 2% (range, 4%-10%), before G-CSF administration (Figure 3C).
G-CSF modulation of CD34+ cell adhesion to laminin
We studied the ability of 67LR to support CD34+ cell adhesion to laminin by in vitro cell-adhesion assays. CD34+ KG1 leukemic cells, highly purified unstimulated normal BM CD34+ cells, and highly purified G-CSF–mobilized PB CD34+ cells were able to adhere to laminin (Figure 4A). KG1-cell adhesion to laminin was mediated by different receptors; indeed, preincubation with anti-67LR and anti–α6-integrin antibodies caused a 53% and a 56% reduction of cell attachment to laminin, respectively. Preincubation with anti-67LR antibodies did not affect the adhesion to the same substrate of normal unstimulated BM CD34+ cells, expressing very low levels of 67LR, whereas anti–α6-integrin antibodies caused a 67% reduction of their laminin-dependent cell adhesion. Interestingly, G-CSF–mobilized PB CD34+ cells adhered to laminin to the same extent as BM CD34+ cells; however, such cell adhesion to laminin was almost completely inhibited by anti-67LR antibodies. Of note, anti–α6-integrin antibodies did not affect PB CD34+ cell adhesion to laminin because expression of α6 integrin was strongly down-regulated during G-CSF–induced HSC mobilization. Indeed, flow cytometry analysis performed on BMMNCs obtained from BM specimens of 10 healthy donors and on PBMNCs obtained from 10 healthy donors after G-CSF stimulation showed that the mean percentage ± SEM of α6-expressing CD34+ cells was 27.3% ± 3.2%, (range, 9.6%-36.3%) in unstimulated BM CD34+ cells and 4% ± 1.1% (range, 0%-10%) in G-CSF–mobilized PB CD34+ cells.
67LR expression increases in circulating CD34+ and CD34+/CD38– cells during G-CSF administration. (A) Western blot analysis with an anti-67LR polyclonal antibody of PBMNCs collected from 2 representative donors before (day 0) or at different time points (days 3 and 5) of G-CSF administration, of highly purified circulating CD34+ cells collected from the same donors at day 5 of G-CSF administration, and of highly purified BM CD34+ cells collected from 2 different donors. Treatment by G-CSF increases 67LR production, especially in CD34+ cells. (B) 67LR expression in CD34+/CD38– progenitor cells during G-CSF–induced stem-cell mobilization from a representative case. Immunophenotyping of 67LR-expressing cells was performed on enriched CD34+/CD38– cells (see “Materials and methods”) collected at day 0 (top panel) and day 5 (bottom panel) of G-CSF administration on a mononuclear gate by triple staining with a PerCP-conjugated anti-CD34, a PE-conjugated anti-CD38, and an anti-67LR polyclonal antibody detected by a FITC-conjugated antirabbit antibody. CD34+/CD38– cells expressing 67LR were evaluated within the gate of CD34+CD38– cells (circled). Treatment by G-CSF increases 67LR production even in CD34+/CD38– cells.
67LR expression increases in circulating CD34+ and CD34+/CD38– cells during G-CSF administration. (A) Western blot analysis with an anti-67LR polyclonal antibody of PBMNCs collected from 2 representative donors before (day 0) or at different time points (days 3 and 5) of G-CSF administration, of highly purified circulating CD34+ cells collected from the same donors at day 5 of G-CSF administration, and of highly purified BM CD34+ cells collected from 2 different donors. Treatment by G-CSF increases 67LR production, especially in CD34+ cells. (B) 67LR expression in CD34+/CD38– progenitor cells during G-CSF–induced stem-cell mobilization from a representative case. Immunophenotyping of 67LR-expressing cells was performed on enriched CD34+/CD38– cells (see “Materials and methods”) collected at day 0 (top panel) and day 5 (bottom panel) of G-CSF administration on a mononuclear gate by triple staining with a PerCP-conjugated anti-CD34, a PE-conjugated anti-CD38, and an anti-67LR polyclonal antibody detected by a FITC-conjugated antirabbit antibody. CD34+/CD38– cells expressing 67LR were evaluated within the gate of CD34+CD38– cells (circled). Treatment by G-CSF increases 67LR production even in CD34+/CD38– cells.
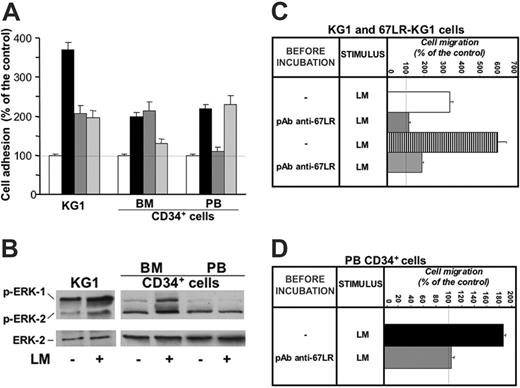
67LR mediates in vitro cell adhesion and migration to laminin of G-CSF–mobilized CD34+ cells and affects MAPK phosphorylation in these cells. (A) In vitro cell adhesion to laminin of KG1, unstimulated BM, and G-CSF–mobilized PB CD34+ cells. KG1 cells, highly purified BM CD34+ cells from 3 donors and highly purified G-CSF–mobilized PB CD34+ cells from 3 donors were plated in wells coated with human placental laminin (5 μg/well), after preincubation with nonimmune rabbit antibodies (▪), an anti-67LR polyclonal antibody (dark gray bars), an anti-α6 antibody (light gray bars), and allowed to adhere for 16 hours at 37°C. As negative controls, heat-denatured BSA was used for coating. Attached cells were fixed and stained with crystal violet; the absorbance (OD) of the eluted stain was measured by a spectrophotometer at 540 nm. All experiments were performed in triplicate and reported as a percentage of controls. The 100% values represent cell adhesion to heat-inactivated BSA (□). Results are presented as mean ± SD. 67LR supports cell adhesion to laminin of KG1 and G-CSF–mobilized CD34+ cells, whereas adhesion to laminin of unstimulated marrow CD34+ cells is 67LR independent and mostly mediated by α6 integrins. (B) MAPK activation in CD34+ cell adhered to laminin. KG1 cells, highly purified unstimulated BM CD34+ cells, and highly purified G-CSF–mobilized PB CD34+ cells were plated in 35-mm wells coated with human placental laminin and allowed to adhere for 16 hours at 37°C. As negative controls, heat-denatured BSA was used for coating. Cells were then lysed and subjected to Western blot with anti–phospho-ERKs and anti–ERK-2 (as a loading control) polyclonal antibodies. Adhesion to laminin increased MAPK phosphorylation in both KG1 and BM CD34+ cells, whereas PB CD34+ cell adhesion to laminin, mostly mediated by 67LR engagement, did not increase MAPK activation. (C) In vitro cell migration to laminin of wild-type (□) and 67LR-transfected (▥) KG1cells. After incubation with nonimmune antibody (---) and a polyclonal anti-67LR antibody (pAb anti-67LR), KG1 cells were plated in Boyden chambers and allowed to migrate toward human placental laminin (25 μg/mL). The 100% values represent cell migration in the absence of chemoattractant. Values are the mean ± SD of 3 experiments performed in triplicate. Laminin-induced migration is increased in 67LR-transfected KG1 cells and is strongly reduced by blocking 67LR. (D) In vitro cell migration to laminin of G-CSF–mobilized PB CD34+ cells (▪). After incubation with nonimmune antibody (---) and a polyclonal anti-67LR antibody (pAb anti-67LR), highly purified G-CSF–mobilized CD34+ cells were plated in Boyden chambers and allowed to migrate toward human placental laminin. 100% values represent cell migration in the absence of chemoattractant. Values are the mean ± SD of 3 experiments performed in triplicate. Laminin-induced migration of G-CSF–mobilized PB CD34+ cells is almost totally abolished by blocking 67LR.
67LR mediates in vitro cell adhesion and migration to laminin of G-CSF–mobilized CD34+ cells and affects MAPK phosphorylation in these cells. (A) In vitro cell adhesion to laminin of KG1, unstimulated BM, and G-CSF–mobilized PB CD34+ cells. KG1 cells, highly purified BM CD34+ cells from 3 donors and highly purified G-CSF–mobilized PB CD34+ cells from 3 donors were plated in wells coated with human placental laminin (5 μg/well), after preincubation with nonimmune rabbit antibodies (▪), an anti-67LR polyclonal antibody (dark gray bars), an anti-α6 antibody (light gray bars), and allowed to adhere for 16 hours at 37°C. As negative controls, heat-denatured BSA was used for coating. Attached cells were fixed and stained with crystal violet; the absorbance (OD) of the eluted stain was measured by a spectrophotometer at 540 nm. All experiments were performed in triplicate and reported as a percentage of controls. The 100% values represent cell adhesion to heat-inactivated BSA (□). Results are presented as mean ± SD. 67LR supports cell adhesion to laminin of KG1 and G-CSF–mobilized CD34+ cells, whereas adhesion to laminin of unstimulated marrow CD34+ cells is 67LR independent and mostly mediated by α6 integrins. (B) MAPK activation in CD34+ cell adhered to laminin. KG1 cells, highly purified unstimulated BM CD34+ cells, and highly purified G-CSF–mobilized PB CD34+ cells were plated in 35-mm wells coated with human placental laminin and allowed to adhere for 16 hours at 37°C. As negative controls, heat-denatured BSA was used for coating. Cells were then lysed and subjected to Western blot with anti–phospho-ERKs and anti–ERK-2 (as a loading control) polyclonal antibodies. Adhesion to laminin increased MAPK phosphorylation in both KG1 and BM CD34+ cells, whereas PB CD34+ cell adhesion to laminin, mostly mediated by 67LR engagement, did not increase MAPK activation. (C) In vitro cell migration to laminin of wild-type (□) and 67LR-transfected (▥) KG1cells. After incubation with nonimmune antibody (---) and a polyclonal anti-67LR antibody (pAb anti-67LR), KG1 cells were plated in Boyden chambers and allowed to migrate toward human placental laminin (25 μg/mL). The 100% values represent cell migration in the absence of chemoattractant. Values are the mean ± SD of 3 experiments performed in triplicate. Laminin-induced migration is increased in 67LR-transfected KG1 cells and is strongly reduced by blocking 67LR. (D) In vitro cell migration to laminin of G-CSF–mobilized PB CD34+ cells (▪). After incubation with nonimmune antibody (---) and a polyclonal anti-67LR antibody (pAb anti-67LR), highly purified G-CSF–mobilized CD34+ cells were plated in Boyden chambers and allowed to migrate toward human placental laminin. 100% values represent cell migration in the absence of chemoattractant. Values are the mean ± SD of 3 experiments performed in triplicate. Laminin-induced migration of G-CSF–mobilized PB CD34+ cells is almost totally abolished by blocking 67LR.
Therefore, 67LR up-regulation during G-CSF–induced HSC mobilization does not increase CD34+ cell adhesion to laminin. However, unstimulated BM CD34+ cell adhesion to laminin is mostly mediated by α6-integrin receptors, whereas G-CSF–mobilized CD34+ cell adhesion to the same substrate is selectively mediated by 67LR, because α6 integrins down-regulated during mobilization and replaced by 67LR.
A laminin-promoting effect on KG1 and BM CD34+ cell adhesion and migration, largely mediated by α6-integrin receptors, has been already reported.35 Laminin also exerts mitogenic activity on the same cell types.34 Indeed, we found increased MAPK phosphorylation after KG1 and BM CD34+ cell adhesion to laminin (Figure 4B); on the contrary, PB CD34+ cell adhesion to laminin, mostly mediated by 67LR engagement, did not increase MAPK activation. Therefore, 67LR binding to laminin transduces a signal different from that mediated by α6 integrins.
67LR-dependent migration of KG1 and G-CSF–mobilized CD34+ cells
We also investigated by in vitro chemotaxis assays whether 67LR up-regulation could be responsible for increased laminin-dependent CD34+ cell migration. To this end, leukemic KG1 cells were transfected with 67LR cDNA; transfected cells showed a 3.8-fold increase in 67LR expression (mean fluorescence index: 170.4 and 634.96 before and after transfection, respectively). CD34+ KG1 cells migrated toward human laminin, and preincubation with a polyclonal anti-67LR antibody strongly reduced their migratory response (Figure 4C). 67LR-transfected KG1 cells showed increased migration toward laminin, compared with wild-type cells (Figure 4C). 67LR overexpression in transfected KG1 cells did not increase their adhesion to laminin (not shown), demonstrating that 67LR is involved mainly in mediating CD34+ cell migration rather than adhesion.
Then, we investigated by in vitro chemotaxis assays whether 67LR could mediate G-CSF–mobilized CD34+ cell migration to laminin; BM CD34+ cells were not tested because of their weak 67LR expression and activity (Figures 1 and 3A). Highly purified PB CD34+ cells from 3 G-CSF–treated donors migrated toward human laminin, and cell preincubation with anti-67LR antibodies strongly reduced the migratory response to laminin (Figure 4D). Thus, after G-CSF stimulation, laminin-dependent CD34+ cell migration occurs mostly through 67LR engagement.
Laminin concentrations in human sera during G-CSF–induced mobilization
We investigated whether laminin serum concentrations were increased in donors after G-CSF administration, thus creating a chemotactic signaling toward peripheral blood. ELISA procedures of 15 donor sera obtained before (day 0) and after (day 4 or 5) G-CSF–induced CD34+ cell mobilization showed that laminin concentrations in sera were not modified by the G-CSF treatment, that is, the mean ± SEM of serum laminin concentration was 256 ± 78 ng/mL and 268.2 ± 66 ng/mL before and after G-CSF administration, respectively.
Thus, it seems that increased 67LR expression in circulating CD34+ cells during G-CSF–induced HSC mobilization participates in their migration toward laminin, even though a laminin gradient between BM and PB is not created.
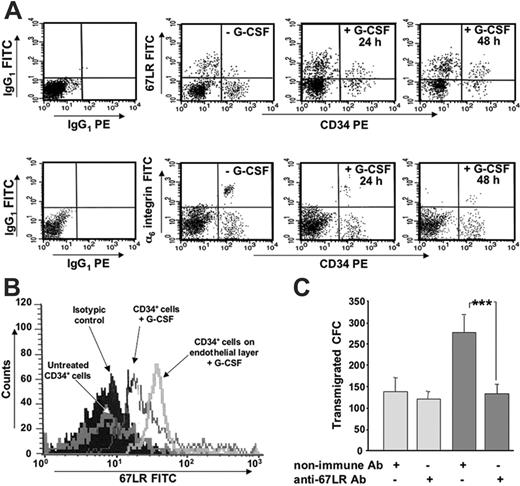
67LR is up-regulated by G-CSF and by exposure to BM-derived endothelial cells in human BM CD34+ cells and is involved in their transendothelial migration. (A) 67LR and α6-integrin expression on BM CD34+ cells after in vitro G-CSF treatment, in one representative experiment. BMMNCs from healthy donors were cultured for 24 and 48 hours with medium alone or in presence of 200 ng/mL G-CSF and analyzed by 3-color flow cytometry on mononuclear cells gated for side light scatter (SSC-H) and CD45+, with anti-67LR and anti-α6 polyclonal antibodies or nonimmune polyclonal antibodies, as a negative control, and detected by a FITC-conjugated antirabbit antibody. 67LR expression on human BM CD34+ cells is increased by in vitro G-CSF treatment, whereas α6-integrin expression decreases. (B) 67LR expression on BM CD34+ cells after in vitro G-CSF treatment and exposure to BM-derived endothelial cells, in one representative experiment. BMMNCs from healthy donors were cultured for 24 hours with medium alone, in the presence of 200 ng/mL G-CSF, or on confluent endothelial-cell layers in the presence of 200 ng/mL G-CSF, and analyzed by 3-color flow cytometry, as described. 67LR expression of human G-CSF–stimulated BM CD34+ cells is further increased by exposure to BM-derived endothelial cells. (C) Transendothelial migration of G-CSF–stimulated BM CD34+ cells. After incubation with a polyclonal anti-67LR antibody or a nonimmune antibody, CD34+ cells, stimulated for 72 hours with 200 ng/mL G-CSF, were plated in Transwell plates coated with confluent endothelial monolayers and allowed to migrate toward 200 ng/mL SDF1 (dark gray bars) or medium alone for 4 hours (light gray bars). Cells migrating into the lower compartment were collected and analyzed for their CFC content by methylcellulose colony assay. Data are the mean ± SEM of 3 separate experiments, ***P < .001. G-CSF–stimulated BM CD34+ cell transendothelial migration is significantly inhibited by anti-67LR antibodies.
67LR is up-regulated by G-CSF and by exposure to BM-derived endothelial cells in human BM CD34+ cells and is involved in their transendothelial migration. (A) 67LR and α6-integrin expression on BM CD34+ cells after in vitro G-CSF treatment, in one representative experiment. BMMNCs from healthy donors were cultured for 24 and 48 hours with medium alone or in presence of 200 ng/mL G-CSF and analyzed by 3-color flow cytometry on mononuclear cells gated for side light scatter (SSC-H) and CD45+, with anti-67LR and anti-α6 polyclonal antibodies or nonimmune polyclonal antibodies, as a negative control, and detected by a FITC-conjugated antirabbit antibody. 67LR expression on human BM CD34+ cells is increased by in vitro G-CSF treatment, whereas α6-integrin expression decreases. (B) 67LR expression on BM CD34+ cells after in vitro G-CSF treatment and exposure to BM-derived endothelial cells, in one representative experiment. BMMNCs from healthy donors were cultured for 24 hours with medium alone, in the presence of 200 ng/mL G-CSF, or on confluent endothelial-cell layers in the presence of 200 ng/mL G-CSF, and analyzed by 3-color flow cytometry, as described. 67LR expression of human G-CSF–stimulated BM CD34+ cells is further increased by exposure to BM-derived endothelial cells. (C) Transendothelial migration of G-CSF–stimulated BM CD34+ cells. After incubation with a polyclonal anti-67LR antibody or a nonimmune antibody, CD34+ cells, stimulated for 72 hours with 200 ng/mL G-CSF, were plated in Transwell plates coated with confluent endothelial monolayers and allowed to migrate toward 200 ng/mL SDF1 (dark gray bars) or medium alone for 4 hours (light gray bars). Cells migrating into the lower compartment were collected and analyzed for their CFC content by methylcellulose colony assay. Data are the mean ± SEM of 3 separate experiments, ***P < .001. G-CSF–stimulated BM CD34+ cell transendothelial migration is significantly inhibited by anti-67LR antibodies.
67LR expression in BM CD34+ cells after in vitro G-CSF treatment and its involvement in transendothelial migration
We investigated by in vitro experiments whether G-CSF was able to directly increase 67LR expression in normal BM CD34+ cells. Three-color flow cytometric analysis of BM CD34+ cells from 3 healthy donors, cultured for 24 and 48 hours in medium with or without 200 ng/mL G-CSF, showed increased 67LR expression after in vitro G-CSF treatment (mean percentages ± SEM of 67LR-expressing CD34+ cells: 8% ± 4% versus 25% ± 3% and 33 ± 4% without and with G-CSF at 24 and 48 hours, respectively; both P < .001; Figure 5A). By contrast, α6-integrin expression decreased after in vitro G-CSF treatment (mean percentages ± SEM of α6-integrin–expressing CD34+ cells: 33.3% ± 3.1% versus 14.7% ± 4% and 7.1% ± 2% without and with G-CSF at 24 and 48 hours, respectively; both P < .001; Figure 5B). Interestingly, in vitro G-CSF–treated BM CD34+ cells, expressing high levels of 67LR, showed a further increase of this receptor after contact with BM-derived endothelial-cell layers (mean percentages ± SD of 67LR-expressing G-CSF–treated CD34+ cells: 25% ± 4% and 45% ± 5% before and after coculture with endothelial cells, respectively; P < .001; Figure 5B), suggesting that marrow CD34+ cells 67LR up-regulation in response to G-CSF can be further increased by adhesion to endothelium.
Thus, G-CSF–induced 67LR expression in BM CD34+ cells could participate in their egress from BM by mediating adhesion and transmigration through laminin, as formerly documented for T lymphocytes and cancer cells.18,19,30,31 Therefore, we investigated whether 67LR could promote transendothelial migration of in vitro G-CSF–stimulated BM CD34+ cells, expressing high levels of 67LR (Figure 5A). SDF-1–dependent transendothelial migration of G-CSF–stimulated BM CD34+ cells was strongly affected by preincubation with polyclonal anti-67LR antibodies (Figure 5C), thus suggesting that G-CSF–mediated 67LR up-regulation contributes to CD34+ cell migration across the BM endothelium, an important step in CD34+ cell trafficking from and to BM.13,41,42
67LR expression in mouse BM CD34+ cells after G-CSF–induced HSC mobilization
We investigated whether 67LR up-regulation in response to G-CSF could occur in BM CD34+ cells also in vivo, thus possibly playing a role in their egress from BM. Male Balb/c mice were treated daily for 4 days with intraperitoneal injections of 300 μg/kg G-CSF or saline, as a control; 4 hours after the last injection, PB and BM were analyzed by flow cytometry. Murine mobilization experiments were performed 4 times, each group being composed of 4 mice. As already observed in human CD34+ cell mobilization (Figure 1), G-CSF treatment induced 67LR up-regulation in mouse PB CD34+ cells (mean percentage ± SEM of 67LR+ PB CD34+ cells before and after G-CSF treatment: 11.5% ± 2% versus 47.3% ± 8.2%, respectively; P < .001). Interestingly, G-CSF increased 67LR expression also in mouse BM CD34+ cells (mean percentage ± SEM of 67LR+ BM CD34+ cells before and after G-CSF treatment: 12.5% ± 0.3% versus 62% ± 4.1%, respectively; P < .001; Figure 6).
These findings demonstrate that G-CSF administration increases 67LR expression in BM CD34+ cells, confirming what observed by in vitro stimulation experiments.
Effects of 67LR inhibition on G-CSF–induced HSC mobilization
We then investigated whether the increased 67LR expression in BM CD34+ cells could be involved in their egress from BM, during G-CSF–induced mobilization. To interfere with 67LR function, we injected a neutralizing anti-67LR antibody into Balb/c mice on days 3 and 4 of mobilization, immediately after each G-CSF stimulation, and examined its effect on mobilization. We observed a significantly reduced number of mobilized CD34+ cells (Figure 7A) and of circulating progenitor cells, evaluated as CFCs (Figure 7B). In a control group of G-CSF–treated mice, nonimmune antibodies did not significantly affect the number of circulating CD34+ cell and progenitor cells (Figure 7A-B). Total BM cellularity did not change in MLuC5-treated mice as compared to controls. In addition, percentages of 67LR+ CD34+ cells in the BM of MLuC5-treated and nonimmune antibody-treated mice were similar, demonstrating that the inhibition of CD34+ cell mobilization occurred without affecting BM-resident CD34+ cells (Figure 7C). The attenuation of HSC mobilization in mice was obtained by using the neutralizing anti-67LR antibody MLuC5, which is an IgM; therefore, we had to exclude that circulating 67LR+ CD34+ cells could have been removed by complement-mediated lysis. Our results were confirmed in the C5-deficient Mba/2J strain, in which we also observed a significant reduction of CD34+ cell mobilization after 67LR inhibition. The efficiency of mobilization in this strain was evaluated by comparing the mean ± SEM of PB CD34+ cells in saline-treated mice (20.7/μL ± 3.3%) to that of G-CSF–treated mice (99.8/μL ± 24.2%); P < .05). MluC5 treatment strongly decreased mobilization; indeed, the mean ± SEM of PB CD34+ cells in mice treated with G-CSF plus control antibody was 67.1/μL ± 11.2%, whereas it was 39.2/μL ± 7.6% in mice treated with G-CSF plus MLuC5 (P < .05). Nonimmune antibody did not affect the efficiency of G-CSF–induced mobilization because the slight decrease in mobilized CD34+ cells, as compared to G-CSF alone (67.1/μL versus 99.8/μL), was not statistically significant (P = .134).
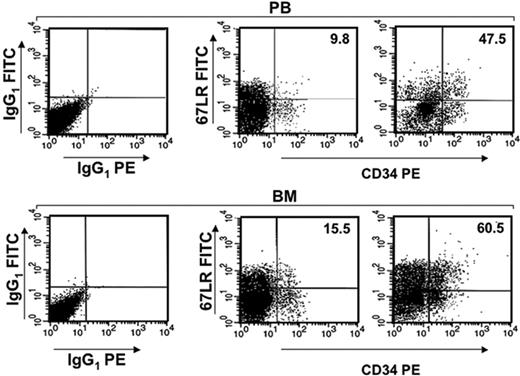
G-CSF increases 67LR expression in mouse BM and PB CD34+ cells. 67LR expression on PB CD34+ cells and on BM CD34+ cells before (left panels) and after G-CSF administration (right panels) from one representative mouse. BALB/c mice were treated daily for 5 days with 300 μg/kg G-CSF or with saline, as a control. Four hours after the last injection, PB and BM CD34+ cells were analyzed by flow cytometry, with a mouse-specific anti-CD34–PE antibody and with an anti-67LR polyclonal antibody, detected by a FITC-conjugated antirabbit antibody.
G-CSF increases 67LR expression in mouse BM and PB CD34+ cells. 67LR expression on PB CD34+ cells and on BM CD34+ cells before (left panels) and after G-CSF administration (right panels) from one representative mouse. BALB/c mice were treated daily for 5 days with 300 μg/kg G-CSF or with saline, as a control. Four hours after the last injection, PB and BM CD34+ cells were analyzed by flow cytometry, with a mouse-specific anti-CD34–PE antibody and with an anti-67LR polyclonal antibody, detected by a FITC-conjugated antirabbit antibody.
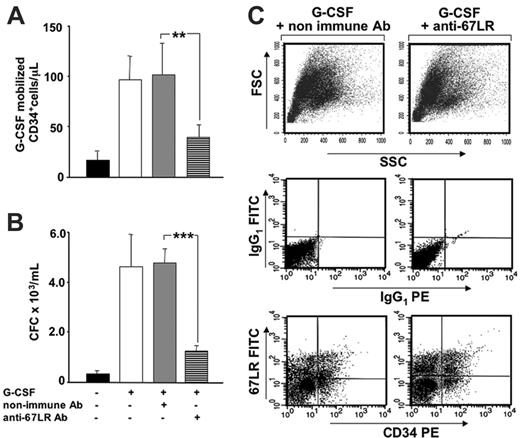
Increased 67LR expression in BM CD34+ cells is involved in G-CSF–induced mobilization of murine HSCs. (A) G-CSF–mobilized CD34+ cells after treatment with anti-67LR antibodies. Balb/c mice were treated daily for 4 days with 300 μg/kg G-CSF (□) or saline (▪), as a control. Nonimmune (▦) or the MLuC5 neutralizing anti-67LR mAbs (▤) were injected into G-CSF–treated mice on days 3 and 4 of mobilization, immediately after each G-CSF stimulation. Four hours after the last injection, PB CD34+ cells were evaluated by flow cytometry, with a mouse-specific anti-CD34–PE antibody. Data are mean ± SEM of 3 separate experiments, each group being composed of 4 mice; **P < .001. (B) G-CSF–mobilized progenitors after treatment with anti-67LR antibodies. Balb/c mice were treated daily for 4 days with 300 μg/kg G-CSF (□) or saline (▪), as a control. G-CSF-treated mice were injected with nonimmune (▦) or MLuC5 neutralizing anti-67LR antibodies (▤) on days 3 and 4 of mobilization, immediately after each G-CSF stimulation. Four hours after the last injection, the number of progenitors mobilized into the circulation was evaluated by colony assays and reported as the number of CFC × 103/mL. Data are mean ± SEM of 3 separate experiments, each group being composed of 4 mice; ***P < .001. Mobilization of murine progenitor cells, evaluated both as circulating CD34+ cells and as CFCs, is significantly inhibited by anti-67LR antibodies. (C) 67LR expression BM CD34+ cells in a representative mouse treated with nonimmune (left column) and MLuC5 (right column) antibodies. Total BM cellularity (top row) as well as the percentage of 67LR+ BM CD34+ cells (middle and bottom rows) in nonimmune antibody and MLuC5-treated mice were similar, thus demonstrating that the inhibition of mobilization occurred without affecting BM CD34+ cells.
Increased 67LR expression in BM CD34+ cells is involved in G-CSF–induced mobilization of murine HSCs. (A) G-CSF–mobilized CD34+ cells after treatment with anti-67LR antibodies. Balb/c mice were treated daily for 4 days with 300 μg/kg G-CSF (□) or saline (▪), as a control. Nonimmune (▦) or the MLuC5 neutralizing anti-67LR mAbs (▤) were injected into G-CSF–treated mice on days 3 and 4 of mobilization, immediately after each G-CSF stimulation. Four hours after the last injection, PB CD34+ cells were evaluated by flow cytometry, with a mouse-specific anti-CD34–PE antibody. Data are mean ± SEM of 3 separate experiments, each group being composed of 4 mice; **P < .001. (B) G-CSF–mobilized progenitors after treatment with anti-67LR antibodies. Balb/c mice were treated daily for 4 days with 300 μg/kg G-CSF (□) or saline (▪), as a control. G-CSF-treated mice were injected with nonimmune (▦) or MLuC5 neutralizing anti-67LR antibodies (▤) on days 3 and 4 of mobilization, immediately after each G-CSF stimulation. Four hours after the last injection, the number of progenitors mobilized into the circulation was evaluated by colony assays and reported as the number of CFC × 103/mL. Data are mean ± SEM of 3 separate experiments, each group being composed of 4 mice; ***P < .001. Mobilization of murine progenitor cells, evaluated both as circulating CD34+ cells and as CFCs, is significantly inhibited by anti-67LR antibodies. (C) 67LR expression BM CD34+ cells in a representative mouse treated with nonimmune (left column) and MLuC5 (right column) antibodies. Total BM cellularity (top row) as well as the percentage of 67LR+ BM CD34+ cells (middle and bottom rows) in nonimmune antibody and MLuC5-treated mice were similar, thus demonstrating that the inhibition of mobilization occurred without affecting BM CD34+ cells.
In C5-deficient mice, C3-mediated clearance of 67LR+ CD34+ cells might still occur. However, white blood cell (WBC) counts in PB were not significantly modified by MLuC5 antibody treatment as compared to controls (not shown). Because many types of circulating WBCs, such as monocytes, T lymphocytes, and neutrophils, are 67LR+,30-32,36 a nonspecific C3-mediated cell clearance can be excluded.
Discussion
Mobilization of HSCs into the blood following treatment with chemotherapy or cytokines mimics the enhancement of the physiologic stem-cell release in response to stress signals4 and is believed to result from changes in the adhesion profile of HSCs, facilitating their egress from BM.6,7
HSC mobilization resembles leukocyte recruitment to inflammatory sites and cancer-cell migration; all these phenomena likely share common biochemical mechanisms. Inflammatory cells, metastasizing cells, and mobilized HSCs have to migrate through the blood-vessel wall; subendothelial basement membrane proteins, such as laminin, fibronectin, and collagen, regulate cell migration and responsiveness to cytokines by interacting with cell-surface adhesion receptors, including 67LR.43-46
We studied the expression and function of 67LR, a nonintegrin cell-surface receptor for laminin that plays a key role in tumor invasion and metastasis,47-50 during G-CSF–induced CD34+ HSC mobilization. Our data document that exposure to G-CSF increases 67LR expression in circulating CD34+ cells as compared with unstimulated BM CD34+ cells. We also found that both numbers and percentages of 67LR+ circulating CD34+ cells after G-CSF administration significantly correlate with the degree of CD34+ cell mobilization; indeed, poor mobilizing donors did not show 67LR increase in circulating CD34+ cells.
We investigated whether 67LR up-regulation in response to G-CSF could increase CD34+ cell adhesion to laminin, as reported for lymphocytes, leukemic cells, cancer cells, and endothelial cells.30-33,19-21,51,52 Unexpectedly, unstimulated BM adhered to laminin to the same extent as G-CSF–mobilized CD34+ cells, even though the latter expressed a higher level of 67LR. However, different receptors were involved in transducing laminin effects. Adhesion to laminin of unstimulated BM CD34+ cells occurred mostly via α6 integrins, whereas G-CSF–mobilized CD34+ cells adhered to laminin exclusively through 67LR. Thus, it seems that unstimulated CD34+ cell anchoring to laminin in the BM microenvironment and their local migration and proliferation during steady-state conditions are mediated by integrin receptors.35 After G-CSF stimulation, CD34+ cell release from BM into the circulation requires the expression of 67LR, which replaces down-regulated α6 integrins in mediating CD34+ cell attachment to laminin. In fact, we demonstrated that 67LR overexpression in transfected KG1 cells did not modify cell adhesion to laminin but increased their migration toward the same substrate.
The observation of α6-integrin down-regulation in circulating CD34+ cells, as compared to steady-state BM CD34+ cells, is in agreement with previous reports showing decreased expression and activity of other integrins, such as α4 and α5, during HSC mobilization.7,10 Interestingly, it has been recently reported that α6 integrins are not involved in HSC mobilization, whereas they promote progenitor-cell homing and engraftment to BM of lethally irradiated mice.53
67LR up-regulation could be involved in HSC mobilization through a mechanism recently proposed for tumor cells. 67LR binding to laminin enhances tumor-cell motility18,19 by determining a conformational modification of laminin, which increases its degradation rate and the release of chemotactic fragments.20 In addition, 67LR overexpression in cancer cells increases their invasiveness by up-regulating the expression and the activity of proteolytic enzymes able to degrade the extracellular matrix, such as membrane type 1 matrix metalloproteinase (MT1-MMP), stromelysin 3, cathepsin L, and the matrix metalloproteinase MMP-2.21 Cancer-cell migration, as well as HSC mobilization, correlates directly with the expression of several proteolytic enzymes6,7 and the proteolytic degradation of extracellular matrix components is a key step in both processes. G-CSF–induced 67LR up-regulation could generate changes in BM CD34+ cells similar to those acquired by tumor cells; indeed, matrix metalloproteinases, such as MT1-MMP and MMP-2, are expressed by human CD34+ progenitors and are needed for in vivo mobilization54,55 ; they also could be secreted by circulating but not BM CD34+ cells.56
Interestingly, circulating CD34+ cells are mostly quiescent and in the G0/G1 phase of the cell cycle, even during G-CSF administration.57 G-CSF–induced 67LR up-regulation in BM CD34+ cells could be involved in transducing migratory signals on binding to laminin, without inducing cell proliferation, unlike integrins.35 Indeed, we found that adhesion to laminin determined a reduced activation of MAPK in PB CD34+ cells, as compared to KG1 and BM CD34+ cells, a phenomenon that could be related to 67LR-mediated activation of dual-specificities phosphatases, as already reported in tumor cells19 and by 67LR in vitro interaction with protein phosphatase-1.58 However, the mechanism of the individual or combined involvement of α6 integrins and 67LR in G-CSF–induced HSC mobilization still needs to be clarified.
We also provide evidence that G-CSF–induced 67LR up-regulation on CD34+ cells mediates their migration toward laminin. Although we could not document the creation of a laminin gradient from BM to blood during mobilization, we cannot exclude the possibility that it may be generated locally or transiently by laminin degradation, due to the increased proteolytic enzyme production in the BM, after G-CSF administration.
In vitro exposure to G-CSF increased 67LR expression in normal human BM CD34+ cells, confirming direct involvement of G-CSF in the modulation of 67LR expression during in vivo administration. We also found that G-CSF–treated human BM CD34+ cells, expressing high levels of 67LR, showed a further increase of such a receptor after adhesion to BM-derived endothelial-cell layers, thus suggesting a possible role of 67LR in BM CD34+ cell intravasation, in response to G-CSF. Indeed, 67LR regulated G-CSF–stimulated human BM CD34+ cell transendothelial migration toward SDF-1, a key chemokine in HSC trafficking from and to BM.13,14
These observations led us to investigate the involvement of 67LR in the mobilization process by injection of G-CSF and anti-67LR antibodies into BALB/c mice. After G-CSF administration, 67LR expression was increased in circulating CD34+ cells, as observed in humans. In addition, 67LR was also up-regulated in BM CD34+ cells and strongly contributed to their migration into the circulation; indeed, anti-67LR antibodies significantly reduced G-CSF–induced CD34+ and progenitor-cell mobilization. These data provide the first in vivo evidence that 67LR plays an important role in stem-cell egress from BM in response to G-CSF.
All together, our data document that 67LR expression is increased in G-CSF–mobilized CD34+ cells as compared with unstimulated BM CD34+ cells. Of note, the level of 67LR expression in circulating CD34+ cells significantly correlates with the mobilization efficiency. Up-regulated 67LR, which replaces α6-integrin receptors after G-CSF stimulation, is required for an efficient HSC mobilization. Indeed, G-CSF administration increases 67LR expression in BM HSCs and contributes to their migration into the circulation most probably by mediating their transendothelial migration.
Interestingly, 67LR overexpression, which is peculiar to metastatic cancer cells,17,59 occurs also in BM and circulating CD34+ stem cells after G-CSF stimulation, as well as in BM and circulating CD34+ leukemic cells (C.S. and N.M., unpublished observation, July 2005). Thus, an intriguing parallel can be drawn between cytokine-stimulated HSCs, leukemic cells, and metastatic cells from solid tumors60 ; indeed, a similar signaling pathway, led by 67LR activation, is involved in cell adhesion, motility, and dissemination in all these different cell types. These findings further support a model in which HSC mobilization could represent a physiologic counterpart of leukemic and metastatic cell spread.
Prepublished online as Blood First Edition Paper, June 20, 2006; DOI 10.1182/blood-2005-11-012625.
Supported by grants from the Ministero dell'Università e della Ricerca Scientifica e Tecnologica (MURST), from Associazione Italiana contro le Leucemie-Linfomi e Mieloma (AIL)–Salerno, and from EU-FP6 2003: “CANCERDEGRADOME” (LSHC-CT-2003-503297).
C.S. and P.R. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal