Growth and survival of hematopoietic cells is regulated by growth factors and cytokines, such as interleukin 3 (IL-3). When cytokine is removed, cells dependent on IL-3 kill themselves by a mechanism that is inhibited by overexpression of Bcl-2 and is likely to be mediated by proapoptotic Bcl-2 family members. Bad and Bim are 2 such BH3-only Bcl-2 family members that have been implicated as key initiators in apoptosis following growth factor withdrawal, particularly in IL-3-dependent cells. To test the role of Bad, Bim, and other proapoptotic Bcl-2 family members in IL-3 withdrawal-induced apoptosis, we generated IL-3-dependent cell lines from mice lacking the genes for Bad, Bim, Puma, both Bad and Bim, and both Bax and Bak. Surprisingly, Bad was not required for cell death following IL-3 withdrawal, suggesting changes to phosphorylation of Bad play only a minor role in apoptosis in this system. Deletion of Bim also had no effect, but cells lacking Puma survived and formed colonies when IL-3 was restored. Inhibition of the PI3 kinase pathway promoted apoptosis in the presence or absence of IL-3 and did not require Bad, Bim, or Puma, suggesting IL-3 receptor survival signals and PI3 kinase survival signals are independent.
Introduction
Regulation of apoptosis by growth factors and cytokines is critical to maintain health and avoid neoplastic disease. During development and in adulthood, excess and unwanted cells are removed when cells that do not receive sufficient growth factor signals activate their death program by default.1 On the other hand, the ability of a mutant cell to survive in the absence of growth factors is an important step leading to its malignant transformation. Loss of cytokine signaling results in cell death by a mechanism characterized by activation of caspases that is regulated by the Bcl-2 family of proteins.2,3 Some members of the Bcl-2 family, such as Bcl-2 and Bcl-xL, can prevent apoptosis induced by withdrawal of growth factor when overexpressed.3,4 Bax and Bak, on the other hand, are proapoptotic Bcl-2 family members, and deletion of both renders cells highly resistant to apoptosis following growth factor withdrawal,5,6 emphasizing the pivotal role these proteins play in this pathway.
Bad, Bim, and Puma belong to a third, proapoptotic subgroup of the Bcl-2 family, which share homology only in a BH3 domain, the so-called BH3-only proteins. These proteins promote apoptosis when overexpressed7,8 probably by antagonizing the prosurvival antiapoptotic Bcl-2 family members.9
Regulation of cell survival by cytokines has often been studied in interleukin 3 (IL-3)-dependent hematopoietic cell lines such as FDC-P1, FL.5, and Ba/F3 cells.10-12 Several studies have implicated the BH3-only protein Bad as an important mediator of apoptosis following IL-3 withdrawal. When phosphorylated by kinases such as AKT, Bad binds to the tau form of 14-3-3 protein.13,14 In the absence of IL-3, unphosphorylated Bad is able to bind to, and inhibit, Bcl-xL,11,15 suggesting this is the mechanism by which cytokines regulate survival of hematopoietic cells. Consistent with this model, experiments using specific kinase inhibitors indicated that the PI3 kinase pathway that activated AKT is responsible for phosphorylation of Bad in response to signals from IL-3 receptors.11,15
The BH3-only protein Bim has also been implicated in signaling cell death following cytokine withdrawal because increased numbers of hematopoietic colonies grew when cytokines were restored to bone marrow cultures from Bim-/- mice, compared to bone marrow cultures of wild-type mice.16
Puma, on the other hand, was first identified as a BH3-only protein that is transcriptionally regulated by p53.8,17,18 However, deletion of Puma also confers resistance to apoptosis in response to some p53-independent apoptotic stimuli. For instance, thymocytes from Puma-/- mice are resistant to apoptosis induced by growth factor deprivation or treatment with dexamethasone or phorbol ester and not only apoptosis induced by γ irradiation. Moreover, myeloid progenitors from Puma-/- mice survive longer in culture in the absence of cytokines than do those from wild-type mice.19,20
To determine the importance of these BH3-only proteins in IL-3 withdrawal-induced apoptosis, we generated multiple IL-3-dependent cell lines from mice lacking Puma, Bad, Bim, both Bad and Bim, or both Bax and Bak. These cells were then tested for both short-term survival and clonogenic survival following IL-3 withdrawal.
Cells lacking both Bax and Bak showed no signs of death when cultured in the absence of IL-3, as previously reported,6 but, surprisingly, only loss of the BH3-only protein Puma, but not loss of Bad or Bim or both, allowed clonogenic survival after IL-3 deprivation. We were unable to demonstrate a significant role for Bad or for changes in Bad phosphorylation, in IL-3 survival signaling, or any role for Bim in regulating Bax translocation to mitochondria. Furthermore, because PI3 kinase inhibitors promoted apoptosis both in the presence and absence of IL-3, most if not all of the survival signals from IL-3 receptors appear to be independent of the PI3 kinase axis.
Materials and methods
Cell lines
IL-3-dependent (factor-dependent myeloid [FDM]) cell lines were generated by coculturing E14.5 fetal liver single-cell suspensions with fibroblasts expressing a HoxB8 retrovirus in the presence of high levels of IL-3, as previously described.21 Data on mice bearing gene deletions for Bad, Bim, Puma, and Bak have been previously published.19,22-24 Bax-/- mice were obtained from Jackson Laboratories (Bar Harbor, ME). Mice lacking both Bad and Bim were generated by Bad-/-;Bim+/- intercrosses and Bax-/-;Bak-/- were generated by Bax+/-;Bak-/- intercrosses. All mice were derived from C57BL/6 embryonic stem (ES) cells or from 129SV-derived ES cells but had been back-crossed onto a C57BL/6 background for at least 10 generations. Cell lines were tested for IL-3 dependence by determining their ability to proliferate in the absence of IL-3 in liquid culture and soft agar. No cell lines were able to generate colonies under such conditions. All cells were cultured in DMEM with 10% fetal calf serum (FCS; JRH Laboratories, Lenexa, KS) supplemented with 2% to 3% IL-3.
Assays for cell viability assays, clonogenic survival, and cytochrome c staining
IL-3 was removed by washing cells 3 times in PBS and then culturing cells in DMEM with 10% FCS with or without IL-3. Viability was determined by staining cells with FITC-coupled annexin V (produced in one of our laboratories) in balanced salt solution including 5 mM CaCl2 and propidium iodide (PI; 1 μg/mL) followed by flow cytometric analysis (Becton Dickinson, San Jose, CA). Q-VD-Z-OPh (Imgenex, San Diego, CA) was made up as a stock solution in DMSO at a concentration of 10 mM and used at a final concentration of 100 μM.
Clonogenic assays were performed as previously described.21 Briefly, 1 × 105 cells/mL were plated with or without IL-3. At the various time points, predetermined numbers of cells were plated in 36-mm Petri dishes in DMEM/20% FCS/10% IL-3 supernatant/0.3% agar. After 21 days the number of colonies was counted and expressed as a percentage relative to the number of colonies generated per 1000 cells plated immediately before culture without IL-3 (days IL-3 withdrawal = 0). For example, if a clone generated 200 colonies/1000 cells plated at day 0 and 50 colonies/1000 cells after culture without IL-3 for 1 day, the relative clonogenicity at day 0 was 100% and 25% at day 1. At least 3 independent clones of each genotype were used in each experiment.
3,3′-dihexyloxacarbocyanine iodide (DiOC6(3); Molecular Probes, Eugene, OR) stock solution (10 mM in methanol) was diluted 1:25 000 in DMEM with FCS. Cells were washed once in PBS and then resuspended in DiOC6(3) in DMEM for 30 minutes at 37°C. Cells were then washed and resuspended in medium and analyzed by fluorescence-activated cell sorting (FACS) in the FL-1 channel.
Intracellular cytochrome c staining was done as previously described.21 Briefly, cells were cultured in the presence or absence of IL-3 for 48 hours and then permeabilized using digitonin for 10 minutes. The cells were then fixed, incubated in blocking solution, and then stained with anti-cytochrome c antibody (catalogue no. 556432; BD PharMingen, San Diego, CA) at a 1:200 dilution overnight followed by an anti-mouse FITC-coupled secondary antibody (Southern Biotechnology, Birmingham, AL) for 1 hour. Cells were analyzed by flow cytometry.
Ly294002 (Calbiochem, San Diego, CA) stock solution (25 mM in DMSO) was used at a final concentration of 25 μM. Wortmannin was used at 0.5 and 5.0 μM.
CFSE staining
CFSE (Molecular Probes) stock solution (5 mM in DMSO) was diluted 1:1000 in DMEM (no FCS). Cells were washed twice in PBS and then resuspended in CFS/DME for 10 minutes at 37°C and resuspended by inversion of the tube every 2 to 3 minutes. Cells were then washed in 5 times volume of cold DMEM with FCS and IL-3 to quench staining and then placed in normal growth media. The following day, the cells were washed 3 times in PBS, counted, and cultured in the presence or absence of IL-3. CFSE fluorescence was determined by flow cytometric analysis (FL1 channel).
Details of Western blotting, cell fractionation, immunoprecipitation, and reverse transcription-polymerase chain reaction (RT-PCR) are given in Document S1 (available at the Blood website; see the Supplemental Materials link at the top of the online article).
Results
Bad and Bim are not required for IL-3 withdrawal-induced apoptosis of immortalized progenitors
To test the requirement for the BH3-only proteins Bad, Bim, and Puma for apoptosis in response to IL-3 withdrawal, we generated immortalized IL-3-dependent lines from mice lacking these genes or combinations of these genes. Multiple independent lines were generated by coculturing E14.5 fetal liver cells with cells producing a HoxB8 retrovirus as previously described.21 Lines lacking genes for various BH3-only proteins were then compared to lines derived from wild-type or Bax-/-;Bak-/- mice.
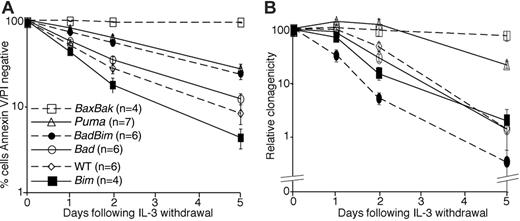
Compared to wild-type and Bim-/- cells, fewer cells lacking Bad, both Bad and Bim, and Puma became positive for annexin V and permeable to PI during the 5-day period of culture without IL-3 (Figure 1A). However, none of the cell lines was as resistant to death following IL-3 withdrawal as Bax-/-;Bak-/- cells, which showed no signs of apoptosis during the 5 days of culture. We also tested cell lines lacking Bid, Hrk, and both Noxa and Puma (Figure S1). Neither deletion of Bid nor Hrk increased the percentage of annexin V/PI- cells following IL-3 withdrawal. Moreover, combined deletion of both Puma and Noxa resulted in no additional protection compared to deletion of Puma alone. Bid processing could be observed in lysates from IL-3-deprived wild-type cells but not in lysates from similarly treated Bax-/-;Bak-/- lysates.
To determine whether loss of BH3-only proteins would allow long-term survival following withdrawal of IL-3, clonogenic assays were performed by starving cells of IL-3 for variable times and then placing the factor-starved cells into soft agar with abundant IL-3, as previously described21 (Figure 1B). Cells lacking Bad, Bim, or both Bad and Bim were indistinguishable from wild-type cells, as their ability to form colonies steadily declined over time, reaching less than 5% after 5 days. In contrast, cells lacking both Bax and Bak retained their full clonogenic potential for at least 5 days. Puma-/- lines were also able to generate significantly more colonies than the wild-type controls following IL-3 deprivation. These results indicate that neither Bad nor Bim is required for cell death following IL-3 withdrawal and that deletion of either or even both genes does not allow clonogenic survival. This is so, even though fewer cells lacking Bad or both Bad and Bim were positive for annexin V/PI at the end of the assays compared with wild-type cells. In contrast, Puma plays a significant role in IL-3 withdrawal-induced cell killing. However, because deletion of Puma did not afford as much protection as deletion of both Bax and Bak, it is likely that other BH3-only proteins, in addition to Puma, activate Bax and Bak following removal of cytokine.
Loss of Puma or both Bax plus Bak promotes clonogenic survival following IL-3 withdrawal. (A) Multiple independent, IL-3-dependent cell lines (n) were starved of IL-3 over a 5-day time course. Cell viability was determined by staining with FITC-conjugated annexin V and propidium iodide (PI) followed by flow cytometric analysis. Results show means ± SEM of 4 independent experiments. (B) Aliquots of the cell lines used in panel A were plated in soft agar with IL-3 after the indicated period of culture without IL-3. Colonies were counted after 21 days and the relative clonogenicity (see “Materials and methods”) determined. Data are the means ± SEM of 4 independent experiments.
Loss of Puma or both Bax plus Bak promotes clonogenic survival following IL-3 withdrawal. (A) Multiple independent, IL-3-dependent cell lines (n) were starved of IL-3 over a 5-day time course. Cell viability was determined by staining with FITC-conjugated annexin V and propidium iodide (PI) followed by flow cytometric analysis. Results show means ± SEM of 4 independent experiments. (B) Aliquots of the cell lines used in panel A were plated in soft agar with IL-3 after the indicated period of culture without IL-3. Colonies were counted after 21 days and the relative clonogenicity (see “Materials and methods”) determined. Data are the means ± SEM of 4 independent experiments.
In the absence of Bad or Bim, cell death due to cytokine withdrawal is still associated with mitochondrial release of cytochrome c and loss of mitochondrial membrane potential
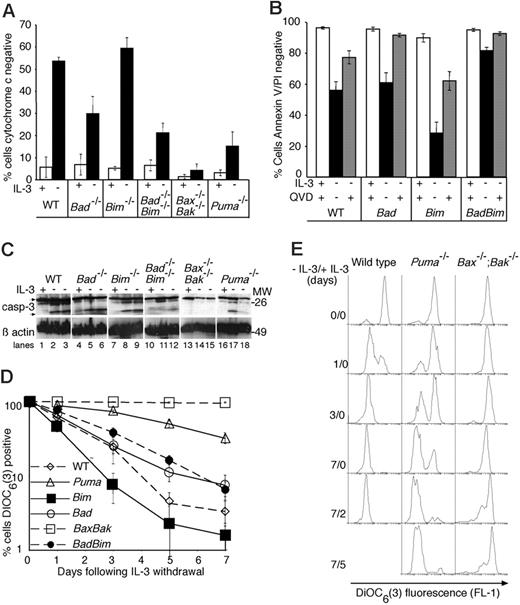
To determine whether IL-3 withdrawal resulted in changes typical of apoptosis in Bad-/-, Bim-/-, or Bad-/-;Bim-/- cell lines, we looked for evidence of cytochrome c release and caspase activation 48 hours after removal of IL-3 (Figure 2A-C). The percentage of cells that had released cytochrome c from their mitochondria by this time was similar in Bim-/-and wild-type lines. In contrast, fewer cells lacking both Bad and Bim or Puma had released cytochrome c at this time. No mitochondrial release of cytochrome c could be detected in IL-3-deprived Bax-/-;Bak-/- cells.
Loss of Bad does not affect mitochondrial release of cytochrome c, caspase processing or loss of mitochondrial membrane potential following IL-3 withdrawal. (A) Cytochrome c release from mitochondria was examined 48 hours following IL-3 withdrawal. Cells were cultured in the presence or absence of IL-3 for 48 hours, then permeabilized, fixed, and stained with antibodies to cytochrome c prior to analysis by flow cytometry to determine the percentage of cells that had lost mitochondrial cytochrome c. Data show the mean ± SEM of 3 independent experiments using 2 independent lines of each genotype. (B) Caspase inhibition diminishes the percentage of cells that are stained by both annexin V and PI following IL-3 withdrawal. Cells were cultured for 24 hours in the presence or absence of IL-3 with or without the broad-spectrum caspase inhibitor Q-VD-Z-OPh (100 μM). Cell viability was determined by annexin V-FITC staining and PI uptake. Columns show the mean ± SEM of 3 independent experiments using 2 independent lines of each genotype. (C) Western blots of lysates from cells cultured for 24 hours with IL-3 (lanes 1, 4, 7, 10, 13, and 16), 24 hours without IL-3 (lanes 2, 5, 8, 11, 14, and 17), and 48 hours without IL-3 (lanes 3, 6, 9, 12, 15, and 18) were probed with an antibody to caspase-3. Arrows show full-length and processed caspase-3, respectively. Probing with an antibody to β-actin was used as a loading control. (D) Changes in mitochondrial membrane potential following IL-3 withdrawal. Cells were cultured without IL-3 for the indicated lengths of time, stained with DIOC6(3), and analyzed by flow cytometry to determine the percentage of cells that had lost their mitochondrial membrane potential. Dead or dying cells have diminished DIOC6(3) fluorescence, which is detected in the FL1 channel. The results show the mean ± SEM of 3 independent experiments, each using 2 independent lines of each genotype. (E) Histograms of DIOC6(3) fluorescence following IL-3 withdrawal and after restoration of IL-3. At each time during factor starvation and following readdition of IL-3 (indicated at the left of each panel), cells were harvested and stained with DIOC6(3). The histograms shown are from a single experiment representative of 3 independent analyses.
Loss of Bad does not affect mitochondrial release of cytochrome c, caspase processing or loss of mitochondrial membrane potential following IL-3 withdrawal. (A) Cytochrome c release from mitochondria was examined 48 hours following IL-3 withdrawal. Cells were cultured in the presence or absence of IL-3 for 48 hours, then permeabilized, fixed, and stained with antibodies to cytochrome c prior to analysis by flow cytometry to determine the percentage of cells that had lost mitochondrial cytochrome c. Data show the mean ± SEM of 3 independent experiments using 2 independent lines of each genotype. (B) Caspase inhibition diminishes the percentage of cells that are stained by both annexin V and PI following IL-3 withdrawal. Cells were cultured for 24 hours in the presence or absence of IL-3 with or without the broad-spectrum caspase inhibitor Q-VD-Z-OPh (100 μM). Cell viability was determined by annexin V-FITC staining and PI uptake. Columns show the mean ± SEM of 3 independent experiments using 2 independent lines of each genotype. (C) Western blots of lysates from cells cultured for 24 hours with IL-3 (lanes 1, 4, 7, 10, 13, and 16), 24 hours without IL-3 (lanes 2, 5, 8, 11, 14, and 17), and 48 hours without IL-3 (lanes 3, 6, 9, 12, 15, and 18) were probed with an antibody to caspase-3. Arrows show full-length and processed caspase-3, respectively. Probing with an antibody to β-actin was used as a loading control. (D) Changes in mitochondrial membrane potential following IL-3 withdrawal. Cells were cultured without IL-3 for the indicated lengths of time, stained with DIOC6(3), and analyzed by flow cytometry to determine the percentage of cells that had lost their mitochondrial membrane potential. Dead or dying cells have diminished DIOC6(3) fluorescence, which is detected in the FL1 channel. The results show the mean ± SEM of 3 independent experiments, each using 2 independent lines of each genotype. (E) Histograms of DIOC6(3) fluorescence following IL-3 withdrawal and after restoration of IL-3. At each time during factor starvation and following readdition of IL-3 (indicated at the left of each panel), cells were harvested and stained with DIOC6(3). The histograms shown are from a single experiment representative of 3 independent analyses.
Application of a broad-spectrum caspase inhibitor (Q-VD-Z-OPh) during the time of cytokine deprivation reduced the numbers of wild-type, Bad-/-, Bim-/-, or Bad-/-;Bim-/- cells that exposed phosphatidylserine and stained with annexin V or became permeable to PI, demonstrating that these late changes are caspase dependent (Figure 2B). When examined by Western blotting, caspase-3 processing was clearly evident in lysates of all cell types starved of IL-3 except for Bax-/-;Bak-/- lines. Variable amounts of caspase-3 processing were observed in the Puma-/- cells, but this was clearly always less than that seen in wild-type, Bad-/-, or Bim-/- cells, suggesting that a proportion of Puma-/- cells die as a result of caspase activation (Figure 2C). These results show that Bax plus Bak and to a lesser extent Puma, but neither Bad alone nor Bim alone, are required for mitochondrial release of cytochrome c and caspase activation following IL-3 withdrawal.
Bax-/-;Bak-/- cells can survive for many weeks in the absence of growth factor, during which time they cease cycling, their metabolic activity declines, and though their mitochondrial transmembrane potential is retained, its level is reduced.6 To determine whether mitochondrial membrane potential is lost following IL-3 withdrawal in the mutant cell lines, we stained growth factor-starved cells with the carbocyanine dye DiOC6(3) that fluoresces in proportion to mitochondrial membrane potential25,26 (Figure 2D-E). Bax-/-;Bak-/- cells maintained membrane potential over the course of the experiment (7 days) with only a slight decrease in DiOC6(3) fluorescence. When IL-3 was restored, there was an increase in the number of cells with normal mitochondrial membrane potential. All other cell lines showed a progressive increase in the percentage of cells in which mitochondrial membrane potential was lost, but the rate at which Puma-/- cells lost membrane potential was significantly slower than that seen in wild-type, Bad-/-, Bim-/-, or Bad-/-;Bim-/- cells (Figure 2D-E). For example, whereas virtually all wild-type cells had lost DiOC6(3) fluorescence after 3 days of culture in the absence of IL-3, less than half the Puma-/- cells had done so. Those Puma-/- cells that had lost mitochondrial transmembrane potential fluoresced with similar intensity to growth factor-starved wild-type cells. Together, these data suggest that although loss of Puma confers a clear survival advantage in IL-3-deprived cells, Puma is not absolutely required for eventual loss of cytochrome c and caspase activation following IL-3 withdrawal. Instead, loss of Puma delays these changes.
Bax translocation is not dependent on Bad or Bim but is delayed by loss of Puma
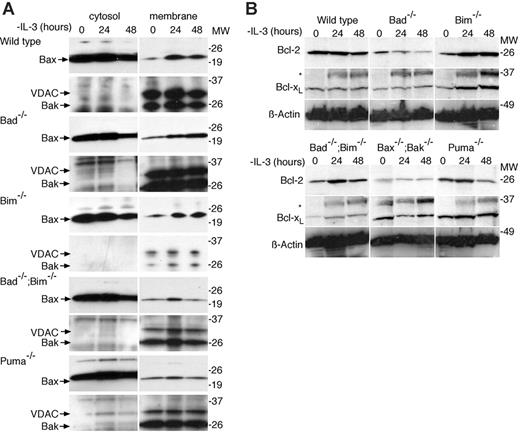
Following growth factor withdrawal, Bax translocates from the cytosol to mitochondrial membranes.27-29 It is thought that BH3-only proteins, such as Bim,12 Bid,28,30,31 and Puma32 may regulate this process by binding to antiapoptotic Bcl-2 family members, thereby liberating Bax (and Bak) to activate the cell death machinery. Alternatively, it has been proposed that Bim and Bid can trigger apoptosis by direct interaction with Bax and Bak.9,24 To determine whether the deletion of Bad, Bim, or Puma affected Bax translocation, we separated cytosolic and membrane fractions of cells cultured in the presence or absence of IL-3 and immunoblotted with antibodies to Bax and Bak (Figure 3A). Bax translocation was observed in IL-3-deprived wild-type, Bad-/-, Bim-/-, and Bad-/-;Bim-/- cells. There appeared to be less (albeit clearly detectable) Bax moving from the cytosolic compartment to the membrane fraction in IL-3-deprived Puma-/- cells, consistent with the delay in apoptosis observed for these cells. Interestingly, the amount of Bax translocation in Puma-/- and Bad-/-;Bim-/- lines was similar, despite their difference in clonogenic survival, suggesting that quantitative differences in Bax translocation do not necessarily predict long-term survival following IL-3 withdrawal. Bak, which is predominantly located in the membrane fraction, remained there. These results show that Bax translocation in response to cytokine withdrawal does not require either the direct or indirect actions of Bad or Bim.
Following IL-3 withdrawal, Bax translocates normally from the cytosolic fraction to the membrane fraction in cells lacking Bad or Bim. (A) Cells of the indicated genotypes were cultured in the absence of IL-3 and then fractionated into cytosolic and membrane fractions using digitonin. Lysates were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and immunoblotted with antibodies to Bax, Bak, and, as a control to demonstrate purity of the fractions, with an antibody to VDAC. (B) Bcl-2 and Bcl-xL expression is maintained following IL-3 withdrawal. Western blots of lysates from cells cultured in the absence of IL-3 for 0, 24, and 48 hours probed with antibodies against Bcl-2 or Bcl-xL. Probing with an antibody to β-actin was used as a loading control. Lysates from equal numbers of cells were loaded in each lane. Asterisks mark a nonspecific band. These blots are the same as shown in Figure 2C, but have been reprobed with the indicated antibodies. The same β-actin controls are shown in Figure 2C and in this panel.
Following IL-3 withdrawal, Bax translocates normally from the cytosolic fraction to the membrane fraction in cells lacking Bad or Bim. (A) Cells of the indicated genotypes were cultured in the absence of IL-3 and then fractionated into cytosolic and membrane fractions using digitonin. Lysates were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and immunoblotted with antibodies to Bax, Bak, and, as a control to demonstrate purity of the fractions, with an antibody to VDAC. (B) Bcl-2 and Bcl-xL expression is maintained following IL-3 withdrawal. Western blots of lysates from cells cultured in the absence of IL-3 for 0, 24, and 48 hours probed with antibodies against Bcl-2 or Bcl-xL. Probing with an antibody to β-actin was used as a loading control. Lysates from equal numbers of cells were loaded in each lane. Asterisks mark a nonspecific band. These blots are the same as shown in Figure 2C, but have been reprobed with the indicated antibodies. The same β-actin controls are shown in Figure 2C and in this panel.
To determine whether deletion of Bad, Bim,or Puma influenced the stability of antiapoptotic Bcl-2 family members following IL-3 withdrawal, we probed Western blots of protein lysates derived from cells starved of IL-3 over 48 hours with antibodies to Bcl-2, Bcl-xL, and Mcl-1 (Figures 3B and S1B). We did not observe an early decline in the levels of these proteins following IL-3 withdrawal but instead saw that the levels of Bcl-xL and Bcl-2 remained unchanged over 48 hours. In contrast, Mcl-1 expression declined markedly in wild-type cells by 48 hours of IL-3 deprivation but was maintained in Bax-/-;Bak-/- cells (Figure S1). The decline in Mcl-1, but not Bcl-2 or Bcl-xL, correlated with the extent of apoptosis following IL-3 deprivation, but we have not yet determined the mechanism or whether it is a cause or consequence of apoptosis.
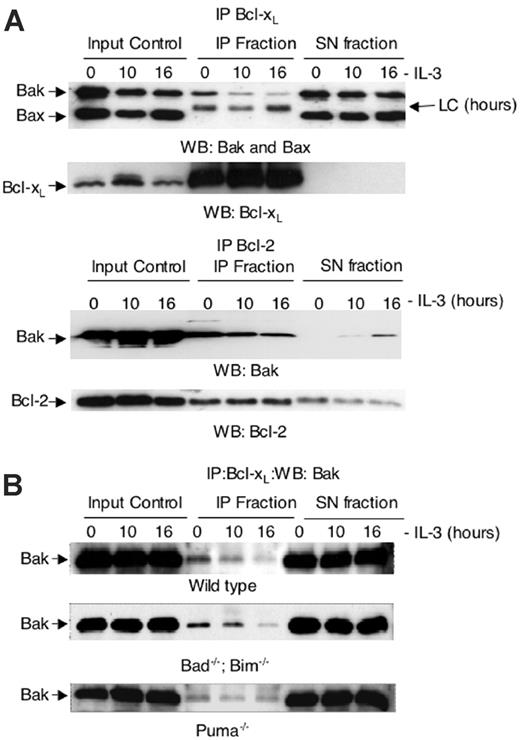
To determine the effect IL-3 deprivation had on binding of Bcl-xL to Bax or Bak we immunoprecipitated Bcl-xL from the mitochondrial fraction of lysates from FDC-P1 cells grown in the presence or absence of IL-3 (Figure 4A). Bcl-xL was able to immunoprecipitate Bak but not Bax, and less Bak remained bound to Bcl-xL following IL-3 withdrawal. These observations indicate that loss of Bak-Bcl-xL binding may be critical for IL-3 withdrawal-induced apoptosis.
To determine whether Bcl-xL binding to Bak was altered in the absence of Puma, we immunoprecipitated Bcl-xL from the mitochondrial fraction of lysates from wild-type, Puma-/-, and Bad-/-; Bim-/- cells cultured in the presence or absence of IL-3 (Figure 4B). Some but not all Bak immunoprecipitated with Bcl-xL in lysates from cells cultured in the presence of IL-3. Following IL-3 removal, progressively less Bak remained bound to Bcl-xL in wild-type and Bad-/-;Bim-/- cells. In contrast, considerable amounts of Bak remained bound to Bcl-xL in IL-3-deprived Puma-/- cells. This would be consistent with Puma acting by disassociating Bak from Bcl-xL following IL-3 withdrawal. The persistence of Bcl-xL binding to Bak is associated with the increased clonogenic survival of Puma-/- cells.
Bak coimmunoprecipitates with Bcl-xL in lysates from FDC-P1 cells, wild-type, and Bad-/-;Bim-/- FDM cells cultured in the presence of IL-3 but not in lysates from cells deprived of IL-3. Bak-Bcl-xL association is retained in IL-3-deprived Puma-/- FDM cells. (A) Anti-Bak and anti-Bax Western blots of anti-Bcl-xL immunoprecipitated (IP) material from the heavy membrane fraction of FDC-P1 cells cultured in the presence (0) or absence of IL-3 for 10 and 16 hours. Anti-Bcl-xL immunoprecipitates, as well as the total input and the supernatant fraction after each immunoprecipitation (SN fraction) are shown. LC indicates immunoglobulin light chain of antibody used for immunoprecipitation. (B) Anti-Bak Western blots of anti-Bcl-xL immunoprecipitated from the heavy membrane fraction of wild-type, Bad-/-;Bim-/-, and Puma-/- FDM cells cultured in the presence (0) or absence of IL-3 for 12 and 24 hours.
Bak coimmunoprecipitates with Bcl-xL in lysates from FDC-P1 cells, wild-type, and Bad-/-;Bim-/- FDM cells cultured in the presence of IL-3 but not in lysates from cells deprived of IL-3. Bak-Bcl-xL association is retained in IL-3-deprived Puma-/- FDM cells. (A) Anti-Bak and anti-Bax Western blots of anti-Bcl-xL immunoprecipitated (IP) material from the heavy membrane fraction of FDC-P1 cells cultured in the presence (0) or absence of IL-3 for 10 and 16 hours. Anti-Bcl-xL immunoprecipitates, as well as the total input and the supernatant fraction after each immunoprecipitation (SN fraction) are shown. LC indicates immunoglobulin light chain of antibody used for immunoprecipitation. (B) Anti-Bak Western blots of anti-Bcl-xL immunoprecipitated from the heavy membrane fraction of wild-type, Bad-/-;Bim-/-, and Puma-/- FDM cells cultured in the presence (0) or absence of IL-3 for 12 and 24 hours.
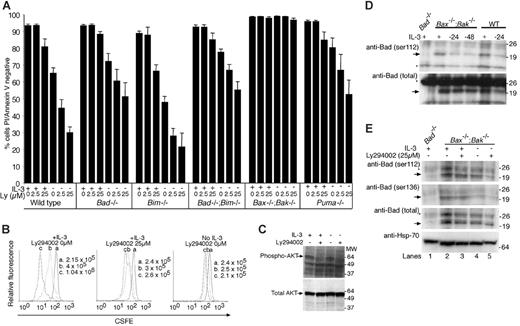
PI3 kinase inhibition by Ly294002 increases apoptosis caused by IL-3 withdrawal in Bad-/-, Bim-/-, and Puma-/- cells
It has been reported that IL-3 promotes cell survival by stimulating PI3 kinase/AKT phosphorylation of Bad on serine residues S112, S136, and S155, because the phosphorylated form of Bad is sequestered by binding to 14-3-3 proteins, and thereby prevented from binding antiapoptotic Bcl-2 family members, in particular Bcl-xL.11,14,33,34 Because deletion of neither Bad or Bim prevented cell death induced by IL-3 withdrawal, it appears unlikely that cell death caused by lack of IL-3 signaling is critically dependent on loss of PI3 kinase phosphorylation of Bad or Bim. It remained possible, however, that cell death induced by inhibition of PI3 kinase was dependent on another BH3-only protein, such as Puma, or that Bad and Bim acted redundantly. To test these possibilities, we treated cell lines with Ly294002, a fairly specific PI3 kinase inhibitor, and cultured the cells in the presence or absence of IL-3 (Figure 5A). Ly294002 promoted cell death in all cell lines except those lacking Bax and Bak, indicating that it caused cell death by a Bax/Bak-dependent mechanism. We observed similar effects when cells were treated with wortmannin, another PI3 kinase inhibitor, in the absence of IL-3 (Figure S2). Fewer annexin V/PI+ cells were observed when cells were cultured with IL-3 and wortmannin compared with culture in IL-3 plus Ly294002. Because Ly294002 (and wortmannin) increased the extent of apoptosis in the absence of IL-3 as well as in its presence, some prosurvival PI3 kinase activity must be independent of IL-3 signaling. Furthermore, because Ly294002 promoted death even in cells lacking Puma, Bim, or Bad, loss of PI3 kinase signaling appears able to cause apoptosis by pathways independent of these BH3-only proteins or does so by activating multiple BH3-only proteins.
The ability of IL-3 to enhance survival of Ly294002-treated cells argues that a significant part of the survival signal from ligated IL-3 receptors is transduced independently of the PI3 kinase axis, provided the dose of Ly294002 used was sufficient to block PI3 kinase activity. To confirm that Ly294002 was biologically active we measured the proliferation of Bax-/-;Bak-/- cells by staining them with the fluorescent marker CFSE, and culturing them in the presence or absence of IL-3 with or without Ly294002 and immunoblotted lysates from Bax-/-;Bak-/- cells with antibodies to phospho-AKT and total AKT (Figure 5B-C). Diminishing CFSE fluorescence results from dilution of the stain as cells divide (left panel) and is widely used to measure proliferation of cells. The intensity of CFSE staining did not diminish in cells starved of IL-3 (right panel), indicating that these cells were not proliferating. In the presence of IL-3, treatment with Ly294002 decreased the proliferation rate of Bax-/-;Bak-/- cells without affecting their viability, indicating that this concentration of drug was active in these cells (middle panel). Further, Ly294002 treatment in the presence or absence of IL-3 diminished the amount of phospho-AKT present in cells. Because inhibiting PI3 kinase and downstream kinases completely stopped cell division induced by IL-3 but only had a small effect on survival in the presence of IL-3, it seems reasonable to conclude that the PI3 kinase axis plays an important role in IL-3-induced proliferation, but not in IL-3 stimulation of survival. Because neither Ly294002 nor wortmannin is completely selective for PI3 kinase, it remains possible that other kinases inhibited by these drugs may play a role in IL-3-induced survival signaling.
PI3 kinase inhibitor Ly294002 causes apoptosis in the presence or absence of IL-3 in all cell lines except those lacking both Bax and Bak. (A) Cells of the indicated genotypes were cultured for 24 hours with or without IL-3 with or without the addition of Ly294002 (25 μM). Cell viability was determined by staining with FITC-coupled annexin V and PI followed by flow cytometric analysis. Data show the mean ± SEM of 3 independent experiments using 2 independent lines of each genotype. (B) Ly294002 inhibits proliferation in Bax-/-;Bak-/- cells. Cells were stained with CSFE and cultured in the presence of IL-3 alone (left panel), IL-3 plus Ly294002 (middle panel), or without IL-3 (right panel). At 0 (a), 24 (b), and 48 hours (c), the cells were counted and CFSE fluorescence determined by flow cytometric analysis. The histograms for each time point are overlaid. CFSE fluorescence declines (shifts to the left) as cell number increases. The results shown are from one experiment that is representative of 3 independent experiments with different Bax-/-;Bak-/- clones. (C) Bax-/-;Bak-/- cells were cultured in the presence or absence of IL-3 and plus or minus Ly294002 (25 μM). Lysates from these cells were run on SDS-PAGE and immunoblotted with antibodies recognizing only phosphorylated AKT (S473) or total AKT. (D) Bad phosphorylated on S112 and total Bad decline following IL-3 withdrawal. Western blot of lysates harvested from Bax-/-;Bak-/- cells cultured with IL-3 (+), or 24 or 48 hours following IL-3 withdrawal (-), and from wild-type cells cultured with IL-3, or 24 hours following IL-3 withdrawal, were probed with antibodies to S112 phospho-Bad or total Bad. Arrows indicate Bad and asterisks mark nonspecific bands. Lysates from equal numbers of cells were loaded in each lane. (E) Ly294002 reduces the level of both phosphorylated Bad and total Bad when combined with IL-3 withdrawal. Western blot of lysates from Bax-/-;Bak-/- cells cultured for 24 hours with or without IL-3 and Ly294002 (25 μM) for 24 hours were probed with antibodies to S112 or S136 phospho-Bad or total Bad. Probing with an antibody to Hsp-70 was used as a loading control. Lysates from equal numbers of cells were loaded in each lane. Arrows indicate Bad and asterisks mark nonspecific bands.
PI3 kinase inhibitor Ly294002 causes apoptosis in the presence or absence of IL-3 in all cell lines except those lacking both Bax and Bak. (A) Cells of the indicated genotypes were cultured for 24 hours with or without IL-3 with or without the addition of Ly294002 (25 μM). Cell viability was determined by staining with FITC-coupled annexin V and PI followed by flow cytometric analysis. Data show the mean ± SEM of 3 independent experiments using 2 independent lines of each genotype. (B) Ly294002 inhibits proliferation in Bax-/-;Bak-/- cells. Cells were stained with CSFE and cultured in the presence of IL-3 alone (left panel), IL-3 plus Ly294002 (middle panel), or without IL-3 (right panel). At 0 (a), 24 (b), and 48 hours (c), the cells were counted and CFSE fluorescence determined by flow cytometric analysis. The histograms for each time point are overlaid. CFSE fluorescence declines (shifts to the left) as cell number increases. The results shown are from one experiment that is representative of 3 independent experiments with different Bax-/-;Bak-/- clones. (C) Bax-/-;Bak-/- cells were cultured in the presence or absence of IL-3 and plus or minus Ly294002 (25 μM). Lysates from these cells were run on SDS-PAGE and immunoblotted with antibodies recognizing only phosphorylated AKT (S473) or total AKT. (D) Bad phosphorylated on S112 and total Bad decline following IL-3 withdrawal. Western blot of lysates harvested from Bax-/-;Bak-/- cells cultured with IL-3 (+), or 24 or 48 hours following IL-3 withdrawal (-), and from wild-type cells cultured with IL-3, or 24 hours following IL-3 withdrawal, were probed with antibodies to S112 phospho-Bad or total Bad. Arrows indicate Bad and asterisks mark nonspecific bands. Lysates from equal numbers of cells were loaded in each lane. (E) Ly294002 reduces the level of both phosphorylated Bad and total Bad when combined with IL-3 withdrawal. Western blot of lysates from Bax-/-;Bak-/- cells cultured for 24 hours with or without IL-3 and Ly294002 (25 μM) for 24 hours were probed with antibodies to S112 or S136 phospho-Bad or total Bad. Probing with an antibody to Hsp-70 was used as a loading control. Lysates from equal numbers of cells were loaded in each lane. Arrows indicate Bad and asterisks mark nonspecific bands.
It has also been suggested that IL-3 promotes survival by causing other kinases such as Pim1/Pim2, p21-activated kinase, or MEK, to phosphorylate Bad on residues 112, 136, or 155.11,35-39 To study regulation of Bad phosphorylation by IL-3, we probed lysates of Bax-/-;Bak-/- and wild-type cells cultured in the presence or absence of IL-3 with antibodies that bind specifically to Bad phosphorylated on S112, and, as a control, with an antibody recognizing total Bad (Figure 5D). Levels of both 112 phosphorylated and total Bad in lysates from Bax-/-;Bak-/- and wild-type cells decreased slightly following IL-3 withdrawal. However, because Bad is not required for cell death caused by IL-3 withdrawal and because the decrease in phosphorylation corresponded to a decrease in total levels of Bad, these changes do not appear to be relevant to apoptosis regulated by this cytokine. To test whether PI3 kinase played an important role in maintaining phosphorylation of Bad in IL-3-stimulated cells, we examined lysates from cells treated with Ly294002 (Figure 5E). Neither removal of IL-3 nor addition of Ly294002 had dramatic effects on the amount of Bad phosphorylation on S112 or S136.
Puma mRNA and protein levels are increased following IL-3 withdrawal
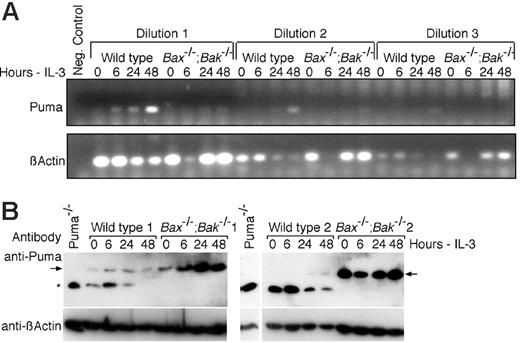
To begin to understand how Puma is regulated by IL-3 signaling, we isolated mRNA and protein from wild-type and Bax-/-;Bak-/- cells cultured in the presence or absence of IL-3. RT-PCR revealed increases in Puma mRNA over the 48-hour time course of IL-3 withdrawal, particularly in wild-type cells. There was also an increase in Puma message in Bax-/-;Bak-/- cells, but the levels amplified by RT-PCR were not as great (Figure 6A). Western blotting revealed that Puma protein levels increased progressively in response to IL-3 withdrawal in both wild-type and Bax-/-;Bak-/- cells (Figure 6B). Interestingly, Bax-/-;Bak-/- cell lines had elevated levels of Puma protein even in the presence of IL-3 even though there appeared to be less Puma mRNA in Bax-/-;Bak-/- cells. These data suggest that Puma may be regulated both transcriptionally and posttranslationally following IL-3 withdrawal. Moreover, Bax-/-;Bak-/- cells appear to be able to tolerate higher levels of Puma expression, presumably because they are resistant to Puma-induced apoptosis, but the mechanism causing elevated Puma levels in these cells is presently unclear. The diminished levels of Puma mRNA but elevated Puma protein in Bax-/-;Bak-/- cells raises the possibility that Puma protein turnover is somehow influenced by either Bax or Bak.
Regulation of Puma by IL-3 signaling. (A) RT-PCR analysis of changes in Puma mRNA levels following IL-3 withdrawal in wild-type and Bax-/-;Bak-/- cells. Total RNA (1.2 μg) extracted from wild-type and Bax-/-;Bak-/- cells cultured in the presence or absence of IL-3 was reverse transcribed and 2.5 μL of the reaction volume (dilution 1) and 1:5 serial dilutions of this (dilutions 2 and 3) were used in a PCR to amplify Puma mRNA. RT-PCR analysis of β-actin mRNA is shown as a control. (B) Lysates from 2 wild-type and 2 Bax-/-;Bak-/- cells cultured in the presence of IL-3 (0 hours) or in the absence of IL-3 for the indicated time periods were immunoblotted with an antibody to Puma. Probing with an antibody to β-actin was used as a loading control. The arrows indicate Puma and the asterisks a nonspecific band that was observed only in cell lines other than Bax-/-;Bak-/- cells. The lysate from Puma-/- cells shown aligned to the wild-type 2 and Bax-/-;Bak-/- 2 clones was run on the same gel, but intervening irrelevant bands have been removed.
Regulation of Puma by IL-3 signaling. (A) RT-PCR analysis of changes in Puma mRNA levels following IL-3 withdrawal in wild-type and Bax-/-;Bak-/- cells. Total RNA (1.2 μg) extracted from wild-type and Bax-/-;Bak-/- cells cultured in the presence or absence of IL-3 was reverse transcribed and 2.5 μL of the reaction volume (dilution 1) and 1:5 serial dilutions of this (dilutions 2 and 3) were used in a PCR to amplify Puma mRNA. RT-PCR analysis of β-actin mRNA is shown as a control. (B) Lysates from 2 wild-type and 2 Bax-/-;Bak-/- cells cultured in the presence of IL-3 (0 hours) or in the absence of IL-3 for the indicated time periods were immunoblotted with an antibody to Puma. Probing with an antibody to β-actin was used as a loading control. The arrows indicate Puma and the asterisks a nonspecific band that was observed only in cell lines other than Bax-/-;Bak-/- cells. The lysate from Puma-/- cells shown aligned to the wild-type 2 and Bax-/-;Bak-/- 2 clones was run on the same gel, but intervening irrelevant bands have been removed.
Discussion
IL-3-dependent cell lines from gene-deleted mice were generated to test the requirement for several BH3-only proapoptotic Bcl-2 family members in the regulation of cell survival and death by IL-3. Cells lacking both Bax and Bak were highly resistant to cell death caused by removal of IL-3, indicating that these multi-BH domain proapoptotic Bcl-2 family members are required for cell death following cytokine removal. Because BH3-only proteins are known to be essential for apoptosis initiation upstream of Bax and Bak,40 we reasoned that after ligand is removed from the IL-3 receptors, 1 or more BH3-only proteins are triggered. We first tested the requirement for Bad because many reports have implicated it as a key mediator of cytokine withdrawal-induced death.11,14 Although there were fewer annexin V/PI+ cells in Bad-/-cell lines following IL-3 withdrawal compared with wild-type cells, we found no absolute requirement for Bad in death of IL-3-deprived myeloid cells. Further, when Bad-/- cells were tested for their ability to proliferate on readdition of IL-3 following periods of cytokine deprivation, they showed no more enhanced ability to form colonies than control wild-type cells. This suggests that although Bad may contribute to the speed with which apoptotic morphology appears, it is not required for these cells to commit to death.
Deletion of Bim, another BH3-only protein implicated in regulation of cell death and survival by cytokines,12,16,41 also had little effect, even when deleted together with Bad. Contrary to expectation, Bim-/- cells appeared significantly more sensitive to IL-3 withdrawal than wild-type cells. The most likely explanation for this observation is clonal variation. In the wild-type IL-3-dependent cells, Bim expression was below the level of detection either in the presence or absence of IL-3. Hrk-/- cells also appeared more sensitive to IL-3 withdrawal-induced apoptosis than wild-type cells; however, this difference was not statistically significant.
The antibodies used to detect phosphorylated forms of Bad readily detect overexpressed protein, but gave weaker signals when detecting endogenous protein, which limited the interpretation of alterations in levels of phosphorylated Bad. Although we could observe modest reductions in the levels of phosphorylated Bad in IL-3-deprived and Ly294002-treated cells compared to untreated cells, these reductions appeared to correspond to changes in the levels of total Bad. Phosphorylation of Bad did not appear to be dynamically controlled, and any changes in Bad phosphorylation appear unlikely to be important in the regulation of cell survival because deleting Bad completely had no effect.
Although Puma was initially identified as a key mediator of cell death activated by p53, we found it also played a significant role in causing cell death when IL-3 was removed from factor-dependent myeloid cell lines. Further, Puma mRNA levels and protein expression increased following IL-3 withdrawal. Deletion of Puma did not protect the cells to the same extent as combined loss of Bax and Bak, suggesting that other BH3-only proteins also act in this pathway. Although it is known that in apoptosis following DNA damage p53 directly promotes transcription of Puma,8,19,20,42 we do not yet know what regulates Puma expression when IL-3 is removed from factor-dependent cells. However, it appears unlikely that the PI3 kinase pathway plays an important role because removal of IL-3 was still able to increase cell death when PI3 kinase was inhibited by Ly294002 or wortmannin.
Our data challenge the widely held model for the major pathway by which removal of IL-3 leads to apoptosis. First, we show that Bad is not the BH3-only protein responsible for inducing cell death following withdrawal of IL-3, consistent with reports showing that apoptosis induced by cytokine withdrawal did not correlate with the phosphorylation status of Bad.43 Our results are also consistent with the lack of a substantial phenotype in Bad knock-out mice, because most cell types derived from them showed no significant resistance to a range of death stimuli, including growth factor withdrawal.22
Second, our results challenge the belief that IL-3 receptors signal survival via activation of PI3 kinase. Inhibition of PI3 kinase prevented proliferation of Bax-/-;Bak-/- cells and blocked AKT phosphorylation induced by IL-3, consistent with observations by Craddock et al.44 We also found that Ly294002 could promote apoptosis in the absence of Bad, Bim, or Puma, and in the presence or absence of IL-3. This suggests IL-3 receptor survival signaling and PI3 kinase survival signals are separable, that neither depend on Bad or Bim, and that PI3 kinase is primarily involved in IL-3-induced cell proliferation.
Third, although we could not demonstrate a role for Bad in apoptosis induced by IL-3 withdrawal, we found that Puma plays an important role in this pathway, as it does in p53-dependent and -independent pathways in other cell types.18-20
Although a similar, slightly reduced, percentage of Puma-/- cells and Bad-/-;Bim-/- cells stained with annexin V following IL-3 withdrawal, and comparable amounts of Bax translocated from cytosolic to membrane fractions, only the Puma-/- cells retained the capacity to form clones when IL-3 was restored. The enhanced clonogenic survival of Puma-/- cells was associated with persistent binding of Bcl-xL to Bak, but not Bax, consistent with published results.24 This suggests that one key function of Puma in IL-3 withdrawal-induced apoptosis is to displace Bak from Bcl-xL, thereby allowing Bak to activate the death effector machinery. Because the clonogenic survival advantage conferred by loss of Puma was similar to the effect of overexpressing Bcl-2 in wild-type IL-3-dependent cells,21 it is likely that deletion of Puma will cooperate with oncogenes to promote transformation, as does overexpression of Bcl-2.3,45-47 As a key mediator of cell death caused by p53, as well as a major determinant of cell death regulated by cytokines, Puma is likely to be a potent tumor suppressor gene.
Prepublished online as Blood First Edition Paper, May 16, 2006; DOI 10.1182/blood-2006-03-014209.
Supported by National Health and Medical Research Council (NHMRC) Project Grant 384404 (P.G.E.), NHMRC Program Grant 257502, Australian Research Council Discovery Grant DP0343431, and the Juvenile Diabetes Research Foundation (JDRF)/NHMRC and the Leukemia and Lymphoma Society. P.G.E. is supported by an NHMRC Career Development Grant, and D.L.V. by an Australian Research Council (ARC) Federation Foundation Fellowship. J.S. is supported by an NHMRC Career Development Grant. C.B. is supported by a grant from the Deutsche Forschungsgesellschaft (DFG; BO-1933), and C. B. and T. Y. are supported by José Carreras Leukaemia Foundation grant DJCLS R 06/09.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Drs D. Huang, J. Adams, S. Cory, P. Bouillet, L. O'Reilly, H. Puthalakath, and S. Willis for reagents, discussions, and advice.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal