Abstract
Accumulating evidence indicates that, in absence of CD8+ T-cell activation, CD4+ T-cell–mediated allograft rejection is associated with a dominant Th2-cell response and eosinophil infiltrates. In this study, we analyzed the mechanisms by which CD8+ T cells regulate alloreactive CD4+ T-cell priming and differentiation into interleukin 4 (IL-4)–producing cells. We showed that interferon γ (IFN-γ) production by CD8+ T cells was dispensable for the inhibition of Th2-cell development, as well as tissue eosinophilia and type 2 cytokine production in the rejected grafts. Since we noticed that CD8+ T cells not only suppressed Th2 differentiation, but also down-modulated the overall priming of alloreactive CD4+ T cells, we evaluated whether CD8+ T cells act by limiting the accumulation of donor-derived dendritic cells (DCs) in lymph nodes. We found that indeed, alloreactive CD8+ T cells rapidly eliminated allogeneic DCs from T-cell areas of draining lymph nodes, through a perforin-dependent mechanism. Thus, our data demonstrate that cytotoxic T lymphocyte (CTL)–mediated clearance of allogeneic DCs is a negative feedback mechanism that limits the duration of alloantigen presentation in draining lymph nodes, thereby modulating the amplitude and polarization of the primary alloreactive CD4+ T-cell responses.
Introduction
Allograft rejection arises from an immune process resulting from the recognition of alloantigens by a host's T lymphocytes and their subsequent activation and differentiation into effector cells. Graft-derived dendritic cells (DCs) play an essential role in this process by migrating to the draining lymph nodes of the recipients wherein they prime alloreactive T cells through 2 main pathways of alloantigen presentation.1 First, the direct pathway involves the recognition of allogeneic major histocompatibility complex (MHC) molecules on donor DCs by alloreactive T cells present at high frequency in the recipient,2 and is sufficient to mediate acute allograft rejection.3 Second, for the indirect pathway, peptides derived from donor allogeneic MHC molecules are presented on the self-MHC class II molecules expressed by recipient DCs,4 and can also initiate rapid skin graft rejection.1,5
Although experiments in CD4 and CD8 knockout mice have unambiguously shown that CD4+ T lymphocytes, but not CD8+ T cells, are essential in the initiation of allograft rejection,6 it is generally thought that Th1-type CD4+ T cells and cytolytic CD8+ T cells are the critical effectors that lead to graft rejection in healthy individuals. As a consequence, it has been hypothesized that immune deviation favoring the emergence of a Th2 response, and antagonizing a Th1 response, could promote tolerance and long-term survival.7 However, the beneficial role of Th2 responses in allograft survival has been challenged by reports showing that rejection processes could be associated with type-2 responses.8-14 The factors that influence the differentiation of the alloreactive CD4+ T lymphocytes are still poorly known. Although it has been proposed that Th2 responses are confined to the neonatal period,15 it is now largely recognized that Th2 polarization could develop in adults following allogeneic graft transplantation leading to an alternative pathway of allograft rejection characterized by massive eosinophil infiltrates.16 These forms of Th2-associated rejection have been principally observed when donor-specific CD8+ T-cell activities are deficient.8,12,14,17 Indeed, activated CD8+ T cells have been shown to down-regulate Th2-cell development in allergic-type response,18 in response to respiratory syncytial virus19,20 and in allotransplantation.8,21-23 CD8+ T cells secrete interferon γ (IFN-γ), which could act directly on CD4+ T cells by promoting T-bet expression and interleukin 12 (IL-12)/Stat-4–dependent Th1-cell development.24 Alternatively, CD8+ T cells could promote DC activation and IL-12 production, thereby supporting the induction of Th1-type responses through IFN-γ–dependent25 or –independent mechanisms.18
We have previously analyzed the development of alloreactive CD4+ T cells in the absence of CD8+ T-cell activation in vivo. Immunization of adult CD8-deficient mice with semi-allogeneic DCs induced the development of potent donor-specific CD4+ T-cell responses strongly polarized toward the Th2 phenotype.22 This led us to conclude that, in the absence of CD8+ T-cell priming, the default response of alloreactive CD4+ T cells is a sustained production of Th2-type cytokines.22 The aim of this study was to investigate the mechanisms involved in the CD8+ T-cell–mediated regulation of allospecific Th-cell development following skin graft transplantation or subcutaneous immunization with allogeneic DCs.
Materials and methods
Mice
BALB/c (H-2d) and C57BL/6 (B6) (H-2b) mice were purchased from Centre d'Elevage R. Janvier (Le Genest St Isle, France). CD8α–/–, perforin-deficient (Pfp–/–), or IFN-γ–/– B6 mice were obtained from The Jackson Laboratory (Bar Harbor, ME). C57BL/6 CD8α–/– (BALB/c × C57BL/6)F1 (CB6F1), B6 Pfp–/–, and IFN-γ–/– mice were bred and maintained in our specific pathogen-free animal facility.
In vivo anti-CD8 mAb treatment
For in vivo CD8+-cell depletion, the anti-CD8α 53-6.72 rat IgG2a (TIB-105; American Type Culture Collection [ATCC], Manassas, VA) was purified from ascites fluid by caprylic acid precipitation. Mice were injected intraperitoneally with 100 μg 53-6.72 for 3 consecutive days (at days –6, –5, and –4) and with 500 μg the day of immunization (day 0). CD8+ T-cell depletion was almost complete as fewer than 1% of CD8β+ T cells still persisted in the draining lymph nodes (data not shown).
Skin grafting and histologic studies
Skin grafts were performed as previously described.14 For histologic analysis, skin grafts were fixed with formol for 24 hours at 37°C and then tissue sections were stained with hematoxylin and eosin, after paraffin embedding. The percentage of eosinophils among infiltrating mononuclear cells was determined in at least 3 fields per graft.
Quantitative assessment of transcripts
Skin grafts or CD4+ T cells were homogenized in Trizol reagent (Invitrogen, San Diego, CA) and RNA extracted following the manufacturer's instruction. The RNA samples (1 μg) were treated with DNase I (Invitrogen) and transcribed into cDNA using random primers and M-MLV reverse transcriptase (Promega, Madison, WI). The amount of TCRβ, IL-4, IFN-γ, and HPRT mRNA were determined by quantitative PCR analysis. All reactions were performed using Taqman Universal PCR Master Mix or the SYBR Green master mix (Sigma-Aldrich) as previously described.26 Primers and probes specific for TCRβ, IL-4, IFN-γ, and HPRT have been previously described.26,27
CD8+ T-cell purification and adoptive transfer
Pooled lymph node and spleen cells were incubated with anti–MHC class II M5/114 (TIB 120; ATCC), anti-CD11b M1/70 (TIB 128; ATCC), anti–B220 RA3-3A1 (TIB 146; ATCC), and anti–CD4 GK1.5 (TIB 207; ATCC). Cells were then washed and incubated with sheep anti–rat IgG M-450 Dynabeads (Dynal Biotech, Oslo, Norway). Stained cells were then selectively depleted with a magnet (BioSource International, Camarillo, CA). The purity of CD8+ T cells was routinely above 80% as assessed by flow cytometry analysis. CD8+ T lymphocytes were subsequently injected intravenously into mice (3-5 × 106/mouse).
Bone marrow–derived dendritic cells
Mouse bone marrow cells were cultured in complete medium at 2 × 106 in a bacteriologic petri dish (Greiner Bio-one, Frickenhausen, Germany) supplemented with supernantant of J558L cells transfected with a plasmid expressing the murine granulocyte macrophage–colony-stimulating factor (GM-CSF; a kind gift from Dr P. Pierre, Centre d'Immunologie de Marseille Luminy, Marseille, France) as previously described.22,28 The DC preparations, containing more than 80% of CD11cpos MHCIIpos cells, were used at day 8-9 of culture to immunize mice subcutaneously into the hind footpads.
T-cell assays
CD4+ T cells from popliteal and inguinal lymph nodes of DC-immunized mice or inguinal and axillary lymph nodes draining the skin graft were purified by negative selection as described.26 CD4+ T-cell purity was routinely greater than 90%. CD4+ T cells were seeded in culture with irradiated allogeneic splenocytes in HL-1 synthetic medium (Cambrex, Walkersville, MD) supplemented with 2 mM l-glutamine, penicillin (100 UI/mL), and streptomycin (100 μg/mL) (Eurobio, Courtaboeuf, France). Cultures were incubated for 3 days in a humidified atmosphere of 5% CO2 in air. Supernatants were collected after 72 hours for cytokine analysis. IFN-γ and IL-4 were quantified by a 2-site sandwich enzyme-linked immunosorbent assay (ELISA), as previously described.22 For T-cell proliferation assays, cell cultures were pulsed during the last 8 hours of a 72-hour culture with 1 μCi (37 kBq) [3H]TdR (40 Ci/nM [1480 GBq/nM]; Radiochemical Centre, Amersham, United Kingdom). Incorporation of [3H]TdR was measured by direct counting using an automated β-plate counter (Matrix 9600; Packard, Meriden, CT).
DC migration assay
Mice were injected in the hind footpads with 2 × 106 to 3 × 106 BM DCs from either B6 or CB6F1 mice. In some experiments, DCs (3 × 107/mL) were incubated with 100 ng/mL pertussis toxin (Calbiochem, Darmstadt, Germany) in complete medium for 2 hours at 37°C, and washed twice with phosphate-buffered saline (PBS; Eurobio) before injection. For in vivo cytotoxic assay, B6 and CB6F1 DCs were labeled with 1 μM and 10 μM, respectively, of carboxyfluorescein diacetate succinimidyl ester (CFSE; Molecular Probes, Leiden, The Netherlands) in PBS for 10 minutes at room temperature and washed several times in PBS before injection. At the indicated times, draining popliteal lymph nodes were removed and digested with collagenase IV (Sigma-Aldrich) at 400 U/mL for 30 minutes at 37°C. Before staining, cells were incubated for 30 minutes at room temperature in blocking buffer containing 1% NaN3, 5 mM EDTA, FCS 1%, normal mouse serum 3%, normal rat serum 3%, 5 μg/mL 2.4G2 (anti-CD16, HB-197; ATCC) in PBS. The following fluorochrome-conjugated monoclonal antibodies (mAbs) were used: anti–I-E, anti-CD11c (BD PharMingen, San Diego, CA), and anti–MHC class II (e-Bioscience, San Diego, CA).
Flow cytometry analysis of intracellular cytokine synthesis
CD4+ T cells were cultured in complete medium with T-cell–depleted allogeneic BALB/c splenocytes for 8 hours in the presence of 3 μg/mL anti-CD28 mAb (e-Bioscience). During the last 4 hours of culture, brefeldin A (Sigma-Aldrich) was added at 10 μg/mL. For T-cell depletion, splenocytes were incubated with 30-H12 anti-Thy1.2 mAb (TIB107; ATCC) and incubated in Low-Tox-M rabbit complement (Cederlane, Hornby, ON, Canada) according to the manufacturer's procedure. Intracellular staining was then performed as previously described.22 The following fluorochrome-conjugated mAbs purchased from PharMingen were used: anti-CD4, anti-CD69, anti–IL-4, and anti–IFN-γ. All cytometry data were collected on a FACSCalibur and analyzed using Cellquest software (Becton Dickinson, Mountain View, CA).
Confocal microscopy
CB6F1 semi-allogeneic and B6 syngeneic DCs were labeled with 10 μM CFSE and 10 μM orange fluorescent tetramethylrhodamine (CMTMR; Molecular Probes), respectively, for 15 minutes at 37°C. A mixture of 3 × 106 CFSE-labeled DCs and 3 × 106 CMTMR-labeled DCs was injected into the hind footpads of indicated recipient mice. At 72 hours after injection, popliteal draining lymph nodes were harvested and prepared as described elsewhere.29 Frozen sections (10 μm) were stained with unconjugated anti–peripheral lymph node addressin (PNAd) Ab (Meca-79; PharM-ingen) and anti-B220 mAb (RA3-3AI). PNAd and B220 staining were revealed with AlexaFluor647 goat anti–rat IgG antibodies (Molecular Probes). The samples were then mounted in 90% glycerol-PBS containing 2.5% 1-4-diazabicyclo (2.2.2) octane (DABCO; Sigma). Sections were analyzed by using an LSM 510 confocal microscope and acquisition system (Carl Zeiss, Jena, Germany) equipped with a 63×/1.4 oil-immersion plan-apochromat objective lens. Images were then processed with Adobe Photoshop CS2 software version 8.0.1 (Adobe Systems, San Jose, CA).
Statistical analysis
Results are expressed as the mean plus or minus the standard error of the mean (SEM), and overall differences between variables were evaluated by the Mann-Whitney nonparametric U test using Prism GraphPad software (San Diego, CA).
Results
In the absence of CD8+ T cells, semi-allogeneic skin grafting induces strong alloreactive CD4 T-cell responses with sustained production of type-2 cytokines and massive eosinophil recruitment
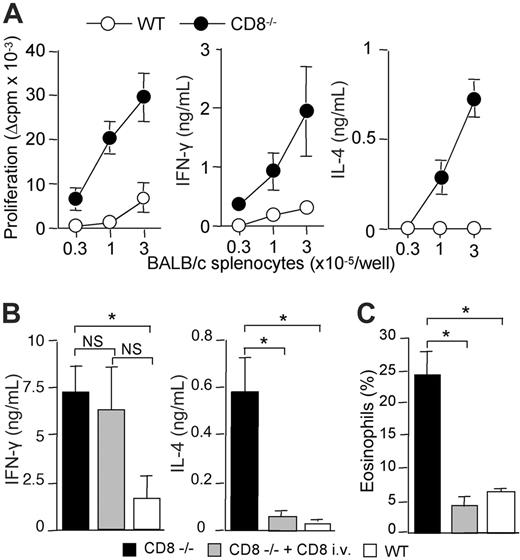
We and others have previously shown that strong alloreactive Th2 response develops in the absence of CD8+ T cells, following subcutaneous immunization with semi-allogeneic DCs.17,22 We therefore determined whether this was also true for skin graft rejection. For this purpose, CD8α-deficient B6 mice received transplants of semi-allogeneic (BALB/c × C57BL/6) F1 (CB6F1) skin. Semi-allogeneic donors were used instead of fully allogeneic donors to prevent host natural killer (NK)–cell activation.14 CD4+ T lymphocytes were purified from lymph nodes draining the graft and were stimulated in vitro with irradiated allogeneic BALB/c- or syngeneic B6-splenocytes, and assessed for proliferation and cytokine production. Figure 1 shows that CD4+ T cells from CD8-deficient B6 mice proliferated strongly in response to allogeneic BALB/c antigen-presenting cells (APCs) and produced IFN-γ and IL-4. By contrast, CD4+ T cells from wild-type (WT) B6 mice exhibited a lower proliferative response and produced IFN-γ but no IL-4. The T-cell responses analyzed were due to the in vivo priming of allospecific CD4+ T cells since lymph node CD4+ T cells from unmanipulated control B6 mice, or mice that had received a transplant of syngeneic skin, failed to proliferate or to produce cytokines in response to allogeneic BALB/c APCs (data not shown).
These data suggested that CD8+ T lymphocytes were required for down-regulating alloreactive Th2-cell development. To directly address this point, we adoptively transferred CD8+ T cells from WT mice into CD8α-deficient mice before transplantation of semi-allogeneic CB6F1 skins. As shown in Figure 1B, the development of IL-4–producing CD4+ T cells was strongly inhibited in CD8–/– mice reconstituted with CD8+ T lymphocytes, whereas IFN-γ production was not affected. Histologic analysis of rejected skin grafts showed dense inflammatory infiltrates in all combinations (not shown). Interestingly, alloreactive Th2-cell priming in CD8-deficient mice was associated with massive eosinophil infiltrates (Figure 1C). Such heavy eosinophil infiltrates were not found in CD8–/– mice reconstituted with normal CD8+ T cells or in WT B6 recipients (Figure 1C). Only rare eosinophils were found within syngeneic skin grafts (not shown). Taken together, our data showed that enhanced expansion of alloreactive CD4+ T cells and Th2 development occurred in CD8-deficient mice following semi-allogeneic skin transplantation, resulting in allograft rejection characterized by massive eosinophilic infiltrates. Using adoptive transfer experiments, we provided the direct demonstration that CD8+ T cells can inhibit Th2-priming and the recruitment of eosinophils at the site of rejection.
CB6F1 skin graft rejection is associated with Th2-cell priming and strong eosinophil infiltrates in the absence of CD8 T cells. WT or CD8-deficient B6 mice received a skin graft from semi-allogeneic CB6F1 mice. (A) At the time of graft rejection (day 10-11), draining lymph nodes were harvested. Purified CD4 T cells were cultured (3 × 105 cells/well) in the presence of allogeneic (BALB/c) irradiated splenocytes for 72 hours. CD4 T-cell proliferation was evaluated by 3H-TdR incorporation. IFN-γ and IL-4 production were measured by ELISA in 72-hour culture supernatants. Results are expressed as mean plus or minus SEM of 6 mice per group and are from 1 representative experiment of 3 performed. (B,C) WT or CD8-deficient B6 mice received a skin graft from semi-allogeneic CB6F1 mice and one group of CD8–/– B6 mice was intravenously injected with WT CD8+ T cells 1 day prior to skin transplantation. Skin grafts and graft-draining lymph nodes were harvested the day the grafts were fully rejected. (B) Purified CD4+ T cells were cultured (2 × 105 cells/well) in the presence of irradiated BALB/c splenocytes (3 × 105 cells/well). IFN-γ and IL-4 production in 72-hour culture supernatants were measured by ELISA. Results are expressed as mean plus or minus SEM of 3 to 4 mice per group and are from 1 representative experiment of 3 performed. (C) Skin graft histology was performed and the percentage of eosinophils among total cell infiltrates was evaluated as described in “Materials and methods” (3-4 mice per group). Statistical significance of difference between groups was evaluated by the Mann-Whitney U test (*P < .05; NS, not significant).
CB6F1 skin graft rejection is associated with Th2-cell priming and strong eosinophil infiltrates in the absence of CD8 T cells. WT or CD8-deficient B6 mice received a skin graft from semi-allogeneic CB6F1 mice. (A) At the time of graft rejection (day 10-11), draining lymph nodes were harvested. Purified CD4 T cells were cultured (3 × 105 cells/well) in the presence of allogeneic (BALB/c) irradiated splenocytes for 72 hours. CD4 T-cell proliferation was evaluated by 3H-TdR incorporation. IFN-γ and IL-4 production were measured by ELISA in 72-hour culture supernatants. Results are expressed as mean plus or minus SEM of 6 mice per group and are from 1 representative experiment of 3 performed. (B,C) WT or CD8-deficient B6 mice received a skin graft from semi-allogeneic CB6F1 mice and one group of CD8–/– B6 mice was intravenously injected with WT CD8+ T cells 1 day prior to skin transplantation. Skin grafts and graft-draining lymph nodes were harvested the day the grafts were fully rejected. (B) Purified CD4+ T cells were cultured (2 × 105 cells/well) in the presence of irradiated BALB/c splenocytes (3 × 105 cells/well). IFN-γ and IL-4 production in 72-hour culture supernatants were measured by ELISA. Results are expressed as mean plus or minus SEM of 3 to 4 mice per group and are from 1 representative experiment of 3 performed. (C) Skin graft histology was performed and the percentage of eosinophils among total cell infiltrates was evaluated as described in “Materials and methods” (3-4 mice per group). Statistical significance of difference between groups was evaluated by the Mann-Whitney U test (*P < .05; NS, not significant).
IFN-γ secretion by CD8+ T cells is dispensable for the prevention of alloreactive Th2-cell development and tissue eosinophilia
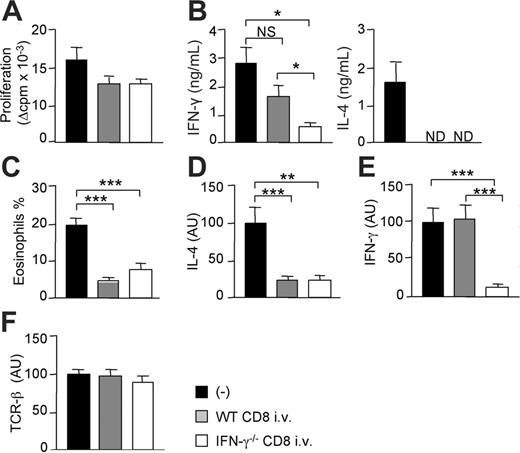
To determine whether the production of IFN-γ by CD8+ T cells was involved in the inhibition of donor-specific Th2-cell development and tissue eosinophilia, we injected CD8-deficient B6 recipients with syngeneic CD8+ T lymphocytes from either WT or IFN-γ–deficient mice prior to semi-allogeneic skin transplantation. Equivalent numbers of CD8+ T cells were found in the graft-draining lymph nodes of mice adoptively transferred with either WT or IFN-γ–/– CD8+ T lymphocytes (not shown). Data in Figure 2A show that allospecific CD4 T cells from mice reconstituted with CD8+ T cells proliferated slightly less than unreconstituted CD8–/– mice. Proliferative responses were equivalent in mice injected with either normal or IFN-γ–deficient CD8+ T lymphocytes. As in Figure 1, in mice lacking CD8+ T cells, CD4 T lymphocytes stimulated in vitro with irradiated BALB/c splenocytes produced IL-4 and IFN-γ (Figure 2B). IL-4 production by alloreactive CD4+ T cells was abolished by adoptive transfer of CD8+ T cells from both WT or IFN-γ–/– mice (Figure 2B). Thus, IFN-γ production by CD8+ T cells was not required to abrogate the development of allospecific IL-4–producing Th2 cells. In contrast, the capacity of alloreactive CD4 T cells to produce IFN-γ was significantly impaired in mice that received IFN-γ–deficient CD8 T cells, as compared with mice reconstituted with WT CD8 T lymphocytes. Altogether, these data indicate that IFN-γ production by CD8 T cells plays a role in the induction of IFN-γ–producing alloreactive Th1 cells, but is dispensable for the inhibition of Th2 priming.
We next quantified eosinophil infiltrates and the expression of cytokine transcripts in the CB6F1 skin grafts during the course of rejection. Data in Figure 2 show that alloreactive Th2-cell development in the CD8-deficient mice was associated with strong eosinophil infiltrations (Figure 2C) together with IL-4 mRNA expression in the semi-allogeneic grafts (Figure 2D). Transcripts coding for IL-4, as well as tissue eosinophilia, were similarly down-regulated in CD8-deficient mice injected with CD8 T cells from either WT or IFN-γ–/– mice. IFN-γ mRNA transcripts were expressed at similar levels in CD8-deficient mice reconstituted or not with normal CD8 T cells (Figure 2E). In contrast, IFN-γ mRNA expression was significantly decreased in mice injected with IFN-γ–deficient CD8+ T cells, in agreement with data in Figure 2B. TCRβ transcript levels were similar in all combinations tested, indicating that rejected skins were infiltrated with equivalent numbers of T lymphocytes (Figure 2F). Altogether, these results demonstrated that IFN-γ production by alloantigen-specific CD8+ T cells was dispensable for the inhibition of alloreactive Th2-cell development, intragraft IL-4 production, and eosinophil infiltration.
CD8 T cells from WT or IFN-γ–deficient mice are equally efficient in suppressing Th2 response and tissue eosinophilia. One day before grafting, CD8-deficient B6 mice were intravenously injected with CD8 T cells from either WT or IFN-γ–deficient B6 mice. Mice then received a skin graft from semi-allogeneic (CB6F1) mice. At day 10, grafts and draining lymph nodes were harvested. (A) Purified CD4+ T cells were cultured (2 × 105 cells/well) in the presence of irradiated allogeneic BALB/c splenocytes (3 × 105 cells/well). Proliferation (A) and cytokine production (B) were measured at 72 hours of culture. Results are expressed as mean plus or minus SEM of 5 to 7 mice per group. (C) The percentage of eosinophils among total cell infiltrates was evaluated as described in “Materials and methods” (mean ± SEM of 3-4 mice per group). Relative levels of IL-4 (D), IFN-γ (E), and TCRβ (F) mRNA were analyzed by real-time quantitative reverse transcriptase–polymerase chain reaction (RT-PCR) from skin graft cDNA and normalized to the HPRT mRNA. Data are pooled from 2 independent experiments with 9 to 12 mice per group. Statistical significance of difference between groups was evaluated by the Mann-Whitney U test (*P < .05; **P < .01; ***P < .005; NS, not significant; ND, not detectable).
CD8 T cells from WT or IFN-γ–deficient mice are equally efficient in suppressing Th2 response and tissue eosinophilia. One day before grafting, CD8-deficient B6 mice were intravenously injected with CD8 T cells from either WT or IFN-γ–deficient B6 mice. Mice then received a skin graft from semi-allogeneic (CB6F1) mice. At day 10, grafts and draining lymph nodes were harvested. (A) Purified CD4+ T cells were cultured (2 × 105 cells/well) in the presence of irradiated allogeneic BALB/c splenocytes (3 × 105 cells/well). Proliferation (A) and cytokine production (B) were measured at 72 hours of culture. Results are expressed as mean plus or minus SEM of 5 to 7 mice per group. (C) The percentage of eosinophils among total cell infiltrates was evaluated as described in “Materials and methods” (mean ± SEM of 3-4 mice per group). Relative levels of IL-4 (D), IFN-γ (E), and TCRβ (F) mRNA were analyzed by real-time quantitative reverse transcriptase–polymerase chain reaction (RT-PCR) from skin graft cDNA and normalized to the HPRT mRNA. Data are pooled from 2 independent experiments with 9 to 12 mice per group. Statistical significance of difference between groups was evaluated by the Mann-Whitney U test (*P < .05; **P < .01; ***P < .005; NS, not significant; ND, not detectable).
CD8+ T-cell–mediated inhibition of alloreactive CD4+ T-cell response and Th2 differentiation is due to DC clearance in draining lymph nodes
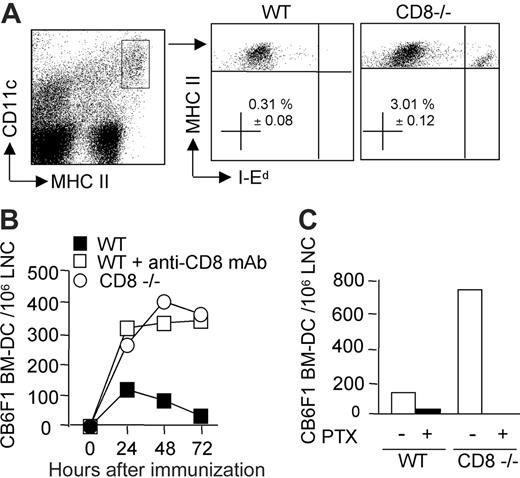
Acute rejection is mainly due to alloreactive T-cell responses primed through the direct pathway of alloantigen presentation following migration of donor-derived DCs to the lymph nodes draining the graft.1 We therefore investigated whether CD8+ T cells could regulate the kinetics of donor DCs' persistence in the draining lymph nodes of mice injected subcutaneously with bone marrow–derived semi-allogeneic DCs. CB6F1 DCs could be distinguished from host CD11c+ MHC class IIhigh DCs through the expression of the allogeneic IEd-MHC class II molecules (Figure 3A). They were readily detectable in CD8-deficient B6 mice, as well as in CD8-depleted B6 mice at 24 hours after injection, and persisted to similar numbers up to 72 hours after injection (Figure 3B). By contrast, in the presence of CD8+ T cells, semi-allogeneic DC numbers started to decrease between 24 to 48 hours after injection, and were almost undetectable by 72 hours (Figure 3B). The reduction in CB6F1 DC numbers observed at 24 hours in the experiment shown was not always observed in repeated experiments, such as that shown in Figure 6. Pretreatment of DCs with pertussis toxin, a Gi protein inhibitor, blocks chemokine receptor signaling and prevents DC migration from skin to lymph nodes.30 Indeed, when WT or CD8-deficient mice were immunized with pertussis toxin–treated CB6F1 DCs, CD11c+ MHC class IIhigh DCs displaying allogeneic IEd-MHC class II molecules were no longer detectable in the lymph nodes (Figure 3C). These data demonstrated that DCs migrated from the injection site to the draining lymph nodes via a Gi protein–dependent mechanism, and that CD8 T lymphocytes controlled the accumulation and persistence of migrant DCs.
The persistence of donor-derived DCs in draining lymph nodes is impaired in the presence of CD8 T cells. Mice were injected in the hind footpads with semi-allogeneic CB6F1 DCs. (A) The presence of injected DCs was monitored by flow cytometry analysis of the draining lymph node cells (pooled from 3 mice per group) after 72 hours. The percentage of I-Ed–positive DCs, gated on CD11c+, MHC IIhigh after propidium-iodide–positive cell exclusion, is shown (mean ± SEM of 4 different experiments). (B) Kinetics of DC recruitment into the draining lymph nodes of WT, CD8–/–, or CD8-depleted mice at 24, 48, and 72 hours after injection. (C) DCs were pretreated or not with pertussis toxin and injected into WT or CD8–/– mice. At 48 hours after injection, draining lymph nodes were analyzed for the presence of injected I-Ed–positive CD11c+, MHCHigh DCs. Data are from 1 representative experiment of 3 performed.
The persistence of donor-derived DCs in draining lymph nodes is impaired in the presence of CD8 T cells. Mice were injected in the hind footpads with semi-allogeneic CB6F1 DCs. (A) The presence of injected DCs was monitored by flow cytometry analysis of the draining lymph node cells (pooled from 3 mice per group) after 72 hours. The percentage of I-Ed–positive DCs, gated on CD11c+, MHC IIhigh after propidium-iodide–positive cell exclusion, is shown (mean ± SEM of 4 different experiments). (B) Kinetics of DC recruitment into the draining lymph nodes of WT, CD8–/–, or CD8-depleted mice at 24, 48, and 72 hours after injection. (C) DCs were pretreated or not with pertussis toxin and injected into WT or CD8–/– mice. At 48 hours after injection, draining lymph nodes were analyzed for the presence of injected I-Ed–positive CD11c+, MHCHigh DCs. Data are from 1 representative experiment of 3 performed.
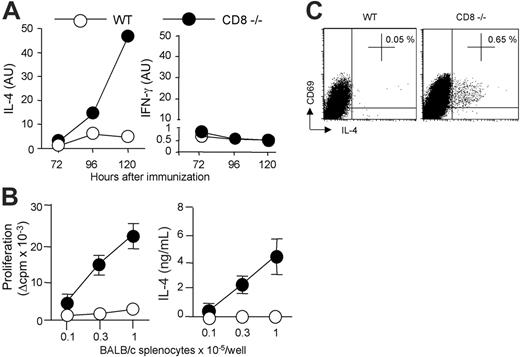
To determine whether allogeneic DC persistence correlated with an enhanced Th2 priming, we assessed the kinetics of cytokine mRNA expression in CD4 T cells draining the immunization site. As shown in Figure 4A, IL-4 transcripts were readily detectable from days 3 to 5 in CD8-deficient mice and their expression was markedly increased as compared with control B6 mice. By contrast, kinetics of IFN-γ mRNA expression were similar in CD4 T cells from either WT or CD8-deficient mice. The differential regulation of IL-4 and IFN-γ was further confirmed by analyzing donor-specific cytokine production by CD4 T cells at day 6 after immunization. As expected, CD4 T cells from CD8-deficient mice immunized with CB6F1 DCs proliferated strongly in response to allogeneic APCs and produced large amounts of IL-4 (Figure 4B), in agreement with our previous works.14,22 In contrast, CD4+ T cells from WT B6 mice immunized under the same conditions exhibited an impaired proliferative response, and produced IFN-γ (not shown) but no IL-4 (Figure 4B). DC migration to lymph nodes was required for T-cell priming since immunization with pertussis toxin–treated DCs abolished alloreactive CD4+ T-cell proliferation and cytokine production in both WT and CD8-deficient mice (not shown). The frequency of IL-4–producing allospecific Th cells was further analyzed by intracellular cytokine staining of lymph node CD4+ T cells stimulated with allogeneic APCs. Data in Figure 4C show that H-2d–specific IL-4–producing CD4+ T cells were present in CD8-deficient mice, but not in WT B6 mice primed with CB6F1 DCs. The frequency of cytokine-producing cells was below 0.05% when CD4 T cells were cultured with syngeneic APCs, or when CD4 T cells from unprimed mice were used as responders (not shown).
Allogeneic DC persistence in situ correlates with an enhanced Th2 priming. (A) WT or CD8–/– B6 mice were injected subcutaneously with 2 × 106 CB6F1 DCs. The kinetics of IL-4 and IFN-γ mRNA expression were performed on CD4+ T cells purified from draining lymph nodes at the indicated times. (B) Six days after immunization, purified CD4+ T cells (2 × 105 cells/well) from pooled lymph node cells (LNCs) (3 mice per group) were stimulated with irradiated BALB/c splenocytes for 72 hours to measure proliferation and cytokine production. Results are expressed as mean plus or minus SEM of triplicate cultures. (C) To evaluate the frequency of ex vivo IL-4–producing cells, purified CD4 T cells were cultured for 8 hours with T-cell–depleted BALB/c splenocytes in the presence of anti-CD28 mAb. Intracytoplasmic staining for cytokines was then performed as indicated in “Materials and methods.” Percentages of CD69pos CD4 T cells producing IL-4 are mentioned. Data are from 1 representative experiment of 3 performed.
Allogeneic DC persistence in situ correlates with an enhanced Th2 priming. (A) WT or CD8–/– B6 mice were injected subcutaneously with 2 × 106 CB6F1 DCs. The kinetics of IL-4 and IFN-γ mRNA expression were performed on CD4+ T cells purified from draining lymph nodes at the indicated times. (B) Six days after immunization, purified CD4+ T cells (2 × 105 cells/well) from pooled lymph node cells (LNCs) (3 mice per group) were stimulated with irradiated BALB/c splenocytes for 72 hours to measure proliferation and cytokine production. Results are expressed as mean plus or minus SEM of triplicate cultures. (C) To evaluate the frequency of ex vivo IL-4–producing cells, purified CD4 T cells were cultured for 8 hours with T-cell–depleted BALB/c splenocytes in the presence of anti-CD28 mAb. Intracytoplasmic staining for cytokines was then performed as indicated in “Materials and methods.” Percentages of CD69pos CD4 T cells producing IL-4 are mentioned. Data are from 1 representative experiment of 3 performed.
CD8 T cells inhibit the expansion of alloreactive Th2 cells through their capacity to rapidly eliminate allogeneic DCs. WT, CD8–/–, or CD8–/– transferred with WT CD8 T cells were injected with CFSElow-labeled syngeneic B6 DCs and CFSEhigh-labeled semi-allogeneic CB6F1 DCs. (A) Draining lymph nodes were harvested 72 hours after injection and analyzed for the presence of CFSE-labeled cells. Cells were gated on CD11cpos, MHC-IIhigh, and CFSEpos cells. Percentages of CFSEhigh CB6F1 cells among total CFSEpos cells are indicated. Results are expressed as mean plus or minus SEM of 4 mice per group. (B) The kinetics of IL-4 mRNA expression were analyzed in purified CD4+ T cells. (C) CD4 T cells (2 × 105/well) were purified from the draining lymph nodes harvested 6 days after immunization, and cultured in the presence of BALB/c APCs. IFN-γ and IL-4 production were measured by ELISA in 72-hour culture supernatants. Results are expressed as mean plus or minus SEM of 5 mice per group. (D) Pooled CD4+ T cells from immunized mice were stimulated with T-cell–depleted BALB/c splenocytes in the presence of anti-CD28 mAb for 8 hours. Intracytoplasmic staining for cytokines was then performed as indicated in “Materials and methods.” Percentages of CD69pos CD4+ T cells producing IFN-γ or IL-4 are mentioned. Data are from 1 representative experiment of 3 performed.
CD8 T cells inhibit the expansion of alloreactive Th2 cells through their capacity to rapidly eliminate allogeneic DCs. WT, CD8–/–, or CD8–/– transferred with WT CD8 T cells were injected with CFSElow-labeled syngeneic B6 DCs and CFSEhigh-labeled semi-allogeneic CB6F1 DCs. (A) Draining lymph nodes were harvested 72 hours after injection and analyzed for the presence of CFSE-labeled cells. Cells were gated on CD11cpos, MHC-IIhigh, and CFSEpos cells. Percentages of CFSEhigh CB6F1 cells among total CFSEpos cells are indicated. Results are expressed as mean plus or minus SEM of 4 mice per group. (B) The kinetics of IL-4 mRNA expression were analyzed in purified CD4+ T cells. (C) CD4 T cells (2 × 105/well) were purified from the draining lymph nodes harvested 6 days after immunization, and cultured in the presence of BALB/c APCs. IFN-γ and IL-4 production were measured by ELISA in 72-hour culture supernatants. Results are expressed as mean plus or minus SEM of 5 mice per group. (D) Pooled CD4+ T cells from immunized mice were stimulated with T-cell–depleted BALB/c splenocytes in the presence of anti-CD28 mAb for 8 hours. Intracytoplasmic staining for cytokines was then performed as indicated in “Materials and methods.” Percentages of CD69pos CD4+ T cells producing IFN-γ or IL-4 are mentioned. Data are from 1 representative experiment of 3 performed.
To ensure that CD8+ T lymphocytes were directly eliminating allogeneic DCs and were inhibiting Th2 priming in vivo in the previous experimental protocol, CD8-deficient mice were reconstituted or not with CD8 T cells. Mice were then immunized with equal numbers of syngeneic B6 and semi-allogenic CB6F1 DCs labeled with different concentrations of CFSE. As shown in Figure 5A, whereas CFSElow syngeneic B6 DCs were readily detectable in draining lymph nodes of both WT or CD8–/– mice, CFSEhigh CB6F1 DCs were found only in mice lacking CD8+ T cells at 72 hours after injection. As in WT mice, CB6F1 DCs were no longer detected in CD8-deficient mice reconstituted with CD8+ T lymphocytes (Figure 5A). In agreement with data in Figure 4A, the persistence of semi-allogeneic DCs, by 72 to 120 hours in CD8-deficient mice, was associated with a strong up-regulation of IL-4-transcripts in ex vivo–isolated lymph node CD4+ T cells (Figure 5B). Adoptive transfer of CD8+ T cells into B6 CD8–/– mice inhibited IL-4 transcripts expression (Figure 5B) and promoted the selective development of alloreactive IFN-γ–producing Th1 cells (Figure 5C). This was confirmed by the ex vivo analysis of cytokine production in alloreactive CD4+ T cells by intracellular staining. The frequency of IFN-γ+ cells was 3-fold higher in mice reconstituted with CD8+ T lymphocytes, whereas IL-4+ cells were undetectable (Figure 5D).
Host CD8 T cells eliminate donor-derived DCs through a perforin-dependent mechanism. WT, IFN-γ–/–, or Pfp–/– B6 mice, treated or not with anti-CD8 mAb, were injected with equivalent numbers of CFSElow-labeled syngeneic B6 DCs and CFSEhigh-labeled semi-allogeneic CB6F1 DCs. Draining lymph nodes were harvested at 24 or 72 hours after injection and analyzed for the presence of CFSE-labeled cells. (A) Cells were analyzed at 72 hours and gated on CD11cpos, MHC-IIhigh, and CFSEpos cells. Numbers indicate the percentage of CFSEhigh CB6F1 cells among total CFSEpos cells for an individual mouse. (B) Results are expressed as mean plus or minus SEM of 3 mice per group and are from 1 representative experiment of 2 performed.
Host CD8 T cells eliminate donor-derived DCs through a perforin-dependent mechanism. WT, IFN-γ–/–, or Pfp–/– B6 mice, treated or not with anti-CD8 mAb, were injected with equivalent numbers of CFSElow-labeled syngeneic B6 DCs and CFSEhigh-labeled semi-allogeneic CB6F1 DCs. Draining lymph nodes were harvested at 24 or 72 hours after injection and analyzed for the presence of CFSE-labeled cells. (A) Cells were analyzed at 72 hours and gated on CD11cpos, MHC-IIhigh, and CFSEpos cells. Numbers indicate the percentage of CFSEhigh CB6F1 cells among total CFSEpos cells for an individual mouse. (B) Results are expressed as mean plus or minus SEM of 3 mice per group and are from 1 representative experiment of 2 performed.
Taken together, these results demonstrated that CD8+ T lymphocytes negatively regulate the expansion of alloreactive IL-4–producing CD4+ T cells in vivo, probably through their capacity to rapidly eliminate allogeneic DCs.
Recipient CD8 T cells eliminate allogeneic DCs from draining lymph nodes through a perforin-dependent mechanism
To determine the effector mechanism implicated in the CTL-mediated killing of allogeneic DCs, normal, IFN-γ–deficient, or perforin-deficient mice were immunized as in Figure 5 with a mix of fluorescent syngeneic (B6) and semi-allogenic (CB6F1) DCs. As shown in Figure 6, by 72 hours after immunization more than 90% of the CB6 F1 CFSEhigh DCs had disappeared from the draining lymph nodes of both WT and IFN-γ–deficient mice. CD8+ T-cell depletion restored CB6F1 DC numbers to similar levels as those recovered in CD8-deficient mice (Figure 6A-B, and data not shown). Interestingly, when we analyzed the kinetics of DC accumulation in draining lymph nodes of perforin-deficient mice, we found that allogeneic DCs still persisted at high numbers even in the presence of CD8+ T cells. In vivo depletion of CD8+ T cells in Pfp–/– mice did not result in any significant increase in the frequency of target DCs, suggesting that perforin-dependent killing was the main pathway accounting for CD8+ T-cell–mediated DC elimination (Figure 6). In vivo cytotoxic elimination of semi-allogeneic DCs was not observed at 24 hours in all the combinations tested (Figure 6B). In agreement with previous works,31,32 these data, together with those in Figure 3B, indicate that the maturation of allospecific CD8+ T cells into CTL effectors able to kill allogeneic DCs through the Pfp-dependent pathway occurs between 24 and 48 hours.
In situ visualization of allogeneic DC persistence in CD8- and perforin-deficient mice. WT, CD8–/–, and perforin–/– B6 mice were injected with a mixture of CFSE-labeled B6 (green) and CMTMR-labeled CB6F1 (red) DCs. At 72 hours after injection, draining lymph nodes were harvested, prepared as described in “Materials and methods,” and stained with anti-B220 and anti-PNAd mAbs (blue). Lymph node stained sections were then analyzed by confocal microscopy.
In situ visualization of allogeneic DC persistence in CD8- and perforin-deficient mice. WT, CD8–/–, and perforin–/– B6 mice were injected with a mixture of CFSE-labeled B6 (green) and CMTMR-labeled CB6F1 (red) DCs. At 72 hours after injection, draining lymph nodes were harvested, prepared as described in “Materials and methods,” and stained with anti-B220 and anti-PNAd mAbs (blue). Lymph node stained sections were then analyzed by confocal microscopy.
In order to directly visualize the persistence of CB6F1 DCs in draining lymph nodes in the absence of host CD8+ T cells or when the host perforin-granzymes pathway was not functional, we performed confocal microscopy analysis. We co-injected WT, CD8–/–, or Pfp–/– B6 mice with CFSE-labeled syngeneic B6 DCs (green) and CMTMR-labeled semi-allogeneic DCs (red). Whereas red CB6F1 DCs were readily detectable 72 hours after injection in the draining lymph nodes of CD8–/– and Pfp–/– mice, they were almost completely absent in WT mice. As expected, the presence of green B6 DCs was not altered in WT mice (Figure 7). These data provide direct evidence of the major role played by the perforin-granzymes pathway in the elimination of allogeneic DCs by host CTLs. We also noticed that injected DCs, after homing to the draining lymph nodes, localized preferentially in the outer paracortex, just beneath the B-cell follicles, where most high endothelial veinules are found, a strategic position to encounter newly homed T cells.29
Discussion
The data presented in this paper indicate that CD8+ T lymphocytes greatly influence not only the magnitude but also the polarization of alloreactive CD4+ T-cell responses that occur following semi-allogeneic skin graft transplantation or DC immunization. Indeed, we show that the absence of CD8+ T lymphocyte activation promotes stronger expansion of alloreactive CD4+ T cells, type-2 cytokine production, and intense eosinophil infiltrates in the transplanted semi-allogeneic skin. Furthermore, we directly demonstrated that IFN-γ secreted by activated CD8+ T cells was dispensable in this regulatory process, suggesting that other mechanisms were involved. Indeed, our results support the conclusion that CD8+ T lymphocytes inhibit an alloreactive CD4+ T-cell response and type-2 development by limiting the life span of donor-derived DCs in the draining lymph nodes. Furthermore, we show that elimination of donor DCs by host CTLs during the course of a primary alloreactive T-cell response in vivo mainly involves the perforin-dependent pathway. We propose that CD8+ T cells that directly recognize allogeneic MHC class I limit CD4+ T-cell expansion and Th2 differentiation through the prevention of donor DC persistence in the T-cell zone of draining lymph nodes. These data demonstrate that elimination of allogeneic DCs by host CTLs is an important mechanism that influences the alloreactive response fate in vivo.
While disappearance of APCs following encounter with antigen-specific T cells has been previously shown in studies of T-cell–APC interaction in vivo,33-37 it was not clear whether CD8+ T-cell–mediated DC clearance could influence CD4+ T-cell priming, expansion, and differentiation during an ongoing primary immune response. In previous works, it was clearly shown that DC loaded with class I–restricted antigenic peptides were rapidly cleared from the draining lymph nodes during the course of secondary CD8+ T-cell responses,35,37 illustrating a “predator-prey” type of interaction between CTLs and DCs.38 However, a small or inconsistent elimination of antigen-loaded DCs was observed in normal mice during primary immunization.35,36 Thus, in contrast to alloreactivity, de novo development of a CTL response to conventional antigen appears to have a minor effect on DC homeostasis during the course of a primary response in normal mice. This could be explained by the fact that allogeneic MHC class I–specific CTL precursors are likely to be present at a higher frequency than conventional antigen-specific CD8+ T cells. Indeed, significant and reproducible elimination of peptide-loaded DCs during primary response were observed in TCR transgenic mice where high numbers of CTL precursors are present.35,36 Herein, we found that killing of allogeneic DCs can indeed occur during the course of a primary alloreactive response, probably because of the presence of high numbers of allospecific CTL precursors, thereby regulating CD4+ T-cell development by limiting the duration of alloantigen presentation in situ.
In agreement with previous studies,35 we found that DC clearance was not observed until 48 hours after immunization, the time required for T cells to acquire cytolytic activity.31,32 This observation suggested that alloreactive CTL-induced killing was the main cellular mechanism involved in allogeneic DC elimination in vivo. Effector CTLs are able to mediate their cytotoxic activity through 2 main pathways, either via the exocytosis of perforin/granzyme-containing granules or through intercellular interactions involving Fas-FasL molecules.39,40 Our data clearly show that the CTL-mediated clearance of allogeneic DCs in vivo mainly involves the perforin pathway since the persistence of allogeneic DCs was not affected in Pfp–/– mice. Indeed, it has been shown that CTL-mediated DC killing in vitro was strictly dependent on perforin rather than Fas.37 Thus, although other pathways, in addition to perforin and Fas,37 may contribute to the regulation of DC survival during the course of established CTL responses in vivo, our data demonstrate that perforin-mediated cytotoxicity plays a central role in the regulation of donor-derived DC homeostasis in draining lymph nodes during the course of a primary T-cell response to transplantation alloantigens.
IFN-γ produced by antigen-specific CD8+ T cells has been considered to be responsible for the control of Th2-cell development in some models of allograft rejection.8,23 However, direct evidence in vivo for a role of IFN-γ secretion by CD8+ T lymphocytes in the inhibition of alloreactive Th2-cell responses was still lacking. In the present study, we showed that IFN-γ production by CD8+ T cells was dispensable for allogeneic DC clearance and for the prevention of alloreactive Th2 responses. Although this observation strongly suggested that CD8+ T cells primarily inhibit Th2-cell development not because they secrete IFN-γ but because they differentiate into CTL effectors that eliminate DCs in the draining lymph nodes, we cannot exclude that once DCs have been eliminated, CD8+ T cells could still influence the differentiation of allospecific Th cells through other mechanisms. However, this hypothesis is unlikely since in vivo CD8-depletion experiments starting at 72 hours after immunization, once allogeneic DCs have been eliminated by allospecific CTLs, no longer affected the Th1 profile of alloreactive CD4+ T cells (data not shown).
On the other hand, the ability of CD8+ T lymphocytes to produce IFN-γ could contribute to the induction of alloreactive Th1-cell development in vivo as suggested by others.8,23 Indeed, we showed that alloantigen-specific Th1 priming was impaired in CD8-deficient mice reconstituted with IFN-γ–deficient CD8+ T lymphocytes. CD8+ T-cell–derived IFN-γ could influence Th1-cell development directly by increasing Stat-1 activation in CD4 T cells, leading to increase T-bet, IFN-γ, and IL-12 receptor expression.24 In addition, the presence of CD8+ T cells during the early phase of the alloreactive response could further support Th1-cell development through their capacity to promote DC activation and IL-12 production, as suggested by others.18 This hypothesis is also unlikely since we found that the CD8+ T-cell–dependent induction of alloreactive Th1 cells still occurred in IL-12Rβ2–/– mice and was not associated with any Th2-cell development (data not shown). Thus, as shown in other situations,24 IL-12 signaling in CD4+ T cells does not seem to be required for the induction of IFN-γ–producing Th1 cells in our model. Although the mechanism of alloreactive Th1-cell development in the presence of CD8 T cells still remains to be characterized, it is possible that, in addition to CD8+ T cells, alloreactive CD4+ T cells could also produce some IFN-γ that could drive their own Th1 polarization.24 Indeed, we found that some Th1 development can occur in the absence of CD8+ T lymphocytes, in agreement with our previous work.22 These IFN-γ–producing alloreactive CD4+ T cells could arise from a memory/effector repertoire already committed to the Th1 pathway.
Altogether, our results indicate that alloreactive Th2-cell development requires the persistence of allogeneic DCs in the draining lymph nodes. In support of this, it has been hypothesized that the kinetics of DC activation may represent a critical factor in the regulation of effector T-cell responses.41 It has been shown that fully mature human DCs become exhausted in their capacity to secrete the Th1-promoting cytokine IL-12 in response to CD40 ligation, whereas IL-6 production was preserved.41,42 It has been proposed that such DCs would preferentially induce Th2 and nonpolarized memory-type T cells.41 In the absence of CD8+ T-cell response, allogeneic mature DCs could accumulate in a so-called “exhausted state” in draining lymph nodes. The preserved capacity of mature DCs to secrete IL-6 could create favorable conditions for enhanced alloreactive CD4+ T-cell priming and Th2 development.41,42 Indeed, it has been shown that IL-6, produced by activated DCs, impaired the regulatory activity of CD4+CD25+ T reg cells43 and could promote Th2 responses.44 Given the importance of donor DC trafficking through the recipient draining lymph nodes in transplant rejection or tolerance,1,45 our results provide new insights into how CD8+ T lymphocytes impact allogeneic DC homeostasis in vivo, thereby regulating the immune responses to transplantation antigens.
Prepublished online as Blood First Edition Paper, January 31, 2006; DOI 10.1182/blood-2005-10-4059.
Supported by a grant from Etablissement Français des Greffes and institutional grants from INSERM, and by fellowships from the Ministère de l'Education Nationale de la Recherche et des Technologies (S.L., J.D.C., A.W.), la Fondation pour la Recherche Médicale (J.D.C.), Ligue Française contre la Sclérose en Plaques (LFSEP) and Association pour la Recherche sur la Sclérose en Plaques (ARSEP) (L.G.), and Association Française contre les Myopathies (AFM) (L.D.).
S.L. and J.D.C. designed, performed research, and analyzed data; L.G., L.D., and C.C. performed research; A.W. contributed analytic tools; C.D. analyzed data; and J.-C.G. designed research, analyzed data, and wrote the paper.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The skillful assistance of the staff of the IFR 30 animal facility is greatly acknowledged. We also thank our colleagues S. Guerder and D. Hudrisier for helpful advice, and S. Mueller and F. Capilla for their technical assistance.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal