Abstract
Plasminogen activators (PAs) are not used for thromboprophylaxis due to rapid clearance, bleeding, and extravascular toxicity. We describe a novel strategy that overcomes these limitations. We conjugated tissue-type PA (tPA) to a monoclonal antibody (mAb) against complement receptor type 1 (CR1) expressed primarily on human RBCs. Anti-CR1/tPA conjugate, but not control conjugate (mIgG/tPA), bound to human RBCs (1.2 × 103 tPA molecules/cell at saturation), endowing them with fibrinolytic activity. In vitro, RBC-bound anti-CR1/tPA caused 90% clot lysis versus 20% by naive RBCs. In vivo, more than 40% of anti-CR1/125I-tPA remained within the circulation (∼90% bound to RBCs) 3 hours after injection in transgenic mice expressing human CR1 (TgN-hCR1) versus less than 10% in wild-type (WT) mice, without RBC damage; approximately 90% of mIgG/125I-tPA was cleared from the circulation within 30 minutes in both WT and TgN-hCR1 mice. Anti-CR1/tPA accelerated lysis of pulmonary emboli and prevented stable occlusive carotid arterial thrombi from forming after injection in TgN-hCR1 mice, but not in WT mice, whereas soluble tPA and mIgG/tPA were ineffective. Anti-CR1/tPA caused 20-fold less rebleeding in TgN-hCR1 mice than the same dose of tPA. CR1-directed immunotargeting of PAs to circulating RBCs provides a safe and practical means to deliver fibrinolytics for thromboprophylaxis in settings characterized by a high imminent risk of thrombosis.
Introduction
Diverse means are used for thromboprophylaxis, including anticoagulation and platelet inhibition.1-4 Yet, although their effectiveness is incomplete, safety concerns limit dosing, frequency, and duration of treatment.5-9 If feasible, means to accelerate the lysis of nascent thrombi shortly after clotting and prevent permanent vascular occlusion may complement existing approaches to thromboprophylaxis.
Pathologic conditions and clinical settings characterized by a high propensity for recurrent thrombosis, in which such adjunct prophylactic thrombolysis would be most helpful, have been identified.10 In theory, plasminogen activators (PAs) could be used prophylactically in such situations to prevent clot extension and recurrence by targeting the interior of thrombi during their formation, thereby negating the problem of inefficient drug penetration into retracted, cross-linked, aged thrombi. Yet, the prophylactic utility of PAs has not been realized due to their unfavorable pharmacokinetics (blood clearance within < 10 minutes) and dose-limiting risk of hemorrhage and tissue damage.11-16
We posited that coupling to carrier red blood cells (RBCs) would endow PAs with prophylactic potential by (1) markedly prolonging PAs circulation time, (2) directing inclusion of RBC-bound PAs into nascent pathogenic thrombi, (3) minimizing PA penetration into existing hemostatic clots, and (4) restricting PA extravasation.17 In support of this concept, we have shown that chemical conjugation of tPA to isolated RBCs generates reinjectible RBC/tPA complex, which prolongs tissue-type PA (tPA) circulation by orders of magnitude without impairing RBC survival or hemostasis, preferentially enhances lysis of nascent versus preexisting clots, and thereby provides effective thromboprophylaxis in animal models of venous and arterial thrombosis.17
Ex vivo tPA conjugation to isolated RBCs followed by reinjection of the RBC/tPA complexes may be applicable to settings in which the risk of thrombosis can be anticipated and transfusion is part of routine clinical care (eg, patients with sickle cell disease). However, phlebotomy, ex vivo loading, and reinfusion of the modified RBCs are impractical in most settings where the use of such therapy could be envisioned. We hypothesized that targeting PAs directly to circulating RBCs would markedly improve the speed, safety, dosing, efficacy, and utility of this drug-delivery strategy.
To achieve this goal, we conjugated tPA to a monoclonal antibody (mAb 7G9) directed against complement receptor type 1 (CR1),18,19 generating an anti-CR1/tPA conjugate. In primates and humans, more than 90% of CR1 is expressed on RBCs at levels of approximately 100 to 1000 copies per cell, providing a well-defined anchorage site for tPA in blood18-21 ; the remaining CR1 is expressed on neutrophils and lymphocytes at levels of 5 × 103 and 2 × 104 copies per cell, but these cell types are found in blood at 1/1000 of the number of RBCs. Previous in vivo studies in nonhuman primates demonstrate that diverse ligands can be coupled to circulating RBCs using anti-CR1 conjugates without damage or shortening their survival.18,22-24
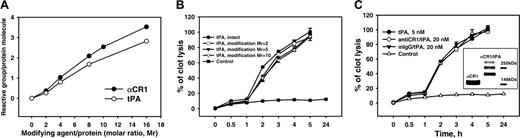
Synthesis and fibrinolytic activity of anti-CR1/tPA and IgG/tPA conjugates. (A) Modification curves of anti-CR1 IgG and tPA at indicated molar ratios (Mr) of SATA and SMCC, respectively, determined with Ellman reagent. (B) In vitro lysis of 125I-fibrinogen clots by 5 nM tPA or SMCC-modified tPA. (C) In vitro fibrinolysis of 125I-fibrinogen clots by indicated amounts of tPA or tPA conjugates. The data in this and the following figures are presented as the mean plus or minus SEM. Unless specified otherwise, in vitro fibrinolysis data show results of triplicates for each condition. The insert shows anti-CR1/tPA analysis using a 4% to 12% gradient SDS-PAGE run in Tris-Glycine buffer under nonreducing conditions. The results show maternal anti-CR1 IgG (left band) and the anti-Cr1/tPA conjugate (right band) containing species with 1, 2, or 3 molecules of tPA per molecule of IgG.
Synthesis and fibrinolytic activity of anti-CR1/tPA and IgG/tPA conjugates. (A) Modification curves of anti-CR1 IgG and tPA at indicated molar ratios (Mr) of SATA and SMCC, respectively, determined with Ellman reagent. (B) In vitro lysis of 125I-fibrinogen clots by 5 nM tPA or SMCC-modified tPA. (C) In vitro fibrinolysis of 125I-fibrinogen clots by indicated amounts of tPA or tPA conjugates. The data in this and the following figures are presented as the mean plus or minus SEM. Unless specified otherwise, in vitro fibrinolysis data show results of triplicates for each condition. The insert shows anti-CR1/tPA analysis using a 4% to 12% gradient SDS-PAGE run in Tris-Glycine buffer under nonreducing conditions. The results show maternal anti-CR1 IgG (left band) and the anti-Cr1/tPA conjugate (right band) containing species with 1, 2, or 3 molecules of tPA per molecule of IgG.
In the present study, we tested the hypothesis that injection of anti-CR1/tPA conjugates will generate fibrinolytically active RBCs in vivo without compromising RBC survival. Using for this goal transgenic mice expressing human CR1 on their RBCs25 (TgN-hCR1), we found that a single prophylactic injection of anti-CR1/tPA (but not nontargeted tPA) does provide specific-loading of RBCs in CR1-positive mice. This maneuver affords rapid dissolution of subsequently forming pulmonary emboli and prevents occlusive arterial thrombi from developing, while causing far less bleeding than nontargeted tPA. These data in animals indicate that tPA immunotargeting to circulating RBCs is, in principle, feasible, effective, and safe, and might provide a practical new approach to prophylactic fibrinolysis.
Materials and methods
Radiolabeling of proteins and RBCs
Proteins were radiolabeled with 125I-Na (Perkin Elmer, Wellesley, MA) using Iodogen (Pierce, Rockford, IL) and RBCs were radiolabeled with 51Cr as described previously.26
Synthesis and characterization of anti-CR1/tPA and mIgG/tPA conjugates
Anti-CR1 mAb 7G9 was produced and purified as described.18,22 tPA (Alteplase; Genentech, San Francisco, CA) was conjugated to anti-CR1 or control mouse IgG (Jackson ImmunoResearch Laboratories, West Grove, PA), using the bifunctional cross-linking pair succinimidyl 4-(N-maleimidomethyl)cyclohexane-1-carboxylate (SMCC) and N-succinimidyl S-acetylthioacetate (SATA) from Pierce (both dissolved at 5 mg/mL in DMSO immediately prior to modification). Briefly, tPA (1 mg/mL, in 300 mM Hepes pH 7.4) was modified by SMCC for 1.5 hours on ice. On average, approximately 1 maleimide group was incorporated per tPA molecule, modified at 5 molar SMCC excess (Figure 1A). Antibodies (4.5 mg/mL, in PBS pH 7.6) were modified by SATA for 1.5 hours on ice. After de-protection for 2 hours at 20°C by adding 1/10 reaction volume of 0.5 M hydroxylamine (Pierce), 3 to 4 sulfhydryl (SH) groups were incorporated per IgG molecule modified at 16 molar SATA excess. The excess SMCC and SATA was removed by centrifugation on Quick-Spin Columns (Roche, Indianapolis, IN). The number of incorporated reactive groups was determined by quantitative analysis of SH groups before and after protein modification using Ellman reagent (Molecular Probes, Eugene, OR) as per manufacturer's instructions.
Conjugation of tPA to mAb 7G9 or control IgG was performed at 6 molar excess tPA for 16 hours at 4°C. Conjugates were purified by gel filtration chromatography (Sephacryl SHR100; Amersham, Arlington Heights, IL) and dialyzed in PBS containing 200 mM arginine buffer (PBS/Arg), pH 7.4. Formulations of tPA and conjugates were kept in PBS/Arg and, unless specified otherwise, were used in PBS/Arg, PBS-BSA (PBS containing 3% BSA), or PBS.
Binding of conjugates to RBCs (loading)
RBCs were washed by centrifugation (1200g) with PBS-BSA, and resuspended to 10% hematocrit. Anti-CR1/125I-tPA and control mIgG/125I-tPA were incubated with human RBCs for 1 hour at 37°C (loading) on a rotator. Unbound ligand was removed by centrifugation26 and RBC-bound radioactivity was measured in a γ-counter (Perkin Elmer). To test the stability of anti-CR1/tPA binding, human RBCs loaded with anti-CR1/tPA (10 μg/mL) for 1 hour at 37°C and washed by centrifugation with PBS-BSA were incubated at 10% hematocrit on a shaker at 37°C. Aliquots of the RBC suspension (100 μL) were removed at 0 to 360 minutes, washed with PBS-BSA, fixed in 1 mL 1:4 dilution Cytofix (Pharmingen, San Diego, CA) for 30 minutes and incubated for 30 minutes with Alexa 488–labeled anti–mouse IgG (H+L; Molecular Probes, Eugene, OR). Samples were analyzed on a FACSCalibur (Becton Dickinson, San Jose, CA) flow cytometer using an erythrocyte gate set on size (forward scatter) and granularity (side scatter). Data (20 000 events/sample) were analyzed using Flow-Jo software.
The specificity of conjugate binding was confirmed by far-Western blotting. Lysates of RBC ghost membranes from human, WT, and TgN-hCR1 mice were prepared as described elsewhere,27 subjected to 4% to 12% gradient gel sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE; Invitrogen) under nonreducing conditions and transferred to nitrocellulose membrane (NitroBind; Osmonics, Minnetonka, MN). The membrane (cut in 3 pieces, each containing proteins from 3 types of RBC lysates), was incubated with either anti-CR1 (4 μg/mL), anti-CR1/tPA, or mIgG/tPA (8 μg/mL each). Anti-CR1 binding was detected with peroxidase-conjugated anti–mouse IgG. Conjugate binding was detected with rabbit anti-tPA polyclonal antibody (2 μg/mL; Molecular Innovations, Southfield, MI) followed by peroxidase-conjugated secondary antirabbit antibody. Immunoblots were developed by enhanced chemiluminescence (Amersham Biosciences UK, Little Chalfont, England).
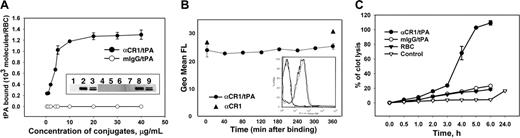
RBC-binding and fibrinolytic capacity of anti-CR1/tPA conjugate. (A) Anti-CR1/125I-tPA (closed symbols) or IgG/125I-tPA (open symbols) binding to washed human RBCs at 37°C, determined after elimination of unbound material (n = 3). Inset: far-Western blotting of RBC plasma membranes. Lysates prepared from RBC ghosts from humans (lanes 3, 6, 9), WT mice (lanes 1, 4, 7), or TgN-hCR1 mice (lanes 2, 5, 8) were subjected to 4% to 12% gradient SDS-PAGE under nonreducing conditions, transferred to nitrocellulose membranes that were cut in 3 pieces, and probed with anti-CR1 (lanes 1-3), mIgG/tPA (lanes 4-6), or anti-CR1/tPA (lanes 7-9). Conjugate binding was detected with polyclonal anti-tPA antibody (2 μg/mL) and anti-CR1 binding was detected with horseradish peroxidase–conjugated anti–mouse IgG. (B) Washed human RBCs loaded with anti-CR1/I-tPA (circles) or anti-CR1 antibody (triangles) for 60 minutes at 37°C were washed with PBS-BSA and incubated in a shaker at 37°C. FACS analysis after staining with labeled anti–mouse IgG was used to monitor detachment of the conjugate or anti-CR1. Inset: typical fluorescent intensity distribution. Two almost overlapping curves on the left depict intact RBC (T = 0 gray solid line, T = 6h dashed black line); 2 almost overlapping curves on the right depict anti-CR1/tPA-RBC complex (T = 0 black dotted line, T = 6h black solid line). (C) 125I-fibrin clots were incubated with human RBCs coated with either anti-CR1/tPA (closed circles) or IgG/tPA (open circles) at 37°C and fibrinolysis was measured as in Figure 1 (n = 3).
RBC-binding and fibrinolytic capacity of anti-CR1/tPA conjugate. (A) Anti-CR1/125I-tPA (closed symbols) or IgG/125I-tPA (open symbols) binding to washed human RBCs at 37°C, determined after elimination of unbound material (n = 3). Inset: far-Western blotting of RBC plasma membranes. Lysates prepared from RBC ghosts from humans (lanes 3, 6, 9), WT mice (lanes 1, 4, 7), or TgN-hCR1 mice (lanes 2, 5, 8) were subjected to 4% to 12% gradient SDS-PAGE under nonreducing conditions, transferred to nitrocellulose membranes that were cut in 3 pieces, and probed with anti-CR1 (lanes 1-3), mIgG/tPA (lanes 4-6), or anti-CR1/tPA (lanes 7-9). Conjugate binding was detected with polyclonal anti-tPA antibody (2 μg/mL) and anti-CR1 binding was detected with horseradish peroxidase–conjugated anti–mouse IgG. (B) Washed human RBCs loaded with anti-CR1/I-tPA (circles) or anti-CR1 antibody (triangles) for 60 minutes at 37°C were washed with PBS-BSA and incubated in a shaker at 37°C. FACS analysis after staining with labeled anti–mouse IgG was used to monitor detachment of the conjugate or anti-CR1. Inset: typical fluorescent intensity distribution. Two almost overlapping curves on the left depict intact RBC (T = 0 gray solid line, T = 6h dashed black line); 2 almost overlapping curves on the right depict anti-CR1/tPA-RBC complex (T = 0 black dotted line, T = 6h black solid line). (C) 125I-fibrin clots were incubated with human RBCs coated with either anti-CR1/tPA (closed circles) or IgG/tPA (open circles) at 37°C and fibrinolysis was measured as in Figure 1 (n = 3).
In vitro fibrinolysis
The fibrinolytic activity of SMCC-modified tPA, conjugates, and RBCs loaded with anti-CR1/tPA was measured using 125I-labeled fibrin clots as described.17,28 Briefly, clots were formed by adding CaCl2 and thrombin to 125I-fibrinogen (6 mg/mL in PBS) and overlaid with 200 μL PBS containing tPA or conjugates. To test the activity of cell-bound conjugates, RBCs were preincubated with either anti-CR1/tPA, control mIgG/tPA, or with PBS; washed and resuspended in PBS-BSA; and mixed with 125I-fibrinogen prior to adding CaCl2 and thrombin. The clots were then overlaid with 200 μL PBS, incubated at 37°C, and the radioactivity in the supernatants was measured in a γ-counter (Perkin Elmer).
Pharmacokinetic analysis of anti-CR1/tPA conjugates
Animal experiments were conducted under protocols approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Pennsylvania. Adult C57BL/6 mice (Jackson Laboratories, Bar Harbor, ME) and TgN-hCR1 mice25 on a C57BL/6xSJL background (purpose bred colony housed at Taconic, Germantown, NY) were used. Anti-CR1/125I-tPA or mIgG/125I-tPA (2 mg/kg in terms of tPA) was injected intravenously into anesthetized mice via the jugular vein. At indicated times, 100 μL to 200 μL of blood was withdrawn in heparin, centrifuged at 1200g, and the radioactivity in the plasma and cell pellets was measured. The animals were killed at 1 hour or 3 hours after injection and the radioactivity in the organs was measured. In a separate set of experiments, RBCs obtained from TgN-hCR1 mice were washed, labeled with 51Cr, loaded with a saturating dose of anti-CR1/tPA, and injected into anesthetized TgN-hCR1 mice; blood samples were collected and assayed as described for 125I.
Lysis of pulmonary emboli by tPA, anti-CR1/tPA, and mIgG/tPA
A suspension of 3 μ to 10 μ 125I-microemboli (ME) was produced by homogenization of 125I-fibrin/plasma clots, as described.29 In all tests of functional activity in vivo, either tPA (2 mg/kg), or mIgG/tPA or anti-CR1/tPA containing identical doses of tPA was injected intravenously into anesthetized TgN-hCR1 or WT mice 30 minutes prior to or 10 minutes after injecting ME. The animals were humanely killed 30 minutes (in prophylactic regimen) or 50 minutes (in therapeutic regimen) after injection of ME, and the radioactivity in the lungs was determined.
Lysis of carotid arterial thrombi by tPA, anti-CR1/tPA, and mIgG/tPA
Thirty minutes after injection of either tPA, IgG/tPA, or anti-CR1/tPA by the procedure identical to that described in “Lysis of pulmonary emboli by tPA, anti-CR1/tPA, and mIgG/tPA” (2 mg/kg tPA), thrombosis was induced in the exposed contralateral carotid artery by applying a piece of filter paper (1 × 2 mm) saturated with 10% FeCl3 to the adventitia.17,30 Blood perfusion was measured using a Doppler ultrasound equipped with a 0.5VB flow probe connected to a recording system (Transonic Systems, Ithaca, NY).
Disruption of hemostatic clots by tPA or anti-CR1/tPA
Anesthetized TgN-hCR1 mice were immobilized and 5-mm segments of the tail were then amputated with a razor blade. The time required to stop spontaneous bleeding was determined by absorption of blood onto filter paper discs at 30-second intervals. Five minutes later, after the bleeding had ceased, the tails were immersed into 10 mL of prewarmed (37°C) saline containing 0.01 mM tri-sodium citrate. Anti-CR1/tPA versus tPA (2 mg/kg tPA) were injected through the jugular vein and the amount of hemoglobin released over the ensuing 60 minutes was determined by measuring light absorption in the buffer at λ405 nm.
Results
Fibrinolytic and RBC-binding activity of anti-CR1/tPA conjugates in vitro
Anti-CR1/tPA and mIgG/tPA conjugates were synthesized using the bifunctional cross-linking reagent pair SATA-SMCC and purified by gel-filtration. Titration curves showed incorporation of approximately 1 maleimide group per tPA and 3 to 4 SH groups per IgG molecule without aggregation at molar ratios of SMCC/tPA and SATA/IgG of 5 and 16, respectively (Figure 1A).
The fibrinolytic activity of SMCC-modified tPA, anti-CR1/tPA, and IgG/tPA conjugates were tested in vitro by measuring the lysis of 125I-fibrin clots (Figure 1B-C). SMCC modification of tPA caused less than 10% loss of fibrinolytic activity (Figure 1B). Several molecular species of conjugates were seen on SDS-PAGE, corresponding to 1, 2, and 3 tPA molecules per IgG (Figure 1C, insert). These data imply that a conjugate population contained, on average, 2 molecules of tPA per IgG. The specific fibrinolytic activity of both conjugates (assuming a 1:2 IgG/tPA ratio) was approximately 8 times lower on a molar basis than that of free tPA (Figure 1C), likely due to steric masking of tPA.
Anti-CR1/125I-tPA, but not IgG/125I-tPA, bound to human RBCs in vitro (Bmax ∼1300 tPA molecules/cell; Figure 2A), in agreement with the known number of CR1 copies per human RBC6,18,19,31,32 and 2 tPA molecules per conjugate. Far-Western blotting of ghost RBC membranes confirmed the specificity of CR1 targeting (Figure 2A, insert): anti-CR1/tPA (lanes 8 and 9), but not mIgG/tPA (lanes 5 and 6) bound to the approximately 200-kDa band revealed by anti-CR1 in human and TgN-hCR1, but not WT mouse RBCs (doublet band corresponds to 2 known human CR1 isoforms). Fluorescence activated cell sorting (FACS) analysis showed no significant detachment of anti-CR1/tPA from human RBCs over at least 6 hours in vitro (Figure 2B). RBCs loaded with anti-CR1/tPA completely lysed 125I-fibrin clots in vitro, whereas RBCs preincubated with mIgG/tPA conferred no additional fibrinolytic activity compared with naive RBCs (Figure 2C).
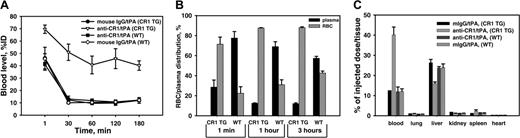
Pharmacokinetics of anti-CR1/125I-tPA and IgG/125I-tPA in TgN-hCR1 mice versus WT mice. (A) Blood clearance of conjugates. Wild-type (WT) and TgN-hCR1 mice (CR1 TG) were injected with 125I-tPA–containing conjugates, and percent of the injected dose (%ID) in the blood was measured. (B) Percent of 125Iodine recovered in plasma (▪) versus RBC pellet (hatched bars) in blood obtained 1 minute, 1 hour, or 3 hours after injection of 125I-labeled conjugates in TgN-hCR1 or WT mice. (C) Organ distribution of anti-CR1/125I-tPA versus IgG/125I-tPA 3 hours after injection in TgN-hCR1 and WT mice. (The number of animals in all experiments is 5 per group.)
Pharmacokinetics of anti-CR1/125I-tPA and IgG/125I-tPA in TgN-hCR1 mice versus WT mice. (A) Blood clearance of conjugates. Wild-type (WT) and TgN-hCR1 mice (CR1 TG) were injected with 125I-tPA–containing conjugates, and percent of the injected dose (%ID) in the blood was measured. (B) Percent of 125Iodine recovered in plasma (▪) versus RBC pellet (hatched bars) in blood obtained 1 minute, 1 hour, or 3 hours after injection of 125I-labeled conjugates in TgN-hCR1 or WT mice. (C) Organ distribution of anti-CR1/125I-tPA versus IgG/125I-tPA 3 hours after injection in TgN-hCR1 and WT mice. (The number of animals in all experiments is 5 per group.)
Anti-CR1/tPA binds safely to circulating RBCs in vivo
We then examined the pharmacokinetics and targeting of anti-CR1/tPA in TgN-hCR1 mice. CR1 is expressed on RBCs only in primates.19,32 Thus, WT mice lacking CR1 provided the ideal specificity control for these studies. mIgG/125I-tPA was eliminated rapidly from the circulation in both WT and TgN-hCR1 mice (Figure 3A, lower curves) with clearance kinetics similar to those of 125I-tPA (data not shown). This implies that tPA conjugated to IgG is accessible to the predominant hepatic clearance pathway. In contrast, the circulation of anti-CR1/125I-tPA was markedly prolonged in TgN-hCR1, but not in WT, mice (Figure 3A). After an initial decrease within 30 minutes (likely due to elimination of a fraction of unbound conjugate), the blood level of anti-CR1/tPA in TgN-hCR1 mice stabilized at values approaching 45% of the injected dose.
Within the first minute after infusion, approximately 75% of circulating anti-CR1/125I-tPA was found in the cellular fraction of blood in TgN-hCR1 mice and approximately 90% of the circulating radioactivity was found in this fraction at 1 hour and 3 hours (Figure 3B). In contrast, approximately 80% of the 125I was found in the plasma after injection of anti-CR1/125I-tPA in WT mice. In WT and TgN-hCR1 mice, approximately 80% of mIgG/125I-tPA was found in plasma (not shown). Thus, approximately 43 μg anti-CR1/tPA was found on blood cells compared with approximately 5 μg in plasma 3 hours after injection of 120 μg of the conjugate in TgN-hCR1 mice. In contrast, approximately 5 and approximately 7 μg of the conjugate was detected in the corresponding fractions in WT mice. TgN-hCR1 mice express approximately 3 times more copies of CR1/RBC (∼3000 molecules per cell) than human RBCs. The detection of 43 μg anti-CR1/tPA on RBCs is consistent with the theoretical RBC binding capacity for the conjugate. Thus, essentially every circulating RBC in TgN-hCR1 mice was saturated with the conjugate.
Both mIgG/125I-tPA and the unbound fraction of anti-CR1/125I-tPA were eliminated from the blood primarily by the liver, the typical predominant site of tPA clearance (Figure 3C). Hepatic uptake of anti-CR1/tPA was significantly reduced in TgN-hCR1 mice due to RBC binding (Figure 3C). Anti-CR1/tPA did not accumulate in the lungs of either TgN-hCR1 or WT mice, indicating that tPA targeting did not cause RBC aggregation and/or retention in the pulmonary capillaries (Figure 3C).
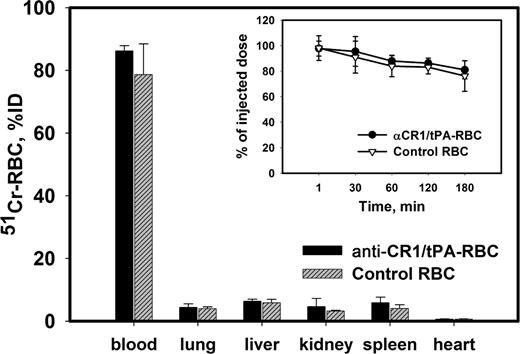
To examine the effect of anti-CR1/tPA targeting on RBC biocompatibility and survival, RBCs were collected from TgN-hCR1 donor mice, 51Cr-labeled, loaded with anti-CR1/tPA, and then injected into naive TgN-hCR1 recipient mice. The kinetics of blood clearance and organ distribution of 51Cr-RBCs loaded with anti-CR1/tPA were identical to control 51Cr-RBCs (Figure 4), indicating that anti-CR1/tPA binding to RBCs did not affect RBC viability, nor caused their aggregation and mechanical retention in any organ, including the lungs.
Prophylactic, but not therapeutic, administration of anti-CR1/tPA facilitates lysis of pulmonary emboli and prevents occlusive clots in the carotid artery
We next examined whether targeting anti-CR1/tPA to RBCs would accelerate the lysis of venous and arterial clots. To do so, we adapted 2 models previously used to test prophylactic fibrinolysis by RBCs carrying chemically conjugated tPA17,28 for use in TgN-hCR1 mice.
Anti-CR1/tPA loading does not damage carrier RBCs.51Cr-labeled RBCs obtained from TgN-hCR1 mice, either naive (▵ and ▨) or loaded with anti-CR1/tPA in vitro (closed symbols), were injected into naive TgN-hCR1 mice. The blood level (inset) was determined at indicated times. The animals were humanely killed 1 hour after injection and the amount of 51Cr in the organs was measured (n = 5).
Anti-CR1/tPA loading does not damage carrier RBCs.51Cr-labeled RBCs obtained from TgN-hCR1 mice, either naive (▵ and ▨) or loaded with anti-CR1/tPA in vitro (closed symbols), were injected into naive TgN-hCR1 mice. The blood level (inset) was determined at indicated times. The animals were humanely killed 1 hour after injection and the amount of 51Cr in the organs was measured (n = 5).
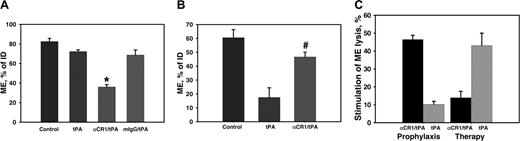
Prophylactic versus therapeutic thrombolysis of pulmonary emboli in TgN-hCR1 mice. tPA, IgG/tPA, or anti-CR1/tPA (2 mg/kg tPA each) were injected intravenously into TgN-hCR1 mice 30 minutes before (A) or 10 minutes after (B) injecting 125I-fibrin microemboli (ME). In panels A and B, the data are shown as the residual 125I radioactivity in the lungs normalized to the injected dose of 125I-ME (P < .005 for anti-CR1/tPA vs both mIgG/tPA and tPA in panel A and P < .002 for anti-CR1/tPA vs tPA in panel B). (C) Stimulation of fibrinolysis in the lungs after prophylactic or therapeutic administration of tPA and anti-CR1/tPA shown as the percent lysis stimulation versus the level of spontaneous lysis in control mice (n = 5 per group).
Prophylactic versus therapeutic thrombolysis of pulmonary emboli in TgN-hCR1 mice. tPA, IgG/tPA, or anti-CR1/tPA (2 mg/kg tPA each) were injected intravenously into TgN-hCR1 mice 30 minutes before (A) or 10 minutes after (B) injecting 125I-fibrin microemboli (ME). In panels A and B, the data are shown as the residual 125I radioactivity in the lungs normalized to the injected dose of 125I-ME (P < .005 for anti-CR1/tPA vs both mIgG/tPA and tPA in panel A and P < .002 for anti-CR1/tPA vs tPA in panel B). (C) Stimulation of fibrinolysis in the lungs after prophylactic or therapeutic administration of tPA and anti-CR1/tPA shown as the percent lysis stimulation versus the level of spontaneous lysis in control mice (n = 5 per group).
To examine the effect of anti-CR1/tPA in pulmonary thromboembolism, a suspension of 125I-labeled fibrin thrombi (3 μ to 10 μ diameter) was injected intravenously. The clots form aggregates with endogenous blood elements and lodge primarily in the lungs where they occlude precapillary vessels.29 Radioactivity detected after necropsy in the lungs reflects the rate of thrombolysis. Equal amounts of tPA, anti-CR1/tPA, or control mIgG/tPA (2 mg/kg tPA) were injected 30 minutes prior (prophylaxis) or 10 minutes after (therapy) injecting 125I-thrombi. Prophylactic administration of tPA minimally (∼10%) augmented endogenous fibrinolysis, whereas preinjection of anti-CR1/tPA augmented fibrinolysis by 50% and thus was significantly more potent than both mIgG/tPA and tPA (P < .005; Figure 5A,C). There was no difference between fibrinolysis by tPA compared with mIgG/tPA, indicating that specific binding to CR1 on circulating RBCs is required for prophylactic thrombolysis (Figure 5A). On the other hand, therapeutic administration of tPA augmented fibrinolysis by 45%, whereas injection of anti-CR1/tPA had little effect (∼12%, P < .002 vs tPA; Figure 5B-C). This implies that the anti-CR1/tPA-RBC complex is less effective in dissolving mature clots compared with tPA and is more effective when incorporated into newly formed ones.
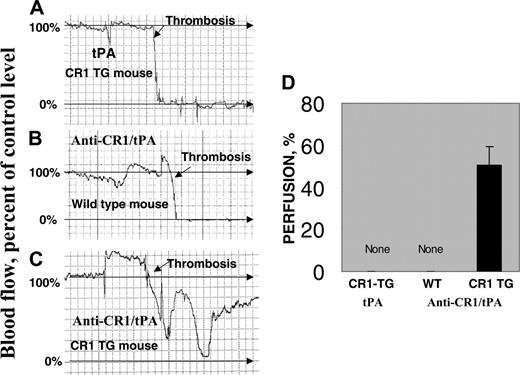
Prophylactic thrombolysis of occlusive arterial clots by anti-CR1/tPA in TgN-hCR1 mice. Occlusive thrombi were formed in the carotid artery of TgN-hCR1 (A, C) or WT (B) mice by applying FeCl3 to the adventitia. Rapid and complete cessation of perfusion was seen in both TgN-hCR1 and WT mice (A, B). Thirty minutes before injury, tPA (A) or anti-CR1/tPA (2 mg/kg tPA) (B, C) was injected. (A-C) Examples of Doppler ultrasound monitoring of blood perfusion. A square unit in the chart corresponds to 160 seconds. (D) Cumulative data of perfusion downstream of the thrombotic site collected in the 3 groups of animals (n = 5 per group). The flow in the vessel prior to thrombosis and the baseline of Doppler signal are considered as 100% and 0% perfusion, respectively. Percent of perfusion in panel D represents the maximal value of flow attained within 30 minutes after thrombosis.
Prophylactic thrombolysis of occlusive arterial clots by anti-CR1/tPA in TgN-hCR1 mice. Occlusive thrombi were formed in the carotid artery of TgN-hCR1 (A, C) or WT (B) mice by applying FeCl3 to the adventitia. Rapid and complete cessation of perfusion was seen in both TgN-hCR1 and WT mice (A, B). Thirty minutes before injury, tPA (A) or anti-CR1/tPA (2 mg/kg tPA) (B, C) was injected. (A-C) Examples of Doppler ultrasound monitoring of blood perfusion. A square unit in the chart corresponds to 160 seconds. (D) Cumulative data of perfusion downstream of the thrombotic site collected in the 3 groups of animals (n = 5 per group). The flow in the vessel prior to thrombosis and the baseline of Doppler signal are considered as 100% and 0% perfusion, respectively. Percent of perfusion in panel D represents the maximal value of flow attained within 30 minutes after thrombosis.
We then examined the effect of anti-CR1/tPA targeting on the evolution of carotid artery thrombotic occlusion using the FeCl3 model in which blood perfusion is monitored by Doppler ultrasound.17 FeCl3 caused complete obstruction of the vessel within 5 to 15 minutes in all untreated WT and TgN-hCR1 mice, with no evidence of reperfusion over the ensuing 30 to 40 minutes. Neither tPA nor anti-CR1/tPA affected thrombus formation or durability in WT mice (Figure 6A-B). In TgN-hCR1 mice preinjected with anti-CR1/tPA, initial reduction of perfusion was similar to other groups, but occlusion was delayed and followed by a rapid and extensive reperfusion within 30 to 40 minutes after the insult (Figure 6C-D).
Targeting anti-CR1/tPA to RBCs reduces tPA-induced lysis of hemostatic clots and bleeding
We then tested whether targeting anti-CR1/tPA to RBCs would spare hemostatic clots from tPA-induced dissolution in a mouse rebleeding model in which blood loss after cessation of bleeding from a severed tail is measured.33 Equal amounts of tPA versus anti-CR1/tPA (2 mg tPA per kg) were injected through the jugular vein immediately after the hemostatic plug was formed and the tail had been immersed into a buffer at 37°C. Profuse bleeding was visible from the wound after injection of tPA, which was confirmed by the amount of hemoglobin released into the buffer (Figure 7). Twenty-fold less rebleeding was seen in TgN-hCR1 mice given injections of anti-CR1/tPA compared with the same amount of soluble tPA (Figure 7A).
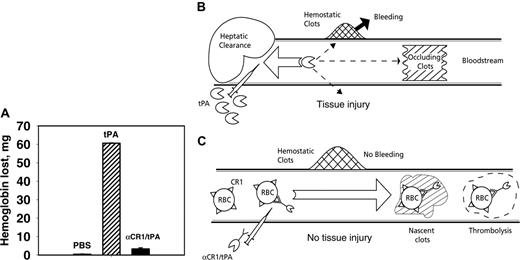
Coupling of tPA to circulating RBCs reduces rebleeding. (A) Soluble tPA caused greater lysis of hemostatic clots than anti-CR1/tPA. Segments from the tails of TgN-hCR1 mice were amputated, and 5 minutes after hemostasis was attained, the tails were immersed into warm saline. PBS, tPA, or anti-CR1/tPA (2 mg/kg tPA each) was injected through the jugular vein. The amount of hemoglobin released from the tail over the ensuing hour was measured. (B, C) Schematic comparison of vascular delivery of tPA and anti-CR1/tPA. (B) Rapid clearance by liver, among other reasons, prohibits prophylactic use and dictates injection of large doses of tPA, which diffuses into hemostatic clots and tissues, causing bleeding and side effects. (C) Injected anti-CR1/tPA binds predominantly to RBCs, circulates for a prolonged time without access to preexisting hemostatic clots and extravascular tissues, while incorporating into and dissolving nascent intravascular clots.
Coupling of tPA to circulating RBCs reduces rebleeding. (A) Soluble tPA caused greater lysis of hemostatic clots than anti-CR1/tPA. Segments from the tails of TgN-hCR1 mice were amputated, and 5 minutes after hemostasis was attained, the tails were immersed into warm saline. PBS, tPA, or anti-CR1/tPA (2 mg/kg tPA each) was injected through the jugular vein. The amount of hemoglobin released from the tail over the ensuing hour was measured. (B, C) Schematic comparison of vascular delivery of tPA and anti-CR1/tPA. (B) Rapid clearance by liver, among other reasons, prohibits prophylactic use and dictates injection of large doses of tPA, which diffuses into hemostatic clots and tissues, causing bleeding and side effects. (C) Injected anti-CR1/tPA binds predominantly to RBCs, circulates for a prolonged time without access to preexisting hemostatic clots and extravascular tissues, while incorporating into and dissolving nascent intravascular clots.
Discussion
Early and sustained recanalization of thrombotic vessels by plasminogen activators reduces mortality after acute myocardial infarction, but therapy is less effective in patients with peripheral arterial occlusion or ischemic stroke.5,6,34,35 Thrombolysis is accompanied by a significant risk of internal bleeding, for example, intracranial hemorrhage (ICH), which occurs in 1% to 2% of patients receiving continuous treatment for 2 hours to 24 hours.12,34 The clinical utility of PAs is also limited by inadequate delivery (rapid elimination, inactivation, and ineffective penetration into thrombi), collateral damage caused by extravasation into tissues, and ischemia-reperfusion injury.11-14,16,36-38
New thrombolytic agents showing longer circulation, greater fibrin specificity, enhanced resistance to PA inhibitors, and reduced side effects have been developed.11,35,39-45 Chimeric molecules have also been constructed (eg, PAs conjugated to clot-targeting ligands) to enhance targeting.46-50 However, no existing formulation prevents PA extravasation or prolongs its circulation sufficiently to permit utility as prophylaxis.
RBCs, the most abundant (> 99%) cellular constituent of blood, circulate for 120 days and provide an attractive vehicle for prolonging the delivery of drugs intended to act intravascularly.51,52 RBCs can serve as carriers to convert PAs into new drugs capable of being used for thromboprophylaxis.17,28 Here we explored targeting tPA to circulating RBCs using CR1 for anchorage. Figure 7 illustrates this strategy: fast hepatic clearance dictates infusion of large doses of tPA that attacks hemostatic clots and causes side effects in the tissues (Figure 7B), while anti-CR1/tPA that binds to and circulates for a prolonged time with RBCs has access to and dissolves predominantly clots formed intravascularly after the treatment (nascent clots), sparing existing hemostatic clots and tissues (Figure 7C).
Anti-CR1/tPA retains stable antigen-binding and fibrinolytic activities (Figures 1 and 2), binds to RBCs in vivo, and circulates for a prolonged time as a stable complex (Figure 3) without damage to carrier RBCs (Figure 4). To test functional activity of the conjugate, we employed analysis of the release of radiolabeled fibrin degradation products from clots. In theory, the enzymatic activity of modified tPA and of RBC-bound tPA could be fairly easily compared with native tPA using a chromogenic assay. However, in the case of RBC-coupled tPA, spectrozyme assay provides less stringent analysis of tPA activity compared with fibrinolysis assay and may overestimate tPA activity due to higher steric accessibility of the RBC/tPA active site to small chromogenic substrates versus plasminogen.28 To avoid overestimation of RBC/PA activity, we used a more stringent fibrinolysis assay both in vitro and in vivo.
A single prophylactic intravenous injection of anti-CR1/tPA facilitates lysis of evolving pulmonary emboli (Figure 5) and prevents stable occlusion of carotid arteries by subsequently formed intravascular clots (Figure 6). Anti-CR1/tPA incorporates preferentially into developing, but not preexisting thrombi (Figure 5B-C), thus sparing hemostatic clots and greatly reducing tPA-induced bleeding (Figure 7). Anti-CR1/tPA was active in TgN-hCR1 mice, but not in WT mice, indicating that CR1 targeting is a prerequisite for these results.
Doppler ultrasounds of the carotid artery in TgN-hCR1 mice preinjected with anti-CR1/tPA show characteristic oscillations during the initial phase of thrombotic occlusion followed by rapid reperfusion (Figure 6C). This agrees with the unstable character of nascent thrombi,53 allowing RBC-bound anti-CR1/tPA to access their interior and cause fibrinolysis, diminishing their stability and leading to their expedited dissolution. The same is true in the ME model (Figure 5), in which small preformed fibrin clots enlarge in vivo by incorporating cellular and plasma elements.54,55 Thus, the difference in the effectiveness of anti-CR1/tPA given as prophylaxis versus therapy in the lung model of ME and on carotid artery perfusion and rebleeding in TgN-hCR1 mice (Figures 5, 6, and 7) imply that RBC-coupled anti-CR1/tPA incorporates selectively into nascent pathologic clots, sparing existing hemostatic clots that are relatively impermeable to RBCs. The unstable nature of the clots early in their development after ferric chloride injury also helps explain why RBC-PA is effective as prophylaxis on the arterial as well as on the venous side of the circulation. This outcome, although counterintuitive in the light of the dominant contribution of platelets in arterial clots (versus fibrin and RBCs in its venous counterpart) is consistent with previous animal studies showing that genetic overexpression of PAs in endothelial and blood cells attenuates arterial thrombosis.55-58
Anti-CR1 provides a viable and efficacious carrier to anchor drugs to circulating RBCs. Anti-CR1 conjugated to pathogen-specific mAbs has undergone preclinical testing as an antiinfectious agent.18,22-24 The utility of CR1 targeting may be enhanced further by using variable fragment single-chain antibodies (scFv),49,50,59 which likely will eliminate Fc-fragment–mediated clearance of the conjugates by macrophages, reduce partial inactivation of conjugated tPA, and provide homogenous formulations amenable to translation into clinics.
The fibrinolytic effects of anti-CR1/tPA in animal models (Figures 5 and 6) are similar to those of RBC/tPA conjugates,17,28 yet these 2 approaches differ in several respects. Almost 6 × 104 copies of tPA can be conjugated to RBCs without damage,17,28 which is approximately 7.5-fold greater than what is achieved using anti-CR1/tPA. The observation that anti-CR1/tPA and RBC/tPA appear to cause comparable effects in both models suggests that the capacity of anti-CR1/tPA to bind to all circulating RBCs offsets the higher loading of RBC/tPA, which is unlikely to invest more than 10% to 15% of the total RBC mass with fibrinolytic activity on a per cell basis (J.-C.M., K.G., D.B.C., V.R.M., unpublished data, October 12, 2002 and May 6, 2004). In addition, RBCs loaded with anti-CR1/tPA might circulate for a longer time than reinjected RBCs, which are more heavily loaded with tPA ex vivo. Additional in vivo studies of efficacy (eg, comparison of dosing and duration of RBC/tPA vs anti-CR1/tPA) are in progress to help optimize these approaches to delivery.
Safety issues, including immune reactions to multiple injections of RBC-bound proteins, must be carefully addressed, although these issues are likely to be resolved by using humanized anti-CR1/PA fusion constructs or PEG-camouflaged conjugates.60 Prolonged retention of plasminogen activators within the circulation by RBCs may, in theory, activate plasminogen and consume fibrinogen. However, plasma fibrinogen was unchanged 1 hour after injection of 2 mg/kg anti-CR1/tPA in TgN-hCR1 mice (data not shown). Moreover, RBC carriage enhances the effectiveness of thrombolysis, permitting considerably lower doses of tPA to be used. In theory, PA inhibitors or other agents to remove or inactivate RBC-PA can be used in case of bleeding.
The utility of anti-CR1/tPA thromboprophylaxis may be refined further by using a pro-drug enzymatically activated within nascent clots. RBC-coupled tPA retains its ability to be activated by fibrin,28 providing a prototype for designing RBC-targeted fibrinolytic pro-drugs that will be activated selectively at sites of nascent thrombosis (eg, via thrombin-mediated activation).42
In summary, our data show that immunotargeting a fibrinolytic agent to circulating RBCs can be achieved by a single intravenous injection of an anti-CR1/tPA conjugate. This approach (summarized in Figure 7B-C) provides rapid and tight binding of tPA to RBCs, markedly prolongs tPA's lifespan in the circulation, accelerates lysis of venous and occlusive arterial thrombi formed subsequent to injection, and reduces bleeding from pre-existing hemostatic clots.
Prepublished online as Blood First Edition Paper, May 30, 2006; DOI 10.1182/blood-2005-11-012336.
Supported by an American Heart Association (AHA) Bugher-Stroke Award, a University of Pennsylvania (PENN) Research Foundation Award, a Sponsor Research Agreement with Elusys Therapeutics, National Institutes of Health grant RO1 HL66442 (V.R.M.), AHA SDG 053525N (S.Z.), research grants from the Spanish government “Ramon y Cajal,” CAM GR/SAL/0325/2004, and FIS PI 040961 (J-C.M.), and National Institutes of Health grants RO1 HL60169 (D.B.C.) and RO1 HL076406.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Drs G. Spitalny, E. Posilico, N. Mohamed, and L. Casey (Elusys) for helpful discussions and Thomas A. Bradley for technical assistance.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal