Abstract
To investigate the role of BCL6 in the pathogenesis of gastric lymphoma, we analyzed the BCL6 promoter region for BCL6 translocations, somatic hypermutations, and deregulating mutations in 43 gastric lymphomas, including 4 extranodal marginal-zone B-cell lymphomas of mucosa-associated lymphoid tissues (MALT lymphomas), 33 diffuse large B-cell lymphomas (DLBCLs), and 6 composite DLBCLs with residual MALT lymphoma (DLCLMLs). BCL6 promoter substitutions by immunoglobulin (Ig) and non-Ig translocation partners, resulting in its deregulation, were frequently involved in DLBCL (36.4%) and DLCLML (50%). Two novel BCL6 translocation partner genes, 28S rRNA and DMRT1, and a new BCL6 translocation breakpoint in intron 2 were also identified. Deregulating mutations were found only in DLBCL (24.2%), which correlated significantly with high BCL6 protein expression. Significantly, high BCL6 expression correlated strongly with longer overall survival (OS), independent of mechanism in gastric DLBCL and DLCLML. Gastric DLBCLs were further subclassified into germinal center B-cell–like (GCB) and non-GCB subgroups immunohistochemically. High BCL6 expression was detected in all GCB cases, irrespective of BCL6 genetic alterations. In the non-GCB subgroup, BCL6-deregulating mutations correlated significantly with high BCL6 expression level. No significant correlation was found between the BCL6 expression level and OS in the non-GCB subgroup, which had significantly poorer prognosis than the GCB subgroup.
Introduction
Primary gastric lymphoma represents about 80% of all extranodal non-Hodgkin lymphomas (NHLs), and is the most common extranodal NHL in the Hong Kong Chinese population. Based on the World Health Organization (WHO) classification, gastric lymphoma can be further classified into MALT lymphoma (extranodal marginal zone B-cell lymphoma of mucosa-associated lymphoid tissue), diffuse large B-cell lymphoma (DLBCL), and composite DLBCL with residual MALT lymphoma (DLCLML; transformed MALT DLBCL).1 In the West, gastric MALT lymphoma is more prevalent, occurring in up to 75% of patients.2 However, gastric DLBCL is more frequent in Chinese patients in Hong Kong, accounting for 60% of gastric NHL.3
MALT lymphomas show histologic features more in common with those of mucosa-associated lymphoid tissue than those of peripheral lymph nodes. They exemplify the close relationship between chronic inflammation and lymphomagenesis, as a strong association has been found between chronic infection with Helicobacter pylori and gastric MALT lymphoma.4 Specific karyotypic alterations characterize MALT lymphomas: trisomies 3 and 18, and translocations t(11;18)(q21;q21), t(1;14)(p22;q32), t(14;18)(q32; q21), t(3;14)(q27;q32), and t(3;14)(p14.1;q32).5 In contrast, gastric DLBCL has a similar histology to its nodal counterpart, but a number of observations suggest that the genetic abnormalities of gastric DLBCL are distinct from those of nodal DLBCL.6-9
DLBCL is a heterogeneous entity both clinically and morphologically. Recently, 3 prognostically important subgroups of DLBCL were identified by cDNA microarray classification (germinal center B-cell–like [GCB], activated B-cell–like [ABC], or type 3); the type 3 group is heterogeneous and behaves in a manner similar to the ABC group.10,11 Hans et al subsequently demonstrated that an immunostain panel of CD10, BCL6, and MUM1 could be used to classify DLBCL into GCB and non-GCB subgroups, with prognostic significance equivalent to that of gene-expression profiling.12
The BCL6 gene was initially identified through its involvement in the DLBCL translocation associating 3q27 with the immunoglobulin (Ig) gene at 14q32.13-15 BCL6 is expressed strongly in B and T cells within the GCs, and is required for the development of GCs and T-helper 2 (Th2)–mediated antigen responses.16-18 BCL6 is a transcription repressor.19 Down-regulation of BCL6 is necessary for lymphocytes within the GC to differentiate into memory B cells or plasma cells, or to undergo selective apoptosis upon antigen stimulation. Among the findings that support this conclusion are the following: (1) BCL6 protein is rapidly degraded upon antigen receptor signaling through the activation of mitogen-activated protein (MAP) kinase phosphorylation of BCL6;20 (2) BCL6 represses PDCD2, a gene associated with programmed cell death in thymocytes;21 (3) BCL6 represses a group of genes involved in B-cell terminal differentiation and cell-cycle control;22 (4) BCL6 represses p53 by directly binding to its promoter and allows GC B cells to tolerate the physiologic DNA breaks required for Ig class-switch recombination and somatic hypermutations (SHMs);23 and (5) BCL6 interacts with the transcriptional activator Miz-1 upon binding to the promoter of CDKN1A, which in turn prevents the p53-independent cell-cycle arrest in GC B cells.24
Structural alterations of BCL6, including promiscuous chromosomal translocations and SHMs, have been identified in several NHLs with various frequencies. BCL6 translocations are found in approximately 40% of DLBCLs and 5% to 10% of follicular lymphomas (FLs).25-28 BCL6 translocations typically juxtapose the 5′ regulatory region of BCL6 to the promoter region of a constitutively expressed partner gene while leaving the coding domain of BCL6 intact, resulting in constitutive BCL6 expression. This, in turn, blocks the normal differentiation of GC B cells and thus contributes to lymphomagenesis.26,29 SHMs in the 5′ noncoding region of BCL6 are found at average frequencies of 1.2 × 10–3/bp in approximately 30% of normal GC B cells and 70% of DLBCLs.30-32 The BCL6 SHM region overlaps with the major translocation breakpoints cluster (MTC) within a 2-kb region encompassing the promoter and the first noncoding exon. Several hotspots within the 2-kb region generate shifted bands in a gel mobility shift assay, suggesting that the region includes 1 or more cis-regulatory elements whose individual mutations may modify the binding of hypothetical BCL6-regulating transcription factors.33-35 Although BCL6 SHMs are found in normal GC B cells, a specific subset of these mutations, BSE1A and BSE1B in BCL6-binding motifs in noncoding exon 1, lead to deregulated BCL6 expression and are found only in patients with DLBCL (15%).36 BCL6 mutations accumulate during high-grade transformation of FL,37,38 and it is suggested that some SHMs (deregulating mutations in this context) may be clonally selected for their effect on the survival of the neoplastic clone.
In this study, we investigated the involvement of BCL6 in the pathogenesis of well-characterized primary gastric MALT lymphoma, DLBCL, and DLCLML by analyzing the BCL6 promoter region for BCL6 translocations, SHMs, and deregulating mutations in BCL6-binding motifs in noncoding exon 1 (BSE1A and BSE1B), and by correlating the results with BCL6 protein expression and prognostic significance. In addition, since BCL6 expression in DLBCL may be more strongly associated with the state of differentiation of the tumor cell (GCB vs non-GCB) than with BCL6 alterations at the genetic level, patients with gastric DLBCL were further subclassified immunohistochemically into GCB and non-GCB phenotypes according to Hans et al,12 and BCL6 protein expression was correlated with the BCL6 genetic alterations and prognostic significance within these 2 subgroups of DLBCL.
Materials and methods
Gastric lymphoma specimens
Paraffin and frozen tumor blocks were retrieved from recent gastrectomy specimens of 43 cases of primary gastric B-cell NHL from Chinese patients, including 10 MALTs (4 low-grade MALTs and 6 DLCLMLs) and 33 DLBCLs. The histologic diagnosis of patients with gastric lymphoma was based on the WHO classification scheme.1 In 6 cases of DLCLML, immunoglobulin light-chain restriction39 of both the residual MALT and DLBCL components by immunostaining indicated a clonal relationship between them (data not shown). Moreover, using DNA extracted from microdissected MALT and DLBCL components, the clonal link between these 2 components was further confirmed by sequence analysis of the rearranged IgH gene40 in 5 of these 6 cases of DLCLML (data not shown). In 1 DLCLML case, it was not practical to microdissect the residual MALT component from the DLBCL lesion. However, immunoglobulin lambda light-chain (Igλ) restriction of both components immunohistochemically was consistent with a clonal relationship (data not shown). The patients were treated with “curative intent” despite the heterogeneity of treatment. Clinical data were available for all of the cases studied; however, no cytogenetic data were available for these cases.
5′ RACE on mRNA and inverse PCR on genomic DNA
The analysis of BCL6 transcripts at the 5′ end was performed by using the RACE (rapid amplification of cDNA ends) kit (Roche Diagnostics, Mannheim, Germany), using the polymerase chain reaction (PCR) conditions and the BCL6-specific primers as described.41 To confirm the presence of chimeric RNA transcripts obtained by 5′ RACE, direct reverse transcription (RT)–PCR was performed on randomly primed cDNA for each case carrying the translocation.
To amplify BCL6 chromosomal translocations involving intron 1, tumor DNA was digested separately with 2 restriction enzymes, BamHI and XbaI, and ligated at low concentration, and inverse PCR was performed as described33 using the Expand Long Template System (Roche Diagnostics). To confirm the presence of BCL6 chromosomal translocations detected by inverse PCR, primers (Table S1, available on the Blood website; see the Supplemental Table link at the top of the online article) were designed across the breakpoint, and direct PCR was performed on genomic DNA for each case carrying the translocation.
Long-range PCR on genomic DNA
Long-range PCR was performed to obtain the chromosomal junction sequences and to identify the breakpoints of all BCL6 translocations that were detected by 5′ RACE on mRNA only. The primers were designed based on the sequence of each fusion transcript (Table S1), and long-range PCR was performed on genomic DNA for each case carrying the translocation using the Expand Long Template System (Roche Diagnostics).
Mutational analysis of BCL6
For mutational analysis of the BCL6 promoter region on the DNA level, the genomic fragment spanning noncoding exon 1 and the first intron (encompassing major mutation cluster [MMC]) was amplified using primers listed in Table S1. To detect somatic mutations in noncoding exon 1 (BSE1A and BSE1B) of the expressed BCL6 mRNA sequence, RT-PCR analysis of BCL6 mRNA spanning exon 1 and part of exon 2 was performed (Table S1) on the random primed cDNA of 43 gastric lymphoma cases. The Expand High Fidelity PCR System (Roche Diagnostics) was used to minimize the PCR amplification errors.
Cloning and DNA sequencing
The final PCR products of 5′ RACE on mRNA, inverse PCR, and long-range PCR were separated on agarose gels. The dominant bands were excised under UV light, and DNA was purified from gel slices using the QIAEX II Gel Extraction Kit (Qiagen, Hilden, Germany). For mutational analysis of the BCL6 promoter region, each PCR product was purified using the High Pure PCR Product Purification Kit (Roche Diagnostics) to avoid spontaneous mutations generated during the excision of PCR bands from the agarose gel under UV light. The purified PCR products were then subjected to direct sequencing in both orientations. In addition, PCR products were also subcloned into pGEM-T Easy vector (Promega, Madison, WI) and sequenced with the forward and reverse pUC/M13 sequencing primers flanking the 2 sides of the inserts. At least 5 clones were sequenced in both orientations for mutational analysis.
Immunostaining
All gastric lymphoma cases (42) were studied for the expression of BCL6 by immunohistochemistry; no adequate paraffin block was available for 1 DLBCL case. For antigen retrieval before immunostaining, sections were subjected to pressure-cooker pretreatment for 10 minutes in citrate buffer (pH 9.0). The mouse monoclonal anti-BCL6 (Dako, Kyoto, Japan) was used as the primary antibody. The LSAB system (Dako) was used for the visualization of the expression of the BCL6 antigen. The level of nuclear BCL6 expression was assessed by a 4-point system based on the proportion of positive cells: less than 10% (–); 10% to 50% (+); 50% to 70% (++); and more than 70% (+++). For the subclassification of 32 gastric DLBCL cases into GCB and non-GCB immunophenotypes according to Hans et al,12 the expression pattern of CD10 and MUM1 was also studied immunohistochemically using mouse monoclonal CD10 (Novacastra Laboratories, Newcastle Upon Tyne, United Kingdom) and MUM1 (Abcam, Cambridge, United Kingdom) antibodies. A heat-induced antigen retrieval step (95°C for 10 minutes) was performed using citrate buffer (pH 6.0) for CD10 and 1 mM EDTA (pH 9.0) for MUM1 antibodies. The ABComplexes system (Dako Japan) was used for the visualization of the expression of these 2 antigens, and their expression levels were assessed by a 2-point system based on the proportion of positive cells: less than 30% (–); and more than 30% (+). Immunohistochemical scoring was done independently on separate occasions by 2 histopathologists (C.C.S. and L.S.), who were blinded to the BCL6 molecular analysis, and was shown to be consistent.
Expression of BCL6 and its translocation partner genes in B-cell lines
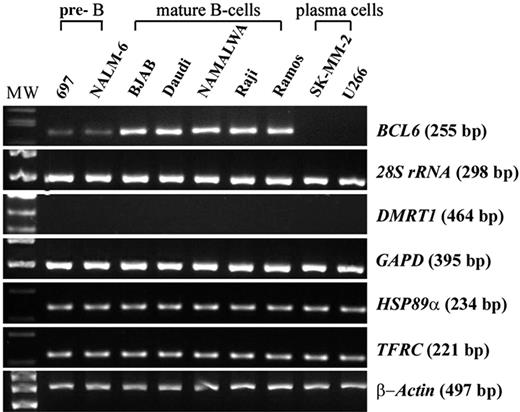
Randomly primed cDNA from a series of B-lineage tumor-cell lines corresponding to different stages of B-cell development was synthesized from 2 μg of total RNA (PE Applied Biosystems, Foster City, CA). For analysis of the expression of BCL6 and its translocation partner genes (28S rRNA, DMRT1, GAPD, HSP89α, and TFRC), semiquantitative RT-PCR was performed for each gene on the cDNA. The β-actin mRNA sequence was also amplified as a control to normalize the amount of cDNA used for each sample.
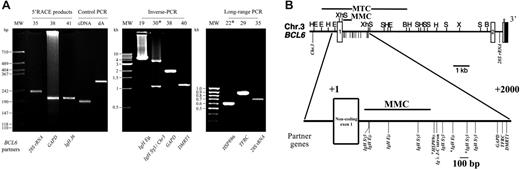
Detection of BCL6 translocations in gastric lymphoma. (A) Ethidium-bromide–stained gel showing BCL6 translocation products detected by various methods in all cases with non-Ig and examples of Ig as BCL6 fusion partner (5′-RACE on mRNA, inverse PCR on genomic DNA, and long-range PCR on genomic DNA). The case number is shown above each lane. cDNA indicates purified control cDNA amplified with control forward and reverse primers; dA, poly dA-tailed control cDNA amplified with oligo-dT anchor primer and the control reverse primer. (B) Top panel shows restriction map of the BCL6 gene, and distribution of the different breakpoints of BCL6 translocations identified in this study. Small vertical lines below the horizontal line indicate the positions of different translocation breakpoints. MTC indicates the major translocation breakpoints cluster; MMC, the major mutation cluster. Restriction sites: E, EcoRI; B, BamHI; H, HindIII; X, XbaI; S, SacI; Xh, Xhol. Lower panel shows the magnified description of top panel showing the region with different translocation breakpoints. The fusion partners of each BCL6 translocation are indicated. *DLCLML cases.
Detection of BCL6 translocations in gastric lymphoma. (A) Ethidium-bromide–stained gel showing BCL6 translocation products detected by various methods in all cases with non-Ig and examples of Ig as BCL6 fusion partner (5′-RACE on mRNA, inverse PCR on genomic DNA, and long-range PCR on genomic DNA). The case number is shown above each lane. cDNA indicates purified control cDNA amplified with control forward and reverse primers; dA, poly dA-tailed control cDNA amplified with oligo-dT anchor primer and the control reverse primer. (B) Top panel shows restriction map of the BCL6 gene, and distribution of the different breakpoints of BCL6 translocations identified in this study. Small vertical lines below the horizontal line indicate the positions of different translocation breakpoints. MTC indicates the major translocation breakpoints cluster; MMC, the major mutation cluster. Restriction sites: E, EcoRI; B, BamHI; H, HindIII; X, XbaI; S, SacI; Xh, Xhol. Lower panel shows the magnified description of top panel showing the region with different translocation breakpoints. The fusion partners of each BCL6 translocation are indicated. *DLCLML cases.
Survival analysis
Statistical analysis was performed using SPSS for Windows Release 13.0. (SPSS, Chicago, IL). Overall survival (OS) of patients was calculated from the date of diagnosis until death or last follow-up. Survival curves were plotted by the Kaplan-Meier method and were compared by the log-rank test.
Results
28S rRNA and DMRT1 are novel translocation partners of BCL6
Two complementary PCR-based approaches, 5′ RACE on mRNA and inverse PCR on genomic DNA, were used in all cases of gastric lymphoma to detect BCL6 translocations (Figure 1A). BCL6 translocations were detected in a total of 15 (34.9%) of 43 cases of gastric lymphoma, including 12 (36.4%) of 33 DLBCL cases and 3 (50%) of 6 DLCLML cases. No BCL6 translocations were detected in MALT lymphoma cases. Among these 15 cases with BCL6 translocations, 9 (60%) of 15 involved Ig: IgH Sγ3 (5 cases), IgH Eμ (3 cases), and Igλ J1/C1(1 case); 5 (33.3%) of 15 involved non-Ig genes: 28S rRNA, DMRT1, GAPD, HSP89α, and TFRC; and 1 DLCLML case (no. 30) was found to harbor 2 independent BCL6 translocations: IgH Sγ3/BCL6 and Chr.3/BCL6. Of the BCL6 non-Ig fusion partners, GAPD was detected by both 5′ RACE and inverse PCR, while 28S rRNA, HSP89α, and TFRC were detected by 5′ RACE, and DMRT 1 was detected by inverse PCR only. 28S rRNA and DMRT1 are novel non-Ig fusion partners of BCL6.
To obtain the chromosomal junctions and breakpoints of the Ig/BCL6 or non-Ig/BCL6 translocations detected by 5′ RACE on mRNA only, but not by inverse PCR on genomic DNA, long-range PCR was performed on genomic DNA (Figure 1A). Case nos. 22 and 29 had been reported previously by our group to have non-Ig/BCL6 chimeric RNA transcripts detected by 5′ RACE, and in the present study, the chromosomal junctions and breakpoints of these 2 translocations were also characterized using long-range PCR.
The distribution of the BCL6 translocation breakpoints identified in gastric lymphoma is shown in Figure 1B. Most of these breakpoints (13 of 15; 86.7%) were located within the MTC region of BCL6, while 5 were identified in the MMC region (Figure 1B). No translocation breakpoint hypercluster region was observed in the MMC region.
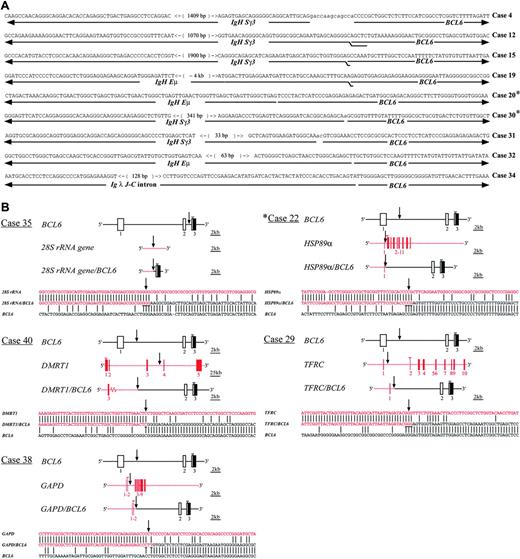
Characterization of the chromosomal junctions of the BCL6 translocations in gastric lymphoma. (A) Nucleotide sequences of the chromosomal junctions and breakpoints of Ig/BCL6 translocations. The overlapping nucleotides of the junction sequences of BCL6 and the fusion partners are doubly underlined. Variable numbers of nucleotides of unknown origin inserted at the breakpoints are shown in lowercase letters and are not underlined. The number within each bracket indicates the distance between the 2 nucleotides. (B) Schematic representation of the non-Ig/BCL6 translocations. ▪ and □ represent exons from BCL6 and the fusion partners, respectively. Vertical arrows indicate the chromosomal junctions and breakpoints of non-Ig/BCL6 fusion genes. The overlapping nucleotides of the junction sequences of BCL6 and the fusion partner genes are underlined. Open arrowheads indicate coding initiation start sites. *DLCLML cases.
Characterization of the chromosomal junctions of the BCL6 translocations in gastric lymphoma. (A) Nucleotide sequences of the chromosomal junctions and breakpoints of Ig/BCL6 translocations. The overlapping nucleotides of the junction sequences of BCL6 and the fusion partners are doubly underlined. Variable numbers of nucleotides of unknown origin inserted at the breakpoints are shown in lowercase letters and are not underlined. The number within each bracket indicates the distance between the 2 nucleotides. (B) Schematic representation of the non-Ig/BCL6 translocations. ▪ and □ represent exons from BCL6 and the fusion partners, respectively. Vertical arrows indicate the chromosomal junctions and breakpoints of non-Ig/BCL6 fusion genes. The overlapping nucleotides of the junction sequences of BCL6 and the fusion partner genes are underlined. Open arrowheads indicate coding initiation start sites. *DLCLML cases.
Identification of a new translocation breakpoint within intron 2 of BCL6
The nucleotide sequences of the chromosomal junctions of Ig/BCL6 translocations are shown in Figure 2A, and the schematic representation of each non-Ig/BCL6 translocation is shown in Figure 2B. In each of the Ig/BCL6 translocations and the non-Ig/BCL6 translocations involving DMRT1, GAPD, HSP89α, and TFRC, the 5′ promoter regions of these genes were juxtaposed to the 5′ end of the first intron of BCL6 in the MTC region, while in the 28S rRNA/BCL6 translocation, the 5′ 1.7 kb of 28S rRNA was juxtaposed to BCL6 intron 2. The involvement of intron 2 in BCL6 translocation has not been described before (Figure 2B).
All of the translocations detected resulted in BCL6 promoter substitution by the corresponding fusion partner genes, thereby placing BCL6 under the regulation of heterologous regulatory sequences. For the GAPD/BCL6 translocation, GAPD coding exon 1 was fused upstream of BCL6 exon 2 in the same orientation (Figure 2B). The chimeric RNA transcript GAPD/BCL6 is expected to code for the first 9 codons of GAPD, followed by a stop codon generated as a consequence of the translocation. This sequence is in turn followed by the BCL6 translation initiation codon (ATG) and the complete BCL6 coding sequence. No BCL6 protein expression is expected from 28S rRNA/BCL6 fusion RNA transcript, as no translation mechanism should act on 28S rRNA.
Somatic mutations in the BCL6 MMC region in gastric lymphoma
All 43 cases of gastric lymphoma were subjected to mutational analysis of the BCL6 genomic fragment spanning the 5′ end of the first intron encompassing the MMC region (Figure 3A) and the noncoding exon 1 (Figure 3B). Mutated clones of the BCL6 MMC region were detected in 24 (55.8%) of 43 gastric lymphoma cases: 2 (50%) of 4 MALT lymphoma cases, 19 (57.6%) of 33 DLBCL cases, and 3 (50%) of 6 DLCLML cases. The frequency of mutations ranged from 1.17 to 24.48 × 10–3/bp. The presence of intraclonal heterogeneity in the BCL6 MMC region, a marker of ongoing somatic mutation in B-cell lymphomas of GC origin, was detected in 6 (18.2%) cases of DLBCL. The distribution of somatic mutations detected within the MMC region is shown in Figure 3A. No specific hotspot of somatic mutations within the MMC region was identified. For the 5 BCL6 translocation breakpoints located within the MMC region, 2 of them, from IgH/BCL6 and HSP89α/BCL6 translocations, were detected at nucleotide positions where somatic mutations were observed (Figure 3A).
The features of the somatic mutations detected in the BCL6 MMC region in 24 cases of gastric lymphoma are summarized in Table 1. Of a total of 170 single base-pair substitutions observed, 105 were transitions and 65 were transversions. The overall transition-transversion ratio was 1.63 (expected ratio was 0.5). In addition to acquired somatic mutations, 2 constitutional single-nucleotide polymorphisms (SNPs) within the MMC region at positions 754 (G/C) and 877 (del T) were detected in 4 and 2 cases of gastric lymphoma, respectively. These inherited heterozygous polymorphisms were not counted as acquired somatic mutations among these cases.
Features of somatic mutations in the BCL6 major mutation cluster (MMC) region (858-bp sequence at 5′ of BCL6 first intron was analyzed) in gastric lymphoma
Feature . | Value . |
|---|---|
| Frequency × 10-3/bp, range* | 1.17-24.48 |
| Single bp substitutions | 170 |
| Deletions | 12 |
| Insertions | 10 |
| Transitions/transversions† | 1.63 |
| Strand polarity†‡ | 1.57 |
| RGYW/WRCY bias§ | 1.43 |
Feature . | Value . |
|---|---|
| Frequency × 10-3/bp, range* | 1.17-24.48 |
| Single bp substitutions | 170 |
| Deletions | 12 |
| Insertions | 10 |
| Transitions/transversions† | 1.63 |
| Strand polarity†‡ | 1.57 |
| RGYW/WRCY bias§ | 1.43 |
The overall frequency of mutations in the BCL6 MMC region in gastric lymphoma is extrapolated from the frequency of mutated cases occurring in this group (24 of 43 cases; 55.8%).
The frequency of substitutions affecting each base was corrected for the base composition of the region analyzed.
Values designate the ratio of A → N vs T → N substitutions.
Normalized frequency of mutated G bases occurring in the context of an RGYW (A/G G C/T A/T) and C bases in its inverse compliment WRCY (A/T A/G C C/T) motif.
It has been shown that the quadruplet motif RGYW (A/G G C/T A/T) and the inverse repeat WRCY (A/T A/G C C/T) are targets for increased mutational activity in the BCL6 MMC region.42 To test whether these phenomena also occurred in gastric lymphoma, we analyzed the somatic mutations occurring in these motifs. Together, RGYW/WRCY motifs represented 18.1% (155 of 858) of nucleotides within the region studied (Table 1). Mutations of RGYW/WRCY accounted for 25.9% (44 of 170) of all nucleotide substitutions, a higher frequency than expected.
Translocation breakpoints are located within mutational hotspot motifs of BCL6 and its partner genes in gastric lymphoma
In Ig/BCL6 translocations, most of the breakpoints on both Ig and BCL6 gene sequences were located within or near the mutational hotspot motif RGYW/RGY or its inverse complement WRCY/RCY (Figure 2A). For the Ig sequence, 7 breakpoints were within RGYW motifs, 2 were within RGY motifs, and 1 was 1 bp away from the WRCY motif. For the BCL6 sequence, 2 breakpoints were within RGY motifs, 2 were within RCY motifs, 2 were within WRCY motifs, and 1 was flanked by RCY and RGY motifs.
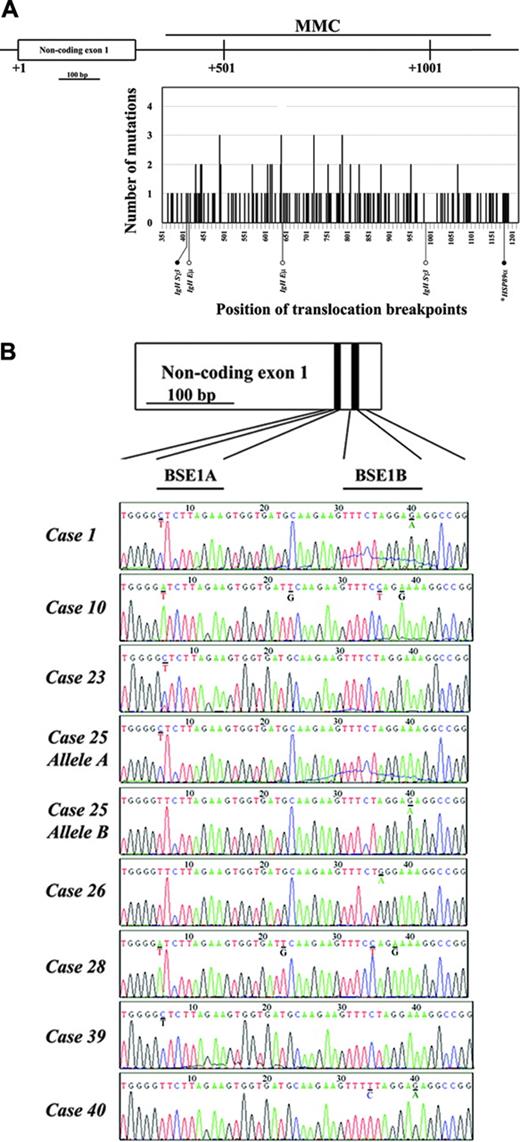
Mutational analysis of the BCL6 first intron and non-coding exon 1 in gastric lymphoma. (A) Distribution of somatic mutations in the BCL6 hypermutation region (nucleotides +358 to +1148 corresponding to the first transcription start site) covering MMC located at 5′ of the first intron detected in 24 cases of gastric lymphoma. The abscissa represents the position along the MMC region, starting from the first nucleotide of BCL6 non-coding exon 1. The ordinates correspond to the number of cases with somatic mutations at each position. Black bars indicate the number of cases with somatic mutations. Constitutional single-nucleotide polymorphisms (SNPs) were not counted. BCL6 translocation breakpoints within this region are indicated below the graph. • indicates the translocation breakpoints overlapping with somatic mutations detected; ○ indicates the translocation breakpoints not overlapping with somatic mutations detected. (B) BCL6-deregulating mutations in the two BCL6 binding motifs (BSE1A and BSE1B) located within the non-coding exon 1 of the gene detected in 8 cases of gastric lymphoma. The positions of the two BCL6 binding motifs are indicated above the case no. 1. The mutated nucleotides are underlined with the wild-type sequences shown below. *DLCLML cases.
Mutational analysis of the BCL6 first intron and non-coding exon 1 in gastric lymphoma. (A) Distribution of somatic mutations in the BCL6 hypermutation region (nucleotides +358 to +1148 corresponding to the first transcription start site) covering MMC located at 5′ of the first intron detected in 24 cases of gastric lymphoma. The abscissa represents the position along the MMC region, starting from the first nucleotide of BCL6 non-coding exon 1. The ordinates correspond to the number of cases with somatic mutations at each position. Black bars indicate the number of cases with somatic mutations. Constitutional single-nucleotide polymorphisms (SNPs) were not counted. BCL6 translocation breakpoints within this region are indicated below the graph. • indicates the translocation breakpoints overlapping with somatic mutations detected; ○ indicates the translocation breakpoints not overlapping with somatic mutations detected. (B) BCL6-deregulating mutations in the two BCL6 binding motifs (BSE1A and BSE1B) located within the non-coding exon 1 of the gene detected in 8 cases of gastric lymphoma. The positions of the two BCL6 binding motifs are indicated above the case no. 1. The mutated nucleotides are underlined with the wild-type sequences shown below. *DLCLML cases.
Similar phenomena also occurred in non-Ig/BCL6 translocation (Figure 2B). The breakpoint on DMRT1 was within a WRCY motif, the GAPD breakpoint was 1 bp away from an RGYW motif, the HSP89α breakpoint was within an RCY motif, the TFRC breakpoint was within an RGYW motif, and the Chr.3 sequence breakpoint was within an RGYW motif. For the BCL6 sequence, the breakpoint on the GAPD/BCL6 allele was within an RGY motif, the breakpoint on the HSP89α/BCL6 allele was within a WRCY motif, the breakpoint on the TFRC/BCL6 allele was within an RGY motif, and the breakpoint on the 28S rRNA/BCL6 allele was flanked by an RGY motif.
High frequency of BCL6 somatic mutations is detected in the BCL6 sequence in Ig/BCL6 rearranged alleles in gastric lymphoma
Figures 4A and 4B show BCL6 somatic mutations detected in Ig/BCL6 rearranged alleles (9 cases) and non-Ig/BCL6 rearranged alleles (4 cases), respectively. For each case with Ig/BCL6 translocation, significantly higher frequencies of BCL6 mutations were detected in the Ig/BCL6-rearranged allele when compared with the nonrearranged allele of the same case. However, for each case with non-Ig/BCL6 translocation, similar frequencies of BCL6 mutations were detected in both the non-Ig/BCL6 rearranged and the nonrearranged alleles. A much higher frequency of BCL6 somatic mutations were detected in the Ig/BCL6 rearranged alleles than in the non-Ig/BCL6 rearranged alleles, or the nonrearranged alleles.
BCL6-deregulating mutations are identified in gastric lymphoma
BCL6-deregulating mutations in the 2 BCL6 binding motifs (BSE1A and BSE1B) were analyzed in 43 cases of gastric lymphoma. Mutations at these sites prevented BCL6 from binding its own promoter, thus disrupting its negative autoregulation. The deregulating mutations were detected in 8 (24.2%) of 33 cases of DLBCL, with 1 case (no. 25) showing these mutations in both alleles (Figure 4B). One DLBCL case (no. 40) showed both deregulating mutation and BCL6 translocation. No BCL6-deregulating mutations were detected in MALT lymphoma and DLCLML cases.
Gastric lymphoma cases with BCL6-deregulating mutations have high BCL6 protein expression
The protein expression of BCL6 in 42 cases of gastric lymphoma was detected by immunohistochemistry using anti-BCL6 monoclonal antibody (mAb). The results of immunostaining for all gastric lymphoma cases with non-Ig/BCL6 translocations, 2 examples of cases with Ig/BCL6 translocations, and 1 example of cases with BSE1 mutation in the BCL6 binding motif are shown in Figure 5A. A low percentage of lymphoma cells showed nuclear BCL6 expression in the case with 28S rRNA/BCL6 translocation. Heterogeneous levels of nuclear BCL6 expression were observed in the cases with non-Ig/BCL6 and Ig/BCL6 translocations, while all the cases with BCL6-deregulating mutations expressed a high level of nuclear BCL6 expression. Low BCL6 protein expression was observed in the 4 gastric MALT lymphoma cases.
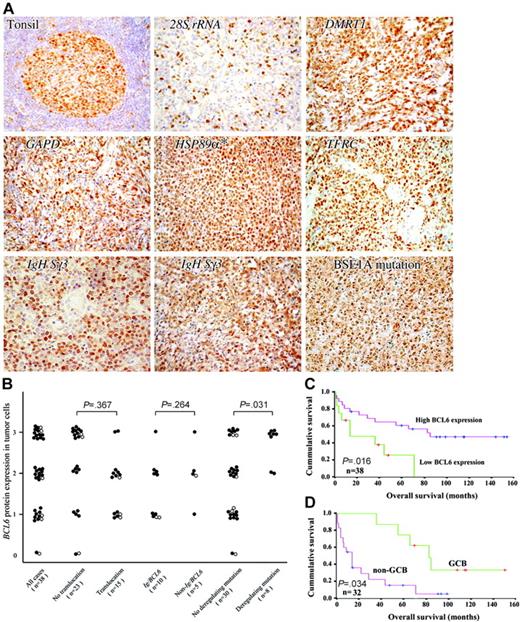
The BCL6 protein expression levels were next correlated between the gastric DLBCL and DLCLML cases (1) with and without BCL6 translocations; (2) with Ig/BCL6 and non-Ig/BCL6 translocations; and (3) with and without BCL6-deregulating mutations. A 2-tailed χ2 test was used for statistical comparison. The BCL6 expression levels of the cases detected with deregulating mutations were significantly higher than the cases without deregulating mutations (P < .05), while no significant differences of BCL6 protein expression were found between the cases with other chromosomal alterations (Figure 5B).
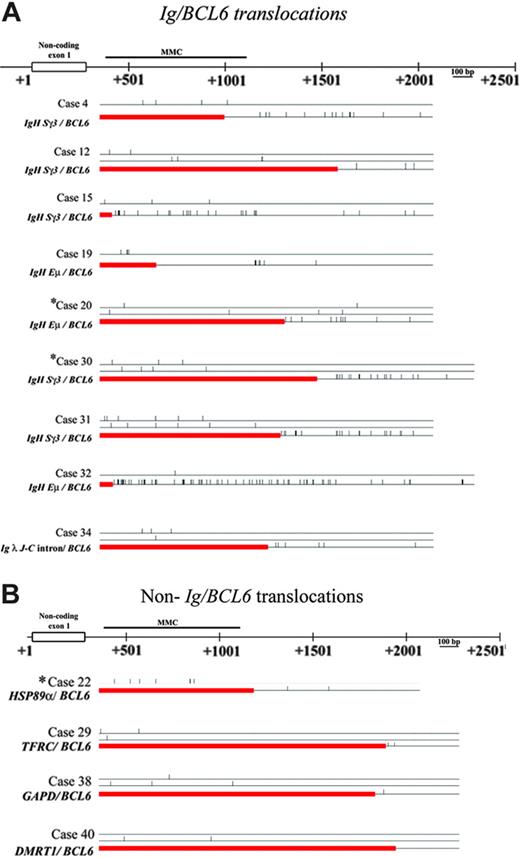
Frequency of somatic mutations in the BCL6 sequence in Ig/BCL6 and non-Ig/BCL6 rearranged alleles in gastric lymphoma. Diagrammatic illustration showing frequency of BCL6 somatic mutations detected in Ig/BCL6 (A) and non-Ig/BCL6 (B) rearranged alleles and their corresponding non-rearranged allele in each case. Straight horizontal lines represent non-rearranged alleles while rectangle boxes represent the fusion partner genes. Small vertical bars represent the mutations detected. *DLCLML cases.
Frequency of somatic mutations in the BCL6 sequence in Ig/BCL6 and non-Ig/BCL6 rearranged alleles in gastric lymphoma. Diagrammatic illustration showing frequency of BCL6 somatic mutations detected in Ig/BCL6 (A) and non-Ig/BCL6 (B) rearranged alleles and their corresponding non-rearranged allele in each case. Straight horizontal lines represent non-rearranged alleles while rectangle boxes represent the fusion partner genes. Small vertical bars represent the mutations detected. *DLCLML cases.
BCL6 protein expression in gastric lymphoma. (A) The results of immunostaining for 5 gastric lymphoma cases with non-Ig/BCL6 translocations, 2 representative examples of cases with Ig/BCL6 translocations, and 1 example of cases with BSE1 mutation in the BCL6 binding motif are shown. Normal lymphoid tissue (tonsil) was used as a control for BCL6 immunostaining. *DLCLML case. (B) The distribution of BCL6 protein expression in tumor cells of the 38 cases of gastric lymphoma. 2-tailed χ2 test was used for statistical comparison. • indicates DLBCL cases; ○, DLCLML cases. (C) Kaplan-Meier curve showing BCL6 protein expression versus OS of gastric DLBCL and DLCLML patients. (D) In gastric DLBCL, the Kaplan-Meier curve showing the OS of the GCB subtype versus the non-GCB subtype.
BCL6 protein expression in gastric lymphoma. (A) The results of immunostaining for 5 gastric lymphoma cases with non-Ig/BCL6 translocations, 2 representative examples of cases with Ig/BCL6 translocations, and 1 example of cases with BSE1 mutation in the BCL6 binding motif are shown. Normal lymphoid tissue (tonsil) was used as a control for BCL6 immunostaining. *DLCLML case. (B) The distribution of BCL6 protein expression in tumor cells of the 38 cases of gastric lymphoma. 2-tailed χ2 test was used for statistical comparison. • indicates DLBCL cases; ○, DLCLML cases. (C) Kaplan-Meier curve showing BCL6 protein expression versus OS of gastric DLBCL and DLCLML patients. (D) In gastric DLBCL, the Kaplan-Meier curve showing the OS of the GCB subtype versus the non-GCB subtype.
Gastric lymphoma cases with high BCL6 protein expression have better prognosis
The correlation of BCL6 chromosomal alterations with OS was next assessed in 43 gastric lymphoma patients. The OS of patients with BCL6-high DLBCL and DLCLML were significantly better than that of the patients with BCL6-low disease (median OS of BCL6-high and BCL6-low patients was 55 and 14 months, respectively; P < .05) (Tables 2, 3; Figure 5C). However, the equality of the survival distributions for other categoric variables showed no significant correlation with the survival value of the gastric lymphoma patients (Table 2).
Log-rank statistics to test the equality of the survival distributions for the different categoric variables in 39 cases of gastric DLBCL and DLCLML
Variable . | n . | P . |
|---|---|---|
| Translocation vs no translocation cases | 15 vs 24 | .333 |
| Ig/BCL6 vs non-Ig/BCL6 translocation cases | 10 vs 5 | .360 |
| Deregulating mutation vs no deregulating mutation cases | 8 vs 31 | .784 |
| Translocation/deregulating mutation vs no translocation/deregulating mutation cases | 22 vs 17 | .315 |
| Ongoing BCL6 somatic mutations in MMC [GCB] vs acquired but not active ongoing BCL6 somatic mutations in MMC [post-GCB] cases | 6 vs 16 | .734 |
| High BCL6 expression vs low BCL6 expression cases* | 26 vs 12 | .016 |
Variable . | n . | P . |
|---|---|---|
| Translocation vs no translocation cases | 15 vs 24 | .333 |
| Ig/BCL6 vs non-Ig/BCL6 translocation cases | 10 vs 5 | .360 |
| Deregulating mutation vs no deregulating mutation cases | 8 vs 31 | .784 |
| Translocation/deregulating mutation vs no translocation/deregulating mutation cases | 22 vs 17 | .315 |
| Ongoing BCL6 somatic mutations in MMC [GCB] vs acquired but not active ongoing BCL6 somatic mutations in MMC [post-GCB] cases | 6 vs 16 | .734 |
| High BCL6 expression vs low BCL6 expression cases* | 26 vs 12 | .016 |
MMC indicates major mutation cluster located at 5′ of the first intron.
BCL6 protein expression was assessed by a 4-point system based on the proportion of positive cells with nuclear staining and was scored as low (- and +) or high (++ and +++). BCL6 expression could not be studied in 1 case due to the unavailability of paraffin block for the case.
Statistical survival data of different subtypes of gastric lymphomas
. | . | Survival . | . | |
|---|---|---|---|---|
| BCL6 expression . | n . | Median, mo . | 5 y, % . | |
| DLBCL*+ DLCLML† | ||||
| High | 26 | 85 | 57.7 | |
| Low | 12 | 14 | 8.3 | |
| DLBCL* | ||||
| High | 23 | 83 | 48.0 | |
| Low | 9 | 14 | 11.1 | |
| DLCLML† | ||||
| High | 3 | NA | 100 | |
| Low | 3 | 36 | 0 | |
. | . | Survival . | . | |
|---|---|---|---|---|
| BCL6 expression . | n . | Median, mo . | 5 y, % . | |
| DLBCL*+ DLCLML† | ||||
| High | 26 | 85 | 57.7 | |
| Low | 12 | 14 | 8.3 | |
| DLBCL* | ||||
| High | 23 | 83 | 48.0 | |
| Low | 9 | 14 | 11.1 | |
| DLCLML† | ||||
| High | 3 | NA | 100 | |
| Low | 3 | 36 | 0 | |
NA indicates not applicable; these 3 patients are still alive.
n = 32.
n = 6.
BCL6 expression and genetic alterations in GCB and non-GCB subgroups of gastric DLBCL
A number of patients with gastric DLBCL (32) were subclassified into GCB or non-GCB subgroups according to Hans et al.12 The expression of CD10 was observed in 6 (18.8%) of 32 patients, and MUM1 in 16 (50%) of 32 patients. Overall, 32 patients with DLBCL were comprised of 10 (31.2%) GCB and 22 (68.8%) non-GCB phenotypes. All 6 patients detected with ongoing BCL6 SHMs were found with the GCB phenotype. The OS of the GCB subtype was significantly better than that of the non-GCB subtype (P = .034; Figure 5D, Table 4). In the GCB subgroup, high BCL6 expression was found in all 10 patients, so no comparison could be made between the BCL6 expression level and BCL6 genetic alterations or OS. However, in the non-GCB subgroup, 9 (40.9%) of 22 patients had low BCL6 expression, while 13 (59.1%) of 22 patients had high BCL6 expression. Ten (76.9%) of 13 of the patients with high BCL6 expression were found to have BCL6 translocations and/or deregulating mutations. All non-GCB patients with BCL6 deregulating mutations were found to have high BCL6 expression. Within the non-GCB subgroup, no significant correlation was found between OS and BCL6 expression level or genetic alterations.
Statistical survival data of different subgroups of gastric DLBCLs classified immunohistochemically according to Hans et al12
. | . | Survival . | . | |
|---|---|---|---|---|
| DLBCL subtypes . | n . | Median, mo . | 5 y, % . | |
| GCB | 10 | 5 | 80.0 | |
| Non-GCB | 22 | 14 | 22.7 | |
. | . | Survival . | . | |
|---|---|---|---|---|
| DLBCL subtypes . | n . | Median, mo . | 5 y, % . | |
| GCB | 10 | 5 | 80.0 | |
| Non-GCB | 22 | 14 | 22.7 | |
Discussion
Most of the primary gastric lymphoma cases in the Hong Kong Chinese population are DLBCLs, while MALT lymphomas and DLCLMLs occur rarely. DLBCL is a heterogeneous group of tumors, varying in clinical features, immunophenotype, and cytogenetics. It is uncertain whether gastric DLBCL is pathogenetically different from its nodal counterpart. For example, BCL2 rearrangements occur in about 30% of nodal DLBCLs,9 but are lacking in gastric DLBCLs.6,7 A high incidence of c-myc (MYC) rearrangements is observed in gastric DLBCL, but involvement of MYC is rare in nodal DLBCL.8
In this study, 36.4% of gastric DLBCLs were identified with BCL6 translocations, and more than half of these BCL6 translocations involved Ig as the translocation partner. The frequency and pattern of BCL6 translocations in gastric DLBCL is largely similar to those found in DLBCL. BCL6 translocations have been reported in about 30% to 40% of DLBCLs,26,27,33,43-45 and chromosomal translocations between BCL6 and Ig genes, especially involving IgH, are the most common.33,46,47
No BCL6 translocations were found in MALT lymphoma cases, but BCL6 translocations were identified in half of the gastric DLCLML cases. Two of these translocations involved IgH Sγ3 and IgH Eμ regions, suggesting that these IgH/BCL6 translocations occurred during isotype class-switching, similar to gastric DLBCL. BCL6 rearrangements similar to these have been previously described in DLCLML.41,48 It is interesting that a proportion of IgM+ gastric MALT lymphomas undergoes aberrant isotype switch recombination.49 This ability might be directly involved in the progression of malignancy to DLCLML (ie, the presence of many reactive lymphoid follicles in acquired MALT lymphoma may provide a presetting for genetic alterations of BCL6, resulting in high-grade transformation to DLCLML). However, these preliminary data on BCL6 chromosomal alterations in half of gastric DLCLML cases will need to be substantiated in a larger series of DLCLML cases before implicating the speculation that BCL6 alterations may be involved in high-grade transformation of gastric MALT lymphoma.
Transgenic mouse models that mimic the common BCL6 translocation, t(3;14), provide evidence that BCL6 translocation may promote lymphomas or predispose the B cell for transformation into a tumor cell.50,51 The genes that we observed to rearrange with BCL6 display a broader pattern of expression. Transcriptional regulation of BCL6 by substituted promoters from rearranging genes may lead to its persistent expression beyond the GC stage (Figure 6). However, the BCL6 translocations involving the 2 novel partner genes, 28S rRNA and DMRT1, suggest total abrogation of BCL6 expression from the rearranged allele, since no translation mechanism would normally work on 28S rRNA, and DMRT1 is expressed specifically in testis.52 The unexpected observation of high BCL6 protein expression in the case with DMRT1/BCL6 translocation (Figure 5A), in which BCL6 fusion mRNA expression was not detected, can be explained by its expression from the nonrearranged allele in which mutation in BCL6 binding site BSE1B was detected. It has been widely observed that not all DLBCL tumors express BCL6;27,53,54 the detection of 28S rRNA/BCL6 translocation may provide clues to some of these DLBCL cases detected with no BCL6 expression.
Identification of a breakpoint within BCL6 intron 2 in 28S rRNA/BCL6 by 5′ RACE, and a new breakpoint cluster55 (called the alternative breakpoint region [ABR]) mapping from 245 to 285 kb 5′ to BCL6 by long-range PCR suggest that BCL6 translocation may be more frequent, as it could be underestimated using traditional detection methods. For identifying the cases with BCL6 gene rearrangement, Southern blot hybridization has been traditionally used on tumor DNA using BCL6 gene arrangement probes to detect BCL6 translocations involving intron 1. More recently, interphase fluorescent in situ hybridization (FISH) has been used on tissue sections using a dual-color breakapart BCL6 rearrangement probe.56 For the cloning of translocation partners of BCL6, long distance inverse (LDI)–PCR on genomic DNA has been used to amplify BCL6 translocations involving intron 1 and 5′ RACE on mRNA to detect the presence of BCL6 chimeric transcripts involving heterologous partners. The benefits of the LDI-PCR and 5′ RACE methods is that the cloning can be performed in the absence of cytogenetic data when only small amounts of archival tumor materials are available for the study. However, interphase FISH using the BCL6 split probe is recommended in identifying novel BCL6 translocations, in parallel with LDI-PCR and 5′ RACE. Interphase FISH can detect BCL6 translocations with novel breakpoints distant from the known breakpoint cluster,50 not usually targeted by LDI-PCR and 5′ RACE.
Expression of BCL6 and its non-Ig translocation partner genes in a series of B-cell lines at different stages of differentiation by semiquantitative RT-PCR. β-Actin mRNA levels were used as control for the normalization of the amount of cDNA used for each sample.
Expression of BCL6 and its non-Ig translocation partner genes in a series of B-cell lines at different stages of differentiation by semiquantitative RT-PCR. β-Actin mRNA levels were used as control for the normalization of the amount of cDNA used for each sample.
The frequency and pattern of SHMs of BCL6 in DLBCL are largely similar to that of Ig. Ig SHMs accumulate at a rate of 10–4 to 10–3/bp and extend 1.5 kb to 2 kb downstream of the transcription initiation site, with preference for certain hotspots.57 Ig SHMs favor transition over transversion and display strand polarity, as inferred from G-over-C bias. It has been recently shown that SHMs in Ig and BCL6 are driven by the same mechanism.58-60 BCL6 SHMs identified in gastric DLBCL display characteristics similar to those harbored by other B-cell neoplasms with GC origin;42 6 cases of gastric DLBCL were detected with BCL6 ongoing somatic mutations which normally occur almost exclusively in the GC. BCL6 SHMs were also identified in half of the gastric MALT lymphoma and DLCLML cases, thus suggesting GC origin of these gastric lymphomas.
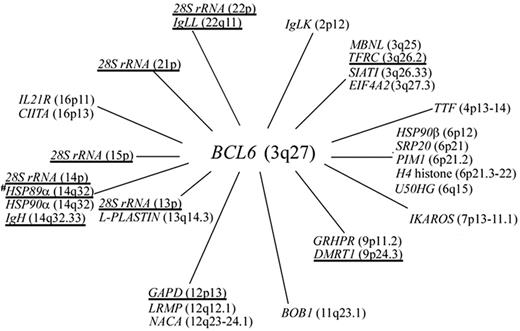
The results of this study and those of previous reports shed light on the mechanisms mediating BCL6 translocations. For the IgH/BCL6 translocations, as the breakpoints were clustered at Sγ3 and Eμ regions, the translocations might be the results of physiologic error during Ig isotype class switching in the centrocytes of GC. On the other hand, BCL6 translocations involving non-Ig partners are likely to be also driven by a definite mechanism(s), according to the following observations: (1) Although BCL6 translocations involve heterologous promoters (Figure 7), the translocations are not random: BCL6 translocations involving α-NAC, CIITA, GAPD, H4 histone, HSP89α, IKAROS, IL-21R, L-plastin, PIM-1, TFRC, and TTF have been frequently detected; and (2) translocation breakpoints of the BCL6 non-Ig fusion partners are localized in a single exon or intron. For GAPD/BCL6 translocations, the breakpoint on GAPD identified in gastric lymphoma is 52 bp 5′ upstream the breakpoint detected in DLBCL of the central nervous system.61 For TFRC/BCL6 translocation, the breakpoint on TFRC identified in gastric lymphoma is 288 bp 3′ downstream of the breakpoint detected by Yoshida et al.47 For HSP89α/BCL6 translocations, the breakpoint on HSP89α identified in the gastric DLCLML case is 109 bp and 258 bp 5′ upstream of the breakpoint detected in DLBCL by Akasaka et al and in DLBCL of the central nervous system by Montesinos-Rongen et al.61 Similarly, BCL6 translocation breakpoints in IL-21R, L-plastin, and H4 histone were also localized in a single intron or exon.33,62,63 These findings suggest that the BCL6 non-Ig fusion partners possess special structural feature(s) that make a region vulnerable to breakage. The most probable mechanism that drives the non-Ig/BCL6 translocations in gastric lymphoma may be a consequence of the aberrant SHM machinery targeting these non-Ig partner genes. This mechanism is suggested by the remarkable detection of single nucleotide substitutions, deletions, and insertions in the HSP89α, GAPD, and TFRC sequences at the breakpoints, and a 55-bp deletion of TFRC sequence at the TFRC/BCL6 genomic junction (results not shown). The deletions or insertions of variable numbers of nucleotides at the breakpoints are comparable with those observed in IgV. A similar type of mutation was also observed in the H4 sequence in 4 H4/BCL6 translocations in DLBCL.64 Moreover, SHM has been shown to act on PIM1, PAX5, TTF, and PAX5 in DLBCL,65 and these 4 hypermutable genes are also susceptible to chromosomal translocations in the same region, consistent with a role for hypermutation in generating translocations by DNA double-strand breaks. These observations suggest that the SHM machinery targets certain non-Ig genes in DLBCL, predisposing the mutated region to translocation with BCL6.
In the present study, the BCL6 translocation breakpoints were mainly located in the MTC region, and approximately half of them were found within the MMC region. Most of the breakpoints in these translocations were located within the SHM hotspot motif RGYW/RGY or its inversion, and there was no difference in the location of the BCL6 breakpoints between the Ig/BCL6 and non-Ig/BCL6 translocations. These findings support a hypothesis that SHM predisposes the mutated region to the subsequent development of translocation.
In this study, somatic mutations were detected at a much higher frequency in the BCL6 sequence adjacent to the translocation junctions in the Ig/BCL6 rearranged allele compared with the corresponding nonrearranged alleles and the non-Ig/BCL6 rearranged alleles. Similar phenomena were previously observed for MYC and BCL2 when translocated to Ig loci in lymphoma.66,67 In both cases, the mutations were attributed to the IgV gene SHM mechanism acting on sequences that had become linked to the Ig locus following chromosomal translocation. Therefore, it is likely that the same mechanism may also act on the BCL6 sequence of an Ig/BCL6 rearranged allele. It has been shown experimentally that SHM is not Ig specific, as non-Ig sequences are mutated when in the context of Ig with the Ig enhancer permitting mutation.68-70
The prognostic significance of BCL6 translocation and expression has been previously analyzed in different series of nongastric DLBCL, and the level of BCL6 expression was reported to depend on 2 main factors: the presence or absence of BCL6 rearrangement, and the specific partners (Ig or non-Ig) involved in BCL6 translocations; these factors have been described as independent markers of favorable clinical outcome in DLBCL and predictive of survival.43-46 However, in this study on gastric lymphoma, we showed that neither BCL6 translocation status nor translocation partners correlated with either BCL6 protein expression or OS in gastric lymphoma. Similarly, in a case with t(3;16)(q27;p11), leading to the fusion of BCL6 with the IL-21R, the level of BCL6 mRNA was unexpectedly low.71 We found that the BCL6-deregulating mutations correlated with its high expression in gastric DLBCL. We also found that high BCL6 expression predicted longer OS, independent of BCL6 translocation status, translocation partner, or BCL6-deregulating mutations, in gastric DLBCL and DLCLML. However, when patients with gastric DLBCL were subclassified immunohistochemically into GCB and non-GCB subtypes,12 all patients in the GCB subgroup had high BCL6 expression independent of BCL6 alterations at the genetic level and had significantly better OS than the non-GCB subgroup. On the other hand, in the non-GCB subgroup, heterogeneous BCL6 expression (low and high) was detected and BCL6 deregulating mutations correlated significantly with high BCL6 expression, but no significant difference in OS was found between the BCL6 expression level or genetic alterations within the subgroup, which had overall poorer prognosis. The results on the gastric DLBCL GCB/non-GCB comparison seemed to indicate that BCL6 expression level was not a significant determinant of OS and that the apparent correlation was not causal but due to the association of GCB with high BCL6 expression. However, these results on the BCL6 expression level and genetic alterations and its correlation with prognosis will need to be substantiated in a larger series of patients of each subgroup (GCB and non-GCB) of gastric DLBCL.
Chromosomal locations of all the BCL6 translocation partners (annotated genes only) described in B-cell NHL.BCL6 fusion partner genes identified by us in gastric lymphoma are underlined.
Chromosomal locations of all the BCL6 translocation partners (annotated genes only) described in B-cell NHL.BCL6 fusion partner genes identified by us in gastric lymphoma are underlined.
Prepublished online as Blood First Edition Paper, June 13, 2006; DOI 10.1182/blood-2006-05-022517.
Supported by a grant from the Michael Kadoorie Cancer Genetics Research Fund (to G.S. and R.H.L.).
Y.-W.C. designed research, performed research, analyzed data, performed clinical correlation, and wrote the manuscript; X.-T.H. performed research and analyzed data; A.C.L. performed research and analyzed data; W.-Y.A. selected gastric lymphoma cases and performed clinical correlation; C.-C.S. analyzed immunohistochemical expression data; M.L.W. performed research; L.S. analyzed immunohistochemical expression data; Q.T. designed research and analyzed data; K.-M.C. provided gastric lymphoma surgical specimens and clinical data of gastric lymphoma patients; Y.-L.K. provided clinical data of gastric lymphoma patients and wrote the manuscript; R.H.L. conceived the research and provided clinical data of gastric lymphoma patients; and G.S. conceived the research, designed research, analyzed data, and finalized the manuscript.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Kai Yau Wong for his expert technical advice throughout this study.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal