Abstract
Interruption of the normal fetal-to-adult transition of hemoglobin expression should largely ameliorate sickle cell and beta-thalassemia syndromes. Achievement of this clinical goal requires a robust understanding of gamma-globin gene and protein silencing during human development. For this purpose, age-related changes in globin phenotypes of circulating human erythroid cells were examined from 5 umbilical cords, 99 infants, and 5 adult donors. Unexpectedly, an average of 95% of the cord blood erythrocytes and reticulocytes expressed HbA and the adult beta-globin gene, as well as HbF and the gamma-globin genes. The distribution of hemoglobin and globin gene expression then changed abruptly due to the expansion of cells lacking HbF or gamma-globin mRNA (silenced cells). In adult reticulocytes, less than 5% expressed gamma-globin mRNA. These data are consistent with a “switching” model in humans that initially results largely from gamma- and beta-globin gene coexpression and competition during fetal development. In contrast, early postnatal life is marked by the rapid accumulation of cells that possess undetectable gamma-globin mRNA and HbF. The silencing phenomenon is mediated by a mechanism of cellular replacement. This novel silencing pattern may be important for the development of HbF-enhancing therapies.
Introduction
Within minutes of birth, the oxygen content of human blood abruptly rises, and the benefit of fetal hemoglobin (alpha2gamma2; HbF) is lost. In humans and some other mammals, HbF is replaced by adult hemoglobin (alpha2beta2; HbA) as the predominant respiratory protein in blood. The expression of HbA improves the efficiency of oxygen delivery to tissues. This hemoglobin switching process begins during fetal development, and is largely completed during infancy. A reduced rate of fetal hemoglobin loss during this developmental period may be caused by placental insufficiency and congenital cyanotic heart disease.1 Elevated levels of butyric acid in diabetic mothers also delay the switching phenomenon in the fetus.2 Paradoxically, those elevated levels of butyric acid have no effects upon HbF in adults with diabetes.3
In addition to its general role in respiration during fetal life, HbF expression during postnatal life is beneficial to patients with beta-hemoglobinopathies. The consideration of a therapeutic function for HbF emerged when it was recognized that blood from infants with sickle cell disease had a low rate of sickling.4 Sickle cell patients with hereditary persistence of fetal hemoglobin expression were later found to have less severe clinical syndromes.5 With the decline in HbF expression, beta-hemoglobinopathy infants manifest their phenotype near the completion of the fetal-to-adult hemoglobin transition at about 6 months of age. In this setting, the beneficial effects of HbF extend beyond the ability to deliver oxygen. HbF possesses the disease-specific functions of interrupting sickle hemoglobin polymerization6 and reducing globin chain imbalances in beta-thalassemic erythrocytes.7 The clinical benefits of HbF expression in adult patients depend largely upon the level and cellular distribution of HbF.8 In adults, therapeutic approaches are being sought to reactivate HbF expression to levels above 20% in a majority of circulating erythrocytes. In order to develop a preventive strategy, a more robust description of the fetal-to-adult switching phenomenon is required. For this purpose, single-cell assays that define the distribution and magnitude of fetal hemoglobin protein and gamma-globin mRNA during perinatal human life are reported here. Unexpectedly, it was discovered that gamma-globin mRNA and protein silencing in humans is almost completely confined to postnatal development.
Patients, materials, and methods
Patients
Peripheral blood samples from 109 anonymous donors (5 newborn umbilical cord, 99 infant, and 5 adult) were obtained using study protocols at the National Institutes of Health, the National Children's Hospital in Washington, and National Naval Medical Center (protocol no. B03-035). The research studies were performed at the National Institutes of Health under an exemption from 45 CFR 46, which is in accord with the principles of Helsinki. High-performance liquid chromatography (HPLC) analyses demonstrated no hemoglobinopathies. The adult blood was obtained from healthy blood donors and the newborn blood from umbilical cord during uncomplicated term deliveries. The infant donors had no history of transfusions and had normal blood parameters9 at the time the samples were studied. A summary of those hematologic parameters for all 109 donors is provided in Table S1 (available at the Blood website; see the Supplemental Table link at the top of the online article). Eighty-six infant donor blood samples were used for hemoglobin protein analyses. The remaining 13 infant donor blood samples were used for reticulocyte sorting and mRNA quantitation.
Flow cytometry analysis and quantitative polymerase chain reaction (qPCR) for gamma- and beta-globin expression
A sorting strategy based upon CD71 expression10 was modified for the isolation of single, nonpermeabilized reticulocytes. A 96-well autoclone algorithm was designed for single-cell isolation using an EPICS ELITE ESP flow cytometer (Beckman Coulter, Hialeah, FL).
The cDNA was prepared from single cells using reagents from the Cells-to-cDNA II kit (Ambion, Austin, TX). Cells were sorted directly into lysis buffer, and the lysates were transferred to PCR reaction tubes (Applied Biosystems, Foster City, CA). The tubes were incubated at 75°C for 15 minutes and then treated with DNase I at 37°C for 15 minutes. The DNase I was heat inactivated at 75°C for 5 minutes, and dNTP and oligo-dT were added. The mixture was incubated at 70°C for 3 minutes and immediately transferred to ice for 1 minute. RT buffer (10×), RNaseOut (Invitrogen, Carlsbad, CA), and Moloney murine leukemia virus (M-MLV) reverse transcriptase were added in sequence to the mixture and incubated at 42°C for 1 hour. The reverse-transcriptase enzyme was heat inactivated at 94°C for 10 minutes.
The cDNA samples were split into duplicates, and then amplified with gamma- and beta-globin–specific reagents as reported previously.11 Transcript copy numbers were calculated from quantitative PCR and standard curve comparisons (20-200 000 copies). Reticulocytes with fewer than 200 copies of total globin (gamma + beta) mRNA were discarded due to excess RNA degradation. At least 45 reticulocytes were examined from each donor. Among those reticulocytes, gamma- or beta-globin gene silencing was identified by amplification below the sensitivity limit of the assay (≤ 20 copies/cell).
Immunostaining for fetal and adult hemoglobin
Immunostaining with antibodies directed against glycophorin A (GPA), CD71 (Beckman Coulter), fetal hemoglobin (HbF-PE; Caltag Laboratories, Burlingame, CA), and adult hemoglobin (HbA-FITC; Perkin Elmer Wallac, Norton, OH) were performed as previously reported.12 A minimum of 30 000 cells was analyzed using an EPICS ELITE ESP flow cytometer (Beckman Coulter). Three-dimensional analyses were performed using Expo software (version 1.2; Beckman Coulter).
Hemoglobin analyses
Cellular lysates of all blood samples were analyzed for HbF and HbA content using a 20 × 4-mm POLYCATA column (Poly LC, Columbia, MD) fitted to a Gilson HPLC system (Gilson, Middleton, WI) as previously described.13
HbF expression and silencing in human erythrocytes. (A) HPLC analyses were performed on peripheral blood of 86 infant donors and plotted as HbF/(HbA + HbF) (expressed as a ratio; y-axis) versus postnatal age (months; x-axis). (B) The distribution of HbF-silenced cells (expressed as a percentage; y-axis) versus postnatal age (months; x-axis) as determined by flow cytometry. The smooth trend lines were drawn using averages for each 10-week interval. The postnatal (PN) age of the donors is shown on the x-axis with vertical gray lines at 6 months. Postnatal ages range from 1 day to 60 months.
HbF expression and silencing in human erythrocytes. (A) HPLC analyses were performed on peripheral blood of 86 infant donors and plotted as HbF/(HbA + HbF) (expressed as a ratio; y-axis) versus postnatal age (months; x-axis). (B) The distribution of HbF-silenced cells (expressed as a percentage; y-axis) versus postnatal age (months; x-axis) as determined by flow cytometry. The smooth trend lines were drawn using averages for each 10-week interval. The postnatal (PN) age of the donors is shown on the x-axis with vertical gray lines at 6 months. Postnatal ages range from 1 day to 60 months.
Results
Peripheral blood samples from 86 of 99 infant donors were examined for hemoglobin profiling. The remaining 13 infant samples were used for globin mRNA quantitation (Table 1). The median age of the infants was 21 weeks (range, 0 days to 5 years). All of the infants had hematologic parameters within normal limits (Table S1) and had received no prior blood transfusions. HPLC analyses and HbF detection using flow cytometry were determined for each donor as displayed as a function of age (Figure 1). The loss of HbF during early infancy among this infant population occurred rapidly over the first 6 months of life with a pattern nearly identical to the classic description.14 After 6 months, the HbF was reduced to levels below 10% of the total for the remainder of life. In addition to measuring the bulk levels of HbF in the infant blood samples, flow cytometry was used to determine the distribution of HbF within the cellular population. Thirty thousand cells were examined from each donor. HbF silencing is defined using flow cytometry as detection of HbF-based fluorescence at levels equivalent to the isotypic controls. As shown in Figure 1B, the percentage of HbF-silenced cells remained below 10% during the initial weeks of postnatal life. Similarly low percentages of silencing were detected in umbilical cord blood samples (not shown). The fraction of HbF-silenced erythrocytes rose rapidly during the next several months to become the predominant phenotype. Considerable variation in the percentage of HbF-silenced cells was noted during the first year of life, but the older infants demonstrated silencing in greater than 70% of their circulating erythrocytes.
Profiles of patient samples used for quantitation of beta- and gamma-globin mRNA
Patient no. . | Gestational age, wk . | Postnatal age, mo . | HbF/(HbF + HbA), % . | Hemoglobin, g/L . | MCV, fL . | ARC, 109/L . |
|---|---|---|---|---|---|---|
| CB 1 | 37 | 0 | 82.2 | 164 | 111 | 172 |
| CB 2 | 38 | 0 | 87.2 | 178 | 117 | 152 |
| CB 3 | 41 | 0 | 78.1 | 140 | 102 | 163 |
| CB 4 | NA | 0 | 67.4 | 163 | 112 | 120 |
| CB 5 | NA | 0 | 81.1 | 157 | 109 | 216 |
| IN 1 | 39 | 0.33 | 81.3 | 143 | 104 | 80 |
| IN 2 | NA | 0.4 | 76.4 | 123 | 99 | 82 |
| IN 3 | 39 | 0.43 | 90.9 | 128 | 93 | 84 |
| IN 4 | 35 | 0.83 | 86.8 | 96 | 99 | 64 |
| IN 5 | NA | 2.3 | 34.6 | 115 | 86 | 53 |
| IN 6 | NA | 2.6 | 29.7 | 104 | 87 | 44 |
| IN 7 | NA | 2.6 | 43.5 | 100 | 85 | 40 |
| IN 8 | 33 | 4 | 26.7 | 110 | 78 | 55 |
| IN 9 | NA | 6 | 7.6 | 114 | 76 | 54 |
| IN 10 | 34 | 8 | 2.8 | 134 | 78 | 39 |
| IN 11 | 42 | 9 | 3.7 | 103 | 75 | 42 |
| IN 12 | NA | 9 | 2.1 | 115 | 79 | 43 |
| IN 13 | 26 | 35 | 2.1 | 133 | 76 | 51 |
| AB 1 | NA | NA | <0.5 | WNL | NA | NA |
| AB 2 | NA | NA | <0.5 | WNL | NA | NA |
| AB 3 | NA | NA | <0.5 | WNL | NA | NA |
| AB 4 | NA | NA | <0.5 | WNL | NA | NA |
| AB 5 | NA | NA | <0.5 | WNL | NA | NA |
Patient no. . | Gestational age, wk . | Postnatal age, mo . | HbF/(HbF + HbA), % . | Hemoglobin, g/L . | MCV, fL . | ARC, 109/L . |
|---|---|---|---|---|---|---|
| CB 1 | 37 | 0 | 82.2 | 164 | 111 | 172 |
| CB 2 | 38 | 0 | 87.2 | 178 | 117 | 152 |
| CB 3 | 41 | 0 | 78.1 | 140 | 102 | 163 |
| CB 4 | NA | 0 | 67.4 | 163 | 112 | 120 |
| CB 5 | NA | 0 | 81.1 | 157 | 109 | 216 |
| IN 1 | 39 | 0.33 | 81.3 | 143 | 104 | 80 |
| IN 2 | NA | 0.4 | 76.4 | 123 | 99 | 82 |
| IN 3 | 39 | 0.43 | 90.9 | 128 | 93 | 84 |
| IN 4 | 35 | 0.83 | 86.8 | 96 | 99 | 64 |
| IN 5 | NA | 2.3 | 34.6 | 115 | 86 | 53 |
| IN 6 | NA | 2.6 | 29.7 | 104 | 87 | 44 |
| IN 7 | NA | 2.6 | 43.5 | 100 | 85 | 40 |
| IN 8 | 33 | 4 | 26.7 | 110 | 78 | 55 |
| IN 9 | NA | 6 | 7.6 | 114 | 76 | 54 |
| IN 10 | 34 | 8 | 2.8 | 134 | 78 | 39 |
| IN 11 | 42 | 9 | 3.7 | 103 | 75 | 42 |
| IN 12 | NA | 9 | 2.1 | 115 | 79 | 43 |
| IN 13 | 26 | 35 | 2.1 | 133 | 76 | 51 |
| AB 1 | NA | NA | <0.5 | WNL | NA | NA |
| AB 2 | NA | NA | <0.5 | WNL | NA | NA |
| AB 3 | NA | NA | <0.5 | WNL | NA | NA |
| AB 4 | NA | NA | <0.5 | WNL | NA | NA |
| AB 5 | NA | NA | <0.5 | WNL | NA | NA |
ARC indicates absolute reticulocyte count; CB, umbilical cord blood; IN, infant donor blood; AB, adult donor blood; NA, not available; and WNL, within normal limits.
To further determine the cellular distribution of HbF and HbA protein expression during infancy, dual-stained flow cytometry was performed and compared with isotypic control antibodies. Umbilical cord and adult samples were also studied for comparison. HbF histograms and HbF-HbA scatterplots from 8 representative donors are shown in Figure 2. In addition, a 3-dimensional view of each analysis is provided to demonstrate the separate cell-population peaks (in red). Adult hemoglobin was detected in more than 95% of cord, infant, and adult cells. In cord blood, fetal hemoglobin was also determined to have a pancellular distribution manifest as a single peak (Figure 2A). As shown in Figure 2B, a small population of HbF-silenced cells was clearly detected at one month of age. That silenced population was separate from the HbF-expressing cells, and the peak grew rapidly to become predominant by 4 to 6 months of age (Figure 2E-2G). In adults, HbF silencing became pancellular with HbF detected in less than 5% of cells (Figure 2H). An additional peak with intermediate-level HbF fluorescence was also detected in the infant samples as highlighted in the 3-dimensional views. That intermediate-level peak was very clearly detected in the blood from the 2-month-old infant (compare Figure 2A, C, and H). No infant possessed a single peak of intermediate fluorescence in the absence of higher-level or silenced peaks. This pattern is consistent with a replacement of those erythrocytes present in umbilical cord blood with cells having distinctly lower or absent levels of HbF.
Flow cytometric analyses of HbF and HbA expression during postnatal development. Representative flow cytometric histograms from (A) umbilical cord blood, (B-G) blood from infants of ages 1 to 6 months, respectively, and (H) adult blood. Cells were dual stained with anti-HbF and anti-HbA antibodies. Thirty thousand cells were analyzed for each sample. Left panels show histogram analyses of HbF expression (x-axis) versus the cell count (y-axis). Center panels show scatterplot analyses with HbF fluorescence (x-axis) versus HbA fluorescence (y-axis). The percentage of HbF-silenced cells is shown in the top left of each center panel. Right panels provide a 3-dimensional, density view of the data shown in the middle panels. The vertical axis represents the cell count at each level of HbF and HbA fluorescence. The HbF and HbA coordinates with cells are shown as peaks with arbitrarily assigned colors (red-colored peaks denote the highest densities of cells). Bars shown in left and center panels denote the upper level of fluorescence among 98% of cells stained with the isotypic controls.
Flow cytometric analyses of HbF and HbA expression during postnatal development. Representative flow cytometric histograms from (A) umbilical cord blood, (B-G) blood from infants of ages 1 to 6 months, respectively, and (H) adult blood. Cells were dual stained with anti-HbF and anti-HbA antibodies. Thirty thousand cells were analyzed for each sample. Left panels show histogram analyses of HbF expression (x-axis) versus the cell count (y-axis). Center panels show scatterplot analyses with HbF fluorescence (x-axis) versus HbA fluorescence (y-axis). The percentage of HbF-silenced cells is shown in the top left of each center panel. Right panels provide a 3-dimensional, density view of the data shown in the middle panels. The vertical axis represents the cell count at each level of HbF and HbA fluorescence. The HbF and HbA coordinates with cells are shown as peaks with arbitrarily assigned colors (red-colored peaks denote the highest densities of cells). Bars shown in left and center panels denote the upper level of fluorescence among 98% of cells stained with the isotypic controls.
To define the patterns of globin gene expression and silencing, methods were also developed to quantitate mRNA levels within individual reticulocytes. Reticulocytes were identified by coexpression of glycophorin A (GPA) and transferrin receptor (CD71). In separate studies, thiazole orange (TO, an RNA dye) staining confirmed that more than 95% of CD71-expressing erythrocytes in the peripheral blood contain RNA (Figure 3A-B). Visual examination of the sorted cells revealed enucleated reticulocytes (not shown). Based upon those results, reticulocytes (GPA+/CD71+) were sorted as single cells directly into lysis buffer contained in a 96-well plate for reverse-transcriptase (RT)–QPCR processing. For these studies, blood from 23 donors (5 umbilical cord, 13 infant, 5 adult) was obtained. Gestational ages were obtained for 10 of the 23 patient samples and ranged from 26 to 42 weeks. The hematologic parameters for each of the donors whose blood was sorted for mRNA analyses are provided in Table 1. Polycythemia was expected in both cord and newborn blood resulting from the relative hypoxia of intrauterine life. The red cell size (MCV) was also higher in cord blood and newborns and then decreased during the first months of life. Furthermore, the levels of fetal hemoglobin decreased rapidly during infancy to levels consistent with the infants studied in Figure 1. In each sample, the values shown fall within the normal age-adjusted range.9
Reticulocyte isolation by flow cytometry. Peripheral blood red cells were dual stained with anti–transferrin receptor (CD71) and a RNA-intercalating dye, thiazole orange (TO). (A) The CD71 fluorescence pattern of the erythrocyte population in peripheral blood. The forward scatter (size) is shown on the x-axis, and the box shows the CD71+ cells. (B) The TO fluorescence of the CD71+ cells in panel A; 97.5% of the CD71+ cells were also positive for TO.
Reticulocyte isolation by flow cytometry. Peripheral blood red cells were dual stained with anti–transferrin receptor (CD71) and a RNA-intercalating dye, thiazole orange (TO). (A) The CD71 fluorescence pattern of the erythrocyte population in peripheral blood. The forward scatter (size) is shown on the x-axis, and the box shows the CD71+ cells. (B) The TO fluorescence of the CD71+ cells in panel A; 97.5% of the CD71+ cells were also positive for TO.
As measured by quantitative PCR, cord and adult blood reticulocytes were found to have distinct patterns of globin mRNA expression (Figure 4). Figure 4A-B show the distribution and copy numbers of gamma-globin expression in cord and adult blood reticulocytes. The average gamma-globin copy numbers shown in Figure 4B are derived from the subset of clones with detectable levels of gamma-globin mRNA (nonsilenced reticulocytes). Betaglobin and total (gamma + beta) globin levels are also shown for comparison (Figure 4C-4F). Among the cord blood reticulocytes, pancellular distributions of gamma-globin (208 [94.5%] of 220) and beta-globin (220 [100%] of 220) mRNA were detected. Among the individual cord blood donors, the percentage of gamma-globin mRNA–silenced reticulocytes ranged from 0% to 9.3%. Cord blood reticulocytes demonstrated an average gamma-globin mRNA copy number of 1740 ± 1350 copies/cell compared with 2380 ± 2180 copies of beta-globin mRNA per cell. Since reticulocytes have a shorter half-life in the circulation, the lower percentage of gamma-globin mRNA in reticulocytes compared with fetal hemoglobin in whole blood (Table 1; whole blood, 79.2% ± 7.4%) at birth is consistent with a more advanced stage of hemoglobin switching in those immature cells.
Adult reticulocytes also demonstrated a pancellular distribution of beta-globin mRNA, but gamma-globin mRNA was not detected (< 20 copies/cell) in 96.6% ± 3.9% of the reticulocytes. This pattern is consistent with the restricted expression pattern of HbF reported previously.15 The average copy number of gamma-globin mRNA was reduced from 1740 ± 1350 copies/cell in the cord blood to 136 ± 165 copies/cell among those rare adult reticulocytes that expressed the gamma-globin genes. In contrast to the profound loss of gamma-globin mRNA, beta-globin mRNA increased to 4970 ± 3710 copies/cell in adult reticulocytes. The total (gamma + beta) globin copy numbers in cord and adult blood were not significantly different (Figures 4E-F).
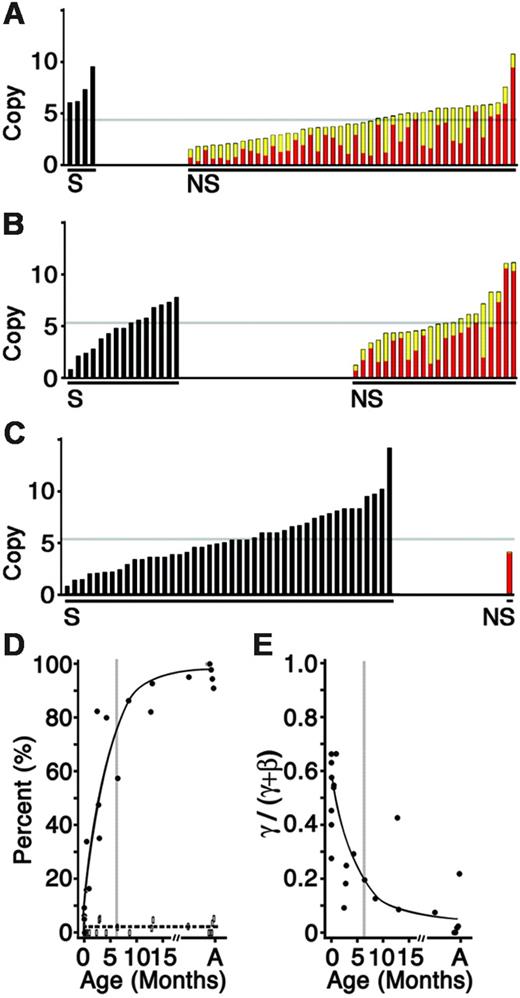
The levels and distribution of gamma-globin mRNA expression in sorted reticulocytes from human infant blood were also studied. The gamma- and beta-globin mRNA profiles of 632 single reticulocytes from the peripheral blood of 13 infants ranging from the ages of 1 day to 35 months were determined. The profiles from individual reticulocytes from an umbilical cord, 2-month-old infant, and adult donor are shown for comparison (Figure 5A-C). As shown in Figure 5A, silencing of the gamma-globin genes was detectable in umbilical cord blood. The average level of gamma silencing in cord blood was 5%. The population of gamma-silenced reticulocytes then increased rapidly after parturition. The silenced population included reticulocytes with a wide distribution of total (gamma + beta) levels at all developmental stages. After 3 to 5 months of extrauterine life, 80% of the reticulocytes demonstrated silencing of the gamma-globin genes. Thereafter, the rate of silencing became more gradual (Figure 5D). By the third year, an adult pattern with more than 90% silenced reticulocytes was seen. Of importance, the percentage of reticulocytes that contained low levels of gamma-globin mRNA remained stable (Figure 5D dashed line), suggesting a bimodal distribution for the silenced and nonsilenced reticulocytes. As predicted by such a bimodal distribution, the percentage of reticulocytes containing higher levels of gamma-globin mRNA (> 100 copies per cell) was reduced as the percentage of gamma-globin mRNA–silenced reticulocytes increased (data not shown). In addition to their rapid replacement with gamma-globin mRNA–silenced reticulocytes, the shrinking population of nonsilenced reticulocytes exhibited fractionally less gamma-globin mRNA with advancing age (Figure 5E). Of interest, during the first 2 to 4 weeks of life, the mean percentage of gamma-globin mRNA among the nonsilenced population was not significantly decreased compared with cord blood. Thereafter, gamma-globin mRNA levels decreased and beta-globin mRNA levels increased with advancing age. By 3 years of age, the level of gamma-globin mRNA among the nonsilenced population (4.9% of the reticulocytes) was reduced to 460 ± 590 copies/cell. The lone adult reticulocyte in Figure 5C demonstrated a gamma-globin mRNA level of only 115 copies compared with 4700 copies of beta-globin mRNA. The pattern of gamma-globin gene silencing shown here in reticulocytes is similar to that demonstrated for HbF (Figure 1) in suggesting that silencing of the fetal gene products is largely confined to postnatal human life.
Levels of gamma- and beta-globin mRNA in umbilical cord and adult blood reticulocytes. The copy number (× 1000) of cDNA for gamma-globin (A-B), beta-globin (C-D), and total (E,F; gamma + beta) in 440 individual reticulocytes collected from umbilical cord (CB) and adult (AB) are shown on each y-axis (Copy no.). Panels on the left (A,C,E) display data from 220 individual cells (x-axis) arranged from lowest to highest copy number. Panels on the right (B,D,F) show the mean copy number with standard deviations from each of the 5 cord blood donors (x-axis). Black bars indicates cord blood; open bars, adult blood; and gray bars, mean of 220 individual cells. AB indicates adult blood; CB, cord blood; and m, mean. Asterisks signify significance between cord and adult mean values (P < .005, paired Student t test).
Levels of gamma- and beta-globin mRNA in umbilical cord and adult blood reticulocytes. The copy number (× 1000) of cDNA for gamma-globin (A-B), beta-globin (C-D), and total (E,F; gamma + beta) in 440 individual reticulocytes collected from umbilical cord (CB) and adult (AB) are shown on each y-axis (Copy no.). Panels on the left (A,C,E) display data from 220 individual cells (x-axis) arranged from lowest to highest copy number. Panels on the right (B,D,F) show the mean copy number with standard deviations from each of the 5 cord blood donors (x-axis). Black bars indicates cord blood; open bars, adult blood; and gray bars, mean of 220 individual cells. AB indicates adult blood; CB, cord blood; and m, mean. Asterisks signify significance between cord and adult mean values (P < .005, paired Student t test).
Distribution of gamma- and beta-globin mRNA during postnatal development. The copy number (× 1000) of cDNA for individual reticulocytes sorted from (A) an umbilical cord, (B) a 2-month old infant, and (C) an adult human. Black bars represent gamma-globin gene–silenced (S) reticulocytes. Colored bars represent the nonsilenced (NS) reticulocytes with beta-globin copy number represented in red and gamma-globin copy number in yellow. The light gray line reflects a mean copy number for each donor. (D) Percentage of gamma-globin gene–silenced reticulocytes (•, solid line; gamma-globin mRNA below detection limit of 20 copies per cell) versus those reticulocytes containing low levels of gamma-globin mRNA (open boxes, dashed line; 21-100 copies of gamma-globin mRNA per cell) arranged according to donor age (x-axis). (E) Gamma-globin level among the nonsilenced populations expressed as a percentage of the total (gamma + beta; y-axis) arranged according to donor age (month; x-axis). The smooth trend lines were drawn using averages for each 10-week interval. In panels D and E, the postnatal (PN) age of the donors is shown on the x-axis with vertical gray lines at 6 months. A indicates adult.
Distribution of gamma- and beta-globin mRNA during postnatal development. The copy number (× 1000) of cDNA for individual reticulocytes sorted from (A) an umbilical cord, (B) a 2-month old infant, and (C) an adult human. Black bars represent gamma-globin gene–silenced (S) reticulocytes. Colored bars represent the nonsilenced (NS) reticulocytes with beta-globin copy number represented in red and gamma-globin copy number in yellow. The light gray line reflects a mean copy number for each donor. (D) Percentage of gamma-globin gene–silenced reticulocytes (•, solid line; gamma-globin mRNA below detection limit of 20 copies per cell) versus those reticulocytes containing low levels of gamma-globin mRNA (open boxes, dashed line; 21-100 copies of gamma-globin mRNA per cell) arranged according to donor age (x-axis). (E) Gamma-globin level among the nonsilenced populations expressed as a percentage of the total (gamma + beta; y-axis) arranged according to donor age (month; x-axis). The smooth trend lines were drawn using averages for each 10-week interval. In panels D and E, the postnatal (PN) age of the donors is shown on the x-axis with vertical gray lines at 6 months. A indicates adult.
Discussion
General patterns for fetal-to-adult hemoglobin switching have been accepted for decades based upon bulk assays using whole blood.14 Microscopy and flow cytometry methods were also developed for single-cell assays of HbF, but quantitation of HbF using those methods continues to be suboptimal. Only recently have quantitative, single-cell methods become available for the direct study of globin gene switching using clinical samples. This study was carried out as the first description of the levels and distribution of gamma-globin protein and gene expression in humans during the period from fetal to adult development.
As noted by the similar patterns of HbF (Figure 1) and gamma-globin mRNA (Figure 5) reduction, HbF expression in vivo is regulated primarily at the genetic level. The pancellular mixture of fetal and adult hemoglobin and mRNA species during later fetal development (cord blood) suggests that fetal hemoglobin switching is half completed at the time of birth in term pregnancies. The absence of significant percentages of HbF-protein–silenced erythrocytes and gamma-globin gene–silenced reticulocytes in cord blood, despite the relatively advanced stage of globin gene switching, was unexpected and novel. It suggests that the genetic program for hemoglobin switching encompasses gradual loss of the gamma-globin gene in the absence of silencing during the entire period of fetal development. The presence of erythrocytes containing both HbF and HbA molecules may be advantageous in the growing fetus for improving both the oxygen supply (HbF advantage; placental uptake) and delivery (HbA advantage; peripheral tissue release). While low levels of gamma-globin gene–silenced reticulocytes were detected in cord blood, a close temporal relationship between birth and the rapid expansion of the silenced population was seen. The steep postnatal rise in silenced cells suggests that respiration or some other stimulus related to birth itself may trigger a cellular program that promotes gamma-globin gene silencing. As such, there is neither theoretical advantage nor experimental evidence that HbF silencing plays any major role in hemoglobin switching before birth. Hence, these data suggest that humans synchronize silencing of the fetal globin genes and proteins with the onset of normoxic respiration.
Since gamma-globin gene silencing was barely detected in cord blood reticulocytes, competition between the gamma- and beta-globin genes is possibly the major genetic mechanism that underlies hemoglobin switching during fetal development.16,17 Gene competition could explain the gradual loss of gamma-globin among nonsilenced cells that progresses with a pattern that is largely independent of parturition. The gradual loss is consistent with an autonomous control mechanism for globin gene switching.18,19 However, the birth-related, rapid expansion of a gamma-globin–silenced population is more consistent with models of inductive reprogramming or clonal replacement.20,21 Considerable controversy exists with regard to which of these models most closely describes hemoglobin switching. Data from transplantation studies are ambiguous in describing the potential for developmental stage-specific microenvironments to reprogram globin gene expression.22,23 Further studies could help determine whether some of the inconsistencies between models and data can be explained, in part, by the developmental distinction of globin gene competition and silencing reported here.
The temporal association between gamma-globin gene silencing and birth also hints that environmental signals may play an important role in fetal-to-adult hemoglobin switching. Embryonic-to-fetal hemoglobin switching correlates well with the onset of erythropoietin production in mammals.24,25 Hormonal metamorphosis is also associated with hemoglobin switching in amphibians.26 However, no hormonal trigger has been identified to date for the fetal-to-adult switch in humans or other mammals. Results from sheep,27 primates,28 and humans29,30 suggest that signal transduction does play a role in the regulation of the gamma-globin gene in adults. In the laboratory, pancellular expression of the gamma-globin gene is achievable via signal transduction by specific cytokines.11 For these reasons, signaling compounds are being considered for pharmaceutical development.31
The realization that significant gamma-globin gene silencing is confined to postnatal development may be important for the interpretation of previously reported data as well as current efforts to increase HbF. The therapeutic potential for butyrate compounds to increase HbF was derived from the observation that diabetic pregnancies are associated with delayed fetal hemoglobin switching as measured in cord blood.2 Despite this reported effect upon hemoglobin modulation in the fetus, diabetes has little or no effect upon fetal hemoglobin levels in adults.3 In model systems, butyrate does not reactivate silenced gamma-globin genes.32,33 Hence, our data suggest that the paradoxic effects of diabetes upon HbF may be due to the absence in the fetus and the presence in adults of gamma-globin gene silencing. As a result of postnatal silencing, butyrate compounds may have an attenuated clinical effect if begun after the silencing phenomenon is completed. Independent of diabetes, children with sickle cell disease have a markedly delayed pattern of HbF silencing, and hydroxyurea has greater effects upon HbF in those children than adults.34,35 This age-related difference in therapeutic effect may also be related to the level of silencing of the gamma-globin genes. Based upon the unexpected realization that gamma-globin silencing is a postnatal phenomenon, consideration should be given to initiating HbF-modulating therapies very early in life before gamma-globin gene silencing becomes predominant.
Prepublished online as Blood First Edition Paper, May 30, 2006; DOI 10.1182/blood-2006-04-015859.
P.A.O. performed research and wrote the paper; N.M.G., J.D.S., N.V.B., and Y.T.L. performed research; J.W.M. and C.H.R. (LCDR MC USN) contributed vital reagents; A.N.S. designed research; N.L.C.L. designed research and contributed vital reagents; and J.L.M. supervised and assisted the research team.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
This research was supported by the Intramural Research Program of the NIH, NIDDK. We would like to acknowledge Eberle Schultz for her assistance in this study.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal