Abstract
Despite the relevance of the c-kit/stem cell factor (SCF) signaling pathway in mast cell (MC) diseases, the exact frequency of KIT mutations in different compartments of bone marrow (BM) hematopoietic cells of individuals with systemic mastocytosis (SM), and its different diagnostic categories, remains unknown. In this study, we prospectively analyzed the presence of KIT mutations in fluorescence-activated cell-sorting (FACS)– purified populations of BM MCs (n = 113) and other BM cell compartments (n = 67) from adults with SM. Our results show the presence of D816V KIT mutation in virtually all adults (93%) with indolent and aggressive forms of SM, except well-differentiated SM (29%), while other KIT mutations were rarely (< 3%) detected. In around one-third of patients with mutated MCs, the KIT mutation was also detected in CD34+ hematopoietic cells and eosinophils, and, to a lesser extent, in monocytic, neutrophil-lineage BM precursor cells and lymphocytes. Most patient with poor-prognosis SM (81%) carried the KIT mutation in 2 or more BM myeloid cell populations, while this was detected in a smaller proportion (27%) of indolent cases. These results would support the notion that KIT mutation is a hallmark of adult SM where it targets a pluripotent hematopoietic stem cell, and may contribute to explaining previously observed discrepancies in the literature.
Introduction
Mast cell diseases (MCDs) are a heterogeneous group of disorders characterized by an abnormal proliferation and accumulation of mast cells (MCs) in different tissues. In a relatively high proportion of cases, the clonal nature of the disease can be established on the basis of the demonstration of gain-of-function mutations involving the tyrosine kinase domain of c-kit in lesional skin and bone marrow (BM) cells.1-9 Typically, the A7176→T substitution is detected, whereby an aspartate is changed for a valine at codon 816 of the c-kit protein sequence. However, other uncommon somatic (V560G,10 D815K,11 D816Y,2,11,12 D816F,2,11 D816H,13 and D820G14 ) and germ-line (F522C,15 A533D,16 K509I,17 and del41918 ) mutations, which may result in a ligand-independent activation of the stem cell factor receptor, have been reported, most frequently in individual cases. Presence of the KIT mutations in patients with systemic mastocytosis (SM) is not restricted to BM MCs, but it has also been sporadically reported in other non-MC hematopoietic lineages,7,19-24 suggesting that expansion of clonal MCs may arise from an uncommitted hematopoietic stem cell. However, attempts to demonstrate the presence of KIT mutation in purified CD34+ hematopoietic precursor cells have failed so far.25
The pathogenetic relevance of the different KIT mutations in MCDs is still not fully understood; however, their identification has become of major prognostic significance26 due to the availability of protein kinase inhibitors such as imatinib (STI571, Gleevec; Novartis, Basel, Switzerland). These new targeted drugs have proven to be of limited efficacy in patients with MCD carrying the D816V-activating KIT mutation, while they are effective in cases displaying other KIT mutations (eg V560G,27,28 F522C,15 K509I,17 and del41918 ). Despite this, the frequency of the D816V KIT mutation in SM is highly variable in the literature,2,3,7,11 even once the analysis is restricted to patients showing the same type of MCD.3,5,9,29 Accordingly, in adult patients, the frequency of the D816V KIT mutation varies from 31%30 to virtually all cases.5 Such variability could be due to the relatively low number of cases included in most studies, to patient selection with predominance of aggressive versus indolent forms of the disease and/or to the sensitivity of the methods used to assess the presence/absence of KIT mutations, particularly in cases with low MC burden.
In the present study, we have prospectively analyzed the presence of KIT mutations in fluorescence-activated cell-sorting (FACS)–purified populations of immunophenotypically aberrant BM MCs from a series of 113 patients with SM. In addition, in a subgroup of 67 patients with SM associated with KIT mutations, the presence of the mutation was also explored in other BM cell compartments.
Patients, materials, and methods
Patients
In the present study, a total of 113 adults who were consecutively diagnosed with SM at the reference centers of the Spanish Network on Mastocytosis (REMA) (Mast Cell Unit of the Hospital Ramón y Cajal, Madrid, Spain; and Department of Cytometry of the Cancer Research Center, Salamanca, Spain) were analyzed. In all cases, informed consent was obtained prior to the study, according to the guidelines of the local ethics committees.
According to the World Health Organization (WHO) criteria,31,32 patients were classified as follows: indolent systemic mastocytosis (ISM), 74 patients; aggressive systemic mastocytosis (ASM), 6 patients; systemic mastocytosis associated with a clonal non–mast-cell lineage hematopoietic disease (SM-AHNMD), 13 patients; and mast cell leukemia (MCL), 6 patients. In addition, 7 patients with well-differentiated systemic mastocytosis (WDSM)15 and 7 patients with systemic mastocytosis without skin involvement associated with recurrent anaphylaxis or vascular collapse (SM-ana)33 were also studied. Diagnosis included a complete clinical and physical work-up, a routine blood cell count and white blood cell (WBC) differential, routine serum biochemistry tests and measurement of serum tryptase levels (Pharmacia, Uppsala, Sweden), abdominal ultrasonography and/or computed tomography (CT) scan, bone densitometry and X-rays of the bones; furthermore, magnetic resonance imaging (MRI) was performed in selected cases. Skin biopsy was performed in all cases with cutaneous lesions. A complete BM study was performed in all cases following previously established criteria for morphology,34 histology, and immunohistochemistry.35,36 In addition, 4-color multiparameter flow cytometry immunophenotypic analysis of BM MCs was performed according to the recommendations of the Spanish Network on Mastocytosis (REMA; Escribano et al37,38 ). Demographics and clinical characteristics of the study population according to disease category are summarized in Table 1.
Systemic mastocytosis: demographics and clinical characteristics of the study population according to disease category
Category . | No. of patients . | Median age, y (range) . | Sex, M/F . | Median WBC count, × 109/L (range) . | Median BM mast cells, % (range) . | UP, % . | Hepatomegaly, % . | Splenomegaly, % . | Eosinophilia, % . | KIT mutation test before treatment, % . |
|---|---|---|---|---|---|---|---|---|---|---|
| MCL | 6 | 69 (37-76) | 4/2 | 4.7 (1.5-13.4) | 36 (25-60) | 0 | 67 | 67 | 50 | 100 |
| ISM | 74 | 45 (21-79) | 34/40 | 6.2 (4.3-15.7) | 0.14 (0.013-1.65) | 99 | 5.4 | 4 | 14 | 100 |
| SM-ana | 7 | 46 (19-70) | 5/2 | 4.9 (3.9-8.2) | 0.15 (0.035-0.26) | 0 | 0 | 0 | 0 | 100 |
| WDSM | 7 | 23 (12-73) | 2/5 | 5.6 (4.0-6.2) | 0.30 (0.008-12) | 100 | 0 | 0 | 0 | 100 |
| ASM | 6 | 55 (25-77) | 5/1 | 4.8 (2.7-20.1) | 1.85 (0.02-19) | 33 | 83 | 100 | 0 | 67* |
| SM-AHNMD | 13 | 72 (37-83) | 8/5 | 6.1 (1.8-21.3) | 0.60 (0.013-7.4) | 62 | 23 | 23 | 23 | 92* |
Category . | No. of patients . | Median age, y (range) . | Sex, M/F . | Median WBC count, × 109/L (range) . | Median BM mast cells, % (range) . | UP, % . | Hepatomegaly, % . | Splenomegaly, % . | Eosinophilia, % . | KIT mutation test before treatment, % . |
|---|---|---|---|---|---|---|---|---|---|---|
| MCL | 6 | 69 (37-76) | 4/2 | 4.7 (1.5-13.4) | 36 (25-60) | 0 | 67 | 67 | 50 | 100 |
| ISM | 74 | 45 (21-79) | 34/40 | 6.2 (4.3-15.7) | 0.14 (0.013-1.65) | 99 | 5.4 | 4 | 14 | 100 |
| SM-ana | 7 | 46 (19-70) | 5/2 | 4.9 (3.9-8.2) | 0.15 (0.035-0.26) | 0 | 0 | 0 | 0 | 100 |
| WDSM | 7 | 23 (12-73) | 2/5 | 5.6 (4.0-6.2) | 0.30 (0.008-12) | 100 | 0 | 0 | 0 | 100 |
| ASM | 6 | 55 (25-77) | 5/1 | 4.8 (2.7-20.1) | 1.85 (0.02-19) | 33 | 83 | 100 | 0 | 67* |
| SM-AHNMD | 13 | 72 (37-83) | 8/5 | 6.1 (1.8-21.3) | 0.60 (0.013-7.4) | 62 | 23 | 23 | 23 | 92* |
UP indicates urticaria pigmentosa.
In those 3 patients tested at time of therapy (1 SM-AHNMD and 2 ASM), all cell populations tested were positive for the D816V KIT mutation.
Isolation of mast cells and other subpopulations of BM nucleated cells
Isolation of BM MCs and other subpopulations of nucleated BM cells was performed using a 4-way fluorescence-activated cell-sorter (FACSAria; Becton Dickinson Biosciences [BDB], San Jose, CA) equipped with the FACSDiva software (BDB). Prior to sorting, cells were stained with a single 4-color combination of monoclonal antibodies (MAbs)—CD14/CD117/CD34/CD45—conjugated with fluorescein isothiocyanate (FITC)/phycoerythrin (PE)/allophycocyanin (APC)/peridinin chlorophyll protein (PerCP)– cyanin 5.5 (Cy5.5) according to well-established stain-and-then-lyse-and-wash procedures, which have been described in detail elsewhere.39 In the first 41 consecutive patients only purified BM MCs were analyzed for the presence of KIT mutation; in those 67 mutated patients out of the remaining 72 cases, the KIT mutation was also analyzed in other BM non-MC cell fractions (Table 2). The following phenotypic characteristics were used for the isolation of the different subpopulations of BM nucleated cells: MCs were identified as being CD117hi/CD34–/CD45+/CD14–; monocytes were CD45hi/CD14hi/CD34–/CD117–; neutrophils were CD45+/CD34–/CD117–/CD14–/sideward light scatter (SSC)hi; eosinophils were CD45+/CD34–/CD117–/CD14–/SSChi with both green and orange autofluorescence; CD34+ hematopoietic progenitor cells (HPCs) were identified as being SSClo/int/CD34+/CD45+/CD14–/CD117–/+; and lymphocytes were SSClo/ CD45hi/CD14–/CD117–/CD34–. In 3 patients, nucleated erythroid precursors (CD45–/SSClo/CD14–/CD34–/CD117–) were also sorted.
Frequency of KIT mutation in different diagnostic subgroups of adult patients with systemic mastocytosis
. | . | Patients with KIT mutation on BM mast cells, no. (%) . | . | . | . | . | . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Category . | Patient mast cells, no. of total (%) . | Mast cells . | CD34+ HPCs . | Eosinophils . | Monocytes . | Neutrophils . | Lymphocytes . | |||||
| MCL* | 5/6 (83) | 2/2 (100) | 2/2 (100) | 1/1 (100) | NA | 1/1 (100) | NA | |||||
| ISM | 72/74 (97)§ | 43/43 (100) | 9/38 (24) | 9/42 (21) | 4/37 (11) | 3/37 (8) | 1/10 (10) | |||||
| SM-ana† | 7/7 (100)∥ | 6/6 (100) | 0/6 (0) | 0/6 (0) | 0/6 (0) | 0/6 (0) | 0/2 (0) | |||||
| WDSM‡ | 2/7 (29) | 2/2 (100) | 1/2 (50) | 0/2 (0) | 0/2 (0) | 0/2 (0) | 0/2 (0) | |||||
| ASM | 6/6§ (100) | 5/5 (100) | 3/4 (75) | 4/5 (80) | 3/4 (75) | 4/5 (80) | 3/4 (75) | |||||
| SM-AHNMD | 13/13 (100)¶ | 9/9 (100) | 6/9 (67) | 6/9 (67) | 5/8 (63) | 4/7 (57) | 2/5 (40) | |||||
| Total | 105/113 (93) | 67/67 (100) | 21/61 (34) | 20/65 (31) | 12/57 (21) | 12/58 (21) | 6/23 (26) | |||||
. | . | Patients with KIT mutation on BM mast cells, no. (%) . | . | . | . | . | . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Category . | Patient mast cells, no. of total (%) . | Mast cells . | CD34+ HPCs . | Eosinophils . | Monocytes . | Neutrophils . | Lymphocytes . | |||||
| MCL* | 5/6 (83) | 2/2 (100) | 2/2 (100) | 1/1 (100) | NA | 1/1 (100) | NA | |||||
| ISM | 72/74 (97)§ | 43/43 (100) | 9/38 (24) | 9/42 (21) | 4/37 (11) | 3/37 (8) | 1/10 (10) | |||||
| SM-ana† | 7/7 (100)∥ | 6/6 (100) | 0/6 (0) | 0/6 (0) | 0/6 (0) | 0/6 (0) | 0/2 (0) | |||||
| WDSM‡ | 2/7 (29) | 2/2 (100) | 1/2 (50) | 0/2 (0) | 0/2 (0) | 0/2 (0) | 0/2 (0) | |||||
| ASM | 6/6§ (100) | 5/5 (100) | 3/4 (75) | 4/5 (80) | 3/4 (75) | 4/5 (80) | 3/4 (75) | |||||
| SM-AHNMD | 13/13 (100)¶ | 9/9 (100) | 6/9 (67) | 6/9 (67) | 5/8 (63) | 4/7 (57) | 2/5 (40) | |||||
| Total | 105/113 (93) | 67/67 (100) | 21/61 (34) | 20/65 (31) | 12/57 (21) | 12/58 (21) | 6/23 (26) | |||||
Patient mast cells indicates the frequency of patients carrying KIT mutation in their mast cells, from all patients analyzed. All other data shows patients with KIT mutation-positive mast cells. Results expressed as number of cases with the KIT mutation from all cases analyzed (N). All but 3 patients had the D816V KIT mutation.
NA indicates not analyzed.
One patient had the D816Y KIT mutation.
One patient had the VI815-816 insertion in the KIT sequence.
One patient had the I817V KIT mutation.
P < .001 compared with the WDSM group.
P < .05 compared with the WDSM group.
P < .01 compared with the WDSM group.
The purity of the isolated cell populations was evaluated after acquiring 3 × 103 cells of each FACS-sorted cell fraction in a FACScalibur flow cytometer (BDB), and it was constantly greater than 90% (mast cells: 94% ± 3.7%; CD34+ HPCs: 96.7% ± 2.1%; eosinophils: 91.7% ± 4.1%; monocytes: 96.4% ± 1.8%; neutrophils: 99.6% ± 0.5%; erythroid precursors: 99% ± 1.2%; and lymphocytes: 98% ± 1.4%) in the absence of both contamination (< 0.01%) by MCs and cross-contamination with other D816V+ cell populations in the sample.
DNA extraction and detection of KIT mutation
Purified subpopulations of BM cells were used to extract genomic DNA (gDNA) using either the QIAamp mini- or micro-DNA extraction kits (QIAGEN, Valencia, CA) according to the instructions of the manufacturer. For those samples containing less than 104 FACS-sorted cells, the REDEx-tract-N-Amp blood polymerase chain reaction (PCR) kit (Sigma-Aldrich, St Louis, MO) was used following the recommendations of the manufacturer, with the 5′-TTC TTT TCT CCT CCA ACC TA-3′ and 5′-TGC AAA TTT TGC TGA AGT AT-3′ primers (Isogen Biosciences, Maarsen, The Netherlands).
For the detection of the somatic activating codon D816V KIT mutation, gDNA was used. In all cases a peptide nucleic acid (PNA)–mediated PCR clamping technique (LightCycler thermocycler, software version 3.5; Roche Diagnostics, Mannheim, Germany) with the DNA Master Hybridization probes Kit (Roche Diagnostics) was used, as previously described in detail.11 Hybridization probes and PNA-oligomer were purchased from TIB MOLBIOL (Berlin, Germany) and Applied Biosystems (Foster City, CA), respectively.
Positivity for the D816V or other KIT mutations localized in the proximity of codon 816 was confirmed by sequencing the PCR products in both directions, using the 5′-CAG CCA GAA ATA TCC TCC TTA CT-3′ and 5′-TTG CAG GAC TGT CAA GCA GAG-3′ primers (Isogen Life Sciences) and the dye-deoxy terminator method, in an ABI Prism 3100 Genetic Analyzer (Applied Biosystems, Foster City, CA).
Statistical methods
In order to evaluate the statistical significance of the differences observed between groups, the nonparametric chi-square and the Fisher tests (SPSS 12.0 software; SPSS, Chicago, IL) were used; P values less than .05 were considered to be associated with statistical significance.
Results
Frequency and type of KIT mutation present in BM mast cells from adults with systemic mast cell disorders
Overall, 105 (93%) of the 113 SM patients studied showed KIT mutations in their BM MCs (Table 2). Upon classifying the patients according to their diagnosis, a high frequency of patients carrying the KIT mutation was found in all different groups of patients except WDSM (97% vs 29%; P < .001) (Table 2). Accordingly, while all ASM (6 of 6 patients), SM-AHNMD (13 of 13 patients), and SM-ana (7 of 7 patients) and most MCL (5 of 6 patients) and ISM (72 [97%] of 74 patients) carried the KIT mutation in their BM MCs, only 2 of 7 patients with WDSM had activating KIT mutation–positive BM MCs. From those 7 cases in which no mutations were detected at the activating loop of c-kit, 1 corresponded to a 76-year-old female with a MCL with 60% BM MCs; another 2 patients (a 52-year-old male and a 40-year-old female) were diagnosed with ISM associated with skin involvement and variable percentages of BM MCs (1% and 0.027%, respectively). The other 5 patients (2 men and 3 women) were diagnosed with WDSM, with variable percentages of BM MCs (median, 0.7%; range, 0.07%-12%). Interestingly, purified BM MCs from all the 5 females with SM in the absence of detectable KIT mutations were shown to have a clonal origin as tested by the human androgen receptor gene assay.40
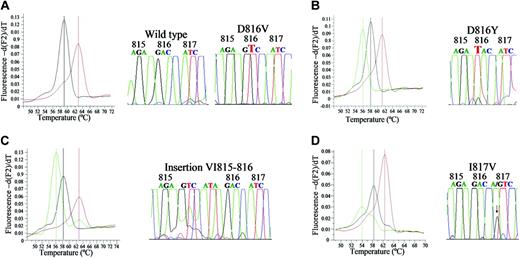
In most of the mutated cases (102 [97%] of 105), PNA-mediated PCR clamping and DNA sequencing showed that the genetic change corresponded to the typical D816V KIT mutation (melting curve at 63°C). Of the other 3 mutated cases, 1 corresponded to a WDSM showing a A7178 → G point mutation that caused a I817V substitution in the KIT sequence (melting curve at 54°C); another was a SM-ana with an insertion of 6 bp (GTC ATA) just before the 816 KIT codon (melting curve at 56°C); the third case with MCL showed a G7175 → T point mutation causing a D816Y substitution (melting curve at 56°C) (Figure 1).
In a subgroup of 11 patients (9 with ISM, 1 with SM-AHNMD, and 1 with WDSM), the same PCR-based approach was used for the detection of KIT mutation in skin-biopsy samples. Comparison between skin and BM results showed a 100% (11 of 11 patients) concordance: 10 patients were positive for the D816V KIT mutation and 1 (the patient with WDSM) was negative.
Distribution of the KIT mutation in different compartments of BM cells
Among those patients carrying a KIT mutation in their BM MCs, 27 (40%) of 67 patients analyzed showed the presence of D816V KIT mutation in gDNA extracted from purified subpopulations of nucleated BM cells other than MCs. Of note, the frequency of patients with KIT mutation in their BM fraction of CD34+ HPCs (21 [34%] of 61 patients) and eosinophils (20 [31%] of 65 patients) was higher than it was in patients with KIT mutation–positive monocytes (12 [21%] of 57 patients) and neutrophils (12 [21%] of 58 patients) (Table 2).
Interestingly, the frequency of cases carrying a KIT mutation in CD34+ HPCs was significantly higher (P < .001) among patients with aggressive forms of mastocytosis, such as MCL (2 of 2 patients), ASM (3 of 4 patients) and SM-AHNMD (6 of 9 patients), than it was in patients with good-prognosis disease categories such as ISM (9 [24%] of 38 patients), SM-ana (0 of 6 patients) and WDSM (1 of 2 patients). Similar results were observed when other nucleated BM myeloid cell populations were analyzed for the presence of KIT mutations. Accordingly, a high proportion of all MCL, ASM, and SM-AHNMD carried the KIT mutation in their BM eosinophils (11 [73%] of 15 patients), monocytes (8 [69%] of 12 patients), and neutrophil lineage cells (9 [64%] of 14 patients), while it was detected in a smaller (P < .001) proportion of all patients with D816V+ ISM (eosinophils, 21%; monocytes, 11%; and neutrophil lineage cells, 8%) and constantly absent in patients with SM-ana and WDSM carrying the KIT mutation in their BM MCs. This also translated into an overall higher frequency of patients carrying the KIT mutation in 2 or more populations of BM myeloid cells in cases with intermediate/poor-prognosis SM (MCL, ASM, and SM-AHNMD) compared with the good-prognosis SM (ISM, SM-ana, and WDSM) (81% vs 27%, P = .02). Interestingly, the presence of KIT mutation was detected in all ISM with hypereosinophilia (5 of 5 patients) but also in patients with normal eosinophil counts (4 of 37 patients).
Melting curves and sequences of PCR products from patients with systemic mastocytosis carrying different KIT mutations. (A) D816V KIT mutation (melting curve at 63°C) found in a patient with ISM. (B) D816Y KIT mutation (56°C) found in a patient with MCL. (C) VI815-816 insertion (56°C) found in a patient with SM-ana. (D) I817V KIT mutation (54°C) found in a patient with WDSM. Comparison with typical D816V KIT mutation and wild-type sequence (melting curve at 59°C) are shown in all panels.
Melting curves and sequences of PCR products from patients with systemic mastocytosis carrying different KIT mutations. (A) D816V KIT mutation (melting curve at 63°C) found in a patient with ISM. (B) D816Y KIT mutation (56°C) found in a patient with MCL. (C) VI815-816 insertion (56°C) found in a patient with SM-ana. (D) I817V KIT mutation (54°C) found in a patient with WDSM. Comparison with typical D816V KIT mutation and wild-type sequence (melting curve at 59°C) are shown in all panels.
In addition, all patients showing involvement of the neutrophil (n = 10), monocytic (n = 10), and/or eosinophilic (n = 13) BM cell compartments in which CD34+ HPCs were simultaneously analyzed also showed the presence of KIT mutation in this latter BM cell compartment, independent of the diagnostic subgroup. In contrast, in 6 patients (1 WDSM, 1 SM-AHNMD, and 4 ISM), CD34+ HPCs carrying the KIT mutation were detected in the absence of involvement of any of the more mature myeloid cell compartments analyzed. Likewise, all 6 patients (3 ASM, 2 SM-AHNMD, and 1 ISM) carrying the D816V KIT mutation in the lymphocyte population also showed this mutation in CD34+ HPCs and all BM myeloid cell populations tested. However, in 1 patient with ISM carrying D816V in all myeloid populations as well as in another 16 patients showing variable involvement of CD34+ HPCs and other BM non-MC myeloid compartments, lymphocytes carried the wild-type KIT gDNA sequence (Table 2).
Analysis of the KIT mutational status of AHNMD associated with SM
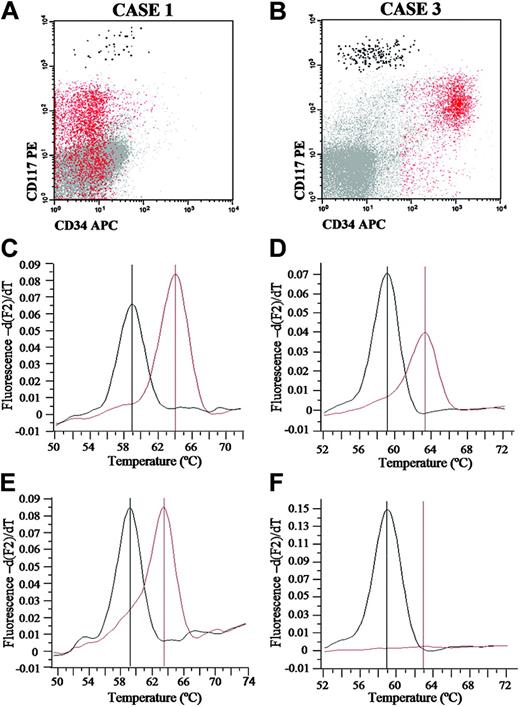
From those 13 patients suffering from systemic mastocytosis associated with other non–mast-cell hematologic disorders, 10 patients had an associated myeloid malignancy and only 3 patients had a lymphoid neoplasia. Six of 13 patients had ASM associated with an AHNMD consisting of a chronic myelomonocytic leukemia (CMML; n = 1), myelodysplastic syndrome (MDS; n = 4) and a B-cell non-Hodgkin lymphoma (NHL; n = 1); the other 7 patients were diagnosed with ISM and AHNMDs consisting of MDS (n = 3), acute myeloid leukemia (AML; n = 1), polycythemia vera (PV; n = 1), B-cell chronic lymphocytic leukemia (B-CLL; n = 1) and lymphoplasmacytic lymphoma (LPL; n = 1). All patients (13 of 13) with SM-AHNMD had the D816V-activating KIT mutation on their BM MCs. In turn, upon analyzing the KIT mutational status of different BM cell populations other than MCs, 9 of these patients showed variable patterns of involvement. Accordingly, while in all patients with ASM associated with myeloid non-MC malignancies, involvement of neoplastic cells from the AHNMD was detected (4 of 4 patients), it was only found in a single patient with ISM associated with an AML (1 of 5 patients with ISM-AHNMD). Interestingly, this latter patient had a germ-line D816V KIT mutation also involving BM lymphoid and erythroid cells, as well as mucosal epithelial cells (Table 3; Figure 2). Despite this, another 2 patients with ISM-AHNMD in which an ISM was associated with B-CLL and PV also showed involvement of either all BM myeloid compartments analyzed or CD34+ HPCs, respectively.
Involvement of different hematopoietic cell lineages in patients with SM-AHNMD as evaluated by the presence or absence of somatic activating KIT mutations at codon 816
. | ISM . | . | . | . | . | ASM . | . | . | . | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | AML . | B-CLL . | MDS . | PV . | LPL . | MDS . | . | . | CMML . | |||||||
| Cell type . | Patient 1 . | Patient 2 . | Patient 3 . | Patient 4 . | Patient 5 . | Patient 6 . | Patient 7 . | Patient 8 . | Patient 9 . | |||||||
| Mast cells, % | 0.7 + | 0.03 + | 0.9 + | 0.1 + | 0.3 + | 7.5 + | 6 + | 0.22 + | 0.2 + | |||||||
| CD34+ HPCs, % | 7.7 +* | 1.6 + | 10 - | 1.9 + | 0.5 - | 0.8 + | 2 + | NA | 1 + | |||||||
| Eosinophils, % | 1.1 + | 1.1 + | 1.7 - | 1.4 - | 5.8 - | 4.8 + | 8.7 + | 7.4 + | 3.2 + | |||||||
| Monocytes, % | 3.7 + | NA | 4.2 - | 3.2 - | 9.2 - | 2.9 + | 12 + | 31 + | 11 + | |||||||
| Neutrophil lineage cells, % | 73 +* | NA | 49 - | 52 - | 54 - | 66 + | NA | 46 + | 67 + | |||||||
| Erythroid precursors, % | 12 + | NA | NA | NA | 7.8 - | NA | NA | NA | NA | |||||||
| Lymphocytes, % | 2.2 + | 19 -† | NA | NA | 6.1 -† | NA | NA | 11.4 + | NA | |||||||
. | ISM . | . | . | . | . | ASM . | . | . | . | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | AML . | B-CLL . | MDS . | PV . | LPL . | MDS . | . | . | CMML . | |||||||
| Cell type . | Patient 1 . | Patient 2 . | Patient 3 . | Patient 4 . | Patient 5 . | Patient 6 . | Patient 7 . | Patient 8 . | Patient 9 . | |||||||
| Mast cells, % | 0.7 + | 0.03 + | 0.9 + | 0.1 + | 0.3 + | 7.5 + | 6 + | 0.22 + | 0.2 + | |||||||
| CD34+ HPCs, % | 7.7 +* | 1.6 + | 10 - | 1.9 + | 0.5 - | 0.8 + | 2 + | NA | 1 + | |||||||
| Eosinophils, % | 1.1 + | 1.1 + | 1.7 - | 1.4 - | 5.8 - | 4.8 + | 8.7 + | 7.4 + | 3.2 + | |||||||
| Monocytes, % | 3.7 + | NA | 4.2 - | 3.2 - | 9.2 - | 2.9 + | 12 + | 31 + | 11 + | |||||||
| Neutrophil lineage cells, % | 73 +* | NA | 49 - | 52 - | 54 - | 66 + | NA | 46 + | 67 + | |||||||
| Erythroid precursors, % | 12 + | NA | NA | NA | 7.8 - | NA | NA | NA | NA | |||||||
| Lymphocytes, % | 2.2 + | 19 -† | NA | NA | 6.1 -† | NA | NA | 11.4 + | NA | |||||||
Results expressed as percentage of cells from all nucleated BM cells.
NA indicates not analyzed; +, presence of KIT mutation; and -, absence of KIT mutation.
Including blast cells.
Including neoplastic B-cells.
Representative analysis of D816V KIT mutation in purified BM MCs and blast cells from 2 patients with SM-AHNMD. (A-B) Immunophenotypic characteristics of blast cells (red dots) and mast cells (black dots) in BM samples from patients 1 and 3 listed in Table 3, prior to FACS sorting. (C-F) Melting curves of wild-type (approximately 59°C; black line) and mutated (approximately 63°C; red line) KIT sequences from mast cells (C-D) and blast cells (E-F) from patients 1 (C-E) and 3 (D-F).
Representative analysis of D816V KIT mutation in purified BM MCs and blast cells from 2 patients with SM-AHNMD. (A-B) Immunophenotypic characteristics of blast cells (red dots) and mast cells (black dots) in BM samples from patients 1 and 3 listed in Table 3, prior to FACS sorting. (C-F) Melting curves of wild-type (approximately 59°C; black line) and mutated (approximately 63°C; red line) KIT sequences from mast cells (C-D) and blast cells (E-F) from patients 1 (C-E) and 3 (D-F).
Discussion
Despite the clinical and pathophysiologic relevance of the c-kit/SCF signaling pathway in MCD,1-4 the exact frequency of KIT mutations in different compartments of BM hematopoietic cells of individuals with SM and its different diagnostic categories remains largely unknown.
At present, it is generally accepted that adult patients usually express activating mutations of c-kit, whereas most cases of childhood-onset and familial mastocytosis would lack these mutations. However, it should be noted that so far, most studies analyzing KIT mutations in SM reported on relatively small series of patients with aggressive forms of the disease2,3,5,7,9,22,29,30 or single case reports.6,15,41 Despite this, recent review papers25,42 estimate that the frequency of the D816V KIT mutation in adult patients with SM could be higher than 80%, while the frequency of KIT mutations in the different forms of SM remains quite variable.2,3,5,7,9,11,29,30,43 In some studies, such variability could be related to differences in the methods used and/or the type of samples used for the identification of KIT mutations. Accordingly, while Pardanani et al,30 through the use of DNA sequencing techniques, have only found 14% (1 of 7) D816V+ cases in BM samples from patients with ISM, others5 using more sensitive methods based on the analysis of mRNA by restriction fragment length polymorphism (RFLP) approaches have found the KIT D816V mutation in all of their 11 patients with ISM. Likewise, the frequency of this mutation in patients suffering from ISM varied from 16% (7 of 43 patients) if analyzed in PB43 to 100% (11 of 11) if assessed in BM samples,5 even though methods with similarly high sensitivity were used. Altogether, these findings suggest that variations in the frequency of D816V+ SM cases may be related to both the sensitivity of the method and the MC burden, and that both factors could be responsible for the impression that aggressive forms of SM are associated with higher frequencies of KIT mutation.3,9,43 Because of this, in the present study we used a method11 with a sensitivity always higher than 1/20, as evaluated in external quality control schemes (European Competence Network on Mastocytosis [ECNM] interlaboratory quality control [round robin]), to detect the D816V KIT mutation in highly purified populations of MCs. An additional advantage of the method used is that it allows detection of mutations in gDNA at codon 816 as well as at codons 815 and 817 of the activating loop of c-kit, enabling us to detect mutations in this region of the KIT gene in purified cell populations independent of whether or not these cells expressed stem cell factor receptor.
Our results show the presence of activating KIT mutations in purified BM MCs from most patients with both indolent and aggressive forms of SM. Interestingly, of the different diagnostic subgroups of SM, only those patients with WDSM showed a relatively low frequency of KIT mutations. These results are in line with the observation that aberrant expression of CD25 and CD2 on BM MCs is frequently absent in WDSM,15 while present in virtually all patients with ISM,37 supporting the notion that aberrant MC phenotypes could be associated with underlying genetic lesions.44 Overall, these results obtained through the analysis of purified MCs could contribute to an explanation of previously observed discrepancies in the literature.2,3,5,7,9,11,29,30,43 As a consequence, our findings would also indicate that treatment with imatinib, which has been shown to be ineffective in vitro and in vivo against MCs carrying the D816V c-kit mutation,27,28,45 would therefore not be indicated in virtually all patients with SM, except most WDSM. However, further studies are required before definitive conclusions can be drawn in this regard.
In the present study we confirm previous observations11,42 that found a low frequency (< 5%) of activating KIT mutations other than D816V in patients with SM. Interestingly, 2 of the 3 patients reported here to carry KIT mutations other than D816V showed mutations (I817V and VI815-816) that had not been previously described in the literature. At present, there is still no information about the functional status of these new mutations; however, it could be speculated that the VI815-816 insertion could cause a ligand-independent activation of c-kit because it led to insertion of a Val at the activating loop of c-kit, at the same position as that of the D816V mutation. Nonetheless, the functionality of this, as well as of the I817V KIT mutation, needs further characterization by functional studies. A third patient had an activating ligand-independent c-kit mutation (D816Y) that had already been identified in patients suffering from mastocytosis2,11 and AML.12 Interestingly, the 2 former cases corresponded to patients suffering from recently described forms of mastocytosis (SM-ana33 and WDSM15 ) in which the presence of KIT mutations has not been systematically explored. Of note, the only KIT mutation described so far in a patient with WDSM corresponded to a F522C15 germ-line mutation; this observation, together with our findings, would support the notion that WDSM could be a genetically heterogeneous and distinct subgroup of mastocytosis.
In 1995, Nagata et al3 described the presence of a D816V mutation in a PB sample from a patient with SM in the absence of detectable circulating MCs. Since then, several reports have identified the D816V mutation in non-MC hematopoietic cell lineages from patients with SM,7,19-24 leading to the notion that SM could arise from a uncommitted hematopoietic progenitor cell.7,25 However, attempts have failed so far to identify the KIT mutation in BM CD34+ HPCs from patients with SM,21 and to the best of our knowledge, this is the first report in which the presence of the KIT mutation in BM CD34+ HPCs of a relatively high proportion of patients with SM is confirmed. In addition, we also showed that involvement of CD34+ HPCs and other non-MC hematopoietic lineages is not restricted to ASM, MCL, and SM-AHNMD, but is also present in a significant proportion of patients with ISM.
Upon comparing the frequency at which involvement of CD34+ HPCs and other more mature CD34– myeloid cell compartments occurred, we found a higher percentage of involvement of the CD34+ HPCs and eosinophils than monocytic and neutrophil lineage cells. Only those patients with the D816V KIT mutation in CD34+ HPCs showed involvement of other more mature myeloid cell populations, while a few patients showed the presence of the mutation in the CD34+ HPC compartment in the absence of an involvement of the other myeloid cell compartments analyzed. Interestingly, the presence of the KIT mutation in the eosinophils was not restricted to patients with eosinophilia associated with SM as previously suggested,19 but it was also found among SM patients with normal PB eosinophil counts. Moreover, in some patients, the presence of the KIT mutation in eosinophils was detected without involvement of the other myeloid cell lineages apart from CD34+ HPCs. In contrast to the common CD34+ CD117+ CD13+ bilineage monocytic-MC precursor proposed by others,46 our results suggest the existence of a common bilineage-restricted committed progenitor for MCs and eosinophils during differentiation of hematopoietic cells.
Most patients with MCL and ASM carried the D816V KIT mutation in almost all myeloid cell populations analyzed as well as the lymphocytes, while this occurred in a significantly smaller proportion of ISM cases and in none of the patients with SM-ana and WDSM. Altogether, these findings suggest that in most aggressive forms of mastocytosis and a minor proportion of ISM, the KIT-mutated HPCs has acquired a clear proliferative advantage involving not only CD117+-expressing mature myeloid cells (eg, MCs) but also other CD117–-maturing myeloid precursors and lymphocytes. The prognostic significance of these observations for predicting clinical outcome in patients with SM showing different forms of the disease requires further investigation.
Regarding SM-AHNMD, we found a slightly different frequency of involvement of the leukemic/neoplastic cells corresponding to the AHNMD in patients with a diagnosis of ASM-AHNMD versus ISM-AHNMD. Accordingly, the D816V KIT mutation, which was detected in BM MCs from all patients studied, was also present in purified neoplastic cells from all ASM-AHNMD patients, but was absent in the AHNMD cells from all except 1 patient with ISM-AHNMD who had a germ-line mutation, indicating the coexistence of 2 different cell clones and, potentially, of 2 different unrelated hematologic disorders in most of the patients from this latter group. Further studies in larger series of patients with SM-AHNMD are necessary to determine the potential clinical significance of these observations.
In summary, in the present study we show the presence of the D816V KIT mutation in almost all SM patients, similar frequencies being observed in all different diagnostic categories of the disease, except WDSM. In most cases with poor-prognosis SM, as well as in a smaller proportion of ISM, involvement of CD34+ HPCs and other hematopoietic cell compartments by the KIT-mutated clone was detected, supporting the origin of SM in a pluripotent hematopoietic stem cell.
Appendix
Contributing members of the Spanish Network on Mastocytosis include the following:
Members: Aldanondo, Isabel1; Alfonso, Amparo2; Almeida, Julia3; Angulo, Miguel3; Azana, Jose M.4; Botana, Luis M.2; Cuevas, Manuela5; De La Hoz, Belen6; Dominguez, Mercedes7; Escribano, Luis8; Fajardo, Ignacio Jose9; Garcia Faroldi, Gianni9; Garcia-Montero Andres C.3; Gonzalez, Marcos10; Hernandez-Campo, Pilar3; Herrero, Sonia11; Jara, Maria3; Löber, Kristin2; Medina Torres, Miguel Angel9; Melgarejo, Esther9; Moreno Iruela, Inmaculada7; Nunez, Rosa8; Orfao, Alberto3; Pernas, Octavio2; Prados, Aranzazu8; Rivas, Rosana3; Rodriguez-Caballero, Arancha3; Rodriguez Caso, Carlos9; Sanchez, Laura8; Sanchez, Maria Luz3; Sanchez-Jimenez, Francisca M.9; Torano, Alfredo7; Urdiales, Jose Luis9.
Centers: 1Servicio de Dermatología, Hospital Ramón y Cajal, Madrid. 2Departamento de Farmacología, Facultad de Veterinaria, Universidad de Santiago de Compostela, Lugo. 3Servicio General de Citometría, Centro de Investigación del Cáncer and Departamento de Medicina, Universidad de Salamanca, Salamanca. 4Servicio de Dermatología, Complejo Hospitalario de Albacete, Albacete. 5Servicio de Inmunología, Hospital Ramón y Cajal, Madrid. 6Servicio de Alergia, Hospital Ramón y Cajal, Madrid. 7Servicio de Inmunología, Centro Nacional de Microbiología, Instituto de Salud Carlos III, Madrid. 8Unidad de Mastocitosis, Hospital Ramón y Cajal, Madrid. 9Departamento de Biología Molecular y Bioquímica, Facultad de Ciencias, Universidad de Málaga, Málaga. 10Servicio de Hematología, Hospital Universitario de Salamanca, Salamanca. 11Servicio de Hematología, Complejo Hospitalario de Albacete, Albacete.
Prepublished online as Blood First Edition Paper, June 1, 2006; DOI 10.1182/blood-2006-04-015545.
A complete list of the contributing members of the Spanish Network on Mastocytosis appears in “Appendix.”
Supported by grants from the Fondo de Investigaciones Sanitarias (FIS) of the Ministerio de Sanidad y Consumo of Spain (03/0770; and REMA, G03/007); from the Ministerio de Ciencia y Tecnología (SAF02-03 096); from the Comunidad Autónoma de Madrid (GR/SAL/0133/2004) and from the Fundación MMA. A.C.G.-M. is supported by a grant from FIS/Fondo Europeo de Desarollo Regional (FEDER) (CP03/00035); C.T. is supported by a grant from the Fundacão Ciência Tecnologica (FCT) of Portugal (SFRH/BD/17545/2004); M.J.-A., R.N., and A.P. are supported by a grant from FIS (G03/007); and L.S. is supported by a grant from FIS (CMO3/0043).
A.C.G.-M. and M.J.-A. have contributed equally to this work. L.E. and A.O. have also contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Professor Frank K. Austen for the critical review of the manuscript.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal