A JAK2V617F mutation is frequently found in several BCR/ABL-negative myeloproliferative disorders. To address the contribution of this mutant to the pathogenesis of these different myeloproliferative disorders, we used an adoptive transfer of marrow cells transduced with a retrovirus expressing JAK2V617F in recipient irradiated mice. Hosts were analyzed during the 6 months after transplantation. For a period of 3 months, mice developed polycythemia, macrocytosis and usually peripheral blood granulocytosis. Transient thrombocytosis was only observed in a low-expresser group. All mice displayed trilineage hyperplasia in marrow and spleen along with an amplification of myeloid and erythroid progenitor cells and a formation of endogenous erythroid colonies. After 3 to 4 months, polycythemia regressed, abnormally shaped red blood cells and platelets were seen in circulation, and a deposition of reticulin fibers was observed in marrow and spleen. Development of fibrosis was associated with anemia, thrombocytopenia, high neutrophilia, and massive splenomegaly. These features mimic human polycythemia vera and its evolution toward myelofibrosis. This work demonstrates that JAK2V617F is sufficient for polycythemia and fibrosis development and offers an in vivo model to assess novel therapeutic approaches for JAK2V617F-positive pathologies. Questions remain regarding the exact contribution of JAK2V617F in other myeloproliferative disorders.
Introduction
Myeloproliferative disorders (MPDs) include 4 main entities: essential thrombocythemia (ET), polycythemia vera (PV), idiopathic myelofibrosis (IMF), and chronic myeloid leukemia (CML). MPDs also include some rare and poorly characterized disorders with common clinical and biological features found in MPDs.1 These diseases are characterized by overproduction of blood cells without obvious blockage in maturation. In CML, hyperplasia of the granulocytic lineage predominates, while in PV or ET and IMF, the erythroid or granulocytic and megakaryocytic lineages are prevalent, respectively. MPDs share the common features of being clonal hematopoietic disorders originating from the transformation of a multipotent progenitor cell and of exhibiting progenitor cells hypersensitive to growth factors.1 In PV, endogenous erythroid colony (EEC) formation2 is commonly used in clinical practice for diagnosis. In addition, hyperresponsiveness to factors such as erythropoietin (Epo), interleukin 3 (IL-3), insulin growth factor 1 (IGF-1), stem cell factor (SCF), and granulocyte-macrophage colony-stimulating factor (GM-CSF) has also been demonstrated.2,3 Similarly, megakaryocytic progenitor cells from ET and IMF are hypersensitive to thrombopoietin (TPO).4
We5 and others6-8 have recently identified in BCR/ABL-negative MPDs a unique and recurrent acquired mutation of the JAK2 protein leading to a valine-to-phenylalanine substitution (JAK2V617F) in the autoinhibitory JH2 (JAK homology) domain. The JAK2V617F mutation is found in most patients with PV and in approximately half of the patients suffering from IMF or ET.6,9 Furthermore, this mutation has been also identified in rare patients suffering from acute myeloid leukemia, other MPDs, and myelodysplastic syndromes.9 Although PV, ET, and IMF exhibit significant phenotypic mimicry and a general pattern of clinical evolution, it still remains puzzling how a unique mutation can be involved in such a diversity of diseases. In order to unravel this question and determine the consequences of this mutation on normal hematopoiesis, we studied the in vivo effects of JAK2V617F expression in hematopoietic cells using an adoptive transfer in recipient mice of marrow cells transduced by a retrovirus expressing JAK2V617F. This expression led to the development of a MPD in 2 stages. The first stage encompassed the major features found in classical human PV, including erythrocytosis, as previously reported,5 neutrophilia, and trilineage hyperplasia. The second stage is characterized by the abatement of polycythemia associated with fibrosis and major features found in human myelofibrosis. This study strongly suggests that JAK2V617F high-level expression in hematopoietic cells is sufficient for both the development of human PV and myelofibrosis.
Materials and methods
Materials
Fetal bovine serum (FBS), murine recombinant TPO, and IL-6 were from Stem Cell Technologies (Meylan, France); liquid cell culture media, including Dulbecco modified Eagle medium (DMEM), were from Invitrogen (Cergy Pontoise, France); and human recombinant Epo was from Amgen (Neuilly, France). Conditioned media from WEHI-3B and SCF cDNA-transfected BHK/MKL cells were used as sources for IL-3 and SCF, respectively. Restriction enzymes were purchased from Fermentas (St Leon-Rot, Germany) and antibodies (Abs) from Upstate Biotechnologies (Lake Placid, NY) for the anti-STAT5a and from Cell Signaling Technology (Ozyme, Saint Quentin, France) for the anti-phospho-STAT5 Tyr 694, anti-phospho-ERK1/2 Thr 202/Tyr 204, anti-ERK1/2 Abs.
Construction and production of retroviruses
Murine JAK2V617F and wild-type JAK2 (JAK2WT) pMEGIX retroviral vector were previously described.5 vesicular stomatitis virus glycoprotein (VSV-G) pseudotyped viral particles were produced into the 293 EBNA cells.10 Ecotropic virus-producing cell lines were then generated from 2 successive infections of the GP+E-86 packaging cell line (kindly supplied by Dr A. Bank, Columbia University, New York, NY)11 with concentrated virus supernatants. High virus-producer clones were selected for their ability to infect a high percentage of EpoR-FDC-P1 cells by measuring green fluorescent protein (GFP) expression. Two independent clones were used to generate 2 different sets of primary recipient mice.
BM transduction procedure
All procedures were approved by the Institut Gustave Roussy ethics committee. C57BL/6J female mice (2-4 months old) were purchased from Janvier (Le Genest, France). Bone marrow (BM) cells were collected 4 days after 5-fluorouracil treatment (150 mg/kg), cocultivated with virus-producing GP+E-86 cells for 4 days in presence of IL-3, IL-6, TPO, and SCF.12 Finally, 2 × 106 transduced cells/animal were injected intravenously into lethally irradiated (9.5 Gy) recipient mice. For secondary transplantation, BM cells collected from primary recipients were injected (2 × 106 cells/animal) into irradiated mice.
Analysis of mice
Hemoglobin (Hb), mean corpuscular volume (MCV), hematocrit, red blood cell (RBC), platelet, and white blood cell (WBC) counts, and the lymphocyte-granulocyte ratio were determined using an automated counter (MS9; Schloessing Melet, Cergy-Pontoise, France) on blood collected from the retro-orbital plexus in citrated tubes. BM cells were removed by flushing both femurs. Spleens were weighed and single-cell suspensions were prepared. Differential cell counts were performed after May-Grünwald-Giemsa staining of blood smears or spleen and BM cell cytospins.
For histopathology analysis, femurs and spleens were fixed in formaldehyde, decalcified, and paraffin embedded. Sections (4.5 μm) were stained with hematoxylin-eosin, periodic acid Schiff, and Giemsa for cytology analysis. Reticulin fibers were revealed by silver staining according to the Gordon Sweet method, and collagen was revealed by trichrome staining.
Flow cytometry (FACS Vantage; Beckman Dickinson, Mountain View, CA) was used to determine GFP expression and cell content of BM and spleen cells with appropriate phycoerythrin (PE)- or allophycocyanin-conjugated antibodies (Gr-1, B220, Mac-1, Ter119, CD43, and CD3 antibodies [Pharmingen, San Diego, CA]), after RBC lysis.
Progenitor cell study
Progenitor cell assays were carried out in 1 mL methylcellulose MethoCult 32/34 (Stem Cell Technologies) without stimulus or maximally stimulated by IL-3, IL-6, TPO, SCF, and EPO. Cultures were scored in duplicate or quadruplicate after different days of incubation: 2 days for CFU-E assays and 7 days for burst-forming unit-erythroid (BFU-E) and GM-CFU assays. Total progenitor cell number was calculated assuming that 1 femur represented 6% of the total marrow and from the number of cells isolated from the spleen.
Cell-signaling stimulation and Western blot analysis
Spleen cells were dissociated from freshly excised spleens and rinsed 3 times with PBS. Then, spleen cells were cultivated in DMEM supplemented with 1% FBS for 16 hours. For stimulation, mIL-3 (100 ng/mL) or EPO (10 U/mL) were added in culture medium for 10 minutes. Whole-cell protein extracts were analyzed by Western blotting with adequate antibodies as previously described.13
QTPCR
BM and spleen RNAs were extracted using Trizol reagent (Invitrogen). Reverse transcription (RT) was carried out with 1 μg of total RNA using random hexamers (Roche Diagnostics, Meylan, France) and Moloney murine leukemia virus reverse transcriptase (Invitrogen). QPCR Mastermix Plus and adequate primers (Eurogentec, Angers, France) were used for quantitative PCR (QTPCR) amplification of the retroviral JAK2WT or JAK2V617F cDNA, the endogenous JAK2 cDNA, the total JAK2 cDNA, and tubulin cDNA as a control (Table 1). QTPCR was performed on a 5700 GeneAmp cycler (Applied Biosystems, Foster City, CA) and analyzed with associated software.
Primers and probes used for quantitative PCR analysis of endogenous and/or retroviral JAK2 mRNA
. | Forward primers 5′-3′ . | Probes 6-FAM/5′-3′/TAMRA . | Reverse primers 5′-3′ . |
|---|---|---|---|
| Retroviral JAK2 | CGTCTCTCCCCCTTGAACCT | TTCGACCCCGCCTCGATCCTC | GAAGGAGTGAGGGCTGGATAAA |
| Total JAK2 | TGTAACTGTCCATAAACAAGATGGTAAAG | TTTGGAGATAGAACTTAGCTCATTAAAAGAAGCCTTGTC | TACCCGTCAATTAATGACACGAA |
| Endogenous JAK2 | GCCTTCACTCAGAGACCAAGCA | ACTTCCAGAACCAGAACAAAGCTCTGTAGCCT | CGTCACAGTTTCTTCTGCCTAGCT |
| Tubulin | GGGTGGAGGCACTGGCT | TGGCATGGGCACCCTGCTCAT | GTCAGGATATTCTTCCCGGATCT |
. | Forward primers 5′-3′ . | Probes 6-FAM/5′-3′/TAMRA . | Reverse primers 5′-3′ . |
|---|---|---|---|
| Retroviral JAK2 | CGTCTCTCCCCCTTGAACCT | TTCGACCCCGCCTCGATCCTC | GAAGGAGTGAGGGCTGGATAAA |
| Total JAK2 | TGTAACTGTCCATAAACAAGATGGTAAAG | TTTGGAGATAGAACTTAGCTCATTAAAAGAAGCCTTGTC | TACCCGTCAATTAATGACACGAA |
| Endogenous JAK2 | GCCTTCACTCAGAGACCAAGCA | ACTTCCAGAACCAGAACAAAGCTCTGTAGCCT | CGTCACAGTTTCTTCTGCCTAGCT |
| Tubulin | GGGTGGAGGCACTGGCT | TGGCATGGGCACCCTGCTCAT | GTCAGGATATTCTTCCCGGATCT |
Southern blot
High-molecular-weight DNAs were prepared from BM and spleen, digested with BamH1 or EcoRI restriction endonuclease, and subjected to Southern blot analysis as previously described.14 Blots were hybridized with a GFP probe isolated from the pMEGIX-JAK2 vector.
Statistical analysis
Results are presented as mean ± SEM and data were analyzed with the 2-tailed Student t test. In figures, asterisks indicate P values less than .05 compared with JAK2WT.
Results
In vitro and ex vivo retroviral JAK2V617F gene activity
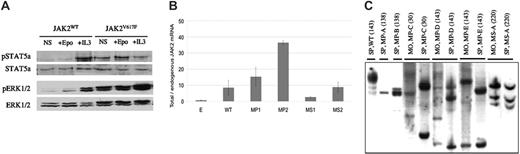
To study the in vivo effect of the JAK2V617F mutation, recipient mice were given transplants of BM cells transduced with a retrovirus expressing murine JAK2V617F. Control viruses were the empty or JAK2WT-expressing virus. Transduction of EpoR-FDC-P1 cells, dependent on GM-CSF, IL-3, or Epo for growth, with the JAK2V617F or JAK2WT virus led to expression of the expected-molecular-size JAK2 proteins and a rapid emergence of growth factor-independent clones only for JAK2V617F virus-transduced cells. Primary spleen cells collected from hosts given transplants of JAK2V617F or JAK2WT virus-transduced BM cells were studied. After growth factor deprivation, spleen cells transduced with JAK2V617F displayed phosphorylation of STAT5a and ERK1/2 proteins. In contrast, JAK2WT virus-transduced cells required the addition of IL3 or Epo to activate these pathways (Figure 1A).
Retroviral gene signaling activity, mRNA expression level, and genomic integration from JAK2V617F virus-recipient mice. (A) Western blot analysis from spleen cells demonstrates constitutive STAT5a and ERK1/2 pathway activation in JAK2V617F recipients in contrast to JAK2WT recipient mice. Total and phosphorylated (p) Stat5a and ERK1/2 are shown in the absence of added growth factor (NS) or the presence of Epo and IL-3. (B) Ratio between total (retroviral and endogenous) and endogenous JAK2 mRNA. Total JAK2 mRNAs were quantified by QTPCR against tubulin (Table 1). Endogenous level was determined from empty virus-recipient mice. Results are mean values from 3 to 4 mice, studied between 3 and 6 months after transplantation with triplicated RNA measurements. WT and E are pooled levels from JAK2WT or empty virus recipient. MP1 and MP2 or MS1 and MS2 are pooled from primary or secondary JAK2V617F-recipient mice, respectively. (C) Identification of proviral insertions in the genome of spleen (SP) and BM samples from JAK2WT (WT) or JAK2V617F virus primary-(MP-A to MP-D) or secondary (MS-A)-recipient mice. Animals were studied at the indicated day after transplantation (in bracket). Proviral integrants were detected using Southern blot analysis with a GFP probe from EcoRI- or BamH1-digested DNA exhibiting 1 enzymatic cut in the proviral DNA and in the genomic DNA. Bands indicate individual proviral integrants. MP-A, MP-B, and MP-D developed a high-grade fibrosis, and MP-E developed a low-grade fibrosis. No fibrosis was detected for MP-C or WT.
Retroviral gene signaling activity, mRNA expression level, and genomic integration from JAK2V617F virus-recipient mice. (A) Western blot analysis from spleen cells demonstrates constitutive STAT5a and ERK1/2 pathway activation in JAK2V617F recipients in contrast to JAK2WT recipient mice. Total and phosphorylated (p) Stat5a and ERK1/2 are shown in the absence of added growth factor (NS) or the presence of Epo and IL-3. (B) Ratio between total (retroviral and endogenous) and endogenous JAK2 mRNA. Total JAK2 mRNAs were quantified by QTPCR against tubulin (Table 1). Endogenous level was determined from empty virus-recipient mice. Results are mean values from 3 to 4 mice, studied between 3 and 6 months after transplantation with triplicated RNA measurements. WT and E are pooled levels from JAK2WT or empty virus recipient. MP1 and MP2 or MS1 and MS2 are pooled from primary or secondary JAK2V617F-recipient mice, respectively. (C) Identification of proviral insertions in the genome of spleen (SP) and BM samples from JAK2WT (WT) or JAK2V617F virus primary-(MP-A to MP-D) or secondary (MS-A)-recipient mice. Animals were studied at the indicated day after transplantation (in bracket). Proviral integrants were detected using Southern blot analysis with a GFP probe from EcoRI- or BamH1-digested DNA exhibiting 1 enzymatic cut in the proviral DNA and in the genomic DNA. Bands indicate individual proviral integrants. MP-A, MP-B, and MP-D developed a high-grade fibrosis, and MP-E developed a low-grade fibrosis. No fibrosis was detected for MP-C or WT.
These results show that the murine JAK2V617F virus leads to constitutive activation of JAK2 downstream effectors in primary cells.
Retroviral gene expression and integration in the different sets of mice
We studied 65 JAK2V617F virus-transplanted/reconstituted mice derived from 3 groups of primary recipients (MP0, n = 4; MP1, n = 14; MP2, n = 15) generated from 3 independent transductions and 2 groups of secondary recipients derived from the transplantation of marrow cells collected from a mouse belonging to the MP0 (MS1, n = 15) or the MP1 group (MS2, n = 17). In parallel, we generated control primary or secondary recipients reconstituted with BM cells transduced with the JAK2WT or empty retroviruses. Mice from the MP0 group are included in this work only for histologic analysis. All engrafted mice expressed GFP in 50% to more than 90% of blood cells. GFP expression levels clearly differed from 1 group to the other but were similar in mice from the same group (unpublished data). Retroviral JAK2 mRNA levels were assessed by QTPCR in marrow and spleen. The lowest levels were found in the MS1 group, and the highest levels were found in the MP2 group. Intermediate levels were seen in the MP1 and MS2 groups, the latest being derived from 1 mouse belonging to the MP1 group (data not shown). The same trend of expression was observed when total (endogenous and retroviral) JAK2 mRNA levels were measured in spleens, using a second QTPCR approach, suggesting that most JAK2 mRNA was from retroviral origin. Indeed, the total JAK2/endogenous JAK2 ratios, using as endogenous JAK2 the level determined in empty virus-recipient mice, were 15 ± 6, 36 ± 1, 2.6 ± 0.5, and 9 ± 3 in the MP1, MP2, MS1, and MS2 groups, respectively (Figure 1B). Verifying that the endogenous JAK2WT level was not significantly different in empty or JAK2V617F virus-recipient mice, we specifically amplified, using a third QTPCR approach, the retroviral and endogenous JAK2 mRNA in the MS1 or MS2 groups and found similar values (2.8 or 8.2, respectively) for the ratio of retroviral JAK2V617F to endogenous JAK2WT. The same trend was observed between the levels of mRNA and GFP (unpublished data) in different groups except for the MP2 group, which expressed a relatively low level of GFP compared with the MP1 and MS2 groups.
Southern blot analysis on EcoRI- or BamH1-digested genomic DNA from BM and spleen cells of JAK2V617F-recipient mice collected from 1 to 7 months after transplantation revealed several virus integration sites (Figure 1C). The intensity of the bands showed the development of a limited number of hematopoietic clones displaying different sites of retroviral integration. This oligoclonal retrovirally marked hematopoiesis was observed early or late after transplantation.
These data show that this strategy essentially leads to a JAK2V617F overexpression model except for the low expresser MS1 group, where some clones may express similar levels of JAK2V617F and JAK2WT. This is particularly relevant with regards to the different states of JAK2V617F zygocity found in human MDS.5
In vivo effects of the JAK2V617 expression
Mice were studied for 6 months after transplantation. Peripheral blood, marrow, spleen, and progenitor cells were analyzed in detail.
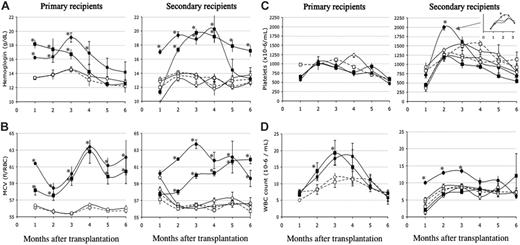
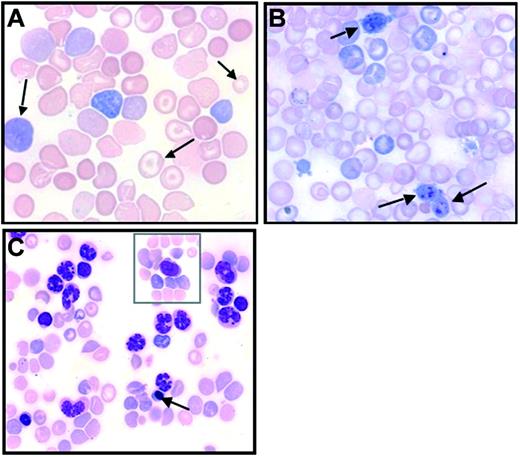
Peripheral blood cells. The most striking feature in primary JAK2V617F-recipient mice was transient polycythemia. All mice displayed high hemoglobin (Hb) levels (Figure 2A) peaking at around 3 months after transplantation and thereafter dropping to reach normal or below-normal levels at around 6 months after transplantation. Abatement of polycythemia did not correlate with a drop in the percentage of GFP-positive cells. Furthermore, as early as day 136 after transplantation, 1 mouse in each group suffered from severe anemia (Hb < 6 g/dL), with high JAK2V617F mRNA expression in spleen cells demonstrating that lessening of the Hb level was not due to disappearance of transduced cells or an extinction of the retroviral gene. Initial polycythemia was associated with elevated hematocrit values (61% ± 7% and 57% ± 7% at 4 months after transplantation in the MP1 and MP2 groups, respectively) with some mice exhibiting values close to 90%. Elevation in the hematocrit value corresponded to an increase in both volume and number of RBCs. The number of RBCs was increased in all mice, peaking at 9.9 ± 0.5 × 106/μL and 7.9 ± 0.5 × 106/μL in the MP1 and MP2 groups 3 months after transplantation (control values: 6.4 ± 0.2 × 106/μL and 6.6 ± 0.1 × 106/μL in the empty and JAK2WT virus-transduced mice). Thereafter, RBC numbers dropped in most mice, reaching normal or below-normal values. Macrocytosis was observed in every mouse during the whole survey as early as 20 days after engraftment (Figure 2B). High MCV was explained by the presence of elevated numbers of newly formed RBCs evidenced by numerous polychromatophilic RBCs or reticulocytes heavily stained by brilliant cresyl blue. After peaking at 4 months, MCV decreased but a rebound was observed in later stages. During these late stages, often associated with anemia, large variations in RBC color (polychromatophilia), size (anisocytosis), or shape (poikilocytosis), including the presence of target cells (Figure 3A) and RBCs with Howell-Jolly bodies, were observed. Therefore, elevated MCV in late stages may be attributed to dyserythropoiesis, or other defects impairing erythropoiesis, featured by these abnormal RBCs.
Blood cell parameters. Primary (left panels) or secondary (right panels) JAK2V617F, JAK2WT, or empty virus-recipient mice were analyzed every 1 to 3 weeks following transplantation, and data collected each month were pooled. Details of platelet counts are indicated for the MS1 group. Depicted are hemoglobin levels (A), MCVs (B), platelet counts (C), and WBC counts (D). • indicates JAK2V617F (MP1/MS1); ▪, JAK2V617F (MP2/MS2); ○, JAK2WT; and □, empty virus. MP or MS correspond to primary or secondary JAK2V617F virus-recipient mice, respectively. Details of platelet counts are indicated for the MS1 group in panel C. The mean numbers of mice studied per determination were 7, 9, 12, and 15 mice for the MP1, MP2, MS1, and MS2 groups, respectively. Around 2 determinations per month were carried out. Similar numbers of matched controls were studied. *P < .05 compared with the JAK2WT-transduced mice.
Blood cell parameters. Primary (left panels) or secondary (right panels) JAK2V617F, JAK2WT, or empty virus-recipient mice were analyzed every 1 to 3 weeks following transplantation, and data collected each month were pooled. Details of platelet counts are indicated for the MS1 group. Depicted are hemoglobin levels (A), MCVs (B), platelet counts (C), and WBC counts (D). • indicates JAK2V617F (MP1/MS1); ▪, JAK2V617F (MP2/MS2); ○, JAK2WT; and □, empty virus. MP or MS correspond to primary or secondary JAK2V617F virus-recipient mice, respectively. Details of platelet counts are indicated for the MS1 group in panel C. The mean numbers of mice studied per determination were 7, 9, 12, and 15 mice for the MP1, MP2, MS1, and MS2 groups, respectively. Around 2 determinations per month were carried out. Similar numbers of matched controls were studied. *P < .05 compared with the JAK2WT-transduced mice.
Platelet numbers, in primary hosts, were usually normal or slightly below normal values (Figure 2C). Thrombocytopenia was seen in mice (< 250 000 platelets/μL) in late stages with the presence of abnormal large size platelets (giant platelets) suggesting dysmegakaryopoiesis (Figure 3B).
WBC counts in primary JAK2V617F-recipient mice were elevated during the first 3 to 4 months after transplantation and returned to normal or below-normal values in late stages (Figure 2D). Nucleated cells reached peak values that were elevated 2-fold from that of control mean values at day 102 after transplantation. Hyperleukocytosis was due to an excess in polynuclear neutrophils (PNs) making up 32% ± 9% of WBCs compared with 13% ± 4% in control mice. At late stages, circulating myeloid precursor cells (up to 9%) that included myeloblasts, metamyelocytes, and band cells were detected along with erythroblasts (up to 35%). Notably, 1 mouse from the MP1 group and 1 mouse from the MP2 group displayed very elevated WBC counts (58 and 102 × 103 cells/mL, respectively) around day 130 after transplantation. One animal died rapidly, while the other was moribund and killed 1 week later. In both cases, most cells were mature granulocytes. Circulating blast cells (< 1%) or precursor cells were observed in low numbers (Figure 3C).
In secondary hosts, identical blood features were recorded (Figure 2A-D), including severe leukocytosis in the MS2 group. However, compared with primary hosts, the MS1 and MS2 groups displayed higher Hb levels and longer duration of polycythemia. Furthermore, neutrophilia was not recorded in the MS2 group, but the percentage of granulocytes was still significantly increased in both secondary groups (25%-26%) compared with the control JAK2WT-matched groups (13%-18%) at 3 or 6 months after transplantation (P < .05). Importantly, and only in the MS1 group, platelet numbers were transiently increased at 2 months after transplantation (P < .05 at days 54 and 64), with values 2 times more elevated than control values.
Abnormal blood cell features. Blood smears from JAK2V617F virus-recipient mice in late posttransplantation stages (May-Grünwald-Giemsa staining). (A) Abnormal RBCs (arrows) showing polychromatophilia, anisocytosis, and the presence of target cells. (B) Giant platelets (arrows). (C) Elevated granulocytosis with erythroblastosis (arrow), and rare blast cells (in inset). Images were obtained using a Zeiss Axiophot microscope, Zeiss Pan-Apochromat 63×/1.40 oil (A) or 40×/1.0 oil (B-C) lenses, a Zeiss AxioCam Mrc camera, and the AxioVision Rel.4.3 acquisition software (all from Zeiss, Oberkochen, Germany).
Abnormal blood cell features. Blood smears from JAK2V617F virus-recipient mice in late posttransplantation stages (May-Grünwald-Giemsa staining). (A) Abnormal RBCs (arrows) showing polychromatophilia, anisocytosis, and the presence of target cells. (B) Giant platelets (arrows). (C) Elevated granulocytosis with erythroblastosis (arrow), and rare blast cells (in inset). Images were obtained using a Zeiss Axiophot microscope, Zeiss Pan-Apochromat 63×/1.40 oil (A) or 40×/1.0 oil (B-C) lenses, a Zeiss AxioCam Mrc camera, and the AxioVision Rel.4.3 acquisition software (all from Zeiss, Oberkochen, Germany).
These data show that overexpression of JAK2V617F induces polycythemia with macrocytosis, mild granulocytosis, and no change in platelet counts in the short term. Long-term expression of the mutated gene leads to abatement of polycythemia with possible anemia, dyserythropoiesis and dysmegakaryopoiesis, circulating precursor cells, and a variable occurrence of mild thrombocytopenia and high neutrophilia. The disease is transplantable in secondary hosts, generating a milder phenotype except for a transient thrombocytosis observed in the low JAK2V617F-expresser MS1 group. These alterations are not seen in mice transduced with the JAK2WT virus, demonstrating that the abnormal features were due to the mutation and not JAK2 overexpression.
BM and spleen. The number of nucleated cells collected from femurs of JAK2V617F-recipient mice was lower than that found in control mice in late stages (> 4 months after transplantation; P < .05) (Figure 4). Differential counts revealed an increase in the percentage of neutrophil precursor cells and a drop in erythroid precursor cells (Figure 4). No shift in the proportions of immature and mature precursor cells were observed, suggesting that no blockage of maturation occurred. The percentage of lymphocytes was decreased. Fluorescence-activated cell sorting (FACS) analysis with lineage markers confirmed these results (data not shown).
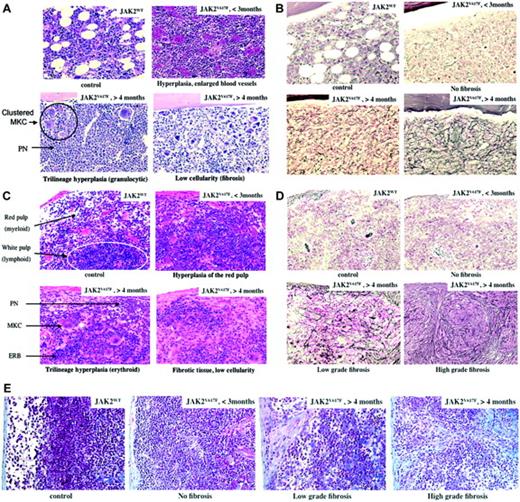
Histological examination of femurs or tibias was carried out from days 52 to 235 after transplantation (Figure 5A-B). Early after transplantation (days 52 to 98, n = 4), the major marrow feature was hyperplasia of the erythroid, granulocytic, and megakaryocytic lineages clearly dominated by mature granulocytic cells. Maturation along the granulocytic or the erythroid lineages was normal. Megakaryocytes were usually clustered and displayed an unusual morphology, including emperipolesis and apoptotic features. Vascular sinuses were enlarged as a result of the erythrocytosis. Late after transplantation (days 131 to 235, n = 9), silver staining showed marked increase in reticulin fibers in all mice (Figure 5B). The extent and density of myelofibrosis was moderate to high, systemic, and mutilating in some areas. Density of cells was particularly low in some mice. Neutrophil hyperplasia was still dominant. Dysmorphic megakaryocytes were seen in all mice, some markedly distorted or present as naked nuclei. Bone cortical thickness was mildly increased, with newly formed bony trabeculae showing mild osteosclerosis.
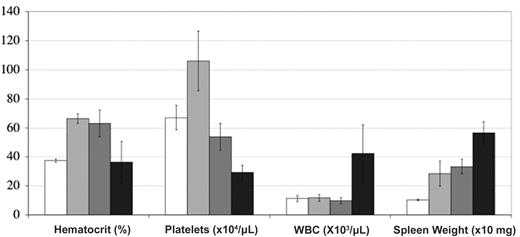
BM and spleen analysis. BM cellularity (A), spleen weight (B) and differential cell count (BM cells [C] and spleen cells [D]) of these organs in recipient mice. BL indicates blast; MY, metamyeloblast/myeloblasts; ME/PO, myelocyte/neutrophil; LY, lymphocyte; MO, monocytes; EO, eosinophil; and ERY, erythroblasts. White, gray, and black histograms are empty, JAK2WT, and JAK2V617F virus-recipient mice (n = 8-12), respectively. For BM cellularity and spleen weight, data from the JAK2V617F virus-recipient mice were pooled (JAK2V617F) or divided into mice studied less (n = 7, days 52-97) or more (n = 5, days 131-143) than 4 months after transplantation (less than or more than 4 months). Differential counts were carried out of cells collected between days 31 and 143 after transplantation (n = 8), with a tendency toward an increase in the percentage of erythroblasts in late transplantation stages. *P < .05 compared with the JAK2WT mice.
BM and spleen analysis. BM cellularity (A), spleen weight (B) and differential cell count (BM cells [C] and spleen cells [D]) of these organs in recipient mice. BL indicates blast; MY, metamyeloblast/myeloblasts; ME/PO, myelocyte/neutrophil; LY, lymphocyte; MO, monocytes; EO, eosinophil; and ERY, erythroblasts. White, gray, and black histograms are empty, JAK2WT, and JAK2V617F virus-recipient mice (n = 8-12), respectively. For BM cellularity and spleen weight, data from the JAK2V617F virus-recipient mice were pooled (JAK2V617F) or divided into mice studied less (n = 7, days 52-97) or more (n = 5, days 131-143) than 4 months after transplantation (less than or more than 4 months). Differential counts were carried out of cells collected between days 31 and 143 after transplantation (n = 8), with a tendency toward an increase in the percentage of erythroblasts in late transplantation stages. *P < .05 compared with the JAK2WT mice.
Spleen weights from recipient mice given transplants of the JAK2V617F virus-transduced cells were always higher than those of control mice. This weight increased over the time of survey (Figure 4). Differential cell counts revealed that splenomegaly was due to an increase in erythroid and myeloid precursor cells (Figure 4). No blockage of maturation was noticed. Morphologic examination of the spleen cells revealed erythroblasts with several nuclei, suggesting occurrence of abnormal erythropoiesis. In a mouse displaying a large increase in platelet numbers (MS1 group), an unusually high number of megakaryocytes was observed. Emperipolesis was usual. FACS analysis revealed high percentages of TER-119-positive (erythroid) and Gr1/Mac-1-positive (granulocyte) cells (data not shown).
Histologic examination of the spleens revealed striking differences between mice studied early (≤ 3 months, 98 days) or late (> 4 months, 131 days) after transplantation (Figure 5C-D). In the early stages, hyperplasia of the red pulp was the dominant feature. Malpighian corpuscles were present in the white pulp. However, they were reduced in size, had no clear germinal center, and were blended by invasion of nonlymphoid cells. Hyperplasia of the red pulp was dominated by normally differentiated erythroid cells. Clustered megakaryocytes with multilobulated nucleus and emperipolesis were observed. Granulocytes were in unusual high numbers near the capsule and around the trabeculae. In later stages, trilineage hyperplasia with a prevalence of erythroid cells was still present in the red pulp. The white pulp was either completely blended or partially preserved. Distorted megakaryocytes were observed including some apoptotic cells and naked nuclei. In contrast to early stages, trabeculae thickness was clearly increased with large deposits of reticulin fibers (Figure 5D) and collagen deposition, particularly in the periphery of the spleen (Figure 5E). Fibrosis was clearly more intense and mutilating in its development and spreading in the spleen than in the marrow cavity. Thick fibrosis was usually associated with accumulation of fibroblasts, pigmented macrophages, plasma cells, and mast cells. It is noteworthy that fibrosis was observed late after transplantation in primary and secondary hosts and was not associated with a particular tendency toward monoclonal retrovirally derived hematopoiesis (Figure 1C).
Histology. Hematoxylin-eosin (A,C), silver stain (B,D), and trichrome (E) colorations of the marrow (A-B) and spleen (C-E) are shown. Control (JAK2WT) and JAK2V617F-recipient mice with posttransplantation time (< 3 months or > 4 months) are indicated with main features: trilineage hyperplasia in early stages and fibrosis/low cellularity in late stages. Images were obtained using a Leica DMRB microscope (Leica, Solms, Germany) with lenses ×20 (0.5 numeric aperture) × 10 = 200 × total magnification, and acquired with a Video 3 charge-coupled device (CCD) Sony Leica Power hole accumulated diode (HAD) camera (Sony, Tokyo, Japan).
Histology. Hematoxylin-eosin (A,C), silver stain (B,D), and trichrome (E) colorations of the marrow (A-B) and spleen (C-E) are shown. Control (JAK2WT) and JAK2V617F-recipient mice with posttransplantation time (< 3 months or > 4 months) are indicated with main features: trilineage hyperplasia in early stages and fibrosis/low cellularity in late stages. Images were obtained using a Leica DMRB microscope (Leica, Solms, Germany) with lenses ×20 (0.5 numeric aperture) × 10 = 200 × total magnification, and acquired with a Video 3 charge-coupled device (CCD) Sony Leica Power hole accumulated diode (HAD) camera (Sony, Tokyo, Japan).
Mice were divided into 3 groups displaying no fibrosis (n = 5; early stages), low-grade fibrosis (n = 3), or high-grade fibrosis (n = 4; late stages). Interestingly, there was a clear trend associating development of fibrosis with anemia (3 of 4 mice were anemic in the high-grade group), thrombocytopenia, high neutrophilia (all high-grade fibrosis mice had the highest WBC counts), and massive splenomegaly (Figure 6).
Hematologic features associated to fibrosis. White, gray, dark gray, and black histograms are JAK2WT (n = 7) or JAK2V617F virus-recipient mice displaying no (n = 4, days 52 to 98 after transplantation), low-grade (n = 5, days 161 to 235 after transplantation), and high-grade fibrosis (n = 4, days 131 to 143 after transplantation), respectively. Error bars indicate SE.
Hematologic features associated to fibrosis. White, gray, dark gray, and black histograms are JAK2WT (n = 7) or JAK2V617F virus-recipient mice displaying no (n = 4, days 52 to 98 after transplantation), low-grade (n = 5, days 161 to 235 after transplantation), and high-grade fibrosis (n = 4, days 131 to 143 after transplantation), respectively. Error bars indicate SE.
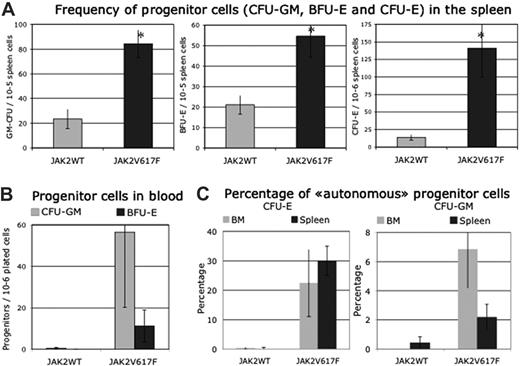
Progenitor cells. (A) Frequencies in spleen (n = 8, days 84 to 143 after transplantation). (B) Frequencies in blood (n = 4, days 138 and 143 after transplantation). (C) Percentage of cells growing in absence of added growth factor (n = 6, days 33 to 143 after transplantation). Error bars indicate SE.
Progenitor cells. (A) Frequencies in spleen (n = 8, days 84 to 143 after transplantation). (B) Frequencies in blood (n = 4, days 138 and 143 after transplantation). (C) Percentage of cells growing in absence of added growth factor (n = 6, days 33 to 143 after transplantation). Error bars indicate SE.
In conclusion, JAK2V617F expression induces trilineage hyperplasia dominated in marrow by granulocytic cells and in spleen by erythroid cells. No sign of blockage in maturation is noticeable. Hyperplasia of the megakaryocytic lineage, as evidenced by clustered cells, is always observed with the presence of dysmorphic cells and emperipolesis. At late stages, increased deposition of reticulin and collagen fibers, sometime associated with mild osteosclerosis, is always observed. Development of fibrosis is associated with anemia, thrombocytopenia, neutrophilia, BM hypocellularity, and massive splenomegaly.
Progenitor cells. In the marrow, no significant difference in the frequency of progenitor cells was detected between JAK2V617F virus-transduced mice and control mice (n = 9, days 52 to 143 after transplantation).
However, due to the decrease in marrow cell recovery, the total number of CFU-E (35% ± 16% compared with JAK2WT virus-transduced mice; P < .05) and BFU-E (36% ± 10% compared with JAK2WT virus-transduced mice; P < .05) was significantly decreased. The number of CFU-GM was only slightly decreased. In spleen, the frequency of all progenitor cells was largely increased in JAK2V617F virus-transduced mice showing marked shift of hematopoiesis to the spleen as observed in stress situation (Figure 7A). Taking into account the spleen cellularity, the number of CFU-E, BFU-E, and GM-CFU was increased 27, 8, and 17 times, respectively, compared with JAK2WT-recipient mice (P < .05). Finally, the cumulative numbers of CFU-E, BFU-E, or CFU-GM in spleen and marrow from the JAK2V617F virus-transduced mice increased 7.6, 2.5, or 1.8 times compared with JAK2WT or empty virus-recipient mice, respectively.
Circulating blood progenitor cells were detected in late stages in unusually high numbers in JAK2V617F mice compared with control JAK2WT mice (Figure 7B).
In the absence of added growth factor, marrow- and spleen-derived CFU-E colonies were always detected in JAK2V617F virus-recipient mice, as well as CFU-GM-derived colonies, but in a low percentage compared with endogenous CFU-E (Figure 7C). Rare endogenous BFU-E colonies were observed.
Discussion
In this study, we show that overexpression of a murine JAK2V617F gene in hematopoietic cells induces a 2-stage MPD in mice. During the initial stage, transplant recipients suffered from rapid erythrocytosis and mild neutrophilia. Transient thrombocytosis was only observed in a low-expresser group. Tissue examinations revealed myeloid hyperplasia with a predominance of granulocytic cells in the marrow and erythroid cells in the spleen and clustered megakaryocytes with emperipolesis features in both organs. The second stage was typically characterized by abatement of polycythemia, abnormal RBCs, dysmegakaryopoiesis, and fibrosis in marrow and spleen. Ultimately, fibrosis development was associated with anemia, thrombocytopenia, severe granulocytosis, marrow hypocellularity, and important splenomegaly. The progenitor pool, which included endogenous CFU-E and circulating progenitors, was amplified mainly due to splenic hematopoiesis. This MPD perfectly mimicks human PV, including its evolution toward the “spent” phase.
This work demonstrates the cause-effect of the JAK2V617F mutation in the development of human PV. The main erythroid phenotype of these mice can be related to the primordial importance of JAK2 signaling in erythropoiesis. Indeed, the lethal mid-gestational phenotype of the JAK2 knock-out (KO) mice has been attributed to a block in definitive erythropoiesis.15,16 Furthermore, the receptor for Epo (EpoR) strictly requires JAK2 for both signaling and trafficking to the cell surface,17 contrasting with other hematopoietic growth factor receptors that use JAK2 but also other JAK family members (JAK1 and Tyk2) either for signaling or for cell-surface expression.18,19 Besides this prevalent activity on erythroid cells, JAK2V617F also favors, to a lesser extent, the granulocytic and megakaryocytic lineages as evidenced by hyperplasia of these cells in organs leading to granulocytosis and possible thrombocytosis. This is in sharp contrast with the myeloid and lymphoid proliferation disease induced by the TEL/JAK2 fusion protein, a disease that is not associated with a polycythemia.20 This result suggests that JAK2V617F activity is dependent to some extent on binding to receptors, especially those controlling the erythroid, granulocytic, and megakaryocytic lineages (EpoR, Mpl, and G-CSF) as recently suggested.21
A surprising finding was the regression of erythrocytosis, with possible occurrence of anemia despite a sustained JAK2V617F expression. This is in contrast with our Epo overproduction model of polycythemia in which erythrocytosis was maintained for up to 1 year.22 Therefore, decline of polycythemia cannot be attributed to the incapacity of the mice to sustain high polycythemia for a prolonged period. Regression of erythropoiesis was associated with myelofibrosis. Similarly, myelofibrosis is observed in patients with PV for only a few years, sometimes 20 years, after diagnosis. This so-called “spent” phase, best characterized by polycythemia decline, spleen enlargement, thrombocytopenia with high leukocytosis, and circulating precursor cells, shares these features in common with our animal model. Occurrence of various grades of fibrosis in all mice studied late after transplantation without evidence of clonal hematopoiesis strongly suggests that myelofibrosis is the direct consequence of long-term sustained JAK2V617F kinase activity rather than additional molecular events.
Myelofibrosis is a common feature of MPD, notably of IMF but also of PV, ET, and CML.23 Animal models for IMF have been previously developed using in vivo overexpression of TPO (TPOhigh)12 or transgenic mice expressing low levels of the transcription factor GATA-1 (GATA-1low mice).24 We have shown in the TPOhigh model that TGF-β1 had a prominent role in fibrosis induction.25 It is therefore tempting to speculate that the release of TGF-β1 by megakaryocytes in JAK2V617F mice may be responsible for myelofibrosis. TGF-β1 is probably released by megakaryocytes as a result of dysfunctional megakaryocytes or pathologic interactions with neutrophils. Dysmegakaryopoiesis and emperipolesis were observed in our model, suggesting that both mechanisms might be involved.
The JAK2V617F mutation is primarily identified in most patients with PV but also in half of patients with IMF and ET, and with a low frequency in other diseases. Our study shows that JAK2V617F causes PV but also induces myelofibrosis and possibly thrombocytosis. The dominance in the first stage of an erythroid phenotype in this mouse model suggests that other mechanisms or events are required to induce ET and IMF. A first hypothesis could be that, in ET and IMF, the JAK2V617F mutation occurs in a different progenitor cell than the primitive stem cells targeted in our model. However, this hypothesis is unlikely because JAK2V617F is found in a myelolymphoid progenitor in IMF and PV (F. Delhommeau, I. Godin, C. Tonetti, S. Dupont, J. P. Le Conedic, N. Debili, P. Saulnier, N. Casaderall, W. V., and S. Giraudier, manuscript in preparation). Another hypothesis would imply the occurrence of a second acquired genetic event in addition to JAK2V617F in ET or IMF that would blend the natural tendency of JAK2V617F to generate PV-like phenotypes. This event could be primary and would explain why no ET-like phenotype appeared in our model. This hypothesis is sustained by recent results in patients with ET who showed monoclonal hematopoiesis even when a minority of JAK2V617F-positive cells was present.26 Alternatively, this event could be secondary to the JAK2V617F mutation. However, this hypothesis is unlikely because it would imply that ET or IMF must occur after a polycythemic stage. A third hypothesis could be that low levels of JAK2 kinase activity would favor a megakaryocytic phenotype, whereas high levels would favor an erythroid phenotype. This “dosage” hypothesis is compatible with our PV model, which is a model of overexpression displaying thrombocytosis only in mice expressing low JAK2V617F/JAK2WT ratios. This hypothesis is mainly supported by the fact that most patients with ET (97%) are heterozygous for the mutation, while approximately 30% of patients with PV or IMF are homozygous. Homozygosity increases the expression of the mutated protein through gene duplication but also increases the effect of the mutated protein through a loss of competition with the wild-type (WT) protein.5 This “dosage” hypothesis can only be verified in heterozygous JAK2V617F/JAK2WT knock-in (KI) mice, where the endogenous promoters are likely to regulate the expression of physiologic and equal amounts of the WT and mutated gene. It predicts that if this mouse model faithfully reproduces the human disease, an ET-like disease will be prevalent.
In conclusion, this work shows that high levels of expression of the JAK2V617F mutant gene in murine hematopoietic cells induces a disease with features similar to those observed in patients with PV. It strongly suggests that levels and durations of JAK2V617F expression directly contribute to the diversity of human MPD where the mutation is found. Further work will be necessary to establish the exact mechanisms or events leading to the development of JAK2V617F-positive pathologies that are not associated with erythrocytosis. Finally, this murine model offers a unique opportunity to assess novel therapeutic approaches for JAK2V617F-positive pathologies, especially PV and myelofibrosis, for which no specific treatment exists.
During the review of this paper, Wernig et al27 demonstrated that this model generates in BALB/c mice a lethal disease with severe leukocytosis and myelofibrosis, suggesting that genetic modifiers are important in the MPD phenotype.
Prepublished online as Blood First Edition Paper, May 2, 2006; DOI 10.1182/blood-2006-02-002030.
Supported by grants from INSERM, IGR, Institut National Contre le Cancer, Cancéropole Ile de France, and by a special funding from La Ligue Nationale Contre le Cancer (labeled team 2004). D.F.P. is an IGR grant recipient.
C.L., D.F.P., M.T., F.M.G., and J.-L.V. designed and performed research and analyzed data; and D.F.P., F.M.G., W.V., and J.-L.V. wrote the paper.
C.L. and D.F.P. contributed equally to this work.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We are grateful to Françoise Wendling for critically reviewing the manuscript, Nicole Denis for Southern experiments, and Stefan Constantinescu and Orianne Wagner-Ballon for signaling and cytology expertise, respectively.

![Figure 4. BM and spleen analysis. BM cellularity (A), spleen weight (B) and differential cell count (BM cells [C] and spleen cells [D]) of these organs in recipient mice. BL indicates blast; MY, metamyeloblast/myeloblasts; ME/PO, myelocyte/neutrophil; LY, lymphocyte; MO, monocytes; EO, eosinophil; and ERY, erythroblasts. White, gray, and black histograms are empty, JAK2WT, and JAK2V617F virus-recipient mice (n = 8-12), respectively. For BM cellularity and spleen weight, data from the JAK2V617F virus-recipient mice were pooled (JAK2V617F) or divided into mice studied less (n = 7, days 52-97) or more (n = 5, days 131-143) than 4 months after transplantation (less than or more than 4 months). Differential counts were carried out of cells collected between days 31 and 143 after transplantation (n = 8), with a tendency toward an increase in the percentage of erythroblasts in late transplantation stages. *P < .05 compared with the JAK2WT mice.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/108/5/10.1182_blood-2006-02-002030/2/m_zh80170600550004.jpeg?Expires=1769140875&Signature=QwZpEZhQ9ceO5cztOJ~vMRSeemVlSQ8-m~gjMUkFIRV9igXQnVcXF1plKTjltY6GbUvaB0fG0CbFi~b6bY4ev7EifoflJAN-GY8F6Ki~SYNBIezc1nRG0ezbhiTdrHYKP10UjiOIlfd7jXqTfbEmeL5WIuVZAW3~fdIS6jjdhcfHLhOtzs9yK-Pit6t1KddHaUfzA0Hl0pOmkXd4yFTOx1GJEZYsh-BFJbCYJ0gD7iSREJxWT9gYjOu78KwpHqjlHx2zIFF6e91AvqP2ZIL-GLd0WnP6MgSKmonoyKdGphskOzV~lVSc7M0dj9CeiGMzQ9ign3Qcfv47RJQsO1he3g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal