The recent literature shows that interest in ocular adnexal lymphomas and their biologic and clinical characteristics—along with their possible association with Chlamydia psittaci infection and therapeutic management with rituximab or anti-Chlamydia psittaci antibiotic therapy—is considerable. These new data have modified the previously reported features of this disease and have made an updated review of the literature necessary. The aims of this review are to present the current knowledge on the biology of these lymphomas, their clinical features and prognostic factors, and the panel of all available treatment options.
Introduction
Lymphoproliferations of the eye, including intraocular and ocular adnexal non-Hodgkin lymphoma (NHL), constitute a heterogeneous group of neoplasms that represent less than 1% of all NHLs1 and 5% to 15% of all extranodal sites.2 First described in 1952,3 they are the most frequent malignant tumors of the eye and ocular adnexae; in fact, in the Florida cancer registry, they represent up to 55% of all orbital tumors.4 As for all NHLs, a marked increase of the incidence of ocular adnexal NHL has also been observed.4 The clinical features and natural history of ocular adnexal lymphoma (OAL) is completely different from those of intraocular lymphoma, which emphasizes the need to distinguish between the therapeutic management of both. Intraocular lymphoma is a subset of primary central nervous system lymphoma,5-7 and intraocular involvement is found in approximately 15% of affected patients.8 The histologic subtype of intraocular lymphoma is almost always high grade9,10 and it can occur in the context of AIDS-related lymphoproliferations. In contrast, OALs—including lesions of the conjunctiva, lacrimal gland, orbit, and eyelids—are mainly low-grade tumors not associated with HIV infection.11 Although combination chemotherapy with high-dose methotrexate is the basis of treatment of primary central nervous system and intraocular lymphomas,10,12 radiotherapy has been the standard treatment for low-grade ophthalmologic lymphomas.13-30 However, other treatment options, such as therapy with monoclonal anti-CD20 antibody,31,32 may constitute a promising alternative to external beam irradiation and its potential toxicity. In addition, some reports have suggested a possible value of anti-Chlamydia psittaci antibiotic therapy.33,34 In this review, which is exclusively focused on ophthalmologic lymphomas, we first present the pathologic, clinical, and prognostic factors of these lymphomas and then discuss all available treatment options in terms of safety and efficacy and their respective indications.
Biology of ophthalmologic lymphomas
Diagnosis of OAL
As for all other forms of nodal and extranodal NHL, confirming a positive diagnosis of OAL is often long and difficult because the tumor is very small in most patients. Positive diagnosis must be based on histologic examination of a sufficient tumor sample obtained by surgical biopsy that is large enough to allow rigorous definition of lymphoproliferative disease according to the World Health Organization (WHO) classification.35 Histopathologic examination consists of morphologic examination of cellular proliferation (cellular appearance and architectural pattern), usually combined with immunohistochemical analysis and molecular analysis.
The exact modalities of biopsy depend on the anatomic site of the lesion and its relation to the eye: biopsy may be performed of the conjunctiva, an eyelid lesion, an orbital mass, or the lacrimal gland. However, sometimes patients have nodal involvement, and surgical biopsy of the node facilitates the diagnosis of malignant lymphoma.
Histopathologic characteristics of OAL
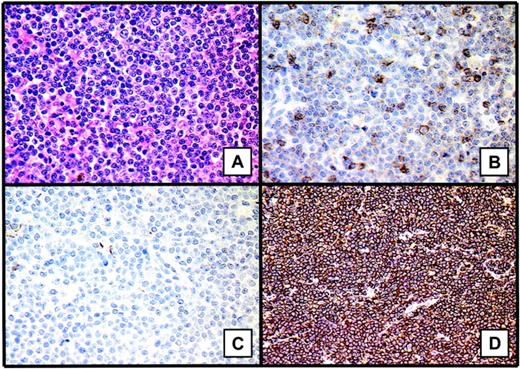
Percentages of the various histopathologic subtypes of lymphoma vary considerably in published series, probably because of the different selection criteria, such as histopathologic subtype and clinical stage.11,13,18,22,23,27,28,36-47 However, a large majority of reported OALs—between 95% and 100% of reported cases— correspond to the B-cell type, and 80% of B-cell lymphomas are low grade. A major characteristic of OAL is the high incidence of marginal zone B-cell lymphoma or mucosa-associated lymphoid tissue (MALT) lymphoma, reported in approximately 50% of patients. These lymphomas are defined by the presence of lymphoepithelial lesions, corresponding to glandular or epithelial structures expanded or distorted by groups of more than 3 lymphoid cells, and by the proliferation of tumor cells composed primarily of small cells. Cytologic features of MALT lymphoma cells are similar to those of marginal zone B-lymphoma cells. They may be larger than small lymphocytes, with irregular folding of the nuclei, and all appear similar. A major immunophenotypic characteristic is the presence of extensive, dense, and packed CD20+ B-cell lymphoid infiltrate. A few CD3+ T-lymphoid cells are interspersed within the B cells. Lymphoid cells are also CD5-, CD10-, Bcl6-, CD23-, and IgD- (Figure 1). A monotypic immunoglobulin can be present and is helpful for the positive diagnosis of lymphoma (frequently IgM-). The other 2 most representative histopathologic subtypes are lymphoplasmacytic and follicular lymphomas. Except for one paper,18 mantle-cell lymphomas represent a median of 5% of patients, as observed for all NHLs.48 Finally, approximately 15% of diffuse large B-cell lymphomas have been observed. The proportion of T-cell lymphoma is low, less than 15%, as reported for nodal NHL.49
Histopathologic features of marginal zone B-cell lymphoma or MALT lymphomas. These lymphomas are defined by the presence of lymphoepithelial lesions and proliferation of tumor cells composed primarily of small cells (A). One of the major immunophenotypic characteristics is the presence of extensive, dense, packed CD20+ B-cell lymphoid infiltrate (D). A few CD3+ T lymphoid cells are interspersed within the B cells (B). The lymphoid cells are also CD10- (C). Images were captured with a Leitz DM-RB microscope equipped with a 40x/0.70 NA objective lens and a JVC 3-CCD camera (KY-F50; Claravision, Massy, France). Images were processed with Claravision software and Adobe Photoshop (Adobe Systems, San Jose, CA). Images in panel A were stained with hematein-eosin-safran staining; those in panel B, with anti-CD3 immunostaining (polyclonal antibody A0452; Dakocytomation, Glostrup, Denmark); those in panel C, with anti-CD10 immunostaining (monoclonal antibody 56C6, NCLCD10; Novocastra, Newcastle upon Tyne, United Kingdom); and those in panel D, with anti0CD20 immunostaining (monoclonal antibody L26, Dakocytomation).
Histopathologic features of marginal zone B-cell lymphoma or MALT lymphomas. These lymphomas are defined by the presence of lymphoepithelial lesions and proliferation of tumor cells composed primarily of small cells (A). One of the major immunophenotypic characteristics is the presence of extensive, dense, packed CD20+ B-cell lymphoid infiltrate (D). A few CD3+ T lymphoid cells are interspersed within the B cells (B). The lymphoid cells are also CD10- (C). Images were captured with a Leitz DM-RB microscope equipped with a 40x/0.70 NA objective lens and a JVC 3-CCD camera (KY-F50; Claravision, Massy, France). Images were processed with Claravision software and Adobe Photoshop (Adobe Systems, San Jose, CA). Images in panel A were stained with hematein-eosin-safran staining; those in panel B, with anti-CD3 immunostaining (polyclonal antibody A0452; Dakocytomation, Glostrup, Denmark); those in panel C, with anti-CD10 immunostaining (monoclonal antibody 56C6, NCLCD10; Novocastra, Newcastle upon Tyne, United Kingdom); and those in panel D, with anti0CD20 immunostaining (monoclonal antibody L26, Dakocytomation).
Molecular and cytogenetic abnormalities of OAL
Few series have focused exclusively on ocular adnexal lymphomas, but a large volume of data have been published on MALT lymphomas, which constitute the leading histopathologic subtype of OAL, including all extranodal sites, particularly ocular adnexal sites. Tumor cell clonality has been well demonstrated in most reports, with IgH rearrangement in more than 80% of patients.38,50,51 In a series of 16 Taiwanese patients with ocular adnexal MALT lymphoma, 13 (81%) patients had rearranged IgH genes, whereas no rearrangement in Bcl-1, Bcl-2, c-MYC, or p53 genes was found, though point mutation of the p53 gene was observed in one patient.51 Similarly, no point mutation was found in Bcl-6 or Bcl-10 genes in biopsy samples of 11 patients with ocular adnexal MALT lymphoma.52
In a series of 37 patients with ocular adnexal MALT lymphoma, Streubel et al53 found trisomy 3 in 38% of patients, trisomy 18 in 13.5% of patients, t(14;18) in 24% of patients, and t(11;18) in 2.7% of patients; no patients had t(1;14)(p22;q32). The frequency of translocations varied considerably with the primary site of the lymphoma; t(14;18)(q32;q21) was most commonly found in lesions of the ocular adnexa, skin, and salivary glands, and t(11; 18)(q21;q21) was mainly detected in pulmonary and gastric tumors. Similarly, trisomy 3 and 18 were more frequently detected in ophthalmologic lymphomas than in gastric and lung lymphomas. More recently, a t(3;14)(p14.1;q32) translocation involving IgH and FOXP1 genes was identified as a novel recurrent chromosomal aberration in MALT lymphomas and was detected in 4 of 20 patients with ocular adnexal lymphoma.54 Two studies on orbital lymphoma using a comparative genomic hybridization technique have been reported: the first study showed 19 gains and 5 losses among the 10 MALT lymphomas (60% of patients), and the most frequent chromosome imbalances involved chromosomes 3 and 6.55 The second study found chromosomal imbalances in 8 (44%) of 18 patients with orbital lymphoma, mostly involving chromosomes 3 and 6.56
Lymphomagenesis of ophthalmologic MALT lymphomas
Although not all ophthalmologic lymphomas are MALT type, this section is devoted to this subset of ocular adnexal lymphoma. As previously mentioned, various cytogenetic abnormalities have been observed in MALT lymphomas, such as t(11;18)(q21;q21), t(14; 18)(q32;q21), t(1;14)(p22;q32), t(3;14)(p14.1;q32), and trisomy 3 and 18.53,57,58 The most frequent translocation, t(11;18)(q21;q21), observed in 15% to 40% of patients, juxtaposes the API2 (apoptosis inhibitor 2) gene on chromosome 11q21 and the MALT1 gene on chromosome 18q21.59-61 The t(14;18)(q32;q21) translocation, found in approximately 10% of patients with MALT, juxtaposes the MALT1 gene and the IgH promoter on chromosome 14, and this abnormality induces constitutive expression of the MALT1 gene.53 The t(1;14)(p22;q32) translocation, present in less than 5% of patients with MALT,53,62 induces a juxtaposition of the Bcl-10 gene on chromosome 1p22 and the IgH promoter on chromosome 14, and this rearrangement is responsible for constitutive expression of the Bcl-10 gene.63 More recently, the t(3;14)(p14.1;q32) translocation was observed to juxtapose the IgH promoter on chromosome 14 and the FOXP1 gene (a gene belonging to a transcriptional factor family) on chromosome 3p14.1.54 Finally, trisomy 3 and 18, observed in 30% to 50% and 10% to 20% of patients, respectively, increase the expression of genes located in the chromosomes.53,62 All these cytogenetic abnormalities have a common effect on the NF-κB complex.64 BCL-10 and API2-MALT1 proteins increase the activity of the IK protein kinase, resulting in the degradation of I-κB and in a nuclear translocation from the cytoplasm of the NF-κB complex.65 NF-κB, involved in the control of immunity, inflammation, and apoptosis,66,67 therefore induces the transcription of several genes responsible for lymphomatous transformation.
A second mechanism of lymphomagenesis of MALT lymphomas has been reported, namely direct inhibition of the cell death process through API2, a member of the inhibitor apoptosis protein (IAP) family, through BCL-10 protein,68 through the induction of Bcl-XL expression by Helicobacter pylori in murine models69 and through escape from the regulation control of Fas/CD95/APO-1 protein by genetic mutation or protein inactivation.70 This hypothesis is based on the absence of T-cytotoxic lymphocyte regulation on H pylori-induced B-cell proliferation.71
This last observation emphasizes the role of bacterial infection in this particular subtype of lymphoma. As previously reported for gastric MALT lymphomas and H pylori infection,72 various authors have investigated a possible association between ophthalmologic lymphomas and infectious agents. Two cases of Toxoplasma gondii DNA detection in primary intraocular B-cell lymphoma have been reported.73 More recently, Ferreri et al33 demonstrated an association between C psittaci and ocular adnexal lymphoma. The presence of C psittaci DNA has been found in 80% of lymphoma samples compared with 12% of reactive lymphadenopathy samples and 0% of nonneoplastic orbital biopsy samples. Moreover, bacterial DNA was found in 43% of peripheral blood mononuclear cells (PBMCs) of patients with NHL but not in healthy donors. However, the genetic alterations induced by C psittaci and the place of this bacterial agent in ophthalmologic lymphomagenesis as a first determinant event of tumor transformation or as a second favorable circumstance promoting tumor proliferation remain to be identified. Moreover, in our series of French patients,74 we did not observe any association between C psittaci and ocular adnexal lymphomas. Additional studies are warranted to elucidate the role of C psittaci in this disease and to identify other microbial agents that could be involved, such as H pylori75 and hepatitis C virus.76
Clinical characteristics and prognostic factors of OAL
Clinical characteristics at initial diagnosis
OAL is frequently responsible for symptoms, though only minor, that can delay specialist consultation. In a large singe-center series, we showed that the presenting symptoms were ophthalmologic in 91% of patients namely, pink conjunctival mass or conjunctival hyperemia in 32% of patients, exophthalmia in 27% of patients, orbital or palpebral mass in 19% of patients, decreased visual acuity and ptosis in 6% of patients, and diplopia in 2% of patients.11 The median interval between onset of the first symptoms and date of diagnosis was 4 months (range, 1 month-10 years). OAL patients may rarely have a history of autoimmune disease, such as thyrotoxicosis77 or Sjögren syndrome,78,79 but pseudolymphoma80,81 and lacrimal gland enlargement82 have occasionally been observed in patients with concomitant autoimmune disease.
A large number of published series have described the clinical features of OAL.11,13,18,23,27,28,30,36,37,39,41-45,83-85 The male-female sex ratio is often less than 1,11,23,27,28,30,36,37,41-45,83,85 which distinguishes ophthalmologic lymphoma from the overall population of NHL, in which the male-female sex ratio is inversed86 but is concordant with the sex ratio reported in MALT lymphoma, the most frequent histologic entity of all ocular NHLs.29,87,88 In all published series except that by Cho et al,42 the median age was approximately 65 years.11,13,18,23,27,28,30,36,37,39,41,43-45,83-85 Few patients have B symptoms or a performance status (PS) greater than 1, though these findings are rarely reported in published series.11,18,28,30,42,84
Lacrimal gland and intraorbital sites, observed in approximately 50% of patients, are the most frequent ophthalmologic sites.11,18,30,37,41,45,83,85 Conjunctival sites are observed in one third of patients and almost always consist of low-grade NHL (96% of patients).89 Eyelid involvement is observed in 0% to 44% of patients in the various published series, with a mean of approximately 10%. This marked heterogeneity may be attributed to different patient selection criteria, such as histologic subtype of lymphoma or stage of disease. Finally, bilateral OAL involvement is observed in 7% to 24% of patients.11,18,23,27,28,30,36,37,39,42-45,83-85
The clinical stage of OAL varies considerably according to published series and includes patients with initial or secondary OAL. This variability can be explained by differences in patient selection criteria because some series only include localized ophthalmologic disease. Few series report any information about the presence of nodal involvement at diagnosis, which ranges between 0% and 24%.11,13,27,30,36,44 Stage IV disease, observed in at least 15% of selected patients, is reported in most series11,13,30,36,37,42,43,45,83,85 and includes bone marrow involvement in approximately 5% to 10% of all studied patients.11,13,36,40,42 Diffuse lymphomatous disease is mainly found in patients with high-grade NHL. In our large series, we observed stage IV disease in 26% and 56% of patients with low-grade and high-grade lymphoma, respectively (P < .01).11 Similarly, as observed for the overall population of patients with NHL, we found elevated serum levels of LDH in 40% of patients with high-grade lymphoma and in 15% of patients with low-grade lymphoma (P < .01).11 Finally, only 2 series have assessed the International Prognostic Index (IPI) score defined for high-grade NHL.11,39,90 Both publications reported a majority of patients in the low-risk and the low-to-intermediate risk groups. Moreover, we found IPI scores of 0 to 1 in 74% and 42% of patients with low-grade and high-grade NHL, respectively, and IPI scores greater than 2 in 5% and 37% of these 2 subgroups of patients. This finding emphasizes that, like nodal NHL, high-grade NHL with ophthalmologic involvement at diagnosis is characterized by a more aggressive pattern based on initial clinical and laboratory features.
All these findings emphasize the importance of an exhaustive staging work-up at diagnosis for patients with OAL, including at least ophthalmologic and clinical examination, with determinations of PS, B symptoms, nodal or extranodal peripheral sites, blood count, erythrocyte sedimentation rate (ESR), liver and kidney function tests, serum protein electrophoresis, serum lactate dehydrogenase (LDH) and β2-microglobulin, chest x-ray, computed tomography (CT), and bone marrow biopsy. This initial evaluation therefore requires multidisciplinary consultations, namely ophthalmologic and hematologic overviews.
Clinical outcome and prognostic factors
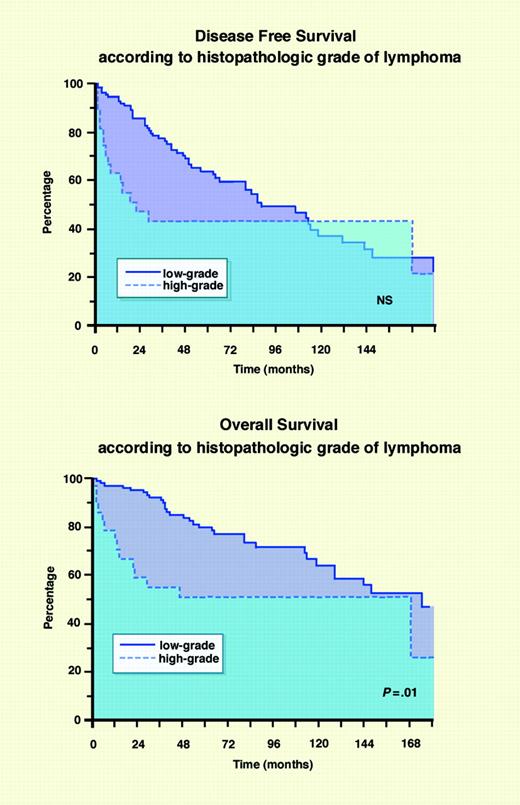
According to the literature, the global outcome of OAL is favorable, and the 5-year overall survival rate ranges between 50% and 94% (Table 1). Similarly, 5-year cause-specific survival, 5-year progression-free survival, and 5-year disease-free survival rates range between 33% and 100% (Table 1). However, these results are significantly influenced by patient selection criteria, particularly the variable proportion of low-grade or localized lymphomas, because few series have reported data exclusively concerning stage I MALT lymphomas.43 In our experience (Figure 2),11 and in accordance with other reports,11,13,36,40,42 the survival of patients with low-grade OAL is significantly better than that of patients with high-grade OAL. The outcome of lymphoma patients with OAL involvement at diagnosis does not differ from that observed for patients with nodal NHL, namely better survival in the low-grade group without stabilization of the survival curves and an OS rate of approximately 50% for the high-grade group. This clearly illustrates the natural history of these lymphomas and the absence of any real prognostic impact of initial OAL involvement.
Clinical outcomes of patients with OAL
Reference, year . | No. of patients . | Low grade, % . | Stage I, % . | DFS/PFS, % . | OS, % . |
|---|---|---|---|---|---|
| Bessell et al,13 1988 | 115 | 84 | 11 | ND | OS5-LG, 80; OS5-HG, 80 |
| White et al,36 1995 | 32 | 100 | 87 | ND | ND |
| Smitt and Donaldson,16 1993 | 25 | 76 | 80 | PFS5, 70 | OS5, 93 |
| Esik et al,18 1996 | 37 | 92 | 95 | CSS5-I, 100 | ND |
| Restrepo et al,37 1998 | 71 | 90 | 61 | ND | ND |
| Bolek et al,20 1999 | 38 | 66 | 53 | CSS5-LG, 89; CSS5-HG, 33 | ND |
| Nakata et al,39 1999 | 57 | 82 | 11 | CSS5, 90 | ND |
| Jenkins et al,40 2000 | 192 | 76 | 64 | CSS5-MALT, 88; CSS5-HG, 52 | ND |
| Auw-Haedrich et al,41 2001 | 46 | 91 | 96 | ND | ND |
| Sasai et al,46 2001 | 32 | 84 | 81 | CSS3-LG, 100; CSS3-HG, 40 | OS3-LG, 90; OS3-HG, 50 |
| Stafford et al,22 2001 | 48 | 94 | 17 | PFS5, 88† | OS5, 69 |
| Bhatia et al,23 2002 | 47 | 70 | 100 | PFS5, 65 | OS5, 74 |
| Cho et al,42 2003 | 68 | 90 | 87 | ND | ND |
| Fung et al,43 2003 | 98 | 81 | 81 | DFS5-MALT-I, 100 | OS5-MALT-1, 94 |
| Martinet et al,27 2003 | 90 | 87 | 91 | PFS5, 87 | OS5, 78 |
| Uno et al,28 2003 | 50 | 100 | 86 | ND | OS5, 91 |
| Zucca et al,88 2003* | 31 | 100 | 87 | CSS5, 87-100 | OS5, 80-100 |
| Coupland et al,10 2004 | 230 | 80 | 83 | ND | ND |
| Lee et al,47 2005 | 37 | 100 | 74 | EFS3, 100 | OS3, 100 |
| Meunier et al,11 2004 | 145 | 82 | 68 | DFS5, 64 | OS5, 79; OS5-LG, 78; OS5-HG, 50 |
| Charlotte et al,84 2006 | 23 | 100 | 78 | DFS3, 85 | OS3, 94 |
| Rosado et al,30 2006 | 62 | 97 | 85 | FFP5, 79 | OS5, 96 |
Reference, year . | No. of patients . | Low grade, % . | Stage I, % . | DFS/PFS, % . | OS, % . |
|---|---|---|---|---|---|
| Bessell et al,13 1988 | 115 | 84 | 11 | ND | OS5-LG, 80; OS5-HG, 80 |
| White et al,36 1995 | 32 | 100 | 87 | ND | ND |
| Smitt and Donaldson,16 1993 | 25 | 76 | 80 | PFS5, 70 | OS5, 93 |
| Esik et al,18 1996 | 37 | 92 | 95 | CSS5-I, 100 | ND |
| Restrepo et al,37 1998 | 71 | 90 | 61 | ND | ND |
| Bolek et al,20 1999 | 38 | 66 | 53 | CSS5-LG, 89; CSS5-HG, 33 | ND |
| Nakata et al,39 1999 | 57 | 82 | 11 | CSS5, 90 | ND |
| Jenkins et al,40 2000 | 192 | 76 | 64 | CSS5-MALT, 88; CSS5-HG, 52 | ND |
| Auw-Haedrich et al,41 2001 | 46 | 91 | 96 | ND | ND |
| Sasai et al,46 2001 | 32 | 84 | 81 | CSS3-LG, 100; CSS3-HG, 40 | OS3-LG, 90; OS3-HG, 50 |
| Stafford et al,22 2001 | 48 | 94 | 17 | PFS5, 88† | OS5, 69 |
| Bhatia et al,23 2002 | 47 | 70 | 100 | PFS5, 65 | OS5, 74 |
| Cho et al,42 2003 | 68 | 90 | 87 | ND | ND |
| Fung et al,43 2003 | 98 | 81 | 81 | DFS5-MALT-I, 100 | OS5-MALT-1, 94 |
| Martinet et al,27 2003 | 90 | 87 | 91 | PFS5, 87 | OS5, 78 |
| Uno et al,28 2003 | 50 | 100 | 86 | ND | OS5, 91 |
| Zucca et al,88 2003* | 31 | 100 | 87 | CSS5, 87-100 | OS5, 80-100 |
| Coupland et al,10 2004 | 230 | 80 | 83 | ND | ND |
| Lee et al,47 2005 | 37 | 100 | 74 | EFS3, 100 | OS3, 100 |
| Meunier et al,11 2004 | 145 | 82 | 68 | DFS5, 64 | OS5, 79; OS5-LG, 78; OS5-HG, 50 |
| Charlotte et al,84 2006 | 23 | 100 | 78 | DFS3, 85 | OS3, 94 |
| Rosado et al,30 2006 | 62 | 97 | 85 | FFP5, 79 | OS5, 96 |
HG indicates high-grade lymphoma; PFSN, N-year progression-free survival; CSSN, N-year cause-specific survival; CSSN-1, N-year cause-specific survival for stage I patients; EFSN, N-year event-free survival; DFSN, N-year disease-free survival; OSN, N-year overall survival; LR, local recurrence; FFPN, N-year failure-free progression; and ND, not done
Data were collected for orbital and conjunctival sites
Five-year PFS for patients with clinical stage I or II disease
Although a number of series have evaluated prognostic factors by univariate analysis, few reports have conducted multivariate Cox proportional hazards model of the characteristics influencing cause-specific survival (CSS), disease-free survival, and overall survival, as shown in Table 2. Various pejorative prognostic factors have been identified in the published studies, such as high-grade subtype lymphomas,11,13,18,20,27,40,41,45,46 and non-MALT lymphomas.39,42 In particular, MALT lymphoma was found not to have any prognostic impact11,29,44 except in one series.18 In many series, advanced-stage disease11,13,18,20,27,40,41,45,46 and age greater than 60 to 64 years,11,18,27,45 the presence of B symptoms (though rarely reported),11 nodal involvement,11,88 nonconjunctival sites,87 and elevated serum LDH level have been identified as factors.11,27,39,88 Finally, a few reports have shown a pejorative prognostic impact of p53 expression41,45 and of p21 and pRb positivity.45
Factors indicating negative OAL prognosis
Reference, year . | No. of patients . | Histoligic subtype of lymphoma . | Univariate analysis . | CSS/DFS Cox proportional hazards model . | OS, Cox proportional hazards model . |
|---|---|---|---|---|---|
| Bessell et al,13 1988 | 115 | Ocular adnexa | High grade | – | – |
| White et al,36 1995 | 42 | Ocular adnexa | Secondary NHL | – | – |
| Esik et al,18 1996 | 37 | Orbital | Older age; advanced stage; high grade | – | – |
| Bolek et al,20 1999 | 38 | Orbital | High grade | – | – |
| Nakata et al,39 1999 | 57 | Ocular adnexa | Non-MALT type; LDH > N; advanced stage | CSS; LDH > N; Non-MALT type | – |
| Jenkins et al,40 2000 | 192 | Ocular adnexa | High grade | – | – |
| Auw-Haedrich et al,41 2001 | 46 | Ocular adnexa | Advanced stage; high grade; p53 expression | – | – |
| Sasai et al,46 2001 | 41 | Ocular adnexa | High grade | – | – |
| Stafford et al,22 2001 | 55 | Orbital | Advanced stage | – | – |
| Cho et al,42 2003 | 68 | Ocular adnexa | Non-MALT type | – | – |
| Fung et al,43 2003 | 98 | Ocular adnexa | Advanced stage | – | – |
| Martinet et al,27 2003 | 90 | Orbital | Age > 64; high grade; ESR > N; muscle infiltration; nonconjunctival site; LDH > N | DFS; age > 64; high grade; ESR > N; nonconjunctival site; LDH > N | Age > 64; high grade |
| Zucca et al,88 2003 | 31 | MALT type | Advanced stage; LDH > N; extranodal site > 1; nodal involvement; IPI score > 2 | CSS; nodal involvement; PFS; IPI score > 2 | Advanced stage |
| Coupland et al,10 2004 | 230 | Ocular adnexa | Age > 60; high grade; advanced stage; p21, p53, and pRB positivity | CSS; age > 60; high grade; advanced stage | – |
| Meunier et al,11 2004 | 145 | Ocular adnexa | High grade; age > 60*; nodal involvement,*; LDH > N*; IPI score > 2* | DFS; age > 60; advanced stage; LDH > N | High grade; B symptoms; age > 60; advanced stage; LDH > N |
| Rosado et al,30 2006 | 62 | Ocular adnexa | Advanced stage | – | – |
Reference, year . | No. of patients . | Histoligic subtype of lymphoma . | Univariate analysis . | CSS/DFS Cox proportional hazards model . | OS, Cox proportional hazards model . |
|---|---|---|---|---|---|
| Bessell et al,13 1988 | 115 | Ocular adnexa | High grade | – | – |
| White et al,36 1995 | 42 | Ocular adnexa | Secondary NHL | – | – |
| Esik et al,18 1996 | 37 | Orbital | Older age; advanced stage; high grade | – | – |
| Bolek et al,20 1999 | 38 | Orbital | High grade | – | – |
| Nakata et al,39 1999 | 57 | Ocular adnexa | Non-MALT type; LDH > N; advanced stage | CSS; LDH > N; Non-MALT type | – |
| Jenkins et al,40 2000 | 192 | Ocular adnexa | High grade | – | – |
| Auw-Haedrich et al,41 2001 | 46 | Ocular adnexa | Advanced stage; high grade; p53 expression | – | – |
| Sasai et al,46 2001 | 41 | Ocular adnexa | High grade | – | – |
| Stafford et al,22 2001 | 55 | Orbital | Advanced stage | – | – |
| Cho et al,42 2003 | 68 | Ocular adnexa | Non-MALT type | – | – |
| Fung et al,43 2003 | 98 | Ocular adnexa | Advanced stage | – | – |
| Martinet et al,27 2003 | 90 | Orbital | Age > 64; high grade; ESR > N; muscle infiltration; nonconjunctival site; LDH > N | DFS; age > 64; high grade; ESR > N; nonconjunctival site; LDH > N | Age > 64; high grade |
| Zucca et al,88 2003 | 31 | MALT type | Advanced stage; LDH > N; extranodal site > 1; nodal involvement; IPI score > 2 | CSS; nodal involvement; PFS; IPI score > 2 | Advanced stage |
| Coupland et al,10 2004 | 230 | Ocular adnexa | Age > 60; high grade; advanced stage; p21, p53, and pRB positivity | CSS; age > 60; high grade; advanced stage | – |
| Meunier et al,11 2004 | 145 | Ocular adnexa | High grade; age > 60*; nodal involvement,*; LDH > N*; IPI score > 2* | DFS; age > 60; advanced stage; LDH > N | High grade; B symptoms; age > 60; advanced stage; LDH > N |
| Rosado et al,30 2006 | 62 | Ocular adnexa | Advanced stage | – | – |
Cox proportional hazards model was used for multivariate analysis
ESR, erythrocyte sedimentation rate; CSS, cause-specific survival
Univariate analysis was performed on patients with low-grade lymphoma
Treatment of OAL
Introduction
The description of the natural history of OAL and the prognostic factors for disease-free survival and overall survival indicate that the management of these lymphomas should comply with the same modalities proposed for nodal lymphoma, with one additional characteristic that must be considered in the final treatment decision—the functional impact of lymphoma on the eye. This assessment, as well as diagnostic biopsy, should be performed by an ophthalmologist, and it illustrates the importance of a multidisciplinary approach to OAL by a team composed of hematologists, radiotherapists, and ophthalmologists. Several major criteria must be considered in the initial assessment of the disease to clearly define optimal treatment: (1) the histopathologic subtype of lymphoma, according to the WHO classification35 ; (2) extension of the disease, inside and beyond the periocular region; (3) prognostic factors related to the patient and to the disease; and (4) the impact of the OAL on the eye(s) and visual function.
Ophthalmologic assessment must evaluate the impact of the tumor on visual function. Examination consists of complete bilateral ophthalmologic examination, including visual acuity, description of functional symptoms, diplopia, visual blurring, evaluation of eye movements, slit lamp examination with anterior chamber examination, determination of intraocular pressure, examination of the conjunctiva, and examination of ocular fundus, to detect any extraocular compression by the tumor.
Various conventional treatment modalities can be proposed for OAL, including single-agent or combination chemotherapy regimens, radiotherapy, and monoclonal anti-CD20 antibody or interferon immunotherapy. More recently, several other options have been proposed, such as anti-C psittaci antibiotic therapy and a wait-and-see policy.
Radiotherapy and OAL
Radiotherapy is clearly the ocular adnexal lymphoma treatment for which immediate and long-term efficacy and toxicity have been most extensively reported, as shown in Table 3. However, in a few series, no distinction was made between low-grade and high-grade lymphoma or between radiotherapy alone and combined radiotherapy/chemotherapy, making it difficult to interpret the final results. Regardless of the histologic subtype of lymphoma, MALT lymphoma,24,28,29 and low-grade or high-grade lymphoma,13-23,25-27,30 radiotherapy induces a very high rate of control of ophthalmologic sites, with local control rates ranging from 86% to 100%, and a local recurrence rate ranging between 0% and 15%. However, these apparently very good results must be interpreted in light of 2 observations. First, radiotherapy is associated with a toxicity consisting primarily of moderate immediately cutaneous or conjunctival reactions and late complications such as constant cataract, or xerophthalmia, and rare ischemic retinopathy, glaucoma, or xerophthalmia-induced corneal ulceration.15,17,19,20,22,23,25-28,30 Second, disseminated recurrences after radiotherapy have been reported in 6% to 50% of patients, with a mean disseminated recurrence rate of 17%.13,15-17,20,23,24-28,30 These findings underscore the fact that radiotherapy of OAL sites induces a high local control rate, with a disseminated recurrence rate of 10% to 25% and mild but frequent ophthalmic toxicity that should be considered before any treatment decision is made.
Radiotherapy for patients with OAL
Reference, year . | No. of patients . | Histology, % low grade . | Local control, % . | Local relapse, % . | Disseminated relapse, % . |
|---|---|---|---|---|---|
| Bessell et al,13 1988 | 112 | NHL, 84 | 100 | 0 | 15 |
| Vitu et al,14 1990 | 14 | NHL, 64 | 86 | 7 | – |
| Letschert et al,15 1991 | 30 | NHL, – | 94 | 0 | 20 |
| Smitt and Donaldson,16 1993 | 25 | NHL, 88 | 100 | 8 | 20 |
| Chao et al,17 1995 | 20 | NHL, 45 | 100 | 0 | 11 |
| Esik et al,18 1996 | 17 | NHL, 92 | 100 | 0 | – |
| Erkal et al,19 1997 | 14 | NHL, 57 | 100 | 14 | – |
| Bolek et al,20 1999 | 38 | NHL, 66 | 100 | 3 | 50 |
| Lau et al,21 1998 | 20 | NHL, – | 100 | 15 | – |
| Stafford et al,22 2001 | 48 | NHL, 94 | 98 | 2 | – |
| Bhatia et al,23 2002 | 52 | NHL, 63 | 100 | 0 | 13 |
| Le et al,24 2002 | 31 | MALT, 100 | 100 | 0 | 16 |
| Liao et al,25 2002 | 20 | NHL, 68 | 100 | 0 | 6 |
| Hasegawa et al,26 2003 | 28 | NHL, 91 | 100 | 0 | 15 |
| Martinet et al,27 2003 | 90 | NHL, 87 | 100 | 3 | 20 |
| Uno et al,28 2003 | 50 | MALT, 100 | 92 | 6 | 6 |
| Tsang et al,29 2003 | 30 | MALT, 100 | 97 | 7 | 20 |
| Meunier et al,11 2004 | 107 | NHL, 91 | 90 | 5 | – |
| Rosado et al,30 2006* | 62 | NHL, 96 | 100 | 0 | 7 |
Reference, year . | No. of patients . | Histology, % low grade . | Local control, % . | Local relapse, % . | Disseminated relapse, % . |
|---|---|---|---|---|---|
| Bessell et al,13 1988 | 112 | NHL, 84 | 100 | 0 | 15 |
| Vitu et al,14 1990 | 14 | NHL, 64 | 86 | 7 | – |
| Letschert et al,15 1991 | 30 | NHL, – | 94 | 0 | 20 |
| Smitt and Donaldson,16 1993 | 25 | NHL, 88 | 100 | 8 | 20 |
| Chao et al,17 1995 | 20 | NHL, 45 | 100 | 0 | 11 |
| Esik et al,18 1996 | 17 | NHL, 92 | 100 | 0 | – |
| Erkal et al,19 1997 | 14 | NHL, 57 | 100 | 14 | – |
| Bolek et al,20 1999 | 38 | NHL, 66 | 100 | 3 | 50 |
| Lau et al,21 1998 | 20 | NHL, – | 100 | 15 | – |
| Stafford et al,22 2001 | 48 | NHL, 94 | 98 | 2 | – |
| Bhatia et al,23 2002 | 52 | NHL, 63 | 100 | 0 | 13 |
| Le et al,24 2002 | 31 | MALT, 100 | 100 | 0 | 16 |
| Liao et al,25 2002 | 20 | NHL, 68 | 100 | 0 | 6 |
| Hasegawa et al,26 2003 | 28 | NHL, 91 | 100 | 0 | 15 |
| Martinet et al,27 2003 | 90 | NHL, 87 | 100 | 3 | 20 |
| Uno et al,28 2003 | 50 | MALT, 100 | 92 | 6 | 6 |
| Tsang et al,29 2003 | 30 | MALT, 100 | 97 | 7 | 20 |
| Meunier et al,11 2004 | 107 | NHL, 91 | 90 | 5 | – |
| Rosado et al,30 2006* | 62 | NHL, 96 | 100 | 0 | 7 |
In this series, 20% of patients had relapses (none in the irradiated area), and 7% had disseminated disease
DFS and OS rates according to histopathologic grade of lymphoma. DFS (A) and OS (B). Adapted with permission from Meunier et al11 by Marie Dauenheimer; original, copyright © 2004 John Wiley & Sons.
DFS and OS rates according to histopathologic grade of lymphoma. DFS (A) and OS (B). Adapted with permission from Meunier et al11 by Marie Dauenheimer; original, copyright © 2004 John Wiley & Sons.
Chemotherapy regimens and OAL
In most reported series, patients were predominantly treated with radiotherapy alone, and only a small proportion was treated by radiotherapy plus chemotherapy or chemotherapy alone. Treatment consisted of single-agent chemotherapy such as chlorambucil or fludarabine for low-grade lymphoma and combined chemotherapy regimens such as cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) or CHOP-like protocols for high-grade lymphoma. In a few patients, neurologic prophylaxis was performed with intrathecal methotrexate or cytarabine. Metastatic involvement of the eye or central nervous system is rarely observed with low-grade ocular adnexal lymphoma, and central nervous system (CNS) prophylaxis with radiotherapy or chemotherapy is considered unnecessary.37 A number of studies11,13,18,16,27,30,36,39,41-47,83,91 have reported treatment with chemotherapy alone or in combination with other modalities, but few have reported the response rates obtained with these treatments.11,46,47,84,85 Lee et al47 reported 3 patients who underwent CHOP chemotherapy, 2 of whom experienced complete remission. Sasai et al46 observed no significant difference in cause-specific or overall survival rates for patients with MALT lymphoma treated with radiotherapy alone or with exclusive single-agent or combination chemotherapy.46 Finally, we reported 3 patients with low-grade lymphoma and one patient with high-grade lymphoma treated with chemotherapy alone; 2 patients with low-grade lymphoma experienced complete remission.11 Although these series showed that high-grade lymphoma should be treated by combination chemotherapy, they did not clearly define the optimal chemotherapy regimen for low-grade lymphoma. They also failed to define the response rate obtained with various treatment regimens and did not evaluate the impact of chemotherapy on specific-cause and overall survival rates.
Surgical excision and wait-and-see policy
Surgical biopsy, which is the first step in the management of OAL, as it is for other histologic subtypes of lymphoma, can consist of apparent complete excision of the tumor, particularly in the case of lacrimal gland tumors, in which encapsulated lesions can be entirely removed. In all instances in which complete surgical excision is suspected, accurate staging of disease must be performed by orbital magntic resonance imaging (MRI) or CT to detect any residual mass. Several publications have reported a total of 80 patients in whom no complementary treatment, and particularly no radiotherapy, was performed after surgical excision.11,13,18,36,37,39,42,44,45,47,91 Some of these studies indicated that local relapse occurred more frequently after simple surgical excision than after radiotherapy.18,42 However, this approach has not been fully evaluated; the outcome was clearly indicated for only 8 patients in these reports.13,36,45,46,91 Two (25%) of these 8 patients did not experience relapse, 3 (37.5%) experienced local recurrence at the initial ophthalmologic site, and 3 (37.5%) contracted disseminated extra-ophthalmologic disease. More recently, Matsuo and Yoshino92 reported a series of 8 patients with MALT lymphoma who did not undergo complementary treatment after surgical biopsy, 7 of whom experienced spontaneous tumor regression between 1 and 5 years. However, some of these patients received local corticosteroid or anti-intracellular bacterial antibiotic therapy. Finally, Tanimoto et al93 evaluated the long-term results of no initial therapy for 36 patients with localized MALT OAL. They showed that 69% of patients did not require any treatment (median follow-up, 7.1 years) and that 6% died of progressive lymphoma. These data indicate that further investigations are required to clearly evaluate a wait-and-see policy after complete surgical excision of OAL.
Immunotherapy of OAL
Immunotherapy of OAL includes IFN-α and rituximab, but few data have been published concerning these 2 modalities. Blasi et al94 reported 5 patients with conjunctival MALT lymphoma treated with 1 500 000 IU IFN-α injected subconjunctivally inside the lesion 3 times a week for 4 weeks. Complete response was obtained in all patients. Four patients had no signs of local recurrence (median follow-up, 21 months; range, 12-36 months), and one patient experienced recurrence after 11 months and systemic progression of the lymphoma. Similarly, few data have been reported on patients with OAL treated with rituximab. Rituximab is a monoclonal chimeric anti-CD20 antibody95 that has been extensively used in the treatment of B-cell NHL, alone or in combination with chemotherapy. Various effector mechanisms for rituximab have been reported: complement-dependent cytolysis,96 antibody-dependent cell-mediated cytotoxicity,96 mAb-triggered induction of B-cell apoptosis,97,98 inhibition of cell proliferation,99 synergistic effect with cytotoxic agents,99,100 and interferon-α.101 Rituximab with combination chemotherapy induces a significant benefit compared with chemotherapy alone in terms of response rates, progression-free survival, and overall survival in patients with follicular102-105 and diffuse large B-cell lymphomas.106-108 Rituximab induces overall and complete response rates in 70% and 42% of patients with relapsed MALT lymphoma, respectively.109,110 Eleven patients with ocular adnexal MALT lymphoma received anti-CD20 antibody therapy; 5 had complete and 3 had partial remissions (73%).30,84,111-113 In light of these preliminary data, the place of rituximab in the treatment of ophthalmologic lymphomas must be more extensively evaluated.
Anti-C psittaci antibiotic therapy
A new therapeutic approach to OAL, particularly conjunctival NHL—anti-C psittaci antibiotic therapy—has recently been proposed. As already mentioned in this review, Ferreri et al33 demonstrated an association between C psittaci and ocular adnexal lymphomas. Nine patients with C psittaci-positive marginal zone B-cell lymphoma of the ocular adnexa were treated with C psittaci-eradicating antibiotic therapy (doxycycline).34 Objective responses were observed in 4 patients with 2 complete remissions, and chlamydial DNA was no longer detectable in PBMCs of all 4 patients with positive findings. These findings were corroborated by a reported combination of adult inclusion conjunctivitis and MALT lymphoma in a young patient114 and by a series of 3 patients treated with antibiotic therapy (2 complete remissions, 1 partial response).115 Inversely, Grünberger et al116 did not find a therapeutic effect of “blind” antibiotic treatment in 11 patients with MALT OAL. Since the first report by Ferreri et al,34 several other series30,74,117-121 have been reported, as shown in Table 4. The prevalence of C psittaci infection in ocular adnexal lymphoma is heterogeneous and ranges from 0% to 80%. Similarly, all samples of extra-ophthalmologic lymphomas, lymphoid hyperplasia, and nonneoplastic orbital tumors were negative. Given that this heterogeneity could be caused by different experimental conditions, we performed C psittaci DNA amplification according to the protocol used by Ferreri et al33 and observed a very low prevalence of bacterial infection (6%). One hypothesis for this apparent discrepancy is that a heterogeneous epidemiologic distribution of C psittaci infection exists worldwide. Moreover, only You et al118 detected C psittaci DNA in 9% of OAL patients and 4.7% of patients of nonneoplastic ocular adnexal disease. This important finding must be evaluated by international crossover studies to confirm the association between C psittaci infection and ocular adnexal lymphoma.
Association between C psittaci and OAL
Reference, year . | OAL, no. (%) . | Extra-OAL, no. (%) . | LH, no. (%) . | Orbital samples, no. (%) . |
|---|---|---|---|---|
| Ferreri et al,33 2004 | 32 of 40 (80) | ND | 3 of 26 (12) | 0 of 20 (0) |
| Gracia et al,117 2005 | 1 of 20 (5) | 0 of 20 (0) | ND | 0 of 10 (0) |
| You et al,118 2005 | 26 of 33 (78) | ND | ND | 5 of 21 (23) |
| Rosado et al,30 2006 | 0 of 57 (0) | ND | 0 of 2 (0) | ND |
| Mulder et al,119 in press | 0 of 20 (0) | ND | ND | ND |
| De Cremoux et al,74 2006 | 1 of 16 (6) | 0 of 10 (0) | 0 of 10 (0) | ND |
| Daibata et al,120 2006 | 0 of 21 (0) | ND | 0 of 3 (0) | ND |
| Liu et al,121 in press | 0 of 21 (0) | ND | ND | ND |
| Total | 60 of 228 (26) | 0 of 30 (0) | 3 of 41 (7) | 5 of 51 (10) |
Reference, year . | OAL, no. (%) . | Extra-OAL, no. (%) . | LH, no. (%) . | Orbital samples, no. (%) . |
|---|---|---|---|---|
| Ferreri et al,33 2004 | 32 of 40 (80) | ND | 3 of 26 (12) | 0 of 20 (0) |
| Gracia et al,117 2005 | 1 of 20 (5) | 0 of 20 (0) | ND | 0 of 10 (0) |
| You et al,118 2005 | 26 of 33 (78) | ND | ND | 5 of 21 (23) |
| Rosado et al,30 2006 | 0 of 57 (0) | ND | 0 of 2 (0) | ND |
| Mulder et al,119 in press | 0 of 20 (0) | ND | ND | ND |
| De Cremoux et al,74 2006 | 1 of 16 (6) | 0 of 10 (0) | 0 of 10 (0) | ND |
| Daibata et al,120 2006 | 0 of 21 (0) | ND | 0 of 3 (0) | ND |
| Liu et al,121 in press | 0 of 21 (0) | ND | ND | ND |
| Total | 60 of 228 (26) | 0 of 30 (0) | 3 of 41 (7) | 5 of 51 (10) |
Values shown are the number of positive results out of the number of samples analyzed.
LH indicates lymphoid hyperplasia; ND, not done.
Because of the small number of treated patients and the heterogeneous results concerning the association between C psittaci and OAL, antichlamydia antibiotic therapy cannot at present be considered standard treatment for ocular adnexal lymphoma. However, the association between C psittaci infection and these lymphomas is impressive in view of the clearly established association between gastric MALT lymphomas and H pylori infection.72 In the light of these findings, urgent biologic and therapeutic investigations are justified.
Which management for OAL?
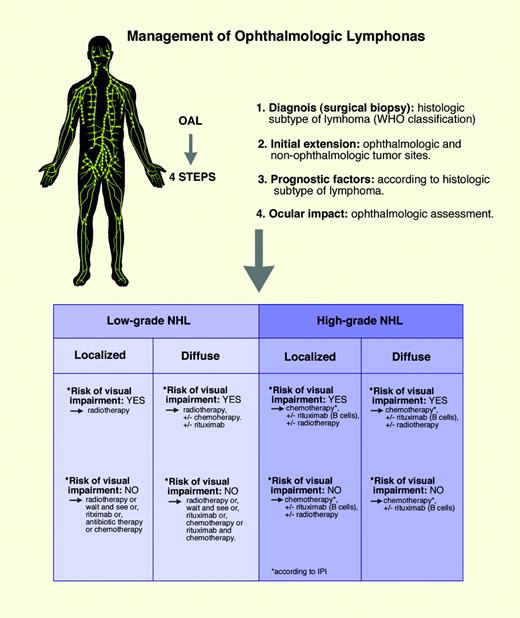
Several guidelines for the management of OAL that represent the author's approach can be proposed on the basis of the published data and our extensive experience, as shown in Figure 3. This figure describes elements to be evaluated at the time of the initial diagnosis, histopathologic subtype of lymphoma, extension of disease, prognostic factors related to the patient and to the disease, ocular impact of the ophthalmologic lymphoma, and new treatment modalities. Proposed treatment guidelines are as follows: (1) Radiotherapy should be performed in patients with low-grade lymphoma who are at risk for visual impairment. In those with high-grade lymphoma, medical treatment consisting of chemotherapy with or without immunotherapy can have a rapid effect on the disease and may delay or suspend the need for radiotherapy. (2) In patients with high-grade OAL, apart from extension of the disease, prognostic factors, and risk for visual impairment, anthracycline-based combination chemotherapy should be administered, together with rituximab monoclonal anti-CD20 antibody for those with B-cell lymphoma. The chemotherapy regimen must be adapted to prognostic factors in term of dose, number of courses, and type of consolidation. (3) In patients with low-grade lymphoma who are not at risk for visual impairment, various treatment options are possible depending on prognostic factors, such as radiotherapy, single-agent chemotherapy, immunotherapy with monoclonal anti-CD20 antibody, antibiotic therapy, such as anti-C psittaci antibiotic therapy, and a wait-and-see policy. However, the place of each of these modalities must be clearly evaluated by multicenter prospective studies.
Conclusions
OAL constitutes a group of malignant lymphoid neoplasms presenting specific characteristics, namely a very high proportion of low-grade B-cell NHL (as many as 80% of patients with approximately 50% of MALT lymphomas), a male-female sex ratio less than 1, orbital and lacrimal gland involvement as the most frequent ophthalmic sites (with bilateral involvement in 10% of patients), and extra-ophthalmologic disease in approximately 33% of patients (with nodal involvement in 20% of patients and bone marrow involvement in 10% of patients). Prognostic factors are not really different from those reported for nodal lymphoma, with a major impact of the histopathologic subtype of the lymphoma. Similarly, the outcome of these patients shows that the OAL site of the tumor does not influence the natural history of NHL. Because of the high proportion of patients with extra-ophthalmologic disease and the need to precisely evaluate the impact of the tumor on ocular function, these patients clearly require concomitant ophthalmologic and hematologic management. In patients with low-grade ophthalmologic lymphoma and no risk for visual impairment, radiotherapy has until now been considered the reference treatment option. However, because of its late toxicity and risk for disseminated relapse, and in light of the recent published data, several other approaches—such as monoclonal antibody immunotherapy and antibiotic therapy—can now be proposed. These new treatments could be an opportunity to more clearly define the mechanisms involved in OAL lymphomagenesis. A wait-and-see policy has also been proposed in some patients with a low tumor burden of low-grade disease. The management of OAL is, therefore, a topical subject that requires the resolution of biologic and clinical issues by means of prospective clinical trials with close collaboration between ophthalmologists and hematologists and that represents an exciting challenge for the next few years.
Management of ocular adnexal lymphomas. Illustration by Marie Dauenheimer.
Prepublished online as Blood First Edition Paper, April 25, 2006; DOI 10.1182/blood-2006-02-005017.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal