Abstract
Thymosin α1 (Tα1), a naturally occurring thymic peptide, primes dendritic cells (DCs) for antifungal T-helper type 1 resistance through Toll-like receptor 9 (TLR9) signaling. As TLR9 signaling also activates the immuno-suppressive pathway of tryptophan catabolism via indoleamine 2,3-dioxygenase (IDO), we examined Tα1 for possible induction of DC-dependent regulatory effects. Tα1 affected T-helper cell priming and tolerance induction by human and murine DCs and induced IDO expression and function in the latter cells. IDO activation by Tα1 required TLR9 and type I interferon receptor signaling and resulted in interleukin-10 production and generation of regulatory T cells. In transfer experiments, functionally distinct subsets of differentiated DCs were required for priming and tolerance to a fungal pathogen or alloantigens. In contrast, Tα1-primed DCs fulfilled multiple requirements, including the induction of T-helper type 1 immunity within a regulatory environment. Thus, instructive immunotherapy with Tα1 targeting IDO-competent DCs could allow for a balanced control of inflammation and tolerance.
Introduction
The induction of tolerance is central to the maintenance of immune homeostasis. Not only are dendritic cells (DCs) key elements in the development of immunity, but they also participate in the generation and maintenance of immune tolerance. Many studies have demonstrated a pivotal role for the enzyme indoleamine 2,3-dioxygenase (IDO) in immune regulation during infection, pregnancy, autoimmunity, transplantation, and neoplasia.1,2 IDO is widely expressed in a variety of human tissues as well as in macrophages and DCs and is induced in inflammatory states via type I or type II IFN signaling. Through localized tryptophan deficiency combined with the release of proapoptotic kynurenines, DCs exert an IDO-dependent homeostatic control over the proliferation and survival of peripheral T cells and promote antigen-specific tolerance.3,4 Murine plasmacytoid DCs (pDCs), which produce and respond to type I IFNs, have been credited with a unique ability to express functional IDO, implying an important role for these cells in the maintenance of peripheral tolerance.5
DCs are now being exploited to improve vaccine efficacy.6 Pathogen-pulsed DCs act, indeed, as a potent fungal vaccine in experimental hematopoietic stem cell transplantation (HSCT).7 Protection is associated with myeloid and T-cell recovery, the activation of CD4+ T-helper type 1 (Th1) lymphocytes, and the concomitant production of IL-10. This cytokine is required for the induction of regulatory T (Treg) cells, which have important functions in immune homeostasis including the onset of transplantation tolerance, inhibition of inflammation, and prevention of graft-versus-host disease (GVHD) lethality and leukemia relapse.8-10 As tolerance is also one major requirement of a successful immune response to fungi,11-13 tolerogenic DCs, including pDCs, may be pivotal in the generation of some form of dominant regulation that ultimately controls inflammation, pathogen immunity, and tolerance in transplant recipients.14
Thymosin α1 (Tα1) is a naturally occurring thymic peptide15 that promotes activation and cytokine production in human and murine mature DCs by signaling through Toll-like receptors (TLRs), including TLR9.16 By influencing the balance of IL-12– and IL-10–producing DCs, Tα1 acts as an immune regulator capable of inducing protective immunity to Aspergillus fumigatus.16 TLR9 stimulation can also lead to IDO activation via mechanisms including autocrine type I IFN signaling17,18 and can promote pDC-mediated generation of CD4+CD25+ cells,19 which are an essential component of the IDO-dependent protective immunity to fungi.11-13 We hypothesized that Tα1 could affect the balance of immunity and tolerance by DCs and the generation of Treg cells. We assessed here the effects of Tα1 on deriving DCs from bone marrow (murine) or peripheral blood (human) precursors cultured in the presence of either GM-CSF/IL-4 (GM-DCs) or FLT3 ligand (FLT3L; FL-DCs), which is known to expand both conventional DCs and pDCs.20 DCs were analyzed for IDO expression and ability to mediate Th1/Treg priming in vitro and in vivo against A fumigatus and alloantigens.
Materials and methods
Mice
Female, 8- to 10-week-old inbred BALB/c and C57BL6 mice were obtained from Charles River/Harlan Breeding Laboratories (Calco, Italy). Homozygous TLR9–/– or IFN-αβR–/– mice on a C57BL6 background (obtained through the courtesy of Dr Manfred Kopf, Swiss Federal Institute of Technology, Zurich, Switzerland) were bred under specific pathogen-free conditions in the Animal Facility of Perugia University (Perugia, Italy). Procedures involving animals and their care were conducted in conformity with national laws and policies.
Donors and patients
Human peripheral blood mononuclear cells were obtained from healthy donors and 7 recipients of T-cell–depleted haploidentical HSC transplants, upon written informed consent. Donor typing, engraftment, and GVHD were assessed as described.21
Experimental HSCT model
Lethally irradiated (8 Gy) C57BL6 mice were infused with T-cell–depleted bone marrow cells from BALB/c mice.7 For GVHD, purified donor CD3+ T splenocytes were added to the graft.22 Individual mice were graded weekly from 0 to 2 for each GVHD criterion (see Figure 3 legend) without knowledge of treatment group.
A fumigatus infection
The strain of A fumigatus as well as culture and infection conditions were as described.16 Mice were anesthetized with 2.5% avertin (Sigma Chemical, St Louis, MO). Quantification of fungal growth in the lungs was done by the chitin assay and results are expressed as micrograms of glucosamine per pair of lungs.16 Periodic acid Schiff (PAS) staining was done as described.16
Reagents
Tα1 and the scrambled peptide (sTα1; both from SciClone Pharmaceuticals, San Mateo, CA) were supplied as purified, endotoxin-free sterile lyophilized acetylated polypeptides.16 The lyophilized powders were reconstituted in sterile water.
DC subset generation and cultures
Human GM-DCs or FL-DCs were obtained from purified CD14+ monocytes, which were from healthy donors or patients who received transplants (1 month after HSCT), cultured in Iscove modified medium for 7 to 9 days in the presence of recombinant GM-CSF (rGM-CSF; Schering-Plough, Milan, Italy) and rIL-4 (Peprotech, Inalco, Milan, Italy) or FLT3L (R&D Systems, Minneapolis, MN).16 DC recovery was between 20% and 30% reduced in cultures from patients who received transplants (data not shown). Tα1 and sTα1 were added to the cultures at 20 ng/mL. Fluorescence-activated cell sorter (FACS) analysis revealed that GM-DCs were CD1a+, CD11c+, CD11b+, CD4+, CD123+, BDCA-2+, and BDCA-4+ in the presence of Tα1. In contrast, FL-DCs were CD123+, CD45RA+, and CD1a–, irrespective of the presence of Tα1. CD11c–CD123+ pDCs were also isolated from peripheral blood with the CD304 (BDCA-4) isolation kit (Miltenyi Biotec, Bergisch Gladbach, Germany). Murine GM-DCs or FL-DCs were obtained from bone marrow cells cultured for 7 to 9 days, as described.16 Tα1 and sTα1 were added to the cultures at 20 ng/mL. In selected experiments, the CD4+ and the anti–mPDCA-1 DC isolation kits (Miltenyi Biotec) were used for isolating CD11c+CD11b+CD4+CD8– (referred to as B220–) and CD11clowB220+Ly-6Chigh pDCs (> 95% pure, referred to as B220+) from Tα1-induced GM-DCs. Splenic DCs (spDCs, > 99% CD11c+ and < 0.1% CD3+; consisting of 90% to 95% CD8–, 5% to 10% CD8+, and 1% to 5% B220+ cells) were purified by magnetic-activated sorting using CD11c MicroBeads and MidiMacs (Miltenyi Biotec). DC populations were further separated into CD8–, CD8+, and B220+ fractions by means of CD8 or B220 MicroBeads (Miltenyi Biotec) in the presence of EDTA to disrupt DC–T-cell complexes.23 The CD8– fraction was about 45% CD4+ and typically contained less than 0.5% contaminating CD8+ cells. Less than 1% CD8+ and less than 5% CD8– DCs expressed the B220 marker. More than 95% of the B220+ cells were stained by 120G8 (data not shown), a recently described monoclonal antibody (mAb) that selectively recognizes B220+Ly6C+CD11c+ pDCs.23 DCs were pulsed in serum-free Iscove medium for 24 hours with live unopsonized Aspergillus conidia or zymosan from Saccharomyces cerevisiae (Sigma) or cytosine-phosphate-guanosine–oligodeoxynucleotide (CpG-ODN) 2006 as described.16 Phagocytosis was done as described.16 Photographs were taken using a high-resolution Microscopy Color Camera AxioCam, using AxioVision Software Rel 3.1 (Carl Zeiss, Milan, Italy).
Flow cytometry
DCs were analyzed for antigen expression with a FACScan flow cytofluorometer (Becton Dickinson, Mountain View, CA) equipped with CELLQuest software and using conjugated mAbs from Pharmingen (San Diego, CA).16
IDO expression and functional analysis
IDO expression and functional activity were assessed as described.24
Generation, purification, and activity of Treg cells
Splenic CD4+ T cells were cocultured with conidia-pulsed DCs for 5 days,7 before flow cytometry or enzyme-linked immunospot (ELISPOT) assay. The 1-methyl-dl-tryptophan (1-MT; Sigma-Aldrich) was used at 2 μM. CD4+CD25+ and CD4+CD25– cells (> 90% pure on FACS analysis) were separated by magnetic cell sorting from lung and thoracic lymph nodes (TLNs).13 For Treg cell inhibition, 5 × 104 TLN Treg cells were added to 3 × 105 CD4+CD25– cells, both from mice that received transplants, stimulated with 3 × 104 autologous Aspergillus conidia-pulsed spDCs or with 1.5 × 105 allogeneic spDCs from naive donor mice for 5 days before H3-thymidine labeling. Purified CD11b+Gr-1+ peritoneal polymorpho-nuclear neutrophils (PMNs; more than 98% pure on FACS analysis; 2 × 106) were exposed to resting conidia in the presence of 4 × 105 CD4+CD25+ for 60 minutes for oxidant production or 24 hours for cytokine production.13
Cytokine and ELISPOT assay
Cytokine content was assessed by enzyme-linked immunosorbent assays (Endogen Human Elisa Kits; R&D Systems and Euroclone, Milan, Italy). AID EliSpot assay kits (Amplimedical, Buttigliera Alta, Turin, Italy) were used on purified splenic CD4+ T cells cocultured with conidia-pulsed DCs for 5 days to enumerate cytokine-producing cells.7 The 1-MT was used at 2 μM.
Adoptive transfer, fungal challenge, and assessment of protection
Mice received 2 intraperitoneal injections of 5 × 105 DCs, 1 week apart, starting 1 day after HSCT, and were infected 1 week after last DC administration. When given as a mixture of different DC subsets, DCs were mixed at the ratio of 10:1 (ie, 5 × 105 CD8+ with 5 × 104 CD8– cells) before infusion. Three days later, lung homogenates, CD4+ T cells (> 98% on FACS analysis), CD4+CD25– T cells (> 98%), or CD4+CD25+ T cells (> 82%) purified with the specific Miltenyi Biotec isolation kits were assessed for patterns of proinflammatory and anti-inflammatory (TNF-α/IL-10 in lung homogenates) Th1 (IFN-γ) or Th2 (IL-4) cytokine production by CD4+ cells stimulated with Aspergillus-pulsed DCs,7 frequency of CD25+ IL-10+ TGF-β+ Treg cells, lymphoproliferation, and gene expression by reverse transcriptase–polymerase chain reaction (RT-PCR). For proliferation, TLN CD4+ T lymphocytes were plated (105 cells/well) with 105 cells/well irradiated allogeneic splenocytes or autologous spDCs pulsed with conidia or 10 μg/mL Con A for 5 days before H3-thymidine labeling.
Generation of Aspergillus-specific human T-cell clones and lymphoproliferation
Aspergillus-specific human CD4+ T-cell clones were generated from peripheral blood CD4+CD45RA+ T cells added at limiting-dilution concentrations to irradiated (20 Gy) feeder autologous peripheral blood cells and stimulated with conidia-pulsed DCs or allogeneic DCs.21 Growing clones were assessed for specificity against fungus-pulsed DCs, allogeneic DCs, autologous irradiated cells (as a negative control), and 0.5% phytohemagglutinin (as a positive control) by H3-thymidine (Amersham Biosciences, Little Chalfont, United Kingdom) labeling or cytokine content in supernatants 2 days later.21
RT-PCR
RNA extraction, synthesis, PCR of cDNA, sequences of gene-specific primers, annealing temperatures, and amplification cycles were done as described.13 Amplification efficiencies were validated and normalized against Gapdh.
Tα1 expands pDCs from bone marrow precursors and activates tryptophan catabolism. (A) Surface expression of CD11c, CD11b, and B220 on DCs derived from bone marrow of C57BL6, TLR9–/–, or IFN-αβR–/– mice and cultured with GM-CSF/IL-4 (GM-DCs) or FLT3L (FL-DCs) in the presence of Tα1(+) or the scrambled peptide (–). Percent of double-positive cells is indicated. In microscopic pictures, cells were stained with Diff-Quik stain (Carlo Erba Reagents, Milan, Italy). Original magnification, × 100 (obtained with a 100×/1.25 oil-immersion objective lens). (B-C) Effect of Tα1 on cytokine production (enzyme-linked immunosorbent assay [ELISA]) by GM-DCs or FL-DCs (B) or purified DC populations from GM-DCs (C) cultured in serum-free medium (1 × 106 cells/mL) with unopsonized Aspergillus conidia (5 × 105/mL) for 24 hours. The IDO inhibitor 1-MT was added at 2 μM. Data are aggregated results from 3 independent experiments. The detection limits (pg/mL) of the assays were less than 16 for IL-12p70 and less than 12 for IL-10. (D) Increased IDO function and expression in DCs derived as in panel A. Cells were assessed for IDO protein expression by immunoblotting and for kynurenine production. Positive and negative controls consisted of IDO protein–expressing MC24 transfectants and mock-transfected MC22 cells, respectively (not shown in the figure). Data are means ± SE of triplicate samples in 1 experiment representative of 3. *P < .05, Tα1-treated versus untreated cells.
Tα1 expands pDCs from bone marrow precursors and activates tryptophan catabolism. (A) Surface expression of CD11c, CD11b, and B220 on DCs derived from bone marrow of C57BL6, TLR9–/–, or IFN-αβR–/– mice and cultured with GM-CSF/IL-4 (GM-DCs) or FLT3L (FL-DCs) in the presence of Tα1(+) or the scrambled peptide (–). Percent of double-positive cells is indicated. In microscopic pictures, cells were stained with Diff-Quik stain (Carlo Erba Reagents, Milan, Italy). Original magnification, × 100 (obtained with a 100×/1.25 oil-immersion objective lens). (B-C) Effect of Tα1 on cytokine production (enzyme-linked immunosorbent assay [ELISA]) by GM-DCs or FL-DCs (B) or purified DC populations from GM-DCs (C) cultured in serum-free medium (1 × 106 cells/mL) with unopsonized Aspergillus conidia (5 × 105/mL) for 24 hours. The IDO inhibitor 1-MT was added at 2 μM. Data are aggregated results from 3 independent experiments. The detection limits (pg/mL) of the assays were less than 16 for IL-12p70 and less than 12 for IL-10. (D) Increased IDO function and expression in DCs derived as in panel A. Cells were assessed for IDO protein expression by immunoblotting and for kynurenine production. Positive and negative controls consisted of IDO protein–expressing MC24 transfectants and mock-transfected MC22 cells, respectively (not shown in the figure). Data are means ± SE of triplicate samples in 1 experiment representative of 3. *P < .05, Tα1-treated versus untreated cells.
Statistical analysis
Student paired t test was used to determine the significance of values in experimental groups (significance was defined as P < .05). Survival data were analyzed using the Mann-Whitney U test. In vivo groups consisted of 6 animals.
Results
Tα1 expands pDCs from bone marrow precursors and activates tryptophan catabolism
To assess whether Tα1 would affect the phenotypic and functional properties of developing murine DCs, bone marrow cells were grown for 7 to 9 days in medium containing GM-CSF/IL-4 or FLT3L, in the presence of Tα1 or a control, scrambled peptide. After maturation, cells were analyzed by flow cytometry and light microscopy (Figure 1A). Contrary to FLT3L, GM-CSF/IL-4 treatment alone would not allow for the emergence of a high fraction of pDCs, as revealed by the percentage of B220+CD11c+ cells on FACS analysis and by morphology examination using light microscopy. However, Tα1, which did not affect total yield of cells (data not shown), greatly increased the occurrence of pDCs in GM-DCs, as revealed by a higher number of B220+CD11c+ DCs and a slightly decreased recovery of conventional CD11b+CD11c+ cells. B220+CD11c+ cells were not increased in FL-DCs treated with Tα1 nor were they in GM-DCs treated with the control peptide. Expansion of pDCs by Tα1 was not observed in GM-DCs from TLR9–/– or IFN-αβR–/– mice, indicating dependency of Tα1 effects on TLR9 and type I IFNR signaling.
Tα1 promotes the induction of IL-12 or IL-10 by mature myeloid DCs or pDCs, respectively.16 We found that Tα1-induced GM-DCs released both IL-12 and IL-10 in response to Aspergillus conidia, whereas Tα1 treatment increased the production of IL-10 more than IL-12, by FL-DCs (Figure 1B). Given the heterogeneity in the Tα1-expanded GM-DCs, CD11c+B220+ and CD11c+B220– DCs were further purified to identify which DC population is responsible for the Tα1-mediated effects. Tα1 induced the release of IL-12 and IL-10 in response to Aspergillus conidia by CD11c+B220+ DCs and increased IL-12, but not IL-10, production by CD11c+B220– DCs (Figure 1C).
Production of IL-10 by DCs in response to fungi is regulated by an IDO-dependent pathway.11,13 In the current setting, IL-10 production by either GM-DCs or FL-DCs in response to Tα1 did not occur with cells from TLR9–/– or IFN-αβR–/– mice and was blocked by the addition of the IDO inhibitor 1-MT to the cultures (Figure 1B). Likewise, 1-MT blocked IL-10 release by purified CD11c+B220+ cells from GM-DCs (Figure 1C). Induction of functional IDO by Tα1 in both FL-DCs and GM-DCs was confirmed by immunoblot analysis and by assessment of enzymic activity in terms of DC conversion of tryptophan to kynurenine. Again, induction of IDO protein and function by Tα1 was not observed in DCs from TLR9–/– or IFN-αβR–/– mice (Figure 1D). These data suggest that Tα1 may act on the development program of DC precursors, which results in the promotion of IDO-expressing pDCs. However, the finding that IDO induction was also observed upon overnight incubation of freshly isolated spDCs or GM-DCs with Tα1 (data not shown) indicates that IDO expression and pDC development program activation by Tα1 may occur independently.
Tα1-induced IDO+ DCs activate Treg cells in vitro
To correlate IDO expression and IL-10 production by DCs with possible regulatory activities, we examined the relative ability of Tα1-induced DCs to induce antigen-specific Th1/Treg priming in vitro by splenic CD4+ T lymphocytes in response to Aspergillus conidia. Figure 2A shows that Tα1 increased priming for IFN-γ–/IL-10–producing CD4+ T cells by GM-DCs and for IL-10–producing cells by FL-DCs. Similar to IDO blockade, depletion of B220+CD11c+ DCs from Tα1-induced GM-DCs abolished Treg cell activation (data not shown). Together with the data in Figure 1C, this finding suggests that CD11c+B220+ DCs are essential components of Tα1-dependent tolerogenic activity. IDO blockade by 1-MT prevented activation of the IL-10–producing CD4+ cells but had no effect on IFN-γ–producing cells, suggesting that IDO is causally and selectively linked to priming for IL-10–producing T cells. Because TLR9 stimulation activates IDO17,18 and also promotes pDC-mediated generation of CD4+CD25+ Treg cells,19 we evaluated the occurrence of CD4+CD25+ T cells expressing markers of Treg activity, such as the forkhead transcription factor Foxp3 and cytotoxic T-lymphocyte antigen 4 (CTLA-4). Cytofluorimetric analysis revealed that CD4+CD25+ T cells were expanded by coculture with DCs (Figure 2B). However, a significant proportion of the CD4+CD25+ T cells stained positive for intracellular Foxp3 and surface CTLA-4 when cultured in the presence of Tα1-induced DCs. The effect was negated by IDO blockade. FL-DCs also induced Foxp3+ Treg cells, although to a lesser degree. In accordance with the data in Figure 1C suggesting that tryptophan catabolism is enhanced by FLT3L, 1-MT appeared to interfere with the expansion of Foxp3+CTLA4+CD25+ T cells cocultured with FL-DCs. Therefore, the development of CD4+CD25+ Treg cells in vitro seemingly occurs through a mechanism involving DC tryptophan catabolism and is promoted by Tα1.
Tα1-induced IDO+ DCs activate Treg cells in vitro. (A) Frequency of IFN-γ–/IL-10–producing splenic CD4+ T cells activated by Aspergillus-pulsed Tα1-treated GM-DCs or FL-DCs. The 1-MT was present in selected cultures. Plates were read with the AID-EliSpot Reader System (Amplimedical). Values are means ± SE per 106 cells of samples from 3 to 5 experiments, calculated using replicates of serial 2-fold dilutions of cells. *P < .05, conidia-exposed versus unexposed cells; **P < .05, thymosin-exposed versus unexposed cells. (B) Phenotypic analysis of CD4+ cells cultured alone (–) or as in panel A. Numbers represent the percentage of double-positive cells.
Tα1-induced IDO+ DCs activate Treg cells in vitro. (A) Frequency of IFN-γ–/IL-10–producing splenic CD4+ T cells activated by Aspergillus-pulsed Tα1-treated GM-DCs or FL-DCs. The 1-MT was present in selected cultures. Plates were read with the AID-EliSpot Reader System (Amplimedical). Values are means ± SE per 106 cells of samples from 3 to 5 experiments, calculated using replicates of serial 2-fold dilutions of cells. *P < .05, conidia-exposed versus unexposed cells; **P < .05, thymosin-exposed versus unexposed cells. (B) Phenotypic analysis of CD4+ cells cultured alone (–) or as in panel A. Numbers represent the percentage of double-positive cells.
Tα1-conditioned GM-DCs protect hosts from aspergillosis in HSCT
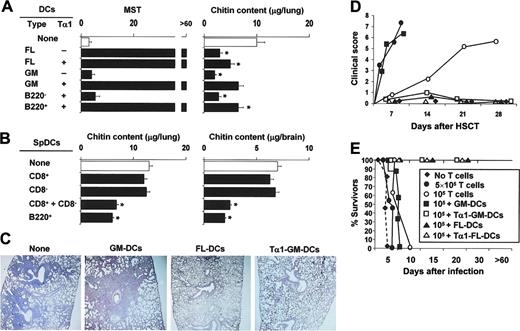
Fungus-pulsed DCs act as a potent fungal vaccine in experimental HSCT.7 Because regulation is absolutely required to balance inflammation and tolerance in HSCT25,26 as well as in antifungal immunity,11 we examined whether Tα1-treated DCs would affect priming and tolerance induction in vivo in an experimental setting of HSCT. Mice that received transplants were infused with fungus-pulsed DCs, infected with Aspergillus conidia, and monitored for survival, fungal growth, and inflammatory pathology in the lungs. Similar to splenic DCs,7 FL-DCs but not GM-DCs conferred resistance to infection in a dose-dependent manner, as mice survived infection and controlled fungal growth after transfer of 5 × 105 (Figure 3A) but not 5 × 104 DCs (data not shown). A paradoxical effect was observed in mice treated with GM-DCs in that mice failed to survive challenge in spite of their effective control of fungal growth. However, Tα1 treatment, which would not affect the vaccinating potential of FL-DCs, dramatically increased that of GM-DCs, as shown by the complete protection afforded by transfer of Tα1-treated GM-DCs (Figure 3A). FL-DCs encompass populations equivalent to mixtures of freshly harvested splenic CD8+, CD8–, and B220+LyC6+ pDCs.20 To dissect the contributions of different subsets to the vaccinating potential of DCs, fractions purified from spDCs were examined, alone or in combination, for ability to induce protection to aspergillosis in HSCT. The results showed that neither CD8– nor CD8+ spDCs alone conferred resistance to infection, as judged by the extensive fungal growth and dissemination. However, protection was observed on combining the 2 subsets and was similar to that observed with B220+ pDCs (Figure 3B) or B220+ pDCs purified from FL-DCs (data not shown). Therefore, the combination of functionally distinct activities is likely responsible for the protective action in vivo of FL-DCs and Tα1-treated GM-DCs in the experimental setting of aspergillosis in HSCT.
Tα1-treated DCs protect from aspergillosis in experimental HSCT. Lethally irradiated C57BL/6 mice received at least 2 × 106 T-cell–depleted allogeneic bone marrow cells from BALB/c mice 2 weeks before the intratracheal injection of 2 × 108/80 μL saline Aspergillus conidia. One and 7 days after transplantation, mice received 5 × 105Aspergillus-pulsed GM- or FL-DCs grown in Tα1 (A) or purified DC populations from spleens (spDCs; B), intraperitoneally. *P < .05, mice receiving DCs versus untreated mice. Resistance to infection was assessed in terms of MST (median survival time in days) and fungal growth in the lung and brain (μg/organ glucosamine content, bars indicating standard errors) 3 days after infection or at the time of death. Also shown in figure are inflammatory lung pathology (C), occurrence of GVHD reactivity (D), and susceptibility to infection (E) in the presence of donor T cells. (C) Periodic acid–Schiff–stained sections were prepared from lungs of mice infected with Aspergillus conidia 3 days earlier either untreated (None) or receiving different types of DCs. Severe signs of bronchial wall damage and necrosis and scarce inflammatory cell recruitment were observed in the lungs of untreated or GM-DC–treated mice, as opposed to mice receiving Tα1-treated GM-DCs or FL-DCs, whose lungs were characterized by few healing infiltrates of inflammatory cells with no evidence of bronchial wall damage or inflammatory response. Original magnification, × 200 (obtained with a 20×/0.45 objective lens). (D) Pathology scores for representative mice receiving, with the graft, different numbers of donor T cells alone or together with different DC types. The degree of systemic GVHD was assessed by a scoring system that sums changes in 5 clinical parameters: weight loss, posture (hunching), activity, fur texture, and skin integrity (maximum index = 10). (E) Survival to infection in mice treated as in panel D.
Tα1-treated DCs protect from aspergillosis in experimental HSCT. Lethally irradiated C57BL/6 mice received at least 2 × 106 T-cell–depleted allogeneic bone marrow cells from BALB/c mice 2 weeks before the intratracheal injection of 2 × 108/80 μL saline Aspergillus conidia. One and 7 days after transplantation, mice received 5 × 105Aspergillus-pulsed GM- or FL-DCs grown in Tα1 (A) or purified DC populations from spleens (spDCs; B), intraperitoneally. *P < .05, mice receiving DCs versus untreated mice. Resistance to infection was assessed in terms of MST (median survival time in days) and fungal growth in the lung and brain (μg/organ glucosamine content, bars indicating standard errors) 3 days after infection or at the time of death. Also shown in figure are inflammatory lung pathology (C), occurrence of GVHD reactivity (D), and susceptibility to infection (E) in the presence of donor T cells. (C) Periodic acid–Schiff–stained sections were prepared from lungs of mice infected with Aspergillus conidia 3 days earlier either untreated (None) or receiving different types of DCs. Severe signs of bronchial wall damage and necrosis and scarce inflammatory cell recruitment were observed in the lungs of untreated or GM-DC–treated mice, as opposed to mice receiving Tα1-treated GM-DCs or FL-DCs, whose lungs were characterized by few healing infiltrates of inflammatory cells with no evidence of bronchial wall damage or inflammatory response. Original magnification, × 200 (obtained with a 20×/0.45 objective lens). (D) Pathology scores for representative mice receiving, with the graft, different numbers of donor T cells alone or together with different DC types. The degree of systemic GVHD was assessed by a scoring system that sums changes in 5 clinical parameters: weight loss, posture (hunching), activity, fur texture, and skin integrity (maximum index = 10). (E) Survival to infection in mice treated as in panel D.
Tα1 conditioning of GM-DCs generates an immune component blunting immunotoxicity
Histopathology revealed that local inflammatory cell recruitment and reaction were high in the lungs of GM-DC–treated mice but low in mice infused with Tα1-treated GM-DCs or with FL-DCs (Figure 3B). These findings suggested that a severe inflammatory toxicity was likely associated with the transfer of GM-DCs, an effect alleviated by Tα1 preconditioning of the GM-DCs. To directly unmask the potential for immunotoxicity of DCs, and its taming by Tα1, the different DC populations were infused into mice receiving different numbers of donor T cells along with the graft. Mice were either left uninfected for the assessment of GVHD or infected for the assessment of susceptibility to infection. Although the initiation of GVHD after stem cell transplantation is dependent on direct antigen presentation by host antigen-presenting cells (APCs),27,28 the indirect antigen presentation by donor APCs has also been described.29 In line with previous findings,22 the severity of GVHD was dependent on the number of infused T cells, signs of GVHD being observed within 10 and 30 days after the respective infusion of 5 × 105 or 1 × 105 T cells. The coadministration of GM-DCs greatly accelerated the induction of GVHD by 105 T cells but, similar to FL-DCs, Tα1-treated GM-DCs totally prevented the effect (Figure 3C). In terms of susceptibility to infection, survival was not modified after the infusion of donor T cells either alone or along with GM-DCs. In contrast, similar to mice given FL-DCs, mice infused with Tα1-treated GM-DCs survived infection (Figure 3D). Together, these results suggested that, like spDCs, FL-DCs are fully competent at inducing antifungal protection after adoptive transfer in HSC transplant recipients. In contrast, GM-DCs are endowed with immunotoxicity, including the promotion of inflammation and GVHD, an activity in the host amenable to regulatory effects initiated by Tα1 in vitro.
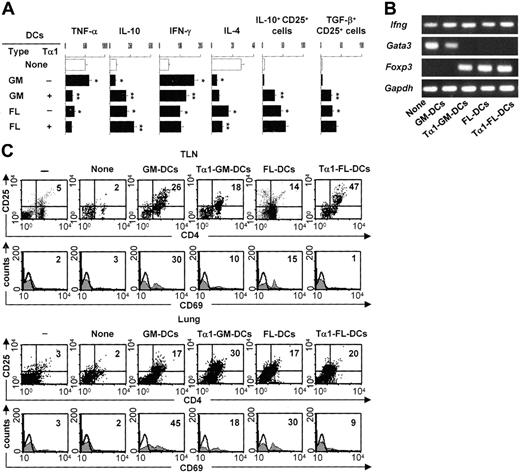
Tα1-induced DCs prime for antifungal Th1/Treg responses
To determine whether Tα1-treated DCs will induce Treg cells in vivo, we assessed levels of TNF-α/IL-10 production in lung homogenates, IFN-γ/IL-4 production by TLN CD4+ T cells, and expression of the genes coding for Ifnγ, the Th2-specific transcription factor Gata3, and Foxp3 in TLN CD4+ T cells. We also assessed the presence of CD4+CD25+ T cells in the lungs and TLNs, as functionally distinct Treg populations are found in the lungs and TLNs of mice with aspergillosis.13 The results showed disparate patterns of TNF-α/IL-10 production in the different groups. TNF-α was high and IL-10 was low in mice either untreated or infused with GM-DCs, the reverse being true in mice receiving FL-DCs and particularly Tα1-treated GM-DCs (Figure 4A). The assessment of the actual IFN-γ/IL-4 production by CD4+ T cells revealed that the amount of IFN-γ was higher and that of IL-4 lower in mice given Tα1-treated GM-DCs or given FL-DCs irrespective of their treatment (Figure 4A). PCR analysis showed that Ifng mRNA expression was always present, Gata3 mRNA was detected in mice either untreated or treated with FL-DCs unexposed to Tα1, and Foxp3 mRNA was expressed in mice given FL-DCs, irrespective of Tα1 exposure, or given Tα1-treated GM-DCs (Figure 4B). We also assessed levels of glucocorticoid-inducible TNF receptor (GITR) expression and found it to be broadly expressed, with no significant differences among experimental groups (data not shown). Cytofluorimetric analysis revealed that the number of CD4+CD25+ T cells increased in TLNs and lungs of mice infused with any type of DC, whether untreated or treated with Tα1 (Figure 4C). Interestingly, some sort of differential compartmentalization was observed in that Tα1-treated GM-DCs induced Treg in the lungs more than TLNs and the opposite was true for Tα1-treated FL-DCs. CD4+CD25+ T cells recovered from mice given Tα1-treated GM-DCs or FL-DCs did not stain positive for the CD69 activation marker, as observed with cells recovered from mice given untreated GM-DCs (Figure 4C). Consistent with the notion that the migration and occupancy of draining lymph nodes is required for graft acceptance,30 CD25+ T cells recovered from mice given FL-DCs or Tα1-treated GM-DCs also stained positive for the CD62L marker (data not shown). TLN Treg cells contained high numbers of IL-10– or TGF-β–producing cells (Figure 4A), whereas lung Treg cells contained more IL-10–than TGF-β–producing cells (data not shown). Altogether, these results suggested that GM-DCs exposed to Tα1 convert an inflammatory/Th1 response to a protective Th1/Treg response upon adoptive transfer in vivo. However, the finding that induced Treg cells home to different compartments could be related to possible phenotypic and functional differences between the different Treg populations. This would be consistent with the finding that a division of labor occurs between the functionally distinct Treg populations that are coordinately activated in the lungs and TLNs of mice exposed to Aspergillus.13 Alternatively, after a first level of activation and priming in lymph nodes by cognate recognition, activated Treg cells may become effector Treg cells capable of trafficking to infected tissues where they control the local inflammatory response.
Tα1-induced DCs prime for antifungal Th1/Treg responses in vivo. Patterns of inflammatory/Th/Treg responses 3 days after the infection in mice treated as described in Figure 2 legend. (A) TNF-α/IL-10 levels were assessed by specific ELISA in lung homogenates, and IFN-γ/IL-4 production was assessed in TLN CD4+ T cells cocultured with Aspergillus-pulsed DCs. Bars indicate standard errors. TLN CD4+CD25+ T cells producing IL-10 or TGF-β were numbered by ELISPOT assay. Results are expressed as the mean number of cytokine-producing cells (±SE) per 2 × 105 cells. *P < .05, DC-treated versus untreated mice. **P < .05, Tα1-treated DCs versus untreated DCs. (B) Total RNA was extracted from freshly purified CD4+ T cells from TLNs of treated or untreated (None) mice. The expressions of the different mRNAs in each cell population were determined by RT-PCR. The expression of a housekeeping gene, Gapdh mRNA, was used as an internal control. The data shown are representative results of 3 experiments. (C) Phenotypic analysis of cells isolated from lung or TLNs of mice infused or not (None) with different types of DCs, (–) indicating uninfected, untreated mice. CD4+ T cells were sequentially reacted with PE-conjugated anti-CD25 (PC61) and FITC-conjugated anti-CD69 (clone HI.2F3) mAbs. Numbers represent the percentage of positive cells over total cells analyzed. Control staining of cells with irrelevant Ab was used to obtain background fluorescence values. Histograms are representative of 1 of 4 independent experiments.
Tα1-induced DCs prime for antifungal Th1/Treg responses in vivo. Patterns of inflammatory/Th/Treg responses 3 days after the infection in mice treated as described in Figure 2 legend. (A) TNF-α/IL-10 levels were assessed by specific ELISA in lung homogenates, and IFN-γ/IL-4 production was assessed in TLN CD4+ T cells cocultured with Aspergillus-pulsed DCs. Bars indicate standard errors. TLN CD4+CD25+ T cells producing IL-10 or TGF-β were numbered by ELISPOT assay. Results are expressed as the mean number of cytokine-producing cells (±SE) per 2 × 105 cells. *P < .05, DC-treated versus untreated mice. **P < .05, Tα1-treated DCs versus untreated DCs. (B) Total RNA was extracted from freshly purified CD4+ T cells from TLNs of treated or untreated (None) mice. The expressions of the different mRNAs in each cell population were determined by RT-PCR. The expression of a housekeeping gene, Gapdh mRNA, was used as an internal control. The data shown are representative results of 3 experiments. (C) Phenotypic analysis of cells isolated from lung or TLNs of mice infused or not (None) with different types of DCs, (–) indicating uninfected, untreated mice. CD4+ T cells were sequentially reacted with PE-conjugated anti-CD25 (PC61) and FITC-conjugated anti-CD69 (clone HI.2F3) mAbs. Numbers represent the percentage of positive cells over total cells analyzed. Control staining of cells with irrelevant Ab was used to obtain background fluorescence values. Histograms are representative of 1 of 4 independent experiments.
Antifungal Treg cells inhibit alloreactivity and inflammation
To assess the suppressive activity of CD25+ Treg cells, TLN cells from mice given the different DC subsets were assessed for proliferative response to allogeneic splenocytes, Aspergillus conidia, or mitogen. The results showed that mice receiving T cells alone or together with GM-DCs showed a robust allogeneic, but not Aspergillus-specific, proliferative response. In contrast, mice receiving FL-DCs or Tα1-treated GM-DCs showed a robust Aspergillus-specific, but not allogeneic, reactivity (Figure 5A). As the response to mitogen was comparable among the different groups, these results suggested that Treg cells directly impact on both allogeneic and pathogen-specific Th1 reactivity. To clarify this issue, purified CD4+CD25+ T cells from TLNs were assessed for ability to block the Aspergillus- or alloantigen-specific proliferation of, and IFN-γ production by, the corresponding CD4+CD25– T cells. While CD4+CD25+ T cells were hyporesponsive to Aspergillus and alloantigens (Figure 5B-C), alloreactivity and the antigen-specific responses of CD25– T cells were both reduced in the presence of CD4+CD25+ T cells from mice receiving FL-DCs or Tα1-treated GM-DCs (Figure 5B-C). As lung Treg cells are endowed with potent anti-inflammatory activity in pulmonary aspergillosis,13 we also examined the suppressive activity of lung CD4+CD25+ T cells on the antifungal effector activity of neutrophils, such as TNF-α and oxidant production, because these functions are exquisitely sensitive to the suppressive activity of Treg cells.13 Both functions were significantly inhibited by lung Treg cells and, particularly, by the Treg cells induced by Tα1-treated GM-DCs (Figure 5D).
Tα1-induced Treg cells inhibit alloreactivity. (A) Murine CD4+ T lymphocytes from TLNs of mice that received transplants were stimulated with irradiated allogeneic splenocytes, autologous splenic DCs stimulated with conidia, or concanavalin A. T-cell proliferation was assessed in a 5-day MLR assay and measured by H3-thymidine incorporation over the last 8 hours. *P < .05, recipient versus donor mice. **P < .05, T-cell– and/or DC-treated mice versus untreated mice. ***P < .05, Tα1-treated DCs versus untreated DCs. (B-C) Proliferative activity and IFN-γ production by purified CD4+CD25– T cells from recipient mice against autologous splenic DCs pulsed with Aspergillus conidia (B) or allogeneic (BALB/c) splenic DCs (C) in the presence of TLN CD4+CD25+ T cells from recipient mice receiving FL-DCs (a) or Tα1–GM-DCs (b). The data shown are representative results from 1 of 3 independent experiments. *P < .05, Aspergillus- or alloantigen-specific reactivity versus unstimulated cells. **P < .05, Tα1-treated versus untreated DCs. (D) Peritoneal neutrophils (PMNs) were exposed to resting conidia in the presence of lung CD4+CD25+ T cells from FL-DC–treated (a) or Tα1-GM-DC–treated mice (b) for 60 minutes for oxidant production (expressed as nanomoles O2–/106 cells) or 24 hours for cytokine production (pg/mL by ELISA). *P < .05, conidia-exposed versus unexposed PMNs. **P < .05, unexposed versus Treg–exposed PMNs. ***P < .05, CD25+ (a) versus CD25+ (b) Treg.
Tα1-induced Treg cells inhibit alloreactivity. (A) Murine CD4+ T lymphocytes from TLNs of mice that received transplants were stimulated with irradiated allogeneic splenocytes, autologous splenic DCs stimulated with conidia, or concanavalin A. T-cell proliferation was assessed in a 5-day MLR assay and measured by H3-thymidine incorporation over the last 8 hours. *P < .05, recipient versus donor mice. **P < .05, T-cell– and/or DC-treated mice versus untreated mice. ***P < .05, Tα1-treated DCs versus untreated DCs. (B-C) Proliferative activity and IFN-γ production by purified CD4+CD25– T cells from recipient mice against autologous splenic DCs pulsed with Aspergillus conidia (B) or allogeneic (BALB/c) splenic DCs (C) in the presence of TLN CD4+CD25+ T cells from recipient mice receiving FL-DCs (a) or Tα1–GM-DCs (b). The data shown are representative results from 1 of 3 independent experiments. *P < .05, Aspergillus- or alloantigen-specific reactivity versus unstimulated cells. **P < .05, Tα1-treated versus untreated DCs. (D) Peritoneal neutrophils (PMNs) were exposed to resting conidia in the presence of lung CD4+CD25+ T cells from FL-DC–treated (a) or Tα1-GM-DC–treated mice (b) for 60 minutes for oxidant production (expressed as nanomoles O2–/106 cells) or 24 hours for cytokine production (pg/mL by ELISA). *P < .05, conidia-exposed versus unexposed PMNs. **P < .05, unexposed versus Treg–exposed PMNs. ***P < .05, CD25+ (a) versus CD25+ (b) Treg.
Tα1 promotes mobilization and Th1/Treg antifungal priming of human DCs
To assess whether Tα1 may affect the Th1/Treg cell priming potential of human DCs, GM- or FL-DCs were derived from peripheral CD14+ cells from healthy donors in the presence of Tα1. As with murine DC cultures, Tα1 promoted the mobilization of CD123+ pDCs while decreasing that of CD1a+ DCs in GM-CSF/IL-4–treated cultures. No such effects were observed in FLT3L cultures (Figure 6A). Because CD4+ monocytes poorly express TLR931 but Tα1 is a potent inducer of TLR9 expression on human mature DCs,16 we assessed whether Tα1 also up-regulated the expression of TLR9 on CD4+ cells. We found that TLR9 surface expression and mRNA were both up-regulated as early as 30 or 60 minutes after Tα1 exposure, respectively (data not shown), a finding suggesting that TLR9 could be implicated in human pDC expansion by Tα1.
Tα1 significantly modified the microbial sensing of GM-DCs or FL-DCs in terms of phagocytosis (from 30% to 56% phagocytosis in GM-DCs and from 32% to 58% phagocytosis in FL-DCs). Interestingly, however, Tα1 also promoted the phagocytosis of both GM- and FL-DCs derived from patients 1 month after transplantation (Figure 6B). In terms of functional activity, Tα1 converted inflammatory IL-12–producing GM-DCs into tolerogenic pDCs that, similar to FL-DCs, produced increased levels of IL-10 (Figure 6C) and primed for IL-10–producing CD4+ T cells in vitro (Figure 6D). As type I IFN-producing pDCs are known to participate in the induction and maintenance of tolerance as well as in the tolerogenic effects of Tα1, we also compared the production of IFN-α in response to Aspergillus conidia, zymosan (meant to be a positive control for GM-DCs), or CpG ODN (a positive control for FL-DCs). IFN-α was produced mainly by FL-DCs or Tα1-treated GM-DCs (Figure 6C) and was comparable to that produced by freshly isolated blood pDCs stimulated with CpG (1703 ± 109 pg/mL) or conidia (876 ± 56 pg/mL). Finally, we examined whether Tα1 treatment would modify the ability of DCs to activate fungus- or alloantigen-specific T-cell reactivity. Figure 6E shows that Tα1 modified neither the antigen-specific T-cell responses induced by DCs nor the allostimulatory capacity of either type of DCs. As a matter of fact, induction of fungus-specific T-cell reactivity was totally absent in DCs from patients who received transplants. These data indicate therefore that Tα1, by harnessing inflammatory DCs, may meet the requirements for successful antifungal Th1/Treg cell priming in the absence of alloreactivity in hematopoietic transplantation.
Discussion
The results of the present study show that Tα1 expands a pDC fraction in GM-DCs that is competent for IDO function, and that IDO+ pDCs are necessary and sufficient to mediate antimicrobial immunity and alloantigen tolerization in experimental HSCT. Differently from CpG ODNs, this activity of pDCs involves the promotion of T-helper type 1 immunity within a regulatory environment mediated by the induction of CD4+CD25+ Treg cells. Given the presence of Tα1 not only in the thymus but also in lymphoid and nonlymphoid peripheral tissues,15 the present findings suggest that Tα1 may act as a natural hormone contributing to the induction and maintenance of peripheral tolerance in physiology and pathophysiology. It is not surprising therefore that levels of Tα1 are elevated in pregnancy and dysregulated in other immune disorders.15
Different categories of pDCs have been described in both experimental and human settings.31 Type I IFN-producing pDCs not only mediate Th1 and Th2 type responses but also participate in the induction and maintenance of tolerance by promoting the development of Treg cells with suppressive activity.31 Expansion of pDCs is contingent upon the hematopoietic growth factor FLT3L,32,33 which acts on FLT3-expressing myeloid and nonmyeloid hematopoietic precursors.33 Tα1 did not promote pDC expansion in FLT3L-exposed cultures but did promote the expansion of murine CD11c+B220+ pDCs and human CD123+ pDCs in the myeloid differentiation pathway. Because development of pDCs and conventional DCs may not fit into a deterministic “lymphoid” or “myeloid” lineage,34 it will be of interest to define which developmental programs critical for DC lineage fate decision are activated by Tα1. Meanwhile, it is worth mentioning that DCs expanded by Tα1 also stained positive for the natural killer 1.1 (NK1.1) marker (L.R., unpublished observation, January 2006), a finding suggesting that Tα1 could be involved in the activation of the third, recently described DC lineage, which is distinct from conventional DCs and pDCs, has the molecular expression profile of both NKs and DCs, and, incidentally, is highly sensitive to TLR9 stimulation.35
Tα 1 promotes mobilization and Th1/Treg antifungal priming of human DCs. (A) Surface expression of CD11c, CD1a, and CD123 on DCs derived from peripheral CD14+ cells of different donors with GM-CSF/IL-4 (GM-DCs) or FLT3L (FL-DCs) in the presence of Tα1. Percentage of positive cells is indicated. (B) Phagocytosis of conidia by GM- or FL-DCs exposed (+) or not (–) to Tα1 from 7 recipients of T-cell–depleted haploidentical HSC transplants. The data are the means ± SE and are expressed as percentage internalization (numbers within panels). *P < .05, Tα1-treated versus untreated cells. Visualized with a 100×/1.25 oil-immersion objective lens. (C) Cytokine production (pg/mL by ELISA) by Tα1-induced DCs, from healthy donors, cultured in serum-free medium (1 × 106 cells/mL) with unopsonized Aspergillus conidia (5 × 105/mL), 10 μg/mL zymosan, or 2 μg/mL CpG-B ODN 2006 for 24 hours. The data shown are aggregated results from 3 independent experiments and are presented in the mean ± SD. The detection limits of the assays were as follows: less than 3 for IL-12p70, less than 5 for IL-10, and less than 3 for IFN-α. (D) Cytokine production by peripheral blood Aspergillus-specific CD4+ T-cell clones from healthy donors in response to Aspergillus-pulsed Tα1-treated DCs as in panel A. Bars indicate standard errors. The detection limits (pg/mL) of the assays were less than 0.5 for IL-4 and IFN-γ.*P < .05, conidia-stimulated versus unstimulated cells. **P < .05, Tα1-exposed versus unexposed cells. (E) Frequency of Aspergillus-specific or alloreactive T-cell clones responding to the different types of fungus-pulsed DCs or unpulsed DCs, respectively, from healthy donors or transplant recipients. Growing clones were assessed for specificity after 2 days of stimulation with DCs. *P < .05, GM-DCs versus peripheral blood cells (–). **P < .05, HSCT-DCs versus all other DCs. nd indicates not done.
Tα 1 promotes mobilization and Th1/Treg antifungal priming of human DCs. (A) Surface expression of CD11c, CD1a, and CD123 on DCs derived from peripheral CD14+ cells of different donors with GM-CSF/IL-4 (GM-DCs) or FLT3L (FL-DCs) in the presence of Tα1. Percentage of positive cells is indicated. (B) Phagocytosis of conidia by GM- or FL-DCs exposed (+) or not (–) to Tα1 from 7 recipients of T-cell–depleted haploidentical HSC transplants. The data are the means ± SE and are expressed as percentage internalization (numbers within panels). *P < .05, Tα1-treated versus untreated cells. Visualized with a 100×/1.25 oil-immersion objective lens. (C) Cytokine production (pg/mL by ELISA) by Tα1-induced DCs, from healthy donors, cultured in serum-free medium (1 × 106 cells/mL) with unopsonized Aspergillus conidia (5 × 105/mL), 10 μg/mL zymosan, or 2 μg/mL CpG-B ODN 2006 for 24 hours. The data shown are aggregated results from 3 independent experiments and are presented in the mean ± SD. The detection limits of the assays were as follows: less than 3 for IL-12p70, less than 5 for IL-10, and less than 3 for IFN-α. (D) Cytokine production by peripheral blood Aspergillus-specific CD4+ T-cell clones from healthy donors in response to Aspergillus-pulsed Tα1-treated DCs as in panel A. Bars indicate standard errors. The detection limits (pg/mL) of the assays were less than 0.5 for IL-4 and IFN-γ.*P < .05, conidia-stimulated versus unstimulated cells. **P < .05, Tα1-exposed versus unexposed cells. (E) Frequency of Aspergillus-specific or alloreactive T-cell clones responding to the different types of fungus-pulsed DCs or unpulsed DCs, respectively, from healthy donors or transplant recipients. Growing clones were assessed for specificity after 2 days of stimulation with DCs. *P < .05, GM-DCs versus peripheral blood cells (–). **P < .05, HSCT-DCs versus all other DCs. nd indicates not done.
Similar to the action of CpG-ODN on murine spCD11c+B220+ pDCs,23,36 pDC expansion and activation of the tryptophan catabolic pathway by Tα1 required signaling through TLR9 and type I IFNR. TLR9 is apparently involved in the activation of both the immunogenic16 and tolerogenic programs (this study) of DCs by Tα1, a finding that rather than being contradictory, has recently been explained on the basis of site- and cell-dependent specificity of TLR9 signaling pathways.18,37 Like CpG-ODN, Tα1 may exploit different TLR9 signaling pathways and this may account for its wide range of bioactivities, including the control of microbial (particularly viral) immunity, autoimmunity, and cancer.15
The recent finding that granulopoiesis and lymphopoiesis in bone marrow are specifically and reciprocally coupled by inflammation38 makes the control of inflammation a central issue in transplantation. Although Tα1 activates innate cells, including DCs, to an antimicrobial16 and antitumor state,39 the attenuation of the immunogenic/inflammatory activity of myeloid DCs by Tα1 through IDO induction qualifies Tα1 as a unique immunoregulatory molecule capable of fine-tuning and controlling the quality of the immune response, which may result in the control of inflammatory response and restoration of protective antimicrobial immunity in the relative absence of GVHD, as seen in mice that received transplants. As chronic inflammation may be linked to cancer development, Tα1 would be expected to work in conditions in which prolonged inflammation may promote cancer. In this regard, it is intriguing that Tα1-conditioned GM-DCs also initiated tumor-specific Th1 priming in vivo in the face of regulatory effects mediated by IDO induction (L.R., unpublished observation, January 2006).
Inflammation and allergy to Aspergillus are regulated by the coordinate activation of distinct Treg cell populations, with IDO acting both at the inductive and effector phases of Treg cell activity. However, donor CD4+CD25+ Treg cells have also been shown to play a pivotal role in preventing GVHD and in tolerance induction in experimental HSCT.10 The finding that Aspergillus-induced Treg cells were capable of inhibiting alloreactivity while sparing responsiveness to mitogen or to third-party cells (data not shown) suggests that pathogen-induced Treg cells may be associated with minimal bystander suppression and is in line with the observation of a positive effect on posttransplantation immunity of antigen exposure at the time of transplantation.40 From a mechanistic perspective, this implies that the function of Treg cells in transplantation can be controlled by the specificity of the T-cell receptor expressed on Treg cells.41-43 Several studies have addressed the effect that fungal infection has on transplantation tolerance, and the overall view is that both prior and concurrent exposure to fungal pathogens can prevent tolerance induction. However, much less attention has been paid to the effect that fungus-directed tolerance based on active T-cell regulation might have on transplantation tolerance. To our knowledge, this is the first study to examine the effects of pathogen-driven CD25+CD4+ Treg cells on responses to alloantigens in transplantation.
Increasing evidence suggests that DCs are the target of Treg cell activity.44 The fact that costimulatory-deficient DCs failed to protect mice from infection and GVHD (L.R., unpublished observations, January 2006) may suggest that regulation of costimulatory molecules on DCs could represent a downstream effector function of Treg cells. In this regard, as IDO expression is causally involved in inhibition of alloreactivity,45 and IDO expression on DCs is regulated by Treg cells through costimulation,44 an “infectious tolerance” mechanism would appear to be at work. Interestingly, we have also constantly observed an increased cellularity (mainly in CD4–/CD8– lymphocyte fractions) in the thymus of mice infused with FL-DCs or Tα1-treated DCs (L.R., unpublished observation, January 2006). Along with the migration of injected DCs to the thymus (data not shown), this finding may indicate the possible contribution of the thymic-dependent pathway in immune reconstitution in HSCT after DC vaccination. Irrespective of whether a single subset or functionally distinct subsets mediate peripheral tolerance and inflammatory control, the relative contribution of the peripheral generation of Treg cells versus their intrathymic selection by cross-reaction within the self-reactive populations remains to be elucidated.
At a time when vaccination of stem cell transplant recipients is highly recommended,46 developing new strategies to expand and modulate the functions of distinct DCs associated with specific regulation of host immunity may provide novel immune-based therapies in HSCT.6 Tα1 is approved in 30 countries for treatment of some viral infections and as an immune adjuvant.15 Within the instructive model of DC-mediated regulation of the Th repertoire, it is conceivable that an improved understanding of the Tα1/DC interaction through IDO will improve the efficacy of Tα1 in chronic inflammatory conditions, from autoimmune diseases to cancer to transplantation.
Prepublished online as Blood First Edition Paper, June 1, 2006; DOI 10.1182/blood-2006-02-004762.
Supported by the National Research Project on AIDS, contract 50F.30, “Opportunistic Infections and Tuberculosis,” Italy.
L.R. and P.P. devised the study, critically evaluated the data at regular intervals, and drafted the paper. L.R. takes responsibility for integrity of the work as a whole. K.P. developed the pathogen-specific T-cell cloning procedure under the supervision of A.V.. C.M., R.G., and S.B. conducted studies of pathogen-specific and alloreactive T-cell responses in mice. P.B. performed all DC cell studies in vitro. F.F. and P.P. were a major source of conceptual and technical information on IDO. G.B. contributed with ELISA and ELISPOT assays. G.R. and E.G. provided major intellectual and practical support with thymosin. F.B., as the Head of the Microbiology Section, made a major intellectual and critical contribution to the overall study.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Lara Bellocchio for dedicated editorial assistance, Dr Paolo Mosci for histology, and Dr Manfred Kopf (Swiss Federal Institute of Technology Zurich) for providing us with mice.

![Figure 1. Tα1 expands pDCs from bone marrow precursors and activates tryptophan catabolism. (A) Surface expression of CD11c, CD11b, and B220 on DCs derived from bone marrow of C57BL6, TLR9–/–, or IFN-αβR–/– mice and cultured with GM-CSF/IL-4 (GM-DCs) or FLT3L (FL-DCs) in the presence of Tα1(+) or the scrambled peptide (–). Percent of double-positive cells is indicated. In microscopic pictures, cells were stained with Diff-Quik stain (Carlo Erba Reagents, Milan, Italy). Original magnification, × 100 (obtained with a 100×/1.25 oil-immersion objective lens). (B-C) Effect of Tα1 on cytokine production (enzyme-linked immunosorbent assay [ELISA]) by GM-DCs or FL-DCs (B) or purified DC populations from GM-DCs (C) cultured in serum-free medium (1 × 106 cells/mL) with unopsonized Aspergillus conidia (5 × 105/mL) for 24 hours. The IDO inhibitor 1-MT was added at 2 μM. Data are aggregated results from 3 independent experiments. The detection limits (pg/mL) of the assays were less than 16 for IL-12p70 and less than 12 for IL-10. (D) Increased IDO function and expression in DCs derived as in panel A. Cells were assessed for IDO protein expression by immunoblotting and for kynurenine production. Positive and negative controls consisted of IDO protein–expressing MC24 transfectants and mock-transfected MC22 cells, respectively (not shown in the figure). Data are means ± SE of triplicate samples in 1 experiment representative of 3. *P < .05, Tα1-treated versus untreated cells.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/108/7/10.1182_blood-2006-02-004762/4/m_zh80190601680001.jpeg?Expires=1767749797&Signature=SKps5exbn9LYV7V6vkw19VSVseU1WdjHBsDVe3-xGw0nIzJ0b4pTrO99XvW3YwuS5kGBekaHY1D8fQbmioHTIgqDDqBQLgFHg-TUF1Z-RjrgCAwJFO2~OJYBplyoWCSLb5Tt6~MOxi-uohUmMdalOn37sZO5~~OwcW5vZAO8ZtOHd52efn~vBtxu6jojeA6Q0edTc6DAEFPxHpL2jVcXUQUk4J7u7vCEHeYyxnczykneuSaWgJ2JLU9xtXmhenwiAToWbz9~BhitXsrfjSF6aBB07IDbxigld5nIdiiJFrDQYQE5T~~fMPYH0oL45HagS0wS3Ngiji~L0dE9Yy5~cw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal