Vascular endothelial growth factor165 (VEGF165) and semaphorin3A (SEMA3A) elicit pro- and antiangiogenic signals respectively in endothelial cells (ECs) by binding to their receptors VEGFR-2, neuropilin-1 (NRP1), and plexin-A1. Here we show that the VEGF165-driven angiogenic potential of multiple myeloma (MM) ECs is significantly higher than that of monoclonal gammopathy of undetermined significance (MGUS) ECs (MGECs) and human umbilical vein (HUV) ECs. This is probably due to a constitutive imbalance of endogenous VEGF165/SEMA3A ratio, which leans on VEGF165 in MMECs but on SEMA3A in MGECs and HUVECs. Exogenous VEGF165 induces SEMA3A expression in MGECs and HUVECs, but not in MMECs. Moreover, by counteracting VEGF165 activity as efficiently as an anti-VEGFR-2 antibody, exogenous SEMA3A restrains the over-angiogenic potential of MMECs. Our data indicate that loss of endothelial SEMA3A in favor of VEGF165 could be responsible for the angiogenic switch from MGUS to MM.
Introduction
Multiple myeloma (MM) remains an incurable disease, despite conventional and high-dose chemotherapies.1 Molecules targeting not only plasma cells, but also the bone marrow (BM) microenvironment are needed to overcome drug resistance.
Pathologic angiogenesis is a constant component of the MM microenvironment.2 The vascular endothelial growth factor (VEGF)/VEGF receptor-2 (VEGFR-2) pathway greatly contributes to MM angiogenesis and growth,3 and mediates proliferation and capillarogenesis in MM endothelial cells (MMECs) through an autocrine loop.4 VEGF165 is the most abundant and effective isoform.5 It binds simultaneously to its cognate receptors VEGFR-1 and VEGFR-2 and to the coreceptor neuropilin-1 (NRP1), a cell-surface glycoprotein expressed on axons in the developing nervous system as well.6 NRP1 is the ligand-binding subunit of the receptor complex for class 3 semaphorins, a family of secreted proteins that mediate neuronal guidance.7 Binding of secreted semaphorin 3A (SEMA3A) to NRP1 induces the collapse of neuronal growth cones8 by activating the signal-transducing subunit(s) of the receptor complex, that is, class A plexins, a family of transmembrane proteins whose cytoplasmic domain is endowed with an R-Ras GAP activity that inhibits integrin function.9 NRP1 is also expressed on ECs,10 where it acts as an isoform-specific receptor for VEGF165, and enhances by 4- to 6-fold its affinity for VEGFR-2, as well as VEGF165-induced cell chemotaxis and proliferation.11 By competing with VEGF165 for binding to NRP112 and by activating class A plexins,13 SEMA3A inhibits integrin-based EC adhesion and migration and capillary sprouting. Autocrine loops of endothelial SEMA3A play a self-limiting role in angiogenesis and regulate EC behavior during its physiologic development.13
Here we analyze the expression levels of VEGF165, SEMA3A, and their receptors NRP1 and plexin-A1 in BM ECs isolated from patients with MM and MGUS (MMECs and MGECs), and from the human umbilical vein (HUVECs). We show that overangiogenic MMECs display a high VEGF165/SEMA3A ratio and behave like MGECs and HUVECs upon exposure to exogenous SEMA3A, which seems as effective as an anti-VEGFR-2 antibody. Our observations point to SEMA3A as a potential antiangiogenic drug that could be employed to re-establish a physiologic VEGF165/SEMA3A ratio in MMECs.
Patients, materials, and methods
Patients
Thirty-two patients fulfilling the International Myeloma Working Group diagnostic criteria14 for MM (n = 18) and MGUS (n = 14) were studied at diagnosis. The MM patients (12 male, 6 female) were aged 44 to 81 years (median 68.5 years) and staged15 as IIA (n = 4), IIB (n = 2), IIIA (n = 10), and IIIB (n = 2); the M-component was IgG (n = 12), IgA (n = 4), and κ or λ (n = 2). The MGUS patients (8 male, 6 female) were aged 42 to 79 years (median 70.6 years) and were IgG (n = 10) or IgA (n = 4). The study was approved by the local ethics committee of the University of Bari Medical School, Italy, and all patients gave their informed consent in accordance with the Declaration of Helsinki.
Separation and culture of ECs and plasma cells
BM MMECs and MGECs were obtained as described.16 Centrifugation on Ficoll gradient of heparinized aspirates was followed by polystyrene flask adherence to isolate stromal cells, removal of suspended cells (hence plasma cells), detachment of adherent cells with a trypsin/EDTA solution, immunodepletion of macrophages and possible residual plasma cells with CD14 and CD38 (macrophage and plasma cell markers, respectively) monoclonal antibody (mAb)-coated flasks (both mAbs were from Immunotech, Coulter, Marseilles, France), absorption to magnetic microbeads (Oxoid Dynal, Oslo, Norway) coated with Ulex europaeus-1 lectin (whose receptor is highly and specifically expressed by ECs), and transfer of beads with bound ECs to plates in complete medium (RPMI-1640 medium supplemented with 10% heat-inactivated fetal calf serum [FCS] and 1% glutamine) to allow cells to spread to the plate surface and grow. Plasma cells were harvested from suspended cells by positive immunomagnetic sorting, using the CD38 mAb-coated beads, transfer of beads with bound cells to plates in complete medium, and bead detachment.17
The purity and viability of EC preparations (more than 95% viable ECs) were assessed by fluorescence-activated cell sorting (FACS; FACScan, Becton Dickinson, San Jose, CA) with double positivity for factor VIII-related antigen (FVIII-RA, a highly specific EC marker) and CD105 (or endoglin, a molecule selectively expressed by ECs),18 and negativity for CD14 and CD38 mAbs, followed by reverse transcriptase-polymerase chain reaction (RT-PCR) for mRNA of FVIII-RA, CD38, CD105, and IgH VDJ region, and by trypan blue viable staining.16 Purity was also evaluated in a transmission electron microscope following conventional embedding and staining.16 Plasma cells were checked for purity and viability (more than 95% viable cells) by FACS positivity for CD38 mAb and negativity for CD3 (a pan-T-cell marker) and CD14 mAbs (< 2% of T cells and macrophages), and by morphology in May-Grünwald-Giemsa staining or by immunocytochemical staining with anti-κ or anti-λ antibody (Dako, Glostrup, Denmark) according to the light chain of the M-component.17 The viability was assessed by trypan blue staining.
HUVECs were obtained from 15 samples following parental consent, and grown in gelatin-coated plates in their own complete medium (M199 medium supplemented with 10% FCS, 0.02% extract of bovine brain, and 0.015% porcine heparin).18 All cell populations were cultured at 37°C in a humidified atmosphere of 5% CO2 in air.
Treatment with VEGF165 and SEMA3A: preparation of conditioned media (CM) and total RNA
ECs at 90% confluence were cultured in duplicate in specific serum-free medium (SFM), alone or supplemented with recombinant VEGF165 (10 ng/mL; Sigma Chemical, St Louis, MO) and collected after 24, 48, and 72 hours to obtain CM and total RNA. MMECs were also exposed to recombinant SEMA3A (150 ng/mL; R&D Systems, Minneapolis, MN). Plasma cells were cultured in duplicate in SFM, and CM collected after 24 hours. RNA was extracted with Trizol reagent (Invitrogen, Life Technologies, Carlsbad, CA), purified using the RNeasy total RNA Isolation Kit (Qiagen, Valencia, CA), and verified for integrity with an Agilent Bioanalyzer (Agilent Technologies, Waldbronn, Germany).
RT-PCR
Total RNA (2 μg) was reverse transcribed by Moloney murine leukemia virus-reverse transcriptase (MMLV-RT; Invitrogen). Next, 1 μg cDNA of VEGF165-untreated and -treated ECs was subjected to RT-PCR for VEGF165, SEMA3A, NRP1, and plexin-A1. The primers (Invitrogen), the PCR conditions, and the length of the products are summarized in Table 1. The products were separated by electrophoresis on 1.5% agarose gels and stained with ethidium bromide. The intensity of bands was measured with a Kodak trans-illuminator (EDAS 290) and the Kodak 1-D Image Analysis Software (Eastman Kodak, Rochester, NY), which converts the band area into numbers of pixels.16
RT-PCR: primers, amplification, and products
Gene . | Sequence of primers, 5′ to 3′ . | Conditions of amplification . | Product length, bp . |
|---|---|---|---|
| VEGF165 | Forward: GCTGCACCCATGGCAGAAGG* | 30 cycles at 95°C 1 minute and 30 seconds, 95°C 1 minute, 65°C 30 seconds, 72°C 30 seconds | 367 |
| Reverse: GAGCAAGGCCCACAGGGATTT† | |||
| SEMA3A | Forward: GGCATATAATCAGACTCACTTGTACGC | 1 cycle at 94°C 10 minutes, 30 cycles at 94°C 30 seconds, 50°C 30 seconds, 72°C 30 seconds, and 1 cycle at 72°C 7 minutes | 450 |
| Reverse: CTTGCATATCTGACCTATTCTAGCGTG | |||
| NRP1 | Forward: GGCTCCAAATAGACCTGGGG | 40 cycles at 95°C 1 minute and 30 seconds, 95°C 1 minute, 60°C 30 seconds, 72°C 30 seconds | 600 |
| Reverse: GGTGCTGTCTATGACCGTGG | |||
| Plexin-A1 | Forward: CCTCGAGAGCAAGAACCACCCCAAGCTGCT | 1 cycle at 94°C 10 minutes, 30 cycles at 94°C 30 seconds, 68°C 30 seconds, 72°C 45 seconds, and 1 cycle at 72°C 7 minutes | 150 |
| Reverse: CCCTTCACCGGCACCTCAGGTGCATT | |||
| GAPDH | Forward: CCCTCCAAAATCAAGTGGGG | 20 cycles at 95°C 1 minute and 30 seconds, 95°C 45 seconds, 60°C 45 seconds, and 72°C 30 seconds | 347 |
| Reverse: CGCCACAGTTTCCCGGAGGG |
Gene . | Sequence of primers, 5′ to 3′ . | Conditions of amplification . | Product length, bp . |
|---|---|---|---|
| VEGF165 | Forward: GCTGCACCCATGGCAGAAGG* | 30 cycles at 95°C 1 minute and 30 seconds, 95°C 1 minute, 65°C 30 seconds, 72°C 30 seconds | 367 |
| Reverse: GAGCAAGGCCCACAGGGATTT† | |||
| SEMA3A | Forward: GGCATATAATCAGACTCACTTGTACGC | 1 cycle at 94°C 10 minutes, 30 cycles at 94°C 30 seconds, 50°C 30 seconds, 72°C 30 seconds, and 1 cycle at 72°C 7 minutes | 450 |
| Reverse: CTTGCATATCTGACCTATTCTAGCGTG | |||
| NRP1 | Forward: GGCTCCAAATAGACCTGGGG | 40 cycles at 95°C 1 minute and 30 seconds, 95°C 1 minute, 60°C 30 seconds, 72°C 30 seconds | 600 |
| Reverse: GGTGCTGTCTATGACCGTGG | |||
| Plexin-A1 | Forward: CCTCGAGAGCAAGAACCACCCCAAGCTGCT | 1 cycle at 94°C 10 minutes, 30 cycles at 94°C 30 seconds, 68°C 30 seconds, 72°C 45 seconds, and 1 cycle at 72°C 7 minutes | 150 |
| Reverse: CCCTTCACCGGCACCTCAGGTGCATT | |||
| GAPDH | Forward: CCCTCCAAAATCAAGTGGGG | 20 cycles at 95°C 1 minute and 30 seconds, 95°C 45 seconds, 60°C 45 seconds, and 72°C 30 seconds | 347 |
| Reverse: CGCCACAGTTTCCCGGAGGG |
This primer is located in exon 2 (part of the conserved region of all VEGF splice variants)
This primer spans the boundaries of exons 5 and 7
Real-time RT-PCR
Total RNA (1 μg) was reverse transcribed to total cDNA. Real-time RT-PCR was performed in a Smart Cycler (Cepheid, Sunnyvale, CA) using the OmniMix HS (TaKaRa Bio, Otsu, Japan) supplemented with SYBR Green I (Sigma Chemical). To compensate for differences in RNA quality or RT efficacy, each sample was processed with parallel assays for the ABL housekeeping gene, using the following primers (Invitrogen): 5′-AAAACCTTCTCGCTGGACCC-3′ (forward) and 5′-GGGCTTCACACCATTCC-3′ (reverse). The absolute values of VEGF165, SEMA3A, NRP1, and plexin-A1 mRNA were thus normalized to the ABL mRNA content.19 The primers used for RT-PCR were applied on duplicate RNA samples. Measurements were taken at the end of the 72°C extension step in each cycle, and the second-derivative method was used to calculate threshold cycle (Ct). Melt-curve analysis showed a single sharp peak for all samples. The average Ct of the ABL gene was subtracted from the average Ct of VEGF165, SEMA3A, NRP1, and plexin-A1 gene to yield the ΔCt. To evaluate the VEGF165-induced expression of each gene, the ΔCt of ECs at time 0 (SFM alone) was subtracted from the ΔCt of the VEGF165-stimulated ECs at 24, 48, and 72 hours to obtain the ΔΔCt.20 The absolute values of each mRNA were obtained by including serial known concentrations (as ng/μL) of the human cDNA expressing the target gene. By plotting the Ct versus the concentration values, a linear standard curve was generated, and used as a reference for extrapolating the absolute values.19
Western blot
Western blot was performed to evaluate the VEGF165 and SEMA3A content of EC CM and the NRP1 and plexin-A1 content of cell extracts (both are not secreted), with β-actin as control, as described21 : 40 μg of proteins were subjected to 8% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) under reducing conditions, transferred electrophoretically to a polyvinylidene difluoride membrane (NEN, Life Science, Boston, MA), incubated with the antibody to VEGF165,22 SEMA3A,23 NRP1,24 and plexin-A125 (all 1:200 [vol/vol] diluted in tris-buffered saline [TBS]-Tween 0.1% and milk 5%), and with the secondary antibody (all from Santa Cruz Biotechnology, Santa Cruz, CA) for 1 hour, then with enhanced chemiluminescence (NEN), and revealed for signal by Kodak Biomax film (Eastman Kodak). The band intensity was expressed as fold expression of the lowest value by arbitrary optical density (OD) taken as unit.
Functional studies
Proliferation assay. In the first series of experiments, the VEGF165-induced growth of MMECs, MGECs, and HUVECs was evaluated by seeding in triplicate 3 × 103 cells/well on 96-well plates in SFM alone or admixed with 10% FCS, both supplemented with 10 ng/mL VEGF165. The cell number was estimated on day 8 by the crystal violet colorimetric method of Kueng et al,26 and data were expressed as mean plus or minus 1 standard deviation (SD) per group of patients and HUVECs. In the second series, MMEC proliferation was evaluated in the same culture conditions in the presence of 10, 50, 150, and 300 ng/mL SEMA3A, or 30 μg/mL anti-NRP1 mAb (Santa Cruz Biotechnology) and/or 5 μg/mL anti-VEGFR-2 mAb (R&D Systems). The function blocking activity of the NRP1 antibody was checked in preliminary experiments in which the antibody (30 μg/mL) antagonized the effect of SEMA3A (150 ng/mL) on MMECs. Data were expressed as mean plus or minus 1 SD.
Chemotaxis assay. The chemotaxis assay was performed in triplicate according to the Boyden microchamber technique27 toward SFM alone (negative control) or admixed with 10 ng/mL VEGF165 alone (positive control) or supplemented with SEMA3A, anti-NRP1, or anti-VEGFR-2 mAbs at the same concentrations as in the proliferation assay. Cells were counted on ×1000 5 oil-immersion field/membrane and given as mean plus or minus 1 SD per group of patients and HUVECs.
Matrigel capillarogenesis assay. The Matrigel capillarogenesis assay assesses the ability of ECs to produce “spontaneous angiogenesis in vitro, ± that is, a 3-D vascular tube and cordlike structure connecting “cellular nodes ± and resembling an organized capillary mesh.18 ECs were plated in duplicate in 24-well plates (2 × 105 cells/well) precoated with Matrigel (300 μL/well; Becton Dickinson) in 1 mL/well in SFM alone or supplemented with VEGF165 (10 ng/mL) alone or together with SEMA3A (150 ng/mL), or anti-NRP1 (30 μg/mL), or anti-VEGFR-2 (5 μg/mL) mAbs. After a 12-hour incubation, the 3-D organization was examined under a reverted, phase-contrast photomicroscope, and after skeletonization of the mesh its topologic parameters were measured by computed image analysis28 : (1) “mesh areas” as the number of empty regions of the field delimited by tubules and cell clusters; (2) “length” as the total length (in micrometers) per field of the cell network; (3) “branching points” as the number of nodes where branches meet. The final results were given as mean plus or minus 1 SD per group of patients and HUVECs.
Results
A constitutive imbalance of endogenous VEGF165/SEMA3A ratio in MMECs, but not in MGECs
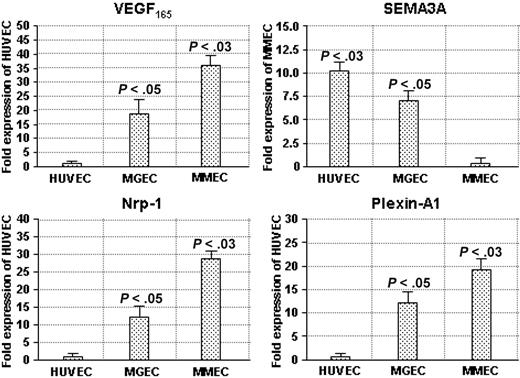
First, we analyzed the transcription of VEGF165, SEMA3A, NRP1, and plexin-A1 genes by semiquantitative RT-PCR on RNA extracted from HUVECs (n = 15) and ECs isolated from the BM of patients with MGUS (n = 14) and MM (n = 18), and found that MMECs displayed the highest constitutive expression of VEGF165, NRP1, and plexin-A1 mRNAs, and the lowest levels of SEMA3A transcript (Figure S1 and Table S1, available at the Blood website; see the Supplemental Materials link at the top of the online article). Next, the expression of VEGF165, SEMA3A, NRP1, and plexin-A1 mRNA was more precisely quantified by real-time RT-PCR (Figure 1). The average expression level of HUVECs, which transcribed VEGF165, NRP1, and plexin-A1 genes at the lowest rate, was taken as the reference value with an arbitrary score of 1, whereas the lowest levels of SEMA3A mRNA expressed by MMECs were the point-1 reference. In MMECs and MGECs, VEGF165 mRNA displayed, respectively, a 36- and 18-fold increase compared with HUVECs, whereas NRP1 transcription was 28 and 12 times higher and plexin-A1 mRNA 19- and 12-fold increased. Notably, the SEMA3A gene was transcribed 11 and 7 times more efficiently in HUVECs and MGECs than in MMECs. The average VEGF165/SEMA3A ratio was 5.4 in MMECs, but 0.4 and 0.3 in MGECs and HUVECs, respectively (P < .005 by Wilcoxon-Wilcox test).
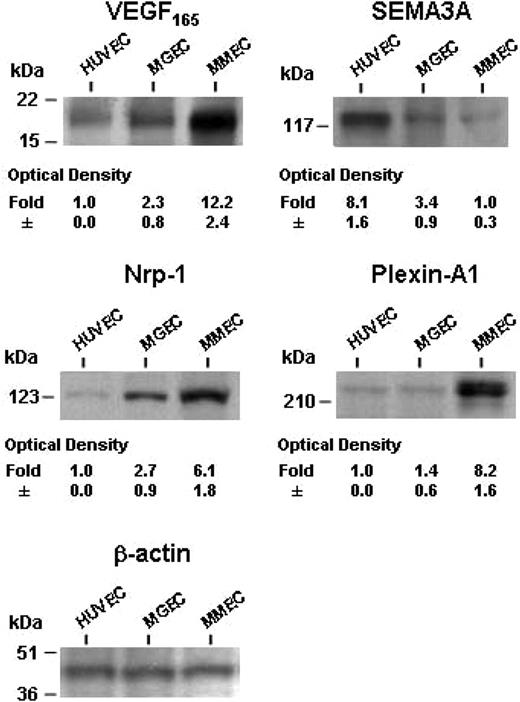
We argue that mRNAs were efficiently translated, since Western blot analysis showed a comparable modulation of protein expression; VEGF165, NRP1, and plexin-A1 levels were higher and SEMA3A protein was lower in MMECs compared with HUVECs and MGECs (Figure 2).
Comparison between plasma cells and ECs
Real-time RT-PCR (Figure 3A) showed that plasma cells of patients with MM expressed 8.3-fold higher levels of VEGF165 mRNA than paired MMECs, and expression in MGUS plasma cells was 7.2-fold higher than paired MGECs. Contrary to VEGF165, SEMA3A mRNA was only 4.1-fold increased in MM plasma cells compared with MMECs, whereas transcription was 10.2-fold higher in MGUS plasma cells compared with MGECs. Accordingly, the average VEGF165/SEMA3A ratio in MM and MGUS plasma cells was 11.3 and 0.2, respectively, hence similar to that of paired ECs. Western blot analysis showed, again, comparable variations between plasma cells and their ECs as the VEGF165 and SEMA3A expression (Figure 3B).
Expression levels of VEGF165, SEMA3A, NRP1, and plexin-A1 genes in the EC types, as evaluated by real-time RT-PCR. Data are shown as fold expression of the lowest transcribed value. The absolute values (as ng/μL) of VEGF165, NRP1, and plexin-A1 in HUVECs were as follows: 380 ± 55, 540 ± 75, and 420 ± 58, respectively; the absolute value of SEMA3A in MMECs was 213 ± 46. Significance of changes determined by the Wilcoxon-Wilcox test.
Expression levels of VEGF165, SEMA3A, NRP1, and plexin-A1 genes in the EC types, as evaluated by real-time RT-PCR. Data are shown as fold expression of the lowest transcribed value. The absolute values (as ng/μL) of VEGF165, NRP1, and plexin-A1 in HUVECs were as follows: 380 ± 55, 540 ± 75, and 420 ± 58, respectively; the absolute value of SEMA3A in MMECs was 213 ± 46. Significance of changes determined by the Wilcoxon-Wilcox test.
Protein levels of VEGF165 and SEMA3A in CM and of NRP1 and plexin-A1 in EC lysates of patients and HUVECs as measured by Western blot analysis. Data are expressed as mean plus or minus 1 SD in each group. The gel is from the patients' and HUVEC samples shown in Figure 1.
Protein levels of VEGF165 and SEMA3A in CM and of NRP1 and plexin-A1 in EC lysates of patients and HUVECs as measured by Western blot analysis. Data are expressed as mean plus or minus 1 SD in each group. The gel is from the patients' and HUVEC samples shown in Figure 1.
Overangiogenic response and lack of SEMA3A induction in VEGF165-treated MMECs
In addition to the robust VEGF165-based autocrine loop we previously reported,4,18 constitutive low levels of endogenous SEMA3A in MMECs may account for their growth advantage and angiogenic ability over MGECs and HUVECs. To further clarify this issue, we assessed the effect of exogenous recombinant VEGF165 on the growth and chemotaxis of HUVECs, MGECs, and MMECs, as well as on the modulation of VEGF165, SEMA3A, NRP1, and plexin-A1 mRNA and protein levels.
Expression levels of VEGF165 and SEMA3A in MGUS and MM plasma cells compared with paired ECs. (A) Real-time RT-PCR shows data as fold expression of the lowest transcribed value. (B) Western blot analysis of cell CM shows that VEGF165 and SEMA3A protein levels vary in parallel with their mRNA in 2 representative patients with MGUS and MM, respectively. Significance of changes determined by the Wilcoxon-Wilcox test.
Expression levels of VEGF165 and SEMA3A in MGUS and MM plasma cells compared with paired ECs. (A) Real-time RT-PCR shows data as fold expression of the lowest transcribed value. (B) Western blot analysis of cell CM shows that VEGF165 and SEMA3A protein levels vary in parallel with their mRNA in 2 representative patients with MGUS and MM, respectively. Significance of changes determined by the Wilcoxon-Wilcox test.
Overangiogenic response and lack of inhibitory SEMA3A induction in VEGF-treated MMECs compared with MGECs and HUVECs. (A) Mitogenic and chemotactic response to VEGF165 by MMECs, compared with MGECs and HUVECs. Parallel expression of VEGF165, SEMA3A, and NRP1 production at mRNA (B) and protein (C) level by the indicated ECs at different times of VEGF165 stimulation.
Overangiogenic response and lack of inhibitory SEMA3A induction in VEGF-treated MMECs compared with MGECs and HUVECs. (A) Mitogenic and chemotactic response to VEGF165 by MMECs, compared with MGECs and HUVECs. Parallel expression of VEGF165, SEMA3A, and NRP1 production at mRNA (B) and protein (C) level by the indicated ECs at different times of VEGF165 stimulation.
MMECs displayed a greater proliferative and chemotactic rate in response to VEGF165 than MGECs and HUVECs (Figure 4A). Since an imbalance between endogenous VEGF165 and SEMA3A could account for such differences, we analyzed their expression by real-time RT-PCR upon cell stimulation with VEGF165 for 24, 48, and 72 hours (Figure 4B). VEGF165 induced transcription of its own mRNA in a time-dependent fashion and peaked at 72 hours with a higher increase (vs day 0) in MMECs than MGECs and HUVECs: 4.1- ± 1.1-fold induction in MMECs; 1.5- ± 0.3- and 2.1- ± 0.5-fold induction in MGECs and HUVECs, respectively. Importantly, VEGF165 did not or only marginally enhanced the expression of SEMA3A mRNA in MMECs (0.9- ± 0.2-fold), whereas a pronounced rise in MGECs (3.4- ± 0.8-fold) and HUVECs (4.6- ± 1.2-fold; P < .02, respectively, Wilcoxon-Wilcox test) was observed. NRP1 transcript was markedly increased at 72 hours only in MMECs (3.8- ± 0.9-fold; P < .03) whereas variations of plexin-A1 were negligible in all the EC types. Accordingly, Western blot analysis (Figure 4C) confirmed the persistent low levels of SEMA3A in MMECs after stimulation with exogenous recombinant VEGF165, which nonetheless induced a time-dependent increase of SEMA3A in MGECs and HUVECs (2.1- ± 0.4-fold and 2.9- ± 0.9-fold, respectively; P < .05). Remarkably, NRP1 protein was greatly increased by exogenous VEGF165 stimulation only in MMECs (3.1- ± 1.2-fold vs 1.4 ± 0.4 of MGECs and 0.9 ± 0.1 of HUVECs), whereas plexin-A1 protein did not vary in any EC types.
The over-angiogenic response of MMECs versus MGECs (and HUVECs) to exogenous VEGF165 can be determined by their respectively low and high constitutive levels of SEMA3A, hence regardless of the expression level of endogenous VEGF165.
Antiangiogenesis by SEMA3A in MMECs
Constitutive expression of SEMA3A in MGECs and HUVECs and its lack of up-regulation by VEGF165 in MMECs provided the rationale for challenging MMECs with exogenous recombinant SEMA3A in order to restrain and counteract VEGF165 activity.
Inhibition of proliferation. Proliferative response of MMECs to VEGF165 was significantly reduced by recombinant SEMA3A in a dose-dependent fashion, and peaked at 150 ng/mL (-37% of untreated cells, P < .01; Wilcoxon rank test). MMEC proliferation was less efficiently inhibited by an anti-NRP1 mAb (-25%), whereas the inhibitory activity of an anti-VEGFR-2 mAb overlapped that of SEMA3A (-40%, P < .01) (Figure 5A).
Inhibition of chemotaxis. Recombinant SEMA3A inhibited VEGF165-induced chemotaxis in MMECs (Figure 5B) in a dose-dependent manner (-67% of the positive control at 150 ng/mL, P < .01), whereas anti-NRP1 mAb was less effective (-21% of the control, P < .05) and anti-VEGFR-2 mAb was as efficient as SEMA3A (-73%, P < .01). Of note, endogenous SEMA3A in MMECs acts a limiting factor for their migratory activity. In fact, knockdown of endogenous SEMA3A by siRNA unleashed both basal and VEGF165-stimulated MMEC migratory activity, which on average was 3 times higher than that of control cells treated with nontargeting siRNA (Figures S2-S3).
VEGF165-induced proliferation of MMECs (with or without FCS 10%) in the presence of different SEMA3A doses, or anti-NRP1 and/or anti-VEGFR-2 antibodies. Data are presented as mean plus or minus 1 SD (A). Effect of VEGF165, alone or in the presence of SEMA3A, on MMEC chemotaxis. Bars represent the mean plus or minus 1 SD of the number of migrated cells per 5 high power fields in triplicate wells (B). Significance of changes by the Wilcoxon-Wilcox test. Gene modulation in presence of exogenous SEMA3A by RT-PCR and real-time RT-PCR (C) at different times of collection. Data are expressed as fold expression of baseline (time 0) value.
VEGF165-induced proliferation of MMECs (with or without FCS 10%) in the presence of different SEMA3A doses, or anti-NRP1 and/or anti-VEGFR-2 antibodies. Data are presented as mean plus or minus 1 SD (A). Effect of VEGF165, alone or in the presence of SEMA3A, on MMEC chemotaxis. Bars represent the mean plus or minus 1 SD of the number of migrated cells per 5 high power fields in triplicate wells (B). Significance of changes by the Wilcoxon-Wilcox test. Gene modulation in presence of exogenous SEMA3A by RT-PCR and real-time RT-PCR (C) at different times of collection. Data are expressed as fold expression of baseline (time 0) value.
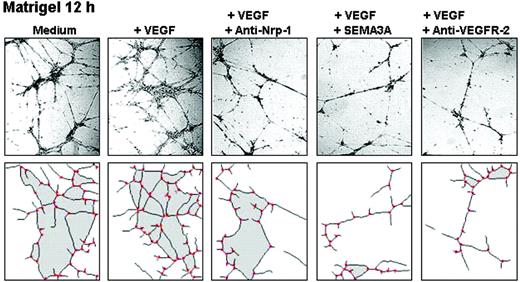
Inhibition of capillarogenesis on Matrigel. MMECs exposed for 12 hours to VEGF165 self-assembled into a closely knit capillary-like plexus with thin, branching tubes linked through numerous junctions: the number of mesh areas was 29.3 ± 9.1; vessel length was 6460 ± 980 μm; branching points were 50 ± 5 (Figure 6). When exposed to VEGF165 and SEMA3A, MMECs gave rise to a poorly organized plexus with few narrow and irregular tubes, and few junctions: mesh areas 7.4 ± 2.1, P < .01; vessel length 782 ± 213 μm, P < .01; branching points 23 ± 8, P < .01. Anti-VEGFR-2 mAb effects were similar: mesh areas 5.3 ± 2.6, P < .01; vessel length 688 ± 243 μm, P < .03; branching points 25 ± 8, P < .01. The anti-NRP1 mAb was less inhibitory: mesh areas 12.8 ± 7.6; vessel length 1870 ± 350 μm; branching points 32 ± 7.
Gene modulation by SEMA3A. RT-PCR showed that exposure of VEGF165-stimulated MMECs to SEMA3A (150 ng/mL) resulted in a time-dependent decrease of endogenous VEGF165 mRNA, which was maximal at 72 hours (Figure 5C). This lowering was further confirmed by real-time RT-PCR and ranged from a 2- to 5-fold (vs time 0) decrease of VEGF165 transcript (P < .02). SEMA3A mRNA was up-regulated with the highest effect at 48 hours, and ranged from 1.9- to 3.1-fold (P < .05). NRP1 gene transcription was time-dependently down-regulated from 1.7- to 5-fold (P < .02).
Discussion
BM angiogenesis increases with progression from MGUS to MM: microvessel density (MVD) is 5- to 6-fold higher in MM, indicating a switch in the angiogenic process partly sustained by endothelial VEGF165.2,29 To elucidate its mechanisms, we analyzed MMECs, MGECs, and HUVECs for (1) endogenous production of VEGF165 and SEMA3A (competing ligands for NRP1, the coreceptor of VEGFR-2 and plexin-A1), which are respectively involved in pro- and antiangiogenic signaling; (2) NRP1 and plexin-A1 expression; (3) responsiveness to exogenous VEGF165 (mimicking what happens in the BM milieu of MMEC); (4) the possible relationship between endogenous VEGF165 and SEMA3A in MMECs. Overall, the study aimed at identifying and testing a potential antiangiogenic role of SEMA3A that could suggest its clinical employment. MMECs were used as overangiogenic ECs as opposed to MGECs and HUVECs, the norm-angiogenic counterpart.18
Capillarogenesis on Matrigel. MMECs of a representative patient were seeded on Matrigel in serum-free medium (SFM). After a 12-hour incubation, their 3-D organization in response to the indicated agents was examined through a Leitz Labiovert inverted phase-contrast light microscope (Leitz, Wetzlar, Germany), using a 20×/0.50 numeric aperture objective. Images were processed and analyzed using a Leica QWin computed image analysis software (Leica Imaging Systems, Cambridge, United Kingdom). The areas are shaded, the vascular branching points are red.
Capillarogenesis on Matrigel. MMECs of a representative patient were seeded on Matrigel in serum-free medium (SFM). After a 12-hour incubation, their 3-D organization in response to the indicated agents was examined through a Leitz Labiovert inverted phase-contrast light microscope (Leitz, Wetzlar, Germany), using a 20×/0.50 numeric aperture objective. Images were processed and analyzed using a Leica QWin computed image analysis software (Leica Imaging Systems, Cambridge, United Kingdom). The areas are shaded, the vascular branching points are red.
We respectively showed the highest and the lowest expression of VEGF165 and SEMA3A in MMEC both at mRNA and protein level, whereas the opposite occurred in MGECs and HUVECs. We also showed the highest expression of NRP1 and plexin-A1 mRNA and protein in MMECs. Therefore, we postulate that the constitutive angiogenic state of MMECs, in terms of growth advantage and higher capillarogenic ability over MGECs and HUVECs, is probably mediated by opposite autocrine loops (VEGF165 stimulator vs SEMA3A inhibitor) competing for binding to NRP1, as shown in carcinoma cells.30 Coexpression of VEGFR-24 and NRP1 at high levels on MMECs (differently from MGECs and HUVECs) may allow VEGF165 binding with high affinity and enhance its downstream signaling; this has been observed in tumor cells endowed with high proliferative and low apoptotic rates.31,32 It has also been shown that the VEGF165 binding to VEGFR-2/NRP1 coexpressing carcinoma cells leads to enhanced phosphorylation of the mitogen-activated protein (MAP) extracellular signal-regulated kinases (ERK)1/233 and phosphatidylinositol 3′-kinase (PI 3-kinase)/Akt (protein kinase B).34 Whether VEGF165 signals through the MAP kinase pathway in MMECs too, will be an object of future studies. Plasma cells behaved like their respective ECs both in MM and MGUS patients: high VEGF165/SEMA3A ratio in MM, the opposite in MGUS. Moreover, plasma cells of MM patients produced more VEGF165 than paired MMECs, thus entailing a major source of this cytokine in the disease.
Here, we characterize the enhanced responsiveness of MMECs to exogenous VEGF165, which is present at high levels in the BM milieu of MM patients.35 We show that exogenous VEGF165 produces a higher increase in MMEC proliferation and chemotaxis than in MGECs and HUVECs. This can be explained by the constitutive low versus high levels of SEMA3A expression in MMECs versus the other EC types, regardless of the endogenous expression level of VEGF165.
Moreover, we find that exogenous VEGF165 stimulates a net increase of endogenous VEGF165 but not SEMA3A in MMECs, whereas it determines a minimal increase of VEGF165 and a strong increase of SEMA3A in MGECs and HUVECs. Thus, it is conceivable that VEGF165 and SEMA3A are integrated via a reciprocal negative feedback pathway in norm-angiogenic MGECs and HUVECs, which is, however, inactivated during malignant progression in over-angiogenic MMECs. This hypothesis is also supported by studies on normal and malignant mesothelial cells36 that behave, respectively, like MGECs (or HUVECs) and MMECs. In addition, the fact that VEGF165 induces its own and NRP1 expression at higher levels in MMECs than in MGECs and HUVECs may also be one of the causes that support a more intense angiogenic response, as previously shown.37 The induction of endogenous VEGF-A (ie, of the VEGF121 and VEGF165 splice variants) through the VEGFR-2 triggering by exogenous VEGF-A has also been shown in breast carcinoma cells33 and pro-myelocytic leukemia cells.38
It is plausible that during malignant progression MMECs acquire high expression/activation of VEGF165 signaling, which contributes to pathologic and exuberant tissue neo-vascularization, and overcomes the inhibitory effect of the SEMA3A autocrine loops usually activated to self-limit physiologic angiogenesis. Kumar et al29 hypothesized an inhibitory (antiangiogenic) activity in MGUS that is lost with the angiogenic switch and transition to MM. Accordingly, we suggest that the high expression of SEMA3A by MGECs may be a self-limiting feature of MGUS angiogenesis, which is lost and overcome by intense expression of VEGF165 with the angiogenic switch when normal ECs are driven toward an MMEC phenotype by cancer cells.
The exposure of MMECs to exogenous SEMA3A appears to re-equilibrate the abnormal excess of endogenous VEGF165, and leads MMECs to a norm-angiogenic state like MGECs and HUVECs. Indeed, we show that recombinant SEMA3A is effective in suppressing VEGF165-mediated angiogenic effects in MMECs: it significantly decreases in a dose-dependent fashion the VEGF165-induced growth, a culture condition mimicking the in vivo VEGF-mediated paracrine loops fostered by the activated microenvironment in MM.2 VEGF165-induced proliferation, migration, and capillarogenesis on the Matrigel surface were also significantly inhibited by recombinant SEMA3A, as much as the anti-VEGFR-2 blocking mAb. At the molecular level, addition of SEMA3A down-regulates VEGF165 expression and up-regulates its own expression, and thus restores the constitutive ratio of these ligands, as observed in MGECs and HUVECs. In contrast, the MMEC proliferation, chemotaxis, and capillarogenesis were less efficiently inhibited by the NRP1 antibody. This can be explained by the fact that VEGF165 binds to VEGFR-2 independently of NRP1, and NRP1 is not required for those VEGF165-mediated EC functions which, instead, are more closely dependent on the VEGFR-2 integrity.39
Overall, our data suggest that SEMA3A is part of a negative loop that acts to restrain VEGF165 signaling in order to self-limit excessive and unscheduled EC growth. It is likely that other semaphorins function as modulators of vasculogenesis and angiogenesis in MM as well.40 SEMA3F, a semaphorin that signals through Nrp-2, inhibits both VEGF165 and basic fibroblast growth factor (bFGF)-induced proliferation of HUVECs.41 Its overexpression markedly reduces the invasive, angiogenic, and metastatic phenotype of melanoma, so that it reverts to a benign, necrotic, and encapsulated tumor.41 Hence, recombinant SEMA3A as an agent aimed at blocking excessive VEGF165 activity and re-establishing physiologic VEGF165/SEMA3A ratio may be a possible future area of research in the antiangiogenic treatment of MM.
Prepublished online as Blood First Edition Paper, May 9, 2006; DOI 10.1182/blood-2006-04-014563.
Supported by Associazione Italiana per la Ricerca sul Cancro (AIRC, National and Regional Funds), Milan, Istituto Superiore di Sanità (Programma Nazionale AIDS), Ministry of Education, Universities and Research (MIUR, 60% and PRIN 2004 and 2005 Projects), Telethon Italy, grant no. GGP04 127 (G.S.), and Ministry for Health, Regione Puglia (grant BS2 and “Convenzione n. 131/Ricerca Finalizzata IRCCS”), Rome, Italy. F.B. belongs to the European Vascular Genomics Network (http://www.evgn.org), supported by the European Community (LSHM-CT-2003-503 251). T.C. is recipient of a fellowship from Fondazione Italiana per la Ricerca sul Cancro (FIRC), Milan, Italy.
A.V. designed the study and supervised all experiments; he also wrote the paper in collaboration with F.D. and has all responsibility for this manuscript; C.S. and G.D.P. performed proliferation and chemotaxis of endothelial cells, Western blot, RT-PCR, and real-time RT-PCR experiments; G.S. and F.B. provided HUVECs, performed siRNA experiments, and helped with data analysis and writing the manuscript; T.C. and F.M. performed cell cultures, isolated the endothelial cells from the bone marrow of patients, and prepared conditioned media; D.R. and D.G. performed the Matrigel in vitro assays and image analysis; G.P. and A.B. provided endothelial cells of some patients and helped with data analysis; F.D. supervised the experiments and data analysis, contributed to writing the paper, and provided critical revision of the manuscript.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The technical assistance of Mr Milena Rizzi is greatly appreciated.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal