Abstract
Clinical use of purified hematopoietic stem cells in myeloablated patients requires cotransplantation of short-term repopulating cells (STRCs) to ensure timely count recovery. Here, we investigated the flow fluorescence-based side population (SP) phenotype of mobilized human peripheral blood (mPB) cells that rapidly repopulate the highly permissive nonobese diabetic/severe combined immunodeficient (NOD/SCID)–β2 microglobulin–/– mouse. No SP cells from this source regenerated detectable progeny in these mice before 8 weeks, although by 12 weeks human B-lymphoid cells were seen in some recipients of SP mPB cells. All myeloid reconstituting activity, including that seen within 3 weeks after transplantation, was associated with the non-SP fraction. Isolation of SP cells depletes human mPB of the rapid myeloid reconstitution capacity provided by myeloid-restricted STRCs which are vital for early hematologic recovery in clinical transplant recipients.
Introduction
Rapid and sustained multilineage hematopoietic reconstitutions are both important requirements for the safe use of clinical hematopoietic cell transplants. We have previously shown that the progeny of human and primate cells with rapid but short-term repopulating activity (STRCs) dominate hematopoiesis early after their transplantation into permissive hosts, and these cell types are therefore likely to control the pace of hematopoietic recovery in patients who receive a transplant with cultured stem cells.1-3 Consequently, strategies aimed to deplete stem cell transplants from leukemic or cancer stem cells or to enrich normal hematopoietic stem cells for modification in vitro need to be tested for their STRC content, as can be efficiently achieved by intravenous or intrafemoral transplantation of highly immunodeficient mice.1,4-7
Side population (SP) cells are defined by their ability to rapidly efflux the fluorescent DNA-binding dye Hoechst 33 342. This methodology has been widely used to enrich stem cells from murine muscle, skin, kidney, liver, and hematopoietic tissue,8,9 as well as normal stem cells from human fetal liver10 and acute myeloid leukemia samples.11,12 Here, we asked whether normal adult human STRCs also have an SP phenotype.
Study design
Cell purification
White blood cells were collected by apheresis from 2 patients with lymphoma after mobilization chemotherapy followed by daily injections of human granulocyte colony-stimulating factor (G-CSF) and from 7 healthy allogeneic stem cell donors after their treatment exclusively with G-CSF according to protocols approved by the Institutional Review Board of the Freiburg University School of Medicine after obtaining informed consent according to the Declaration of Helsinki. Low-density (LD; < 1.077 g/mL) mobilized human peripheral blood (mPB) cells were isolated by Ficoll-Hyqaque (PAA Laboratories, Linz, Austria) density gradient centrifugation. Hoechst staining was done as reported previously.13,14 Briefly, cells were suspended in Hanks balanced salt solution (Gibco, Paisley, United Kingdom) plus 10 mM HEPES buffer (Gibco) and 2% FBS (StemCell Technologies, Vancouver, BC, Canada) at 2 × 107 cells/mL and then stained for 30 minutes on ice with FITC-conjugated anti-CD3 antibody (Becton Dickinson, San Jose, CA). Viability after the Hoechst 33342 staining and isolation procedure was 79.8% ± 4.2% (mean ± SEM) with a total cell recovery of 15.5% ± 1.4%. CD3– SP, and CD3– non-SP cells were sorted on a dual laser MoFlow (Cytomation, Fort Collins, CO).
Progenitor assays
Progenitor assays were done as previously described.9 Myeloid (ie, erythroid, granulopoietic, and mixed erythroid-granulopoietic) colony-forming cell (CFC) content was assessed either directly or after 8 days in suspension culture in DMEM containing 10% FCS, 100 ng/mL SCF, 100 ng/mL Flt-3 ligand, 50 ng/mL TPO, 30 ng/mL IL-3, and 20 ng/mL IL-6 (R&D Systems, Wiesbaden-Nordenstadt, Germany).
Xenotransplantation
CD3– SP and CD3– non-SP cells were injected intravenously at limiting dilutions into sublethally irradiated (325 cGy) NOD/LtSz-PrkdcscidPrkdcscid-β2 microglobulin–/– (NOD/SCID-β2m–/–) mice together with 106 irradiated (15 Gy) human mononuclear mPB cells as carrier cells. Human CD45/71+, CD19/20+, CD15/66b+, CD33+, CD34+, CD41+, and glycophorin-A+ cells present in the bone marrow of the mice that received a transplant were assessed 3, 8, and 12 weeks later by fluorescence activated cell sorting (FACS) analysis (FACSCalibur; Becton Dickinson, Heidelberg, Germany) of bone marrow aspirates.1 In specified cases, cells were also assessed for human CD3+ and CD56+ cells. All antibodies were purchased from Becton Dickinson except anti–glycophorin A (Immunotools, Friesoythe, Germany). The STRC-M activity was defined by detection of human myeloid (CD15+, CD33+, CD41+, and/or glycophorin-A+) cells in bone marrow samples taken 3 weeks after transplantation, and the STRC-ML activity was defined by detection of human myeloid and human B-lymphoid (CD19/20+) cells in bone marrow samples taken after 8 weeks. The criterion used for identifying mice as positive was more than 10 CD45/71+ human cells per 2 × 104 PI– cells analyzed. Gates were set to exclude greater than 99.99% of PI– cells incubated with irrelevant isotype-matched control antibodies labeled with the corresponding fluorochromes. Frequencies of human repopulating cells were calculated using Poisson statistics and the method of maximum likelihood (using L-calc software, StemCell Technologies).
Results and discussion
A small but distinct verapamil-sensitive SP population (0.023% ± 0.007%) was detected in 9 mPB samples analyzed. SP cell staining and sorting did not affect the clonogenicity of the sorted cells; however, greater than 95% of all CFCs were recovered in the non-SP fraction. Nevertheless, a small fraction of CFCs were found to have an SP phenotype, and these CFCs constituted 16% of the total SP fraction (n = 3). Moreover, the SP cells also contained cells that were able to generate myeloid CFCs after 8 days in culture in the presence of SCF, flt-3 ligand, TPO, IL-3, and IL-6, as shown by the maintenance of CFCs at a frequency of 18% in the cultured cells and a 4.4-fold net increase in their numbers (n = 3). In contrast, when 104 cultured non-SP cells were assayed for CFCs, none were detected (n = 3), demonstrating an enrichment of immature myeloid CFC-precursors in the SP fraction.
CD3– SP and CD3– non-SP cells were also injected intravenously at limiting dilutions into a total of 104 sublethally irradiated NOD/SCID-β2m–/– mice to investigate the phenotype of STRCs. The results of these experiments are summarized in Table 1. After 3 weeks, human cells (mostly myeloid) were detected in 10 of 56 mice that received a transplant with non-SP cells. After 8 weeks, both lymphoid and myeloid human cells were detected in 21 of 49 mice injected with non-SP cells which did not change for up to 12 weeks (as determined in 3 of the 6 experiments, data not shown). From these data, we calculated STRC-M and STRC-ML frequencies of 1/3.4 × 10–5 and 1/2 × 10–5 in the CD3– non-SP fraction of human mPB cells. However, analysis of the cell lineages produced by these non-SP STRCs showed fewer megakaryocytic and granulomonocytic cells as compared with unsorted fresh mPB cells (P < .05; data not shown), suggesting that the staining or sorting procedure for isolating non-SP separate from SP cells has a deleterious effect on the expression of these potentialities. There was no indication of any measurable STRC activity in the SP fraction, even though the experimental design and the number of cells tested would have been sufficient to detect less than 10% of all STRCs present in the unseparated samples.
NOD/SCID-β2-microglobulin-/- engraftment data for human CD3- SP and CD3- non-SP LD mPB cells
Cells transplanted, no. of cells per mouse . | Proportion of repopulated mice . | . | |
|---|---|---|---|
| . | STRC-Ms . | STRC-MLs . | |
| CD3- non-SP | |||
| Experiment no. 1 | |||
| 1.2 | 0/3 | 3/3 | |
| 2.12 | 0/3 | 2/3 | |
| 8.6 | 0/3 | 2/3 | |
| 28.7 | 2/2 | 4/4 | |
| Experiment no. 2 | |||
| 4.0 | 0/3 | 1/3 | |
| 1.0 | 0/2 | 0/1 | |
| 42.0 | 0/3 | 1/3 | |
| Experiment no. 3 | |||
| 1.2 | 0/3 | 1/2 | |
| 2.8 | 1/3 | 1/3 | |
| 11.2 | 3/3 | 2/3 | |
| Experiment no. 4 | |||
| 2.6 | 0/3 | 0/3 | |
| 6.3 | 0/3 | 0/2 | |
| 25.0 | 0/3 | 0/3 | |
| Experiment no. 5 | |||
| 1.2 | 0/3 | 0/1 | |
| 5.1 | 1/2 | 0/2 | |
| 20.5 | 3/3 | 1/1 | |
| Experiment no. 6 | |||
| 2.5 | 0/3 | 2/3 | |
| 7.5 | 0/3 | 1/3 | |
| 30.0 | 0/3 | 0/2 | |
| Total | 10/56 | 21/49 | |
| CD3- SP | |||
| Experiment no. 1 | |||
| 0.52-12.5 | 0/13 | 0/13 | |
| Experiment no. 2 | |||
| 4.00-39.0 | 0/9 | 0/3 | |
| Experiment no. 3 | |||
| 3.90-29.67 | 0/9 | 0/7 | |
| Experiment no. 4 | |||
| 2.45-23.55 | 0/8 | 0/8 | |
| Experiment no. 5 | |||
| 0.42-3.60 | 0/9 | 0/7 | |
| Total | 0/48 | 0/38 | |
Cells transplanted, no. of cells per mouse . | Proportion of repopulated mice . | . | |
|---|---|---|---|
| . | STRC-Ms . | STRC-MLs . | |
| CD3- non-SP | |||
| Experiment no. 1 | |||
| 1.2 | 0/3 | 3/3 | |
| 2.12 | 0/3 | 2/3 | |
| 8.6 | 0/3 | 2/3 | |
| 28.7 | 2/2 | 4/4 | |
| Experiment no. 2 | |||
| 4.0 | 0/3 | 1/3 | |
| 1.0 | 0/2 | 0/1 | |
| 42.0 | 0/3 | 1/3 | |
| Experiment no. 3 | |||
| 1.2 | 0/3 | 1/2 | |
| 2.8 | 1/3 | 1/3 | |
| 11.2 | 3/3 | 2/3 | |
| Experiment no. 4 | |||
| 2.6 | 0/3 | 0/3 | |
| 6.3 | 0/3 | 0/2 | |
| 25.0 | 0/3 | 0/3 | |
| Experiment no. 5 | |||
| 1.2 | 0/3 | 0/1 | |
| 5.1 | 1/2 | 0/2 | |
| 20.5 | 3/3 | 1/1 | |
| Experiment no. 6 | |||
| 2.5 | 0/3 | 2/3 | |
| 7.5 | 0/3 | 1/3 | |
| 30.0 | 0/3 | 0/2 | |
| Total | 10/56 | 21/49 | |
| CD3- SP | |||
| Experiment no. 1 | |||
| 0.52-12.5 | 0/13 | 0/13 | |
| Experiment no. 2 | |||
| 4.00-39.0 | 0/9 | 0/3 | |
| Experiment no. 3 | |||
| 3.90-29.67 | 0/9 | 0/7 | |
| Experiment no. 4 | |||
| 2.45-23.55 | 0/8 | 0/8 | |
| Experiment no. 5 | |||
| 0.42-3.60 | 0/9 | 0/7 | |
| Total | 0/48 | 0/38 | |
Decreasing numbers of CD3- SP (× 105) and CD3- non-SP (× 102) LD mPB cells were transplanted into sublethally irradiated NOD/SCID-β2-microglobulin-/- mice in 6 independent experiments. The STRC-M and STRC-ML activity of the cells injected was detected by FACS of human cells present in the murine bone marrow 3 (STRC-Ms) and 8 (STRC-MLs) weeks later. A positive mouse was defined as one containing more than 10 human CD45/71+ cells in 20 000 PI- cells.
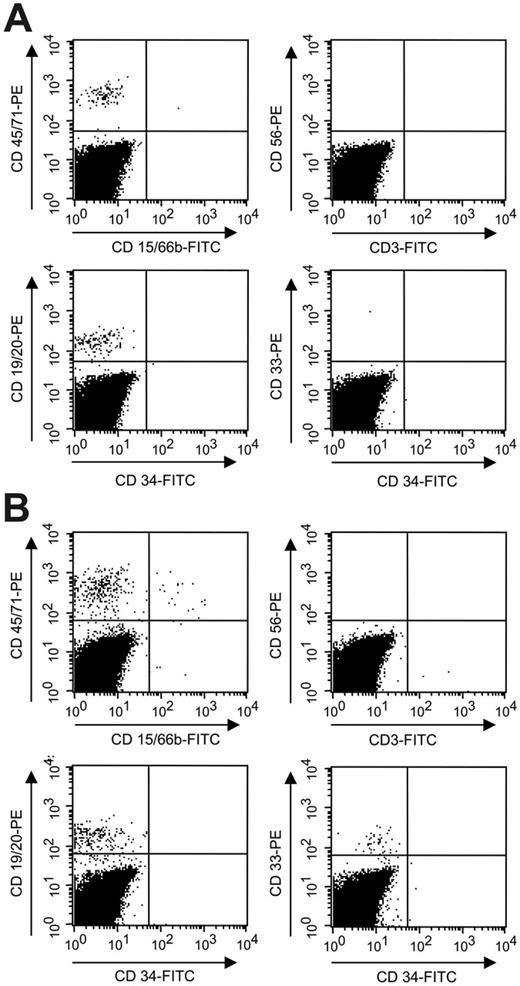
After transplantation of SP cells, no human myeloid cells (CD33+, CD15+, CD66b+, glycophorin A+), T cells (CD3+), natural killer cells (CD56+), or CD34+ cells were detectable in the bone marrow of the mice at any time point (3, 8, or 12 weeks after transplantation). Interestingly, however, a late appearance of human B-lymphoid cells was observed at 12 weeks after transplant in some recipients (Figure 1). These results suggest the existence of an SP repopulating cell that is lymphoid restricted. Because adult NOD/SCID-β2m–/– mice do not support robust human natural killer (NK) or T-cell development, the possibility that these SP cells could have additional lymphoid differentiation abilities cannot be excluded. Evidence of a NK precursor detectable in vitro that lacks myeloid differentiation potential has previously been described in the SP fraction of human cord blood cells,15 and separable cell types with NK or B-lymphoid in vitro differentiation potential have also been reported.16
Human engraftment 12 weeks after transplantation. B-lymphoid–restricted engraftment of human lin– CD3– SP mPB cells (A) and myeloid-lymphoid engraftment of human lin– CD3– non-SP mPB cells (B). FACS analysis of bone marrow cells of mice 12 weeks after transplantation. Cells were stained with anti–human CD45/71-PE, CD15/66b-FITC, CD56-PE, CD3-FITC, CD19/20-PE, CD34-FITC, and CD33-PE. B-lymphoid cells were detected in 4 of 19 mice after transplantation of 300 to 3000 lin– CD3– SP mPB cells in 3 independent experiments.
Human engraftment 12 weeks after transplantation. B-lymphoid–restricted engraftment of human lin– CD3– SP mPB cells (A) and myeloid-lymphoid engraftment of human lin– CD3– non-SP mPB cells (B). FACS analysis of bone marrow cells of mice 12 weeks after transplantation. Cells were stained with anti–human CD45/71-PE, CD15/66b-FITC, CD56-PE, CD3-FITC, CD19/20-PE, CD34-FITC, and CD33-PE. B-lymphoid cells were detected in 4 of 19 mice after transplantation of 300 to 3000 lin– CD3– SP mPB cells in 3 independent experiments.
It should be noted that the present experiments do not provide information about the SP phenotype of human mPB cells with long-term repopulating activity. In fact such experiments are not currently feasible because of the greater than 10-fold lower frequencies of long-term repopulating cells (LTRCs) in mPB harvests,1,18 and these cells would not have been detected in the transplant doses used here which already required maximally practical sorting times (4 hours) and starting cell numbers. We have, however, previously established using the same sorting protocol, that all of the LTRCs in human fetal liver (in which LTRC are > 100 times more prevalent than in mPB) have an SP phenotype.17,18
Our study demonstrates that purification of SP cells depletes human mPB transplants of STRC-M activity. The Hoechst staining and sorting procedure also appears to affect their megakaryocytic and granulocytic differentiation activity. Because Hoechst dye intercalates into DNA, and the sort process involves UV light exposure, potential mutagenic effects of SP sorts need also to be considered. In addition to the difficulty of upscaling this staining and sorting procedure for clinical applications, our results raise concerns about critical cell types that would be eliminated using SP cell–enriched transplants.
Prepublished online as Blood First Edition Paper, May 30, 2006; DOI 10.1182/blood-2006-03-013599.
Supported by grants from the Dr Mildred Scheel Stiftung für Krebsforschung, Bonn, Germany (H.G.), the Deutsche Forschungsgemeinschaft (grant Ka 976/4-1) (C.v.K.), the German Minister for Education and Research (grant D1 KV9527/7) (C.v.K.), the National Institutes of Health (grant P01-HL55435) (C.J.E.), the National Cancer Institute of Canada (grant 013003 with funds from the Terry Fox Run) (C.J.E.), and the Stem Cell Network (C.J.E.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Marie Follow and Klaus Geiger for cell sorting. We also gratefully acknowledge helpful discussions and support from Dr C. Peters and Dr R. Mertelsmann.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal