Abstract
In an acute promyelocytic leukemia (APL)–transplantable mouse model, we previously reported the presence of antibodies recognizing PML-RARα and RARα in the sera of ATRA-treated mice. To evaluate this immune response, we determined the prevalence of anti-RARα antibodies in a cohort of 48 APL mice, treated by ATRA (n = 24) or by placebo pellets (n = 24), and in a preliminary subset of 9 patients with APL using a specific enzyme-linked immunosorbent assay (ELISA). In APL mice, significantly higher antibody levels were observed at the latest time points (day 48 to 58 levels superior to day 15 to 18 or day 28 to 38 levels). Antibody levels were higher in ATRA-treated mice than in placebo-treated mice and were also predictive of better survival. In the patients with APL, anti-RARα antibodies were detected at diagnosis and after maintenance therapy, reminiscent of the ATRA-treated APL mice. Antinuclear or antineutrophil cytoplasmic autoantibodies were also detected. These data reveal for the first time that in patients with APL an immune response may be detected at diagnosis and enhanced after maintenance therapy.
Introduction
Acute promyelocytic leukemia (APL) is characterized by a differentiation block at the promyelocytic stage and by a reciprocal chromosomal translocation, t(15;17)(q24;q21), fusing the promyelocytic leukemia gene (PML) with the retinoic acid receptor alpha (RARA) gene.1 Treatment with all-trans retinoic acid (ATRA) and chemotherapy induces complete remissions in 90% of PML-RARα–positive patients with APL through ATRA-induced differentiation of the leukemic cells and their subsequent elimination by apoptosis.2 Apart from their known effects on cell differentiation, retinoids also play an important role in infection and immune functions.3 In hypovitaminosis A animal models and in vitamin A–deficient children, alterations in antiviral, antibacterial, or antiparasite responses have been shown.3-5 In an APL-transplantable mouse model that shares the promyelocytic features and response to ATRA of human APL,6 we have previously reported the presence of antibodies in ATRA-treated mouse sera.7 By Western blot analysis, we showed that these antibodies recognized mouse and human RARα from spleen and HL-60 cells, respectively, as well as PML-RARα from mouse spleen and NB4 leukemic cells. We hypothesized that such adjuvant effects of ATRA might also participate in the efficacy of ATRA in patients with APL, especially during maintenance therapy when the tumor burden is low and immunocompetency restored.2
The aim of our study was therefore to further characterize anti-RARα antibody production in APL mice as well as in patients with APL. In patients with APL, antibodies to RARα and autoantibodies like antinuclear autoantibodies (ANAs) or antineutrophil cytoplasmic autoantibodies (ANCAs) were analyzed.
Study design
The transplantable APL mouse model of mice undergoing APL transplantation and ATRA therapy was set up as already described.6,7 Data from 3 protocols that did not differ in their response to ATRA were pooled. Placebo (n = 24) and ATRA-treated APL (n = 24) mice died from day 18 to 29 (median, 26 days) and from day 28 to 95 (median, 55 days), respectively, as already reported.6,7 Antibodies against RARα were detected by an enzyme-linked immunosorbent assay (ELISA) at different time intervals as previously described.7 For each mouse serum, specific absorbance (SA) was calculated as the difference between duplicates of mean absorbance with and without GST-RARα. To normalize results between experiments, arbitrary units (AUs) were obtained by dividing the SA obtained in serum dilution by the SA obtained with 1:200 000 dilution of positive control monoclonal anti-RARα antibody (9αF).
An ELISA was performed in sera from 9 patients with APL to detect the presence of anti-RARα antibodies. ANAs and ANCAs were measured by indirect immunofluorescence. We analyzed sera from 9 patients for whom stored sera were available at diagnosis and after 2-year maintenance therapy.2 Patients were enrolled in APL trials: patients 3, 5, and 8 from APL93 and patients 1, 2, 4, 6, 7, and 9 from the APL2000 trial. In these trials informed consent was obtained to use stored samples. For all patients sera were stored in similar conditions at –30°C and thawed once. Eighteen healthy subjects served as controls.
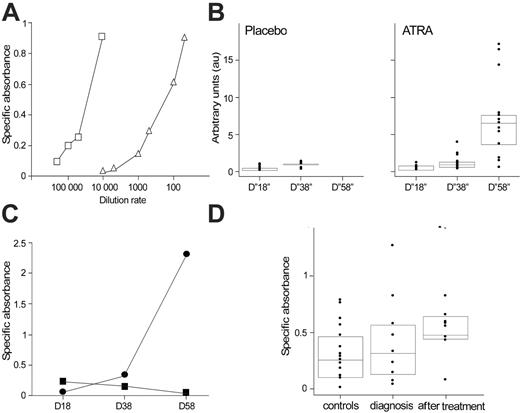
Anti-RARα antibody production in APL mice and patients. (A) Comparative anti-RARα ELISA dose-response curves with a positive mouse serum and an anti-RARα monoclonal antibody control. SA measured by ELISA of a serum from an ATRA-treated mouse serially diluted from 1:50 to 1:10 000 (▵) and from the anti-RARα monoclonal antibody 9αF diluted from 1:10 000 to 1:200 000 (reference curve, □). (B) Time-dependent presence of anti-RARα antibodies in mice with APL. Anti-RARα antibodies were measured by ELISA in 48 mice with APL. On days 15 to 18, all mice were alive, and sera were obtained from only 46 mice, including 23 mice treated by ATRA and 23 treated by placebo. On days 28 to 38, 30 mice were alive, including 24 mice treated by ATRA and 6 treated by placebo. On days 48 to 58, only 13 mice, treated by ATRA, were alive. The box plot in combination with dot plot displays a statistical summary of the data (quartiles and median). Data are expressed as arbitrary units. (C) Time course of the IgG and IgM SA in the anti-RARα ELISA of 1 ATRA-treated APL mouse. For IgG and IgM detection, secondary antibodies specific for mouse IgG and IgM were used in ELISA tests. In 1 ATRA-treated mouse the anti-RARα ELISA was performed in serum collected on days 18, 28, and 38 using specific secondary antibodies for mouse IgG (•) and mouse IgM (▪). Results are expressed as SA. (D) Time-dependent presence of anti-RARα antibodies in APL patient sera. Anti-RARα antibodies (expressed as SA) were measured by ELISA in the serum of 9 patients with APL at diagnosis and after maintenance therapy. ELISA tests were performed in 96-well plates (Nunc; Merck Eurolab, VWR, Fontenay-Sous-Bois, France) coated with either GST or GST-RARα (obtained by fusion of a GST tag to the full-length RARα). After overnight saturation with PBS1X-BSA5%, sera (diluted 1:200 in PBS1X-BSA5%) were incubated 1 hour at room temperature. Peroxidase-conjugated goat anti–human IgG antibody (Sigma-Aldrich, Lyon, France) was added and incubated 1 hour at room temperature, followed by TMB substrate revelation (BD Pharmingen, San Diego, CA). Absorbance was measured at 450 nm (reference filter at 630 nm) using an optical densitometer (Dynatech MR5000; Labsystems, Cergy, France). For each serum, SA was calculated as the difference between duplicates of mean absorbance between GST-RARα and GST. Anti-RARα antibodies were also measured in sera from 18 healthy persons (controls). The box plot in combination with dot plot displays a statistical summary of the data (quartiles and median).
Anti-RARα antibody production in APL mice and patients. (A) Comparative anti-RARα ELISA dose-response curves with a positive mouse serum and an anti-RARα monoclonal antibody control. SA measured by ELISA of a serum from an ATRA-treated mouse serially diluted from 1:50 to 1:10 000 (▵) and from the anti-RARα monoclonal antibody 9αF diluted from 1:10 000 to 1:200 000 (reference curve, □). (B) Time-dependent presence of anti-RARα antibodies in mice with APL. Anti-RARα antibodies were measured by ELISA in 48 mice with APL. On days 15 to 18, all mice were alive, and sera were obtained from only 46 mice, including 23 mice treated by ATRA and 23 treated by placebo. On days 28 to 38, 30 mice were alive, including 24 mice treated by ATRA and 6 treated by placebo. On days 48 to 58, only 13 mice, treated by ATRA, were alive. The box plot in combination with dot plot displays a statistical summary of the data (quartiles and median). Data are expressed as arbitrary units. (C) Time course of the IgG and IgM SA in the anti-RARα ELISA of 1 ATRA-treated APL mouse. For IgG and IgM detection, secondary antibodies specific for mouse IgG and IgM were used in ELISA tests. In 1 ATRA-treated mouse the anti-RARα ELISA was performed in serum collected on days 18, 28, and 38 using specific secondary antibodies for mouse IgG (•) and mouse IgM (▪). Results are expressed as SA. (D) Time-dependent presence of anti-RARα antibodies in APL patient sera. Anti-RARα antibodies (expressed as SA) were measured by ELISA in the serum of 9 patients with APL at diagnosis and after maintenance therapy. ELISA tests were performed in 96-well plates (Nunc; Merck Eurolab, VWR, Fontenay-Sous-Bois, France) coated with either GST or GST-RARα (obtained by fusion of a GST tag to the full-length RARα). After overnight saturation with PBS1X-BSA5%, sera (diluted 1:200 in PBS1X-BSA5%) were incubated 1 hour at room temperature. Peroxidase-conjugated goat anti–human IgG antibody (Sigma-Aldrich, Lyon, France) was added and incubated 1 hour at room temperature, followed by TMB substrate revelation (BD Pharmingen, San Diego, CA). Absorbance was measured at 450 nm (reference filter at 630 nm) using an optical densitometer (Dynatech MR5000; Labsystems, Cergy, France). For each serum, SA was calculated as the difference between duplicates of mean absorbance between GST-RARα and GST. Anti-RARα antibodies were also measured in sera from 18 healthy persons (controls). The box plot in combination with dot plot displays a statistical summary of the data (quartiles and median).
Results and discussion
Anti-RARα antibody production in APL mice
As we have previously reported, anti-RARα antibodies were detected in ATRA-treated APL mice.7 Specificity of the ELISA signal defined previously on immunoblotting experiments with recombinant GST-RARα7 was further assessed in this study by ELISA competition experiments. Preincubation of an anti-RARα–positive serum overnight at 4°C with GST-RARα in a 20-fold excess resulted in an 87% binding inhibition (SA of 0.158 compared with 1.23 with preincubation with buffer alone) (data not shown). The dilution of a positive anti-RARα mouse serum shows a dose-dependent SAsimilar to that obtained with a known anti-RARα monoclonal antibody, 9αF 8 (Figure 1A). In this large cohort of 48 APL mice, it is clear that anti-RARα antibody levels progressively increase with time, from a median AU level of 0.26 on days 15 to 18 (D“18”) to median AU levels of 0.88 on days 28 to 38 (D“38”) and 6.33 on days 48 to 58 (D“58”) (t test, P < .001 between D“18” and D“58”). On D“38,” among 6 surviving placebo-treated mice, only 1 mouse had antibody levels reaching a threshold of 4-fold that of the D“18” median, whereas in ATRA-treated mice 9 of 24 mice reached this level on D“38” and 12 of 13 on D“58,” when all placebo-treated mice were dead (Figure 1B). AUs reached a maximal value of 16.8 on D“58” in ATRA-treated mice. This antibody appearance followed the classic scheme of antibody production, with a time-decreased production of IgM and a time-increased production of IgG from day 18 to day 58 (Figure 1C). To investigate the prognostic value of these antibodies independently of time, we asked whether antibody levels obtained at D“18,” when all mice were alive, were predictive of survival. At this time point, a higher level of antibody was associated with increased survival: Median survival was 28 days in mice with a level lower than the median D“18” AUs (0.26), versus 49 days for mice with a level higher than the median D“18” AUs (log-rank test, P < .001).
Anti-RARα antibody production in patients with APL
To provide relevance to the preclinical data obtained in APL mice, anti-RARα antibodies were retrospectively analyzed by ELISA in 9 patients with APL. All patients were in complete remission at the time of postmaintenance treatment study. For the first time, anti-RARα antibodies were detected both at diagnosis and after maintenance therapy in patients with APL, and the antibody production followed a time-dependent pattern similar to the kinetics of anti-RARα antibodies observed in APL mice (Figure 1D and Table 1). In 5 of 9 patients anti-RARα antibody levels increased with time. Of note, among the 4 patients in whom no increase in anti-RARα antibody levels was seen, 3 already had high levels of antibodies at diagnosis. Interestingly, other autoimmunity manifestations were observed in these patients. At diagnosis, 7 (78%) were either positive for ANAs or ANCAs, and all patients were either ANA or ANCA positive after maintenance therapy (Table 1). This proportion was higher than that expected in the general population (13.3% and 0% to 1.8% of healthy persons may present ANAs9 or ANCAs,10 respectively). Thus, patients with APL present frequent autoantibodies, especially after maintenance therapy.
Anti-RARα antibodies, ANAs, and ANCAs in patients with APL
. | . | Anti-RARα antibody . | . | ANA . | . | ANCA . | . | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Patient no. . | Months after treatment† . | Diagnosis* . | After treatment† . | Diagnosis* . | After treatment† . | Diagnosis* . | After treatment† . | |||
| 1 | 6 | + | + | - | ++ | - | - | |||
| 2 | 6 | +++ | +++ | ++ | ++ | - | - | |||
| 3 | 36 | ++ | ++++ | - | + | - | - | |||
| 4 | 4.7 | ++++ | +++ | ++ | + | - | - | |||
| 5 | 67 | ++++ | ++++ | ++ | ++ | - | - | |||
| 6 | 6 | ++ | ++++ | - | - | + | ++ | |||
| 7 | 4 | + | +++ | + | ++ | - | - | |||
| 8 | 31 | + | +++ | - | + | ++ | + | |||
| 9 | 10 | + | +++ | ++ | ++ | + | + | |||
. | . | Anti-RARα antibody . | . | ANA . | . | ANCA . | . | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Patient no. . | Months after treatment† . | Diagnosis* . | After treatment† . | Diagnosis* . | After treatment† . | Diagnosis* . | After treatment† . | |||
| 1 | 6 | + | + | - | ++ | - | - | |||
| 2 | 6 | +++ | +++ | ++ | ++ | - | - | |||
| 3 | 36 | ++ | ++++ | - | + | - | - | |||
| 4 | 4.7 | ++++ | +++ | ++ | + | - | - | |||
| 5 | 67 | ++++ | ++++ | ++ | ++ | - | - | |||
| 6 | 6 | ++ | ++++ | - | - | + | ++ | |||
| 7 | 4 | + | +++ | + | ++ | - | - | |||
| 8 | 31 | + | +++ | - | + | ++ | + | |||
| 9 | 10 | + | +++ | ++ | ++ | + | + | |||
Results of anti-RARα antibodies are expressed as semiquantitative values according to specific absorbance (SA) value: SA less than 0.2 (+), SA 0.21 to 0.4 (++), SA 0.41 to 0.6 (+++), SA more than 0.61 (++++). Results of ANAs and ANCAs are expressed as negative (-) if no fluorescence was observed and as weakly positive (+) or strongly positive (++) according to the intensity of the signal. Both ANA and ANCA tests were performed according to standard routine procedures of the Laboratoire d'Immunologie by 2 independent investigators. Briefly, ANAs were detected in the same sera (diluted 1:80) by indirect immunofluorescence with commercial Hep2000 cells (Immunoconcepts; BMD, Marne la Vallée, France).9 ANCAs were tested in sera (diluted 1:20) by indirect immunofluorescence with commercial human neutrophils (Inova Diagnostics, San Diego, CA).10
Day 0 of therapy for all patients.
After maintenance therapy (delay in months is given for each patient).
Antibodies against multiple tumor-associated antigens like oncogene proteins (myc,11 ras12 ), gene suppressor proteins (p53 13 ), tumor antigens (MUC1,14 erbB2,15 WT1 16 ), or normal proteins (SPAN-Xb17 ) have been described in patients with solid tumors or hematologic malignancies.18-20 More recently, autoantibodies have been shown to be markers of better prognosis in melanoma patients treated by interferon-α.21 Our results show that in patients with APL, antibodies to RARα and autoantibodies like ANCA, and especially ANA, are detected. An increase in anti-RARα antibodies and ANAs was seen after maintenance therapy that includes ATRA and chemotherapy. In mice,22 as in humans,14 immunotherapy approaches have induced specific antibody production, and in an APL patient vaccinated with an HLA-A2–restricted peptide PR1 derived from proteinase 3, PR1 tetramer–sorted cytotoxic T lymphocytes (CTLs) were obtained after vaccination and showed lysis of the patient's bone marrow cells,23 suggesting that an antitumor response may be elicited in APL. The data of our study support the notion that an immune response elicited by APL cells is enhanced by ATRA therapy and may well be implicated in its efficacy, because immunocompromised APL mice treated by ATRA have reduced survival.24 The immunogenic role played by ATRA in APL is not known and may be related to at least 2 mechanisms. One relates to the now recognized role of vitamin A and its derivatives in the immune response, well seen in vitamin A deficiency3 as in studies on the T helper-2 (Th2) response25 and dendritic cell activation triggered by retinoids.26 The other may relate to the direct effect of ATRA on the leukemic cell itself, where apoptosis and degradation of the oncoprotein PML-RAR could participate in cross-presentation. Ongoing prospective immunomonitoring of patients with APL at diagnosis and throughout treatment will allow us to establish whether the achievement of an efficient immune response is of prognostic value in APL and which criteria may be determinant. If the latter study demonstrates that antibody response correlates with disease-free survival, it will provide, in the setting of minimal residual disease, the basis for vaccine-boosting strategies25-27 that enhance a humoral and/or a cellular immune response.
Prepublished online as Blood First Edition Paper, May 25, 2006; DOI 10.1182/blood-2006-03-013177.
Supported by the French Fondation pour la Recherche Medicale, the Fondation de France, the French Association de Recherche contre le Cancer, the French Ligue Nationale contre le Cancer, the Fondation Saint-Louis, Eli Lilly, and INSERM.
M.R. and J.A.-G. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Cécile Rochette-Egly, UMR-S 596, Strasbourg, France, for providing monoclonal anti-RARα antibody; Dr Felix Agbalika and Dr Catherine Scieux, Laboratoire de Virologie, Hôpital Saint-Louis, Paris, for providing some of the stored sera of patients with APL; Scott Kogan and J. Michael Bishop, University of California, San Francisco, for the APL mouse cells; and Bernard Boursin, Service d'Infographie, IUH, Hôpital Saint-Louis, Paris, for excellent graphic work.
Katerina Pokorna was on leave from the Institute of Molecular Genetics, Academy of Sciences of the Czech Republic, Prague, at the time of this article's writing.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal