Abstract
An inappropriate host response to invading bacteria is a critical parameter that often aggravates the outcome of an infection. Staphylococcus aureus is a major human Gram-positive pathogen that causes a wide array of community- and hospital-acquired diseases ranging from superficial skin infections to severe conditions such as staphylococcal toxic shock. Here we find that S aureus induces inflammatory reactions by modulating the expression and response of the B1 and B2 receptors, respectively. This process is initiated by a chain of events, involving staphylococcal-induced cytokine release from monocytes, bacteria-triggered contact activation, and conversion of bradykinin to its metabolite desArg9bradykinin. The data of the present study implicate an important and previously unknown role for kinin receptor regulation in S aureus infections.
Introduction
Staphylococcus aureus, an important opportunistic Gram-positive human pathogen, is the most common organism isolated from soft-tissue and wound infections. The bacterium can cause a variety of community- and hospital-acquired diseases ranging from relatively benign skin infections, such as furuncles and subcutaneous abscesses, to more severe conditions, including scaled skin syndrome, necrotizing pneumonia, endocarditis, sepsis, and staphylococcal toxic shock syndrome (for reviews, see Lowy1 and Yarwood and Schlievert2 ). In severe conditions, staphylococci may evoke an inappropriate inflammatory host response by modulating so-called host effector systems. For instance, S aureus produces a diverse range of virulence factors contributing to the inflammatory response, among others the enterotoxins and toxic shock syndrome toxin-1 (TSST-1) that form a class of substances also known as pyrogenic toxin superantigens or PTSAgs (for a review, see Balaban and Rasooly3 ). PTSAgs can induce a profound inflammatory reaction by interacting with MHC class II molecules and T-cell antigen receptors disengaged from the normal antigen-specific signal transduction of T cells.4,5 The resulting inflammatory response is by far greater than antigen-specific activation and leads to pathologic levels of proinflammatory cytokines.6
The human contact system, also known as the kallikrein-kinin cascade or intrinsic pathway of coagulation, is another example of a system that can be targeted and affected during infection.7 The contact system consists of 4 factors, 3 serine proteinases (coagulation factors XI and XII, and plasma kallikrein), and 1 nonenzymatic cofactor (high-molecular-weight kininogen). Normally, these factors circulate as zymogens in the bloodstream. Contact activation can occur for instance on newly exposed cellular surfaces and is regulated by limited proteolysis. The initial step is activation of coagulation factor XII, which converts plasma kallikrein into the active form. Active kallikrein in turn amplifies the activation of factor XII, eventually resulting in clot formation, and the release of bradykinin (BK) from the precursor molecule, high-molecular-weight kininogen. Previous studies have shown an interaction between S aureus and the contact system leading to its activation at the bacterial surface.8 As a result, BK is generated and continuously released from the bacterial cell wall over an extended period of time.8 Of interest, this does not apply to all bacterial species. For instance, Streptococcus pneumoniae was not able to activate the contact system in this study.8 BK and its metabolite desArg9BK are potent inflammatory mediators, causing hypotension, increased vascular permeability, edema formation, fever, and pain (for a review, see Mahabeer and Bhoola9 ). Conversion of BK to desArg9BK involves the cleavage of a carboxy-terminal arginine by carboxypeptidases of the N and M type, also known as kininases type I.10 There are 2 kinin receptors described in humans, B1 receptor (B1R) and B2 receptor (B2R) (for a review, see Leeb-Lundberg et al11 ). While BK interacts mainly with B2R, desArg9BK is selective for B1R. The 2 receptors differ also in their expression pattern and pharmacologic profile. B2R is constitutively expressed on most cell types and is rapidly internalized upon agonist binding, followed by its recycling to the cell membrane. B1R, on the other hand, is expressed in very low numbers under physiologic conditions, but is induced upon pathologic insults and autologously in response to agonist binding.12 Upon expression on the cell surface, for instance following stimulation with interleukin 1β (IL-1β) or endotoxin, B1R exhibits high ligand-independent, constitutive activity that is further enhanced by agonist binding.13
The present investigation was undertaken to examine whether S aureus can use the contact system for the induction of inflammatory reactions in the human host. In particular, we wished to analyze the regulation of B1R and B2R at the cellular level in response to treatment with staphylococcal toxins. Our results show that the induction of kinin receptors and their respective ligands is modulated by S aureus and its secreted products. The proposed mechanism may play an important role in severe infections caused by this pathogen.
Materials and methods
Materials
IL-1β was from R&D Systems (Minneapolis, MN); [2,3-Prolyl-3H]BK (2.9×1012 – 3.5×1012 Bq [79-96 Ci]/mmol), des-Arg10-[3,4-prolyl-3,4-3H]kallidin (3.9×1012 Bq [107 Ci]/mmol), and [3H]thymidine (3.0×1012 Bq [80.4 Ci]/mmol) were purchased from PerkinElmer Life Sciences (Wellesley, MA). BK, desArg9BK, desArg10kallidin, BK(1-5), BK(1-6), BK(1-7), and BK(2-7) were from Bachem (Torrance, CA), and staphylococcal enterotoxins A and B (SEA and SEB, respectively) and toxic shock syndrome toxin I (TSST-I) were obtained from Sigma (St Louis, MO). Note that commercially available superantigens are truncated versions of the respective staphylococcal toxins produced in Escherichia coli containing the active domain of the protein.
Cell culture
Human fetal lung fibroblasts (IMR-90 cells) CCL-186 (American Type Culture Collection, Manassas, VA) were cultured in minimum essential medium as described earlier.12 Cells were plated at a density of 1.5 × 105 cells/well in 6-well plates (35-mm well) and used at confluency after 3 to 4 days. All stimulations (and controls) of IMR-90 cells were conducted in culture media containing l-glutamine in the absence of antibiotics and FCS. Human peripheral blood mononuclear cells (PBMCs) were isolated as described.14 Smooth muscle cells were isolated from rabbit superior mesenteric artery and cultured as described.15
Stimulation of PBMCs
PBMCs were incubated with SEA, SEB, or TSST-I at a final concentration of 100 ng/mL or 1% (vol/vol) S aureus Wood supernatants (obtained from overnight cultures of single colonies in 50 mL Todd Hewitt Broth [TH] media [Becton Dickinson, Sparks, MD]) in RPMI 1640 in the presence of 2 mM l-glutamine for 24 hours at 37°C. The cytokine content in PBMC exudates was measured by enzyme-linked immunosorbent assay (ELISA, Quantikine immunoassay kit; R&D Systems). To exclude a possible endotoxin contamination of the purchased purified staphylococcal toxins produced in E coli, toxins were incubated with polymyxin B (PMB; Sigma), a specific LPS antagonist, at a final concentration of 20 μg/mL for 30 minutes before experiments were started. The LPS contents in the stock solutions of the exotoxins (which were diluted 1:10 000 for the assays) were less than 1 ng/mL as determined by the Limulus test.
Intracellular cytokine staining
Purified PBMCs were adjusted to 6 × 106 cells/mL in RPMI medium. Cells were stimulated with 1% of supernatant from an overnight culture of S aureus (Wood 46) in the presence of brefeldin A (3 μg/mL, final concentration). Unstimulated cells and bacterial medium alone were used as controls. Cells were fixed and permeabilized as described16 and then stained with anti–IL-6–FITC, anti–IL-1β–FITC, or anti–TNF-α–FITC (R&D Systems). Samples were analyzed in a FACSCalibur flow cytometer (Becton-Dickinson, Franklin Lakes, NJ). The monocyte population was identified by FSC/SSC characteristics and positive CD14 staining.
SDS–polyacrylamide gel electrophoresis (PAGE), Western blotting, and immunoprinting
Proteins from an overnight culture (S aureus Wood) were precipitated with 5% (wt/vol) trichloro-acetic acid (TCA). The precipitates were dissolved in SDS sample buffer and separated by 12.5% (wt/vol) polyacrylamide gel electrophoresis.17 Commercially available SEA, SEB, and TSST-1 were used as controls. Proteins were transferred onto nitrocellulose membranes as described,18 and after a blocking step, membranes were probed with antibodies against SEA, SEB, and TSST-I (ViroStat, Portland, ME) diluted 1:200 in the blocking buffer,19 and bound antibodies were detected as described.19
RNA isolation, reverse transcription, and quantitative real-time PCR
Total RNA was extracted from IMR-90 cells using the RNA STAT-60 reagent method (Tel-Test, Friendswood, TX) as described by the manufacturer. Isolated RNA was DNAse treated (Ambion, Austin, TX) and reverse transcribed as described before.20 Real-time quantitative polymerase chain reaction (PCR) analyses were performed as described before.21 The primers used were as follows: B1R forward primer, 5′-caactgaacgtggcagaaatctac-3′; B1R reverse primer, 5′-caagcccaagacaaacaccag-3′; B2R forward primer, 5′-gggcacactgcggacct-3′; B2R reverse primer, 5′-gcgtttgctcactgtctgctc-3′; GAPDH forward primer, 5′-gggaaggtgaaggtcggagt-3′; and GAPDH reverse primer, 5′-tccactttaccagagttaaaagcag-3′. The following dual-labeled probes were obtained from BioSearch Technologies (Novato, CA): B1R, 5′-FAM-tggccaacctggcagcctctga-BHQ; B2R, 5′-FAM-tccgtggaacgccagattcacaaac-TAMARA; GAPDH, 5′-FAM-accaggcgcccaatacgaccaa-BHQ.
Radioligand binding
The binding of 1 nM [3H]desArg10kallidin (77.5 Ci/mmol) or 1 nM [3H]BK (90.0 Ci/mmol) (PerkinElmer Life Science Products, Boston, MA) to IMR-90 cells or rabbit smooth muscle cells was performed as described earlier.12 Binding assays were conducted on ice in triplicate, and nonspecific binding was defined as the amount of radiolabeled ligand bound in the presence of 1 μM nonradiolabeled desArg10kallidin or BK.
Thin sectioning and transmission electron microscopy
Thin sections were subjected to immunolabeling as described22 with the modification that Aurion-BSA (Aurion, Wageningen, the Netherlands) was used as a blocking agent. Briefly, sections were incubated with primary antibodies against B1R or B2R, followed by immunodetection with a secondary antibody against rabbit IgG labeled with 10 nm colloidal gold (Agar Scientific, Stansted, United Kingdom). Samples were finally stained with uranyl acetate and lead citrate and observed in a Jeol JEM 1230 electron microscope (JEOL, Tokyo, Japan), operated at 80 kV accelerating voltage. Images were recorded with a Gatan Multiscan 791 CCD camera (Gatan, Pleasanton, CA). For evaluation of the data, numbers of gold particles were determined for 30 cellular profiles in each case.
Determination of bradykinin
Bacteria (2 × 1010 cells/mL in 15 mM Hepes, 135 mM NaCl, 50 μM ZnCl2, pH 7.4) were incubated with plasma as described earlier.8 After 15 minutes of incubation at room temperature, the bacteria were washed, supplemented with new media, and incubated for 15 minutes before being assayed. The bradykinin concentration in the reaction mixture was quantified by the Markit-A kit (Dainippon Pharmaceutical, Osaka, Japan) as described.23
[3H]Thymidine incorporation into rabbit smooth muscle cells
Incorporation of [3H]thymidine into DNA expressed by rabbit vascular smooth muscle cells was performed as described earlier.24 The carboxypeptidase inhibitors DL-2-mercaptomethyl-3-guanidinoethylthiopropionic acid (MGTPA), potato carboxypeptidase inhibitor (PCI), 2-guani-dinoethylmercaptosuccinic acid (GEMSA), and ϵ-aminocaproic acid (EACA) were purchased from Calbiochem (San Diego, CA). The inhibitors were used with a final concentration of 10 μM.
Analysis of BK cleavage products
Rabbit smooth muscle cells grown in 6-well plates were first washed with DMEM to remove any serum. The cells were then incubated with isotopically and chemically pure [3H]BK (PerkinElmer Life Science Products) for various times at 37°C as indicated. The media were then acidified with 50 mL of 2 N HCl/mL, supplemented with 5 nmol BK, and applied on a C18 SepPak cartridge (Waters Associates, Milford, MA). BK cleavage products were then fractionated by high-performance liquid chromatography (HPLC) on a C18 SepPak column as previously described using defined BK fragments as standards.25
Immunohistochemical staining of tissue sections
The study was performed in accordance with the Declaration of Helsinki and ethical approval to obtain the biopsies was granted by the Human Subjects Review Committee of the University of Toronto. Biopsies from a local infection site of a patient with soft-tissue infection caused by S aureus had been collected at surgery and were immediately snap-frozen and stored at –80°C (kindly provided by Prof Donald E. Low, Mount Sinai Hospital, Toronto, ON). The biopsies were designated epicenter or distal tissue based on the clinical assessment and the level of inflammation. Samples were embedded in OCT-compound (Tissue-Tek; Mites, Elkhart, IN), cryostat sectioned to 8 mm, mounted to HTC glass slides (Novakemi, Stockholm, Sweden), and fixed with 2% freshly prepared formaldehyde in PBS. The immunohistochemical staining was performed as previously described26 using 2 μg/mL anti–IL-1β (cocktail of 2-D-8 and 1437-96-15, both murine IgG1, from Dr H. Towbin, Ciba-Geigy, Basel, Switzerland), and 1:1000 dilution of B1R- and B2R-specific antibodies,27 which had been immunopurified from respective polyclonal rabbit antiserum. The color reaction was developed by the addition of 3,3-diaminobenzidine (Vector Laboratories, Burlingame, CT) followed by counterstain of sections with hematoxylin. To control for nonspecific staining, sections that had not been incubated with primary antibodies were included and were always completely negative. Moreover, no staining was observed when the first antibody was replaced by normal rabbit serum or an unrelated antibody (against curli, an E coli surface protein). The immunostainings were evaluated in a RXM Leica microscope (Leica, Wetzlar, Germany) with a 40×/0.55 NA oil objective lens and 10% glycerol in PBS as imaging medium. Samples were located and analyzed by acquired computerized imaging analysis (ACIA) with a Quantimet 550 IW image analysis (Leica) as described earlier.19 The microscope was equipped with a 3-charge couple device color camera (DXC-750p; Sony Sverige, Spanga, Sweden). The whole tissue section was analyzed, which yielded an analyzed cell area (defined by the blue hematoxylin counterstaining) ranging from 1.4 × 105 to 3.4 × 105 mm2. The results are presented as ACIA value, which equals percent positively stained area times mean intensity of positive signal.
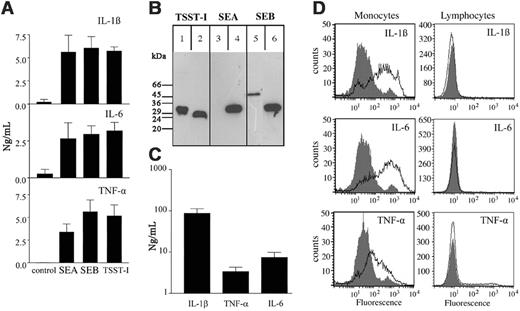
Secretion of proinflammatory cytokines from human peripheral monocytes following stimulation with superantigens fromS aureus. (A) Human PBMCs were treated for 24 hours with 100 ng/mL staphylococcal enterotoxin A (SEA), staphylococcal enterotoxin B (SEB), toxic shock syndrome toxin I (TSST-I), or culture media alone. PBMC exudates were collected and analyzed for their IL-1β, IL-6, and TNF-α content by ELISA. Results show mean values ± SD of 3 independent experiments for each cytokine. (B) Supernatants from an overnight culture of S aureus Wood strain 46 were run on SDS-PAGE. Separated proteins were transferred onto nitrocellulose membranes and probed with antibodies against TSST-I (lanes 1), SEA (lane 3), or SEB (lane 5). Purified toxins were used as controls (lanes 2, 4, and 6). Bound antibodies were detected by peroxidase-conjugated secondary antibodies against rabbit immunoglobulin. It should be noted that size heterogeneity for staphylococcal toxins purified from different isolates has been reported32 and may explain the different apparent molecular weights observed. (C) Human PBMCs were incubated for 24 hours with 1% (vol/vol) supernatants of an overnight culture from S aureus Wood strain 46. Exudates were collected and analyzed for their IL-1β, TNF-α, and IL-6 content by ELISA. Results show the mean ± SD of 3 separate experiments for each cytokine. Background secretion was either below detection level or less than 1% of the stimulated secretion. (D) Human PBMCs stimulated with supernatants from an overnight culture of S aureus (open area) and unstimulated cells (filled area) were fixed, permeabilized, and subsequently stained with fluorescent antibodies against IL-1β, IL-6, and TNF-α. The figure shows the monocyte and lymphocyte population gated on SSC and FSC characteristics.
Secretion of proinflammatory cytokines from human peripheral monocytes following stimulation with superantigens fromS aureus. (A) Human PBMCs were treated for 24 hours with 100 ng/mL staphylococcal enterotoxin A (SEA), staphylococcal enterotoxin B (SEB), toxic shock syndrome toxin I (TSST-I), or culture media alone. PBMC exudates were collected and analyzed for their IL-1β, IL-6, and TNF-α content by ELISA. Results show mean values ± SD of 3 independent experiments for each cytokine. (B) Supernatants from an overnight culture of S aureus Wood strain 46 were run on SDS-PAGE. Separated proteins were transferred onto nitrocellulose membranes and probed with antibodies against TSST-I (lanes 1), SEA (lane 3), or SEB (lane 5). Purified toxins were used as controls (lanes 2, 4, and 6). Bound antibodies were detected by peroxidase-conjugated secondary antibodies against rabbit immunoglobulin. It should be noted that size heterogeneity for staphylococcal toxins purified from different isolates has been reported32 and may explain the different apparent molecular weights observed. (C) Human PBMCs were incubated for 24 hours with 1% (vol/vol) supernatants of an overnight culture from S aureus Wood strain 46. Exudates were collected and analyzed for their IL-1β, TNF-α, and IL-6 content by ELISA. Results show the mean ± SD of 3 separate experiments for each cytokine. Background secretion was either below detection level or less than 1% of the stimulated secretion. (D) Human PBMCs stimulated with supernatants from an overnight culture of S aureus (open area) and unstimulated cells (filled area) were fixed, permeabilized, and subsequently stained with fluorescent antibodies against IL-1β, IL-6, and TNF-α. The figure shows the monocyte and lymphocyte population gated on SSC and FSC characteristics.
Results
Staphylococcal superantigens induce secretion of proinflammatory cytokines from primary human monocytes
To date, more than 10 different superantigens have been reported to be secreted by S aureus,28 of which enterotoxin A (SEA), enterotoxin B (SEB), and toxic shock syndrome toxin I (TSST-I) are the best characterized.29 To investigate a possible role of these toxins in the induction of cytokine secretion from immune cells, purified human peripheral blood mononuclear cells (PBMCs) were incubated with SEA, SEB, TSST-I, or culture media alone. After 24 hours, PBMC exudates were collected and analyzed for their IL-1β, IL-6, and TNF-α content, which are the primary cytokines that are produced by these cells.30,31 Figure 1A shows that the 3 toxins triggered a massive secretion of the analyzed cytokines, resulting in cytokine levels that were 10- to 600-fold higher than the background. Staphylococcal toxins are often encoded by accessory genetic elements, including plasmids, prophages, and mobile pathogenicity islands,5 leading to a pattern of toxin expression that may differ from strain to strain.33 We therefore wished to determine whether the tested staphylococcal superantigens are expressed and secreted by the S aureus Wood strain 46, which was used throughout this study. Thus, supernatants from overnight cultures of this strain were subjected to immunodetection following separation by SDS-PAGE and Western blotting. Figure 1B shows that SEB and TSST-I, but not SEA, were secreted into the culture medium under the growth conditions used. As these 2 toxins are secreted by the Wood strain, we hypothesized that the bacterial supernatants of overnight cultures should also be able to stimulate the secretion of cytokines from PBMCs. To this end, PBMCs were incubated for 24 hours with 1% (vol/vol) supernatants of overnight cultures of S aureus before cytokine measurements were conducted. As shown in Figure 1C, the treatment led to IL-6 and TNF-α levels in the PBMC exudates that were in the same range as those induced by the toxins alone, while the levels of IL-1β were more than 10-fold increased. To confirm whether the 3 cytokines were produced in monocytes and not in copurified B or T cells (“Materials and methods”), cells were stimulated with overnight cultures of S aureus in the presence of brefeldin A. Brefeldin A is a fungal toxin that inhibits the transport of secretory proteins between the ER and the cis-Golgi, which subsequently leads to an intracellular accumulation of de novo synthesized proteins in the endoplasmic reticulum.34 As expected, Figure 1D shows that brefeldin A evoked an increase of the intracellular stored cytokines (IL-1β, IL-6, and TNF-α) in monocytes, but not in the B- and T-cell population. Taken together, these data demonstrate that purified staphylococcal toxins and overnight culture supernatants are able to induce a massive inflammatory response by stimulating monocytes to produce high amounts of proinflammatory cytokines.
Induction of B1R and B2R mRNA expression by exudates from PBMCs stimulated with staphylococcal supernatants
The IMR-90 (human fetal lung fibroblasts) cell line is widely used and well studied for the analysis of kinin receptor regulation in response to inflammatory stimuli.35 IMR-90 cells express B1R and B2R at levels and in a ratio reflecting those in vivo. We therefore used this cell line to investigate B1R and B2R mRNA induction in response to treatment with exudates from PBMCs stimulated with staphylococcal supernatants (subsequently referred to as PBMC exudates). This treatment should mimic an inflammatory environment similar to that found at an infectious site. Prior to extraction of mRNA from IMR-90 cells, cells were incubated for 2 or 6 hours with BK, desArg9BK, IL-1β, or PBMC exudates in the absence or presence of BK or desArg9BK. Expression of mRNA for the B1R and B2R was investigated by quantitative real-time PCR and normalized to the housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH), which was analyzed in parallel. As shown in Figure 2, incubation of cells with BK or desArg9BK for 2 hours did not alter B1R mRNA levels when compared with nonstimulated cells. On the other hand, stimulation with IL-1β or PBMC exudates induced an increase after 2 hours, which was about 7-fold over control (Figure 2A) and even higher (approximately 16-fold) when cells were incubated for 6 hours with PBMC exudates (Figure 2B). Coincubation of PBMC exudates with BK or desArg9BK induced in a time-dependent manner a minor down-regulation of B1R mRNA expression. Figure 2C-D show that exposure to BK, but not to desArg9BK, led to a decrease in B2R mRNA levels, while treatment with IL-1β or PBMC exudates caused an up-regulation that was approximately 3.5 times higher than the control. Incubation with PBMC exudates in the presence of BK or desArg9BK decreased in a time-dependent manner the exudate effect on B2R mRNA expression. Treatment of IMR-90 cells with purified staphylococcal toxins and culture supernatants did not affect the mRNA levels of B1R and B2R (data not shown). The results show that PBMC exudates have the capacity to induce an up-regulation of B1R and B2R mRNA in IMR-90 cells, which is comparable with that seen from an IL-1β–caused induction. It was also noted that BK and desArg9BK down-regulated this effect significantly in the case of B2R.
B1R and B2R mRNA expression in IMR-90 cells. IMR-90 cells were treated with 10 μM BK, 10 μM desArg9BK, 500 pg/mL IL-1β, 1% (vol/vol) PBMC exudates (supernatants from monocytes that had been stimulated for 24 hours with 1% S aureus overnight culture supernatants), 1% PBMC exudates in the presence of 10 μM BK, 1% PBMC exudates in the presence of 10 μM desArg9BK, or media alone in the absence of serum. Incubation times were 2 hours (A,C) and 6 hours (B,D). B1R (A-B) and B2R (C-D) mRNA expression was measured using quantitative real-time PCR and normalized to GAPDH mRNA levels. B1R and B2R mRNA expression in response to treatment with IL-1β was set to 100%. The figure presents the mean ± SEM of 3 independent experiments each performed in triplicate.
B1R and B2R mRNA expression in IMR-90 cells. IMR-90 cells were treated with 10 μM BK, 10 μM desArg9BK, 500 pg/mL IL-1β, 1% (vol/vol) PBMC exudates (supernatants from monocytes that had been stimulated for 24 hours with 1% S aureus overnight culture supernatants), 1% PBMC exudates in the presence of 10 μM BK, 1% PBMC exudates in the presence of 10 μM desArg9BK, or media alone in the absence of serum. Incubation times were 2 hours (A,C) and 6 hours (B,D). B1R (A-B) and B2R (C-D) mRNA expression was measured using quantitative real-time PCR and normalized to GAPDH mRNA levels. B1R and B2R mRNA expression in response to treatment with IL-1β was set to 100%. The figure presents the mean ± SEM of 3 independent experiments each performed in triplicate.
Influence of PBMC exudates on specific B1R and B2R binding
A pathophysiologic effect, in respect to kinin receptor regulation, of S aureus–stimulated PBMC exudates would require a change in surface expression of kinin receptors. Thus, we measured the ability of PBMC exudates to modulate the number of B1Rs and B2Rs at the surface of IMR-90 cells. To this end, radioligand binding assays were performed using receptor-saturating concentrations of [3H]desArg10kallidin, a B1R agonist, and [3H]BK, a B2R agonist, as previously described.12 For these analyses, cells were treated for 6 hours with desArg9BK, BK, IL-1β, or PBMC exudates in the presence or absence of BK or desArg9BK, before the radioligand was added. Figure 3A shows that stimulation with desArg9BK caused a 2-fold increase of B1R agonist binding over control, while incubation with IL-1β or PBMC exudates was more efficacious in triggering an up-regulation of approximately 4.5-fold. The number of binding sites was even more increased when IMR-90 cells were incubated with PBMC exudates in the presence of BK or desArg9BK (6- and 11-fold, respectively). In contrast to B1R, none of the stimuli induced an up-regulation of B2R binding sites (Figure 3B). Indeed, incubation of cells with BK or PBMC exudates in the presence of BK led to a drastic decrease in specific binding of [3H]BK. No other treatment had a significant effect on agonist binding. The results show that the induction of B1R mRNA is reflected in an increased B1R agonist binding capacity of IMR-90 cells that were stimulated with IL-1β or PBMC exudates. Of interest, the combination of PBMC exudate and kinins, especially desArg9BK, induced a more pronounced up-regulation of B1R compared with the PBMC exudate alone (Figure 3A). However, even though stimulation of IMR-90 cells with various substances led to an up-regulation of B2R mRNA, there was no increase in the binding of [3H]BK. Stimulation with toxins SEA, SEB, and TSST-1 as well as S aureus culture supernatants did not cause an up- or down-regulation of B1R or B2R protein expression on IMR-90 cells (data not shown). In order to explain the differences between B1R and B2R up-regulation at the protein level, we used immunoelectron microscopy. Figure 4 shows that treatment of IMR-90 cells with monocyte exudates leads to an up-regulation of the B1R at the cell membrane (approximately 3-fold) and no further enrichment intracellulary, while the B2R accumulates intracellulary (approximately 5-fold), but is not up-regulated at the cell membrane. Thus, the data suggest that even though stimulation of IMR-90 cells leads to increased protein levels of both receptors, only the B1R is up-regulated at the cell surface. The finding can be explained by different trafficking mechanisms that target the receptor to the cell membrane or other yet-unknown mechanism.
Surface expression of B1Rs and B2Rs on IMR-90 cells. IMR-90 cells were incubated for 6 hours with either 10 μM BK, 10 μM desArg9BK, 500 pg/mL IL-1β, 1% (vol/vol) PBMC exudates (supernatants of monocytes that had been stimulated for 24 hours with 1% S aureus overnight culture supernatants), 1% PBMC exudates in the presence of 10 μM BK, 1% PBMC exudates in the presence of 10 μM desArg9BK, or media alone in the absence of serum. After a washing step, cells were assayed for specific [3H]Des-Arg10kallidin (B1R ligand) binding (A) and [3H]BK (B2R ligand) binding (B). Binding of [3H]Des-Arg10kallidin and [3H]BK to nonstimulated cells (control) was normalized to 100% within each experiment. Results represent the mean ± SEM of 3 independent experiments performed in triplicate. **P < .01 by analysis of variance followed by Tukey method for pairwise comparisons.
Surface expression of B1Rs and B2Rs on IMR-90 cells. IMR-90 cells were incubated for 6 hours with either 10 μM BK, 10 μM desArg9BK, 500 pg/mL IL-1β, 1% (vol/vol) PBMC exudates (supernatants of monocytes that had been stimulated for 24 hours with 1% S aureus overnight culture supernatants), 1% PBMC exudates in the presence of 10 μM BK, 1% PBMC exudates in the presence of 10 μM desArg9BK, or media alone in the absence of serum. After a washing step, cells were assayed for specific [3H]Des-Arg10kallidin (B1R ligand) binding (A) and [3H]BK (B2R ligand) binding (B). Binding of [3H]Des-Arg10kallidin and [3H]BK to nonstimulated cells (control) was normalized to 100% within each experiment. Results represent the mean ± SEM of 3 independent experiments performed in triplicate. **P < .01 by analysis of variance followed by Tukey method for pairwise comparisons.
Conversion of BK from a B2R agonist to a B1R agonist
Previous work has shown that S aureus isolates from patients with septic shock activate the human contact system. As a result, BK is generated and continuously released from the bacterial surface at a rate that is sufficient to induce an activation of B2R in transfected CHO cells.8 Similar findings were also observed in the present study when the Wood strain was tested. It is important to mention that so far there have been no reports in the literature showing that staphylococcal proteinases are able to release desArg9BK from the precursor molecules or to remove the carboxy-terminal arginine residue from BK, thereby converting the peptide from a B2R to a B1R agonist. Of interest, staphylococcal secretion products were able to stimulate monocytes to release proinflammatory cytokines followed by a massive up-regulation of B1Rs on IMR-90 cells. Moreover, when the stimulation occurred in the presence of desArg9BK, a synergistic effect was recorded. We therefore investigated whether BK released from the staphylococcal surface may be converted to desArg9BK by a eukaryotic-driven mechanism, for instance due to cleavage by a cell surface–bound endopeptidase. In order to address this question, we used rabbit superior mesenteric artery smooth muscle cells, which have been intensively studied in respect to their pharmacology.15,36 In contrast to IMR-90 cells, the smooth muscle cells are a primary vascular cell line that has retained much of its original phenotype and basally expresses B1Rs and B2Rs at their surface.15,36
For further characterization of the vascular smooth muscle cells, we conducted binding assays with saturating concentrations of [3H]desArg10kallidin and [3H]BK. Figure 5A shows that treatment of cells with desArg9BK, IL-1β, or PBMC exudates led to an increase in the number of B1R binding sites but had no effect on the B2R. Furthermore, stimulation with BK caused a down-regulation of the B2R without any effect on B1R. Taken together, the binding assays show that rabbit vascular smooth muscle cells express basal levels of the B1R and B2R and treatment with various stimuli provokes a pattern of receptor expression that resembles that of IMR-90 cells.
To study the conversion of BK to desArg9BK on rabbit smooth muscle cells, cells were incubated with [3H]BK for 3, 6, and 24 hours. Supernatants were then recovered and analyzed by HPLC. Figure 5B-D demonstrates that when isotopically pure [3H]BK (data not shown) was added to the cells, it was converted to [3H]desArg9BK or [3H]BK(1-8) in a time-dependent manner followed by further degradation to other [3H]metabolites. Maximal [3H]desArg9BK formation occurred within 3 hours, and after 24 hours the primary metabolite was [3H]BK(1-5) (Figure 5D).
We next sought to determine whether the response of rabbit smooth muscles cells to treatment with BK is solely BK mediated or partially caused by the newly formed desArg9BK. To this end, we used a cell proliferation assay, since it has been shown that both kinins (BK and desArg9BK) exert mitogenic effects.37 The assay was also chosen since it requires a relatively long agonist incubation time and, therefore, provides an opportunity for the formation and action of desArg9BK following BK addition. This is in contrast to other methods such as assays of intracellular Ca2+ mobilization, which occurs within seconds. Figure 6A shows that treatment of rabbit smooth muscle cells with desArg9BK or BK caused a significant increase in [3H]thymidine incorporation, which was in the same range as that observed in response to PDGF, one of the most potent vascular smooth muscles mitogens.38 In order to determine which receptor is activated following desArg9BK or BK addition, rabbit smooth muscles cells were incubated with the agonists in the presence or absence of desArg9[Leu8]BK, a selective B1R antagonist, or HOE-140, a selective B2R antagonist. As expected, desArg9BK-induced [3H]thymidine incorporation was significantly reduced in the presence of desArg9[Leu8]BK, while HOE-140 was virtually inactive (Figure 6B). In contrast, [3H]thymidine incorporation caused by stimulation with BK was blocked by more than 50% in the presence of desArg9[Leu8]BK, whereas the effect of HOE-140 was less pronounced. Of note, the antagonists had no effect on [3H]thymidine incorporation in the absence of kinin peptides (data not shown). To investigate whether the conversion of BK to desArg9BK on rabbit smooth muscle cells was triggered by carboxypeptidases of the kininase I type, rabbit smooth muscle cells were incubated with BK in the presence of the carboxypeptidase inhibitors, MGTPA, PCI, GEMSA, EACA, or with a mix of all inhibitors. Figure 6C shows that all inhibitors clearly impaired BK-prompted thymidine incorporation, while having minimal or no effect on basal incorporation. These data provide direct evidence that the mitogenic effect of BK is partially caused by the conversion of BK to desArg9BK, generated upon interaction of BK with carboxypeptidases from smooth muscle cells.
Immunolocalization of B1R and B2R in IMR-90 cells. Ultra-thin sections of unstimulated (A,C) or stimulated with 1% (vol/vol) exudates from monocytes treated with 1% (vol/vol) S aureus supernatant from an overnight culture (B,D). IMR-90 cells were incubated with antibodies against B1R (A-B) or B2R (C-D). Bound antibodies were visualized by secondary antibodies labeled with 10-nm gold particles and processed as described in “Materials and methods.” Examples of intracellular (arrows) and membrane-associated receptors (arrowheads) are indicated. The scale bar indicates 0.5 μm (magnification ×25 000).
Immunolocalization of B1R and B2R in IMR-90 cells. Ultra-thin sections of unstimulated (A,C) or stimulated with 1% (vol/vol) exudates from monocytes treated with 1% (vol/vol) S aureus supernatant from an overnight culture (B,D). IMR-90 cells were incubated with antibodies against B1R (A-B) or B2R (C-D). Bound antibodies were visualized by secondary antibodies labeled with 10-nm gold particles and processed as described in “Materials and methods.” Examples of intracellular (arrows) and membrane-associated receptors (arrowheads) are indicated. The scale bar indicates 0.5 μm (magnification ×25 000).
Expression of kinin receptors in response to PBMC exudates and kinins and chromatographic analysis of the hydrolysis of BK on rabbit smooth muscle cells. Rabbit smooth muscle cells were treated for 6 hours with BK, desArg9BK, IL-1β, 1% (vol/vol) exudates from monocytes stimulated with 1% (vol/vol) supernatant from an overnight culture of S aureus, or culture medium alone (control) in the absence of serum (A). After a washing step, cells were assayed for specific [3H]desArg10kallidin (B1R ligand; open bars) and [3H]BK (B2R ligand; filled bars) binding as described in “Materials and methods.” Specific binding is expressed as percent of control, where 100% is the binding to nontreated cells. The results represent mean ± SEM of at least 3 independent experiments done in triplicate. *P < .05 and **P < .01 compared with control values as determined by Student t test. NS indicates not significantly different from control values. Isotopically pure [3H]BK was incubated with rabbit smooth muscle cells. Supernatants were recovered after 3 hours (B), 6 hours (C), and 24 hours (D) and analyzed by HPLC as described in “Materials and methods.” Results are expressed as percent of total, where total is the total amount of eluted radioactivity. The result is representative of 3 experiments.
Expression of kinin receptors in response to PBMC exudates and kinins and chromatographic analysis of the hydrolysis of BK on rabbit smooth muscle cells. Rabbit smooth muscle cells were treated for 6 hours with BK, desArg9BK, IL-1β, 1% (vol/vol) exudates from monocytes stimulated with 1% (vol/vol) supernatant from an overnight culture of S aureus, or culture medium alone (control) in the absence of serum (A). After a washing step, cells were assayed for specific [3H]desArg10kallidin (B1R ligand; open bars) and [3H]BK (B2R ligand; filled bars) binding as described in “Materials and methods.” Specific binding is expressed as percent of control, where 100% is the binding to nontreated cells. The results represent mean ± SEM of at least 3 independent experiments done in triplicate. *P < .05 and **P < .01 compared with control values as determined by Student t test. NS indicates not significantly different from control values. Isotopically pure [3H]BK was incubated with rabbit smooth muscle cells. Supernatants were recovered after 3 hours (B), 6 hours (C), and 24 hours (D) and analyzed by HPLC as described in “Materials and methods.” Results are expressed as percent of total, where total is the total amount of eluted radioactivity. The result is representative of 3 experiments.
Effect of BK and desArg9BK on [3H]thymidine uptake in rabbit smooth muscle cells. (A) Rabbit smooth muscle cells were treated with BK, desArg9BK, PDGF, or buffer alone (control) for 24 hours and then assayed for [3H]thymidine incorporation as described in “Materials and methods.” The results are presented as percent of basal, where 100% basal is the amount of radioactivity in control. (B) Cells were incubated with BK or desArg9BK in the absence and presence of desArg9[Leu8]BK (DLBK) (B1 receptor antagonist) or HOE140 (B2 receptor antagonist). [3H]Thymidine incorporation was measured after 24 hours. Results are expressed as percent of control, where 100% control is the incorporation in the presence of BK or desArg9BK. The results represent mean ± SEM of at least 3 independent experiments done in triplicate. *P < .05 and **P < .01 compared with the control value as determined by Student t test. (C) Rabbit smooth muscle cells were treated for 24 hours with BK (1 μM) in the presence or absence of carboxypeptidase inhibitors of the kininase I type (DL-2-mercaptomethyl-3-guanidinoethylthiopropionic acid [MGTPA], potato carboxypeptidase inhibitor [PCI], 2-guani-dinoethylmercaptosuccinic acid [GEMSA], ϵ-aminocaproic acid [EACA], or a mix of all inhibitors; black bars). All inhibitors were applied at a final concentration of 10 μM. Control samples in the absence of BK were run in parallel (white bars). The results are presented in percent of thymidine incorporation into the DNA of the rabbit smooth muscle cells, where the incorporation into nontreated cells was set to 100%. The graph represents the mean ± SEM of 2 independent experiments performed in triplicate.
Effect of BK and desArg9BK on [3H]thymidine uptake in rabbit smooth muscle cells. (A) Rabbit smooth muscle cells were treated with BK, desArg9BK, PDGF, or buffer alone (control) for 24 hours and then assayed for [3H]thymidine incorporation as described in “Materials and methods.” The results are presented as percent of basal, where 100% basal is the amount of radioactivity in control. (B) Cells were incubated with BK or desArg9BK in the absence and presence of desArg9[Leu8]BK (DLBK) (B1 receptor antagonist) or HOE140 (B2 receptor antagonist). [3H]Thymidine incorporation was measured after 24 hours. Results are expressed as percent of control, where 100% control is the incorporation in the presence of BK or desArg9BK. The results represent mean ± SEM of at least 3 independent experiments done in triplicate. *P < .05 and **P < .01 compared with the control value as determined by Student t test. (C) Rabbit smooth muscle cells were treated for 24 hours with BK (1 μM) in the presence or absence of carboxypeptidase inhibitors of the kininase I type (DL-2-mercaptomethyl-3-guanidinoethylthiopropionic acid [MGTPA], potato carboxypeptidase inhibitor [PCI], 2-guani-dinoethylmercaptosuccinic acid [GEMSA], ϵ-aminocaproic acid [EACA], or a mix of all inhibitors; black bars). All inhibitors were applied at a final concentration of 10 μM. Control samples in the absence of BK were run in parallel (white bars). The results are presented in percent of thymidine incorporation into the DNA of the rabbit smooth muscle cells, where the incorporation into nontreated cells was set to 100%. The graph represents the mean ± SEM of 2 independent experiments performed in triplicate.
Western blot analysis of toxins secreted from clinical S aureus strains
Next we tested supernatants from overnight cultures of 5 clinical staphylococcal strains for their toxin expression. Figure 7A-B (lanes 2-6) shows that all strains tested produced TSST-1 and SEB, though the amount of secreted toxin seems to vary from strain to strain. Similar results were obtained when the expression of SEA in the clinical isolates was investigated (data not shown). Strain Mu50 (ATCC 700699) whose genome has been completely sequenced, was used as a positive control, and was found to express TSST-1 and SEB (Figure 7A-B lane 1) as well as SEA (data not shown).
Analysis of B1R and B2R expression on tissue biopsies from a patient suffering from a staphylococcal soft-tissue infection
To assess the expression of B1R and B2R in vivo during an infection, we analyzed tissue biopsies collected from a patient with a soft-tissue infection caused by the 9730 strain (Figure 7A-B lane 2). For comparison, samples were collected from 2 sites including the epicenter of the infection and a more distal site. Histologic examination of the biopsies showed that the tissue sections had different morphologies with more signs of inflammation and loss of tissue structure in biopsies obtained from the epicenter of the infectious site (data not shown).
Further immunohistochemical analysis revealed that both B1R and B2R were expressed at the local site of infection. Of interest, the level of B1R expression was more than 3-fold higher at the epicenter compared with the section from the more distal site (Figure 7C). In contrast, equal levels were noted for the B2R in the 2 groups of biopsies. To assess the degree of inflammation in the biopsies, the sections were stained for IL-1β, and in situ imaging analysis revealed a 4-fold higher amount of IL-1β in the epicenter sections. Similar findings were obtained when a biopsy from a patient with staphylococcal-induced erysipelas (an acute superficial form of cellulitis) was analyzed (data not shown). These data are in line with the results obtained from cultured IMR-90 cells and rabbit vascular smooth muscle cells. Taken together, the present investigation shows for the first time that S aureus can induce up-regulation of B1R, which combined with its ability to generate BK that is subsequently converted to a B1R agonist may provide a powerful mechanism to evoke pathologic inflammatory response in the human host.
Detection of TSST-1 and SEB in supernatants from clinical S aureus isolates and expression of B1Rs and B2Rs in a patient suffering from an S aureus soft-tissue infection. (A-B) Supernatants from the clinical isolates 9730, 1878, 2374, 1024, and 15159 (lane 2 to 6) were separated on SDS-PAGE, transferred onto nitrocellulose membranes, and immunostained with antibodies against TSST-1 (A) or SEB (B). The strain ATCC 700699, whose genome has been completely sequenced, was used as a control (lane 1). Note that the apparent molecular weights of toxins vary between the tested strains. Size variation is a common feature of bacterial proteins from different strains that is often caused by homologous recombination between repeated regions within the gene39 and has normally no influence on the activity of the protein. (C) Tissue biopsies from the epicenter of the infection site and from a distal site were obtained from a patient with a soft-tissue infection caused by S aureus. The biopsies were cryosectioned and immunohistochemically stained for IL-1β, B1R, and B2R. Omission of the primary antibody was included as a negative control and was always completely negative. Stainings were quantified by in situ imaging and the results are presented as the imaging value: area and intensity of the positive stain (brown) in relation to the total cell area (blue), as previously described.26
Detection of TSST-1 and SEB in supernatants from clinical S aureus isolates and expression of B1Rs and B2Rs in a patient suffering from an S aureus soft-tissue infection. (A-B) Supernatants from the clinical isolates 9730, 1878, 2374, 1024, and 15159 (lane 2 to 6) were separated on SDS-PAGE, transferred onto nitrocellulose membranes, and immunostained with antibodies against TSST-1 (A) or SEB (B). The strain ATCC 700699, whose genome has been completely sequenced, was used as a control (lane 1). Note that the apparent molecular weights of toxins vary between the tested strains. Size variation is a common feature of bacterial proteins from different strains that is often caused by homologous recombination between repeated regions within the gene39 and has normally no influence on the activity of the protein. (C) Tissue biopsies from the epicenter of the infection site and from a distal site were obtained from a patient with a soft-tissue infection caused by S aureus. The biopsies were cryosectioned and immunohistochemically stained for IL-1β, B1R, and B2R. Omission of the primary antibody was included as a negative control and was always completely negative. Stainings were quantified by in situ imaging and the results are presented as the imaging value: area and intensity of the positive stain (brown) in relation to the total cell area (blue), as previously described.26
Discussion
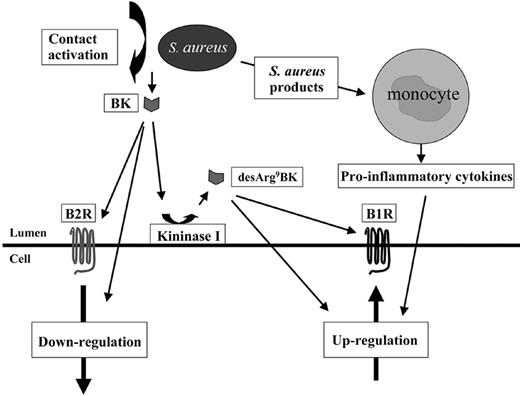
In 1972, Lewis Thomas wrote “The microorganisms.. .turn out.. .to be rather more like bystanders. It is our response to their presence that makes the disease. Our arsenal for fighting off bacteria are so powerful.. .that we are more in danger from them than the invaders.”40 One of the most potent inflammatory mediators we have in the human body is BK. Previous work demonstrated that S aureus is able to activate the contact system and trigger the release of BK.8 Based on this observation, the present study was undertaken to examine the influence of kinin receptor regulation and their subsequent activation by their ligands at an infected site. In order to mimic an inflammatory situation in vitro, PBMCs were stimulated with different staphylococcal products and this was found to trigger a massive cytokine response. Moreover, treatment of IMR-90 cells with exudates from monocytes, after incubation with S aureus overnight supernatant, induces an up-regulation of the B1 receptor, which was further increased in the presence of BK or desArg9BK. The same treatment had no effect on the expression of the B2R when cells were stimulated in the absence of BK, while in the presence of BK the treatment induced a down-regulation of the receptor. Contact activation by S aureus leads to the generation of BK8 specifically. To test whether BK can be converted to a B1R agonist, HPLC analysis and cell proliferation assays were performed. These experiments demonstrated that a significant portion of BK is converted to desArg9BK on vascular smooth muscle cells. In essence, our data present a chain of events (summarized in Figure 8) initiated by S aureus secretion products that leads to the induction of inflammatory reactions through up-regulation of B1Rs on the surface of cells. S aureus uses a number of different host systems (ie, contact system, monocytes, and B1Rs/B2Rs) to cause inflammatory reactions that can take place at different time points during an infectious process and lead to different clinical symptoms.
Proposed mechanism used by S aureus to interact with B1R and B2R. Based on the results of the present study, the following model is suggested. At the infectious site, invading monocytes become activated by staphylococcal toxins and secrete proinflammatory cytokines that induce an up-regulation of the B1R at the infectious focus. Plasma exudation into the infectious site will trigger contact activation and the formation of BK. BK can bind to B2R and trigger its down-regulation or be converted to the B1R agonist, desArg9BK, which subsequently leads to an activation and an additional up-regulation of B1R.
Proposed mechanism used by S aureus to interact with B1R and B2R. Based on the results of the present study, the following model is suggested. At the infectious site, invading monocytes become activated by staphylococcal toxins and secrete proinflammatory cytokines that induce an up-regulation of the B1R at the infectious focus. Plasma exudation into the infectious site will trigger contact activation and the formation of BK. BK can bind to B2R and trigger its down-regulation or be converted to the B1R agonist, desArg9BK, which subsequently leads to an activation and an additional up-regulation of B1R.
Characteristic signs of inflammation are redness and swelling with heat and pain (Cornelius Celcus, first century AD; for a review, see Nathan41 ), symptoms that all can be induced by BK or its metabolite desArg9BK.42 Immunohistologic examination of tissue samples from a patient suffering from a soft-tissue infection caused by S aureus revealed an up-regulation of B1R at the infectious focus, which coincided with increased IL-1β cytokine levels, while the levels of B2R were not increased. These results confirm our in vitro findings and implicate an important pathophysiologic function for B1R regulation at an inflamed site in bacterial infectious diseases.
Bacteria-provoked kinin generation and the subsequent increase in vascular permeability represent an important pathophysiologic mechanism. The increased permeability and the resulting plasma leakage into the infected site can either serve as a source of nutrients or facilitate dissemination of the infection, which eventually can result in more severe conditions such as sepsis and septic shock.43,44 Kinin receptors are therefore an interesting target for the development of novel therapies for the treatment of infectious diseases. In this respect, B1R seems more relevant then B2R, since B1R is thought to have an important role in chronic inflammatory responses, while B2R is down-regulated at an early stage of an inflammatory process. So far, deltibant (CP-0127), a B2R antagonist, is the only kinin antagonist that has been tested for the treatment of bacterial infections. In a multicenter, randomized, placebo-controlled trial, the drug was applied to patients with systemic inflammatory response syndrome and presumed sepsis. Even though the drug had no significant effect on risk-adjusted 28-day survival, posthoc analysis revealed a nonsignificant trend toward improvement.45 Our studies show that S aureus not only evokes an up-regulation of B1R, but also has the ability, via contact activation at the bacterial surface and the help of host carboxypeptidases, to allow a sustained generation of desArg9BK, a B1R agonist. Based on our findings, a strategy using a B1R antagonist alone or in combination with B2R antagonist could represent a promising approach for the development of novel therapies for the treatment of severe S aureus infections.
Prepublished online as Blood First Edition Paper, May 30, 2006; DOI 10.1182/blood-2006-04-016444.
Supported in part by the foundations of Åke Wiberg, AlfredÖsterlund, Crafoord, Tore Nilson, Greta and Johan Kock, the Swedish Foundation for Strategic Research, King Gustaf V's 80-year fund, the Royal Physiographical Society in Lund, the Swedish Heart-Lung Foundation, the Blood and Defense Network and the Vascular Wall Programme at Lund University, the Medical Faculty of Lund University, the Swedish Research Council (projects 7480, 12610, and 13413), National Institutes of Health (NIH) grants GM41659 and AI50498, and Hansa Medical Research AB.
S.H.B. designed and performed research; S.B.P., A.N.-T., L.P., and M.M. performed research; B.L.Z. and L.M.F.L.-L. designed research; and H.H. designed research and wrote the paper.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We wish to thank Monica Heidenholm for excellent technical assistance; Drs Eva Mattsson, Dongsoo Kang, Jane Eddleston, and Astrid Doerner for their scientific advice and advisory help; and Dr James A. Koziol for statistical analysis.


![Figure 3. Surface expression of B1Rs and B2Rs on IMR-90 cells. IMR-90 cells were incubated for 6 hours with either 10 μM BK, 10 μM desArg9BK, 500 pg/mL IL-1β, 1% (vol/vol) PBMC exudates (supernatants of monocytes that had been stimulated for 24 hours with 1% S aureus overnight culture supernatants), 1% PBMC exudates in the presence of 10 μM BK, 1% PBMC exudates in the presence of 10 μM desArg9BK, or media alone in the absence of serum. After a washing step, cells were assayed for specific [3H]Des-Arg10kallidin (B1R ligand) binding (A) and [3H]BK (B2R ligand) binding (B). Binding of [3H]Des-Arg10kallidin and [3H]BK to nonstimulated cells (control) was normalized to 100% within each experiment. Results represent the mean ± SEM of 3 independent experiments performed in triplicate. **P < .01 by analysis of variance followed by Tukey method for pairwise comparisons.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/108/6/10.1182_blood-2006-04-016444/4/m_zh80180601070003.jpeg?Expires=1765883353&Signature=i8RqkUEtl0Dvctw20NZDZoKvoIdV7mM0muilr5O4kJQwekHD4pY3ntY14nfcXT2h7qLhJKbb7MIVOYM6ApmfXzn6nOq3H~sDMFH96C3kIZBVA7DcAgGwIeCEQMgtTSXkqjhg5WRDlvVosJQH6Sip1KizGIyqxX-rWMKfQDpim-YyIQzDt6NCdxk-ich2xGOB8VWQq3jbb-3B6OEQcCzYlHxIoFnbh97xIgUHlACMc9ePlD4QkbEU6b9GuwfhIvabaiLulNbdHB3zC0Sk2DkN7LnneAE3WP88XRWyaud3k4Y57swjKvd23MZfD4cq3Log1hYCzvvpwbogpG4o25323w__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Figure 5. Expression of kinin receptors in response to PBMC exudates and kinins and chromatographic analysis of the hydrolysis of BK on rabbit smooth muscle cells. Rabbit smooth muscle cells were treated for 6 hours with BK, desArg9BK, IL-1β, 1% (vol/vol) exudates from monocytes stimulated with 1% (vol/vol) supernatant from an overnight culture of S aureus, or culture medium alone (control) in the absence of serum (A). After a washing step, cells were assayed for specific [3H]desArg10kallidin (B1R ligand; open bars) and [3H]BK (B2R ligand; filled bars) binding as described in “Materials and methods.” Specific binding is expressed as percent of control, where 100% is the binding to nontreated cells. The results represent mean ± SEM of at least 3 independent experiments done in triplicate. *P < .05 and **P < .01 compared with control values as determined by Student t test. NS indicates not significantly different from control values. Isotopically pure [3H]BK was incubated with rabbit smooth muscle cells. Supernatants were recovered after 3 hours (B), 6 hours (C), and 24 hours (D) and analyzed by HPLC as described in “Materials and methods.” Results are expressed as percent of total, where total is the total amount of eluted radioactivity. The result is representative of 3 experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/108/6/10.1182_blood-2006-04-016444/4/m_zh80180601070005.jpeg?Expires=1765883353&Signature=cJAEGO4s2jm3qxLErGTwI-LlvNi8cjzkEPq23ceCsGElVtBxpXNA21VfSylUwtXaPU0LK95vYz-f~GujmDTE2gfzvNq8leHQGVrnORpI8N9nuUfFfpyNmb0nMLePWiztvCEoTKyEAk2WjKONpMZbkv0ZxMC~qTBK3C18AjxGK6cFM9wpFoGM8zUSNVm2D3ZZ5qIPSgOZp-Pdl3dV7WM6evptgJh2qsLNcbHNIJjUZ8gjZWDuQbnpU~fNOMFI8-tgT6bR6TDASzWOa-hz03pGy9x54PM-X3kXutNAIOIof4awILSm0fpbVmTj6QuvG5BJndXSdggE6041mHlqajetXQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 6. Effect of BK and desArg9BK on [3H]thymidine uptake in rabbit smooth muscle cells. (A) Rabbit smooth muscle cells were treated with BK, desArg9BK, PDGF, or buffer alone (control) for 24 hours and then assayed for [3H]thymidine incorporation as described in “Materials and methods.” The results are presented as percent of basal, where 100% basal is the amount of radioactivity in control. (B) Cells were incubated with BK or desArg9BK in the absence and presence of desArg9[Leu8]BK (DLBK) (B1 receptor antagonist) or HOE140 (B2 receptor antagonist). [3H]Thymidine incorporation was measured after 24 hours. Results are expressed as percent of control, where 100% control is the incorporation in the presence of BK or desArg9BK. The results represent mean ± SEM of at least 3 independent experiments done in triplicate. *P < .05 and **P < .01 compared with the control value as determined by Student t test. (C) Rabbit smooth muscle cells were treated for 24 hours with BK (1 μM) in the presence or absence of carboxypeptidase inhibitors of the kininase I type (DL-2-mercaptomethyl-3-guanidinoethylthiopropionic acid [MGTPA], potato carboxypeptidase inhibitor [PCI], 2-guani-dinoethylmercaptosuccinic acid [GEMSA], ϵ-aminocaproic acid [EACA], or a mix of all inhibitors; black bars). All inhibitors were applied at a final concentration of 10 μM. Control samples in the absence of BK were run in parallel (white bars). The results are presented in percent of thymidine incorporation into the DNA of the rabbit smooth muscle cells, where the incorporation into nontreated cells was set to 100%. The graph represents the mean ± SEM of 2 independent experiments performed in triplicate.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/108/6/10.1182_blood-2006-04-016444/4/m_zh80180601070006.jpeg?Expires=1765883353&Signature=YXxvTXvqcGdw0w24EZHx3eB7Ympgpi729JrdhvxnJmRCXaNtqN4clpWsTMzspcT4Pd4qYKf9GN-L7BFb3CXp9Gv3~kAsROY6Ead5Ro4Ti8YhjcwChS2gVRZVAXP0-JPfkvuv4FqPy7jWjv6bhz9-57GVI8fpZew6Akv8GMHP~JoeyLjphCu0ZIGYuwDXl7Rcua-7LXWZKTTR3gMsWHk6cjrmVFBZTapcxnWc0095dzWuXVdzP7WkneCHXjV7CrkrnhHvY7qXPr9NsnQEFTph1zuXpR10ZJtziNv6i5p4oG1g8c-ObJX8zPtQYlcqidODDL4GUXWAFNSw0r3Q6bzIkw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal