Leukocyte motility is known to be dependent on both β2-integrins and matrix metalloproteinases MMP-2/-9 or gelatinases, which mediate leukocyte adhesion and the proteolysis needed for invasion, respectively. Gelatinases not only play an important role in cell migration, tissue remodeling, and angiogenesis during development, but are also involved in the progression and invasiveness of many cancers, including leukemias. The concept that MMPs associate with integrins, as well as their importance in some physiologic and pathologic conditions, has been advanced previously but has not been examined on leukocytes. This review will examine mainly the function of the MMP-integrin complexes in normal leukocyte migration and the effect of integrin and broad-spectrum MMP inhibitors in tumor progression.
Leukocyte adhesion and migration
Neutrophils, also known as polymorphonuclear leukocytes (PMNs) originate from stem cells in the bone marrow. They represent 60% to 70% of the total circulating leukocytes and are the first cells to be recruited to the sites of infection or injury within minutes to hours after maturation, forming a primary defense against infectious agents or “foreign” substances that invade our body's physical barriers. The initiation of an inflammatory response involves 3 major steps: (1) increased blood flow by dilation of capillaries; (2) escape of plasma proteins from the bloodstream; and (3) extravasation of neutrophils through the endothelium and accumulation at the site of injury. Elimination of invading microorganisms is accomplished by phagocytosis, generation of reactive oxygen metabolites, as well as through release of proteolytic enzymes and microbicidal substances, all stored in intracellular granules of mature PMNs.1
The main functions of neutrophils involve adhesion, extravasation, chemotaxis, phagocytosis, and production of oxidative agents. Like all leukocytes, these functions can be triggered by appropriate stimuli and the synergistic action of different adhesion molecules that are present on the surface of both neutrophils and activated endothelial cells.2 Interactions of neutrophils with the activated endothelium have been extensively studied either under static conditions or under physiologic conditions (flow shear forces). Neutrophil tethering and capture have been shown to be mediated by P-selectin binding to its ligand PSGL-1; neutrophil activation by chemokines, such as IL-8; and firm adhesion by ICAM-1 binding to αLβ2- and αMβ2-integrins.3 Chemokines capable of triggering rapid arrest of T cells, B cells, and monocytes on endothelial cells under physiologic conditions include SLC/CCL21, RANTES, and SDF-1/CXCL12, respectively. Unlike other leukocytes, arrest chemokines for neutrophils have been much more difficult to define, even though the neutrophil adhesion cascade has been studied longer and by more groups.
Role of integrins and MMPs in leukocyte migration
Structure and function of leukocyte β2-integrins
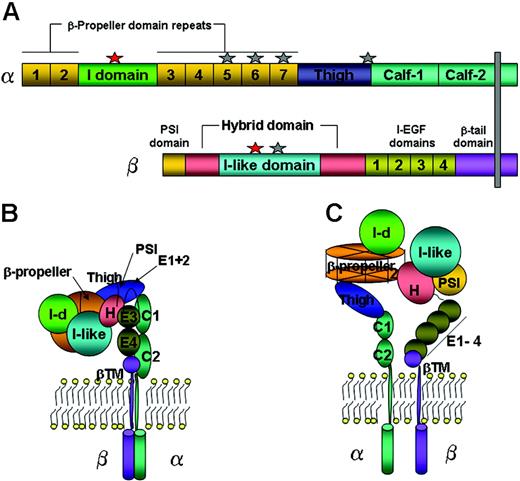
The structural characteristics and functional roles of leukocyte β2-integrins have been extensively reviewed.4,5 The β2-integrins (αLβ2, αMβ2, αXβ2, and αDβ2) consist of α-(1063, 1137, 1144, and 1084 residues, respectively) and β-(747 residues) subunits (Figure 1A). Divalent cations are essential for integrin functions by regulating the integrin structure in a state in which they increase or suppress binding to physiologic ligands. A recent crystal structure of αVβ3-integrin showed that the bent form is capable of binding a physiologic ligand in a Mn2+-dependent manner.6 To date, the primary structures of all 4 β2-integrin α- and β-subunits have been described by molecular cloning.7,8 Each integrin α-subunit contains 7, 60-amino-acid long, homologous segments in the amino-terminal region, and with resemblance to a domain present in the trimeric G protein β-subunit, that are predicted to fold into a 7-bladed β-propeller domain.9 Along with the I-like domain (βA) from the β-subunit, they both interact to form the “head” of the integrin (Figure 1B). Half of all integrin α-subunits contain an additional, 200-amino-acid long, I domain that is inserted between the propeller β sheets 2 and 37,9 and is homologous to (domains within) a plasma glycoprotein von Willebrand factor.10 The 3-dimensional architecture of the extracellular domains of the integrin α- and β-subunits has been revealed by crystallization, electron microscopy, and nuclear magnetic resonance (NMR).11 Based on the crystal structure of the extracellular domains of αVβ3, it has been predicted that the I domain lies on top of the β-propeller domain (Figure 1C).9
Loss of heteromerization of the integrin during biosynthesis caused by mutations in the gene encoding the β-subunit resulted in reduced β2-integrin cell-surface expression and function on leukocytes, leading to a rare human inherited disease called leukocyte adhesion deficiency-I (LAD-I).12 Expression of nonfunctional β2-integrins was also observed in LAD-I patients carrying mutations in the MIDAS motif of the I-like domain in the β-subunit.13 PMNs and monocytes from LAD-I patients fail to migrate through the vascular endothelium or become fully activated because of lack of adherence, actin cytoskeleton rearrangement, and spreading on ICAM-1- or ECM-coated surfaces. This explains why LAD-I patients are susceptible to life-threatening bacterial infections. The same phenotype was observed in β2-integrin knock-out mice.14
Schematic structure of the leukocyte integrin. (A) The integrin's primary structure, including divalent cation-binding sites (Mg2+ as red stars, and Ca2+ as gray stars). (B,C) Schematic representations of the bent (inactive) and straightened (active) conformations of the integrin, respectively. The arrangement of domains is based on the 3-dimensional crystal structure of αVβ3-integrin, with an I domain added between the second and third β-propeller repeats. Each domain is colored as in panel A. I-d indicates I domain; I-EGF, integrin-epidermal growth factor domain; PSI, plexin/semaphorin/integrin; and βTM, β-tail domain.
Schematic structure of the leukocyte integrin. (A) The integrin's primary structure, including divalent cation-binding sites (Mg2+ as red stars, and Ca2+ as gray stars). (B,C) Schematic representations of the bent (inactive) and straightened (active) conformations of the integrin, respectively. The arrangement of domains is based on the 3-dimensional crystal structure of αVβ3-integrin, with an I domain added between the second and third β-propeller repeats. Each domain is colored as in panel A. I-d indicates I domain; I-EGF, integrin-epidermal growth factor domain; PSI, plexin/semaphorin/integrin; and βTM, β-tail domain.
Regulation of cell adhesion and migration
Leukocyte migration is a complex process, controlled by a wide spectrum of leukocyte and endothelial cell adhesion molecules and by the presence of chemotactic molecules. These molecules, as well as growth factors, are responsible for the establishment of a polarized cell migration and there is enough evidence to prove that signaling from both phospholipids and proteins from the Rho family of small GTPases are also involved in directed cell motility.15 Migration of leukocytes is essential for immune responses, tissue repair, and embryonic development.
A polarized morphology of leukocytes was first described to be similar to that of a migrating amebae, with a leading edge at the front and a uropod at the rear of a migrating cell.16 T cells recognize and bind to antigen presenting cells (APCs) through their leading edge. A number of receptors are concentrated at the leading edge, including αVβ3, uPAR, and fMLP-R in neutrophils; CCR2, CCR5, and FAK in T cells; and CXCR4 in B cells, which are able to sense chemotactic gradients, thus guiding leukocytes to migrate in a polarized manner. At the uropod, several reports show localization of ICAMs, L-selectin, αMβ2, PSGL-1, FcγR-IIIb, CD2, CD43, and CD44,17,18 which play a pivotal role in cell adhesion, thus facilitating cell migration. Release of the uropod triggers cell migration. Some of these receptors, when bound to the substratum, become linked to the actin cytoskeleton during cell migration. Interactions between the cytoskeleton and the cell-surface receptors are required for the formation of membrane protrusions, such as lamellipodia (broad, sheetlike structures) and filopodia (thin cylindrical needlelike projections), both structures located at the leading edge.16 Several MMPs, including MT1-MMP and MMP-2, were found to colocalize at membrane protrusions. Interaction of MMPs with their natural inhibitors, TIMPs, at these sites might be the key mechanism for the regulation of cell-surface MMP activation and, eventually, the control of the invasive phenotype of cells.19
Cell-surface association of MMPs and other proteases
Matrix metalloproteinases (MMPs) are a family of structurally related and highly conserved zinc-dependent endopeptidases collectively capable of degrading most components of the basement membrane and ECM.20 MMP substrates also include a wide variety of proteins, such as chemotactic molecules, adhesion molecules, proteinase inhibitors, cell-surface receptors, blood clotting factors, latent growth factors, and growth factor-binding proteins. Most human MMPs can be divided according to their sequence homology, substrate specificity, and cellular location into several subclasses: collagenases, gelatinases, stromelysins, matrilysins, membrane-type MMPs, and others. The basic multidomain structure of MMPs comprises the following: (1) an amino-terminal domain; (2) a catalytic domain; and (3) a carboxy-terminal domain. To date, there are at least 25 secreted or membrane-bound known human MMPs.21 The expression, secretion, and activity of MMPs in normal tissues are subject to tight control. Data generated from intensive studies on MMP activities in different cells and tissues, as well as studies from knock-out animals, witness the importance of these enzymes in many normal physiologic processes (eg, embryonic development, bone resorption, angiogenesis, and wound healing) and pathologic processes (rheumatoid arthritis, multiple sclerosis, periodontal disease, and tumor growth and metastasis).20,22,23
MMPs are secreted as zymogens from inside the cell to the cell surface and into the extracellular environment where they are able to degrade both ECM and non-ECM proteins. It remains unclear how these enzymes make it to the correct location at the cell surface and how the proteolytic activity is controlled at the pericellular space. However, it has been suggested that MMP binding to cell-surface proteins can have an effect on intracellular signaling, facilitate proenzyme localization and activation, mediate cell motility by disruption of cell contacts with the ECM, and promote internalization of the enzyme. For example, integrins are shown to act as receptors for several proteases, including MMPs. Such interactions have been detected in caveolae, in invadopodia, and at the leading edge of migrating cells, where directed proteolytic activity is needed. The first interaction between an integrin (αVβ3) and an MMP (MMP-2) was identified on the surface of melanoma cells and angiogenic blood vessels (Table 1). This complex was shown to be involved in tumor growth and angiogenesis in vivo.82 Caveolae are membrane invaginations known to serve as sites for clustering of various integrins and proteases.83 MT1-MMP was shown to activate αVβ3 through proteolytic cleavage, suggesting that coordinated expression and localization of these molecules may be important for cancer cell invasion and metastasis. Furthermore, there is evidence that the αVβ3-integrin has modulatory properties on MMP-2 activity by binding to its C-terminal domain.28,82 Inhibition of the αVβ3/MMP-2 complex formation by either the MMP-2 C-terminal domain84 or a small molecule inhibitor, TSRI265,85 dramatically suppressed angiogenesis in vivo, demonstrating that this interaction is essential for endothelial cell proliferation and migration. Since then, several other important protease associations with integrins have been reported (Table 1), suggesting that pericellular proteolysis may be activated and targeted by integrins and other cell-surface receptors.
Proteinase association with integrins
Soluble proteases/associated proteins . | Cell-surface expression . | Source . |
|---|---|---|
| MMPs | ||
| MMP-1 | ||
| α1β1 | Myocytes | Stricker et al24 |
| α2β1 | Keratinocytes | Dumin et al25 |
| EMMPRIN | Lung carcinoma | Guo et al26 |
| PAR1 | Breast carcinoma | Boire et al27 |
| MMP-2 | ||
| αVβ3 | Melanoma, endothelial | Brooks et al28 |
| LRP | Fibroblasts | Yang et al29 |
| Collagen chains | Fibroblasts | Steffensen et al30 |
| TSP-2 | Fibroblasts | Yang et al29 |
| TIMP-2 | Malignant cells | Olson et al31 |
| Caveolin-1 | Endothelial | Puyraimond et al32 |
| Hsp90α | Fibrosarcoma | Eustace et al33 |
| MT1-MMP | Fibrosarcoma | Strongin et al34 |
| BS | – | Fedarko et al35 |
| MMP-3 | ||
| Osteopontin | – | Fedarko et al35 |
| MMP-7 | ||
| CD44HSPG | Epithelial | Yu et al36 |
| TM4SF | – | Maecker et al37 |
| CD151 | Rectal carcinoma | Shiomi et al38 |
| MMP-9 | ||
| Collagen chains | Epithelial/fibrosarcoma | Okada et al39 |
| RECK | Fibrosarcoma | Takahashi et al40 |
| CD44 | Melanoma | Yu et al41 |
| ICAM-1 | Leukemias | Fiore et al42 |
| LRP | Fibroblasts | Hahn-Dantona et al43 |
| Ku protein | Macrophages/leukemia | Monferran et al44 |
| TIMP-1 | Fibroblasts | O'Connell et al45 |
| TSP-1 | Malignant cells | Rodriguez-Mazaneque et al46 |
| αL/Mβ2 | Neutrophils/leukemias | Stefanidakis et al47 |
| α5β1 | Epithelial | Wang et al48 |
| α3β1 | Mammary carcinoma | Morini et al49 |
| αVβ5 | Fibrosarcoma | Bjorklund et al50 |
| DMP-1 | – | Fedarko et al35 |
| MT1-MMP | ||
| αVβ3 | Endothelial | Galvez et al51 |
| β1-subunit | Endothelial | Galvez et al51 |
| CD44 | Fibrosarcoma | Mori et al52 |
| TIMP-2 | Breast carcinoma | Imai et al53 |
| Collagen type I | Gingival fibroblasts | Tam et al54 |
| RECK | Fibrosarcoma | Oh et al55 |
| Serine proteases | ||
| uPA | ||
| uPAR* | Malignant | Ellis et al56 |
| αM/Xβ2 | Neutrophils | Xue et al57 |
| αVβ3 | Fibrosarcoma | Xue et al58 |
| αVβ5 | Mammary carcinoma | Carriero et al59 |
| α3β1 | Mammary carcinoma | Wei et al60 |
| Elastase | ||
| αMβ2 | Neutrophils | Cai and Wright61 |
| Seprase | ||
| uPAR | Melanoma | Artym et al62 |
| α3β1 | Melanoma | Monsky et al63 |
| Dipeptidyl peptidase IV | ||
| α3β1 | Fibroblasts | Ghersi et al64 |
| Cathepsin G | ||
| FPR | Leukemias | Sun et al65 |
| HIV-1 gp120 | Leukemias | Avril et al66 |
| Membrane Gp | Platelets/neutrophils | Molino et al67 |
| Proteinase 3 | ||
| αMβ2 | Neutrophils | David et al68 |
| Plasmin | ||
| Annexin II | Kidney cells | MacLeod et al69 |
| Cysteine proteases | ||
| Cathepsin B | ||
| Annexin II | Tumors | Mai et al70 |
| α2-M | Bone metastases | Arkona et al71 |
| Caspase-8 | ||
| α3β1 | Neuroblastoma | Stupack et al72 |
| ADAMs | ||
| ADAM-2 | ||
| α6β1 | Oocytes | Chen et al73 |
| ADAM-7 | ||
| α4β1 | T-cell leukemia | Bridges et al74 |
| α9β1 | T-cell leukemia | Bridges et al74 |
| α4β7 | T-cell leukemia | Bridges et al74 |
| ADAM-9 | ||
| α6β1 | Fibroblasts | Nath et al75 |
| α9β1 | Oocytes | Eto et al76 |
| αvβ5 | Myeloma | Zhou et al77 |
| ADAM-12 | ||
| α9β1 | Hematopoietic | Zhang et al78 |
| ADAM-15 | ||
| αVβ3 | Hematopoietic | Nath et al79 |
| α5β1 | Hematopoietic | Nath et al79 |
| α9β1 | Oocytes | Eto et al76 |
| ADAM-17 | ||
| α5β1 | Epithelial | Bax et al80 |
| ADAM-23 | ||
| αVβ3 | Neuroblastoma | Cal et al81 |
| ADAM-28 | ||
| α4β1 | Lymphocytes | Bridges et al74 |
| α9β1 | T-cell leukemia | Bridges et al74 |
| α4β7 | T-cell leukemia | Bridges et al74 |
| ADAM-33 | ||
| α9β1 | T-cell leukemia | Bridges et al74 |
Soluble proteases/associated proteins . | Cell-surface expression . | Source . |
|---|---|---|
| MMPs | ||
| MMP-1 | ||
| α1β1 | Myocytes | Stricker et al24 |
| α2β1 | Keratinocytes | Dumin et al25 |
| EMMPRIN | Lung carcinoma | Guo et al26 |
| PAR1 | Breast carcinoma | Boire et al27 |
| MMP-2 | ||
| αVβ3 | Melanoma, endothelial | Brooks et al28 |
| LRP | Fibroblasts | Yang et al29 |
| Collagen chains | Fibroblasts | Steffensen et al30 |
| TSP-2 | Fibroblasts | Yang et al29 |
| TIMP-2 | Malignant cells | Olson et al31 |
| Caveolin-1 | Endothelial | Puyraimond et al32 |
| Hsp90α | Fibrosarcoma | Eustace et al33 |
| MT1-MMP | Fibrosarcoma | Strongin et al34 |
| BS | – | Fedarko et al35 |
| MMP-3 | ||
| Osteopontin | – | Fedarko et al35 |
| MMP-7 | ||
| CD44HSPG | Epithelial | Yu et al36 |
| TM4SF | – | Maecker et al37 |
| CD151 | Rectal carcinoma | Shiomi et al38 |
| MMP-9 | ||
| Collagen chains | Epithelial/fibrosarcoma | Okada et al39 |
| RECK | Fibrosarcoma | Takahashi et al40 |
| CD44 | Melanoma | Yu et al41 |
| ICAM-1 | Leukemias | Fiore et al42 |
| LRP | Fibroblasts | Hahn-Dantona et al43 |
| Ku protein | Macrophages/leukemia | Monferran et al44 |
| TIMP-1 | Fibroblasts | O'Connell et al45 |
| TSP-1 | Malignant cells | Rodriguez-Mazaneque et al46 |
| αL/Mβ2 | Neutrophils/leukemias | Stefanidakis et al47 |
| α5β1 | Epithelial | Wang et al48 |
| α3β1 | Mammary carcinoma | Morini et al49 |
| αVβ5 | Fibrosarcoma | Bjorklund et al50 |
| DMP-1 | – | Fedarko et al35 |
| MT1-MMP | ||
| αVβ3 | Endothelial | Galvez et al51 |
| β1-subunit | Endothelial | Galvez et al51 |
| CD44 | Fibrosarcoma | Mori et al52 |
| TIMP-2 | Breast carcinoma | Imai et al53 |
| Collagen type I | Gingival fibroblasts | Tam et al54 |
| RECK | Fibrosarcoma | Oh et al55 |
| Serine proteases | ||
| uPA | ||
| uPAR* | Malignant | Ellis et al56 |
| αM/Xβ2 | Neutrophils | Xue et al57 |
| αVβ3 | Fibrosarcoma | Xue et al58 |
| αVβ5 | Mammary carcinoma | Carriero et al59 |
| α3β1 | Mammary carcinoma | Wei et al60 |
| Elastase | ||
| αMβ2 | Neutrophils | Cai and Wright61 |
| Seprase | ||
| uPAR | Melanoma | Artym et al62 |
| α3β1 | Melanoma | Monsky et al63 |
| Dipeptidyl peptidase IV | ||
| α3β1 | Fibroblasts | Ghersi et al64 |
| Cathepsin G | ||
| FPR | Leukemias | Sun et al65 |
| HIV-1 gp120 | Leukemias | Avril et al66 |
| Membrane Gp | Platelets/neutrophils | Molino et al67 |
| Proteinase 3 | ||
| αMβ2 | Neutrophils | David et al68 |
| Plasmin | ||
| Annexin II | Kidney cells | MacLeod et al69 |
| Cysteine proteases | ||
| Cathepsin B | ||
| Annexin II | Tumors | Mai et al70 |
| α2-M | Bone metastases | Arkona et al71 |
| Caspase-8 | ||
| α3β1 | Neuroblastoma | Stupack et al72 |
| ADAMs | ||
| ADAM-2 | ||
| α6β1 | Oocytes | Chen et al73 |
| ADAM-7 | ||
| α4β1 | T-cell leukemia | Bridges et al74 |
| α9β1 | T-cell leukemia | Bridges et al74 |
| α4β7 | T-cell leukemia | Bridges et al74 |
| ADAM-9 | ||
| α6β1 | Fibroblasts | Nath et al75 |
| α9β1 | Oocytes | Eto et al76 |
| αvβ5 | Myeloma | Zhou et al77 |
| ADAM-12 | ||
| α9β1 | Hematopoietic | Zhang et al78 |
| ADAM-15 | ||
| αVβ3 | Hematopoietic | Nath et al79 |
| α5β1 | Hematopoietic | Nath et al79 |
| α9β1 | Oocytes | Eto et al76 |
| ADAM-17 | ||
| α5β1 | Epithelial | Bax et al80 |
| ADAM-23 | ||
| αVβ3 | Neuroblastoma | Cal et al81 |
| ADAM-28 | ||
| α4β1 | Lymphocytes | Bridges et al74 |
| α9β1 | T-cell leukemia | Bridges et al74 |
| α4β7 | T-cell leukemia | Bridges et al74 |
| ADAM-33 | ||
| α9β1 | T-cell leukemia | Bridges et al74 |
Many of the functions and binding mechanisms of these complexes have not yet been elucidated.
PAR1 indicates protease-activated receptor 1; Hsp, heat shock protein; BS, bone sialoprotein; HSPG, heparan sulfate proteoglycans; RECK reversion-inducing cysteine-rich protein with kazal motifs; DMP-1, dentin matrix protein-1; FPR, formyl peptide receptor; α2-M, α2-macroglobulin; ADAM, a disintegrin and metalloproteinase; Gp, membrane glycoproteins; and –, not studied
uPAR, in turn, interacts with αM/Xβ2, αVβ3, αVβ5, and α3β1
In leukocytes, uPA could bind to its receptor, uPAR, and to αMβ2 simultaneously, forming a trimolecular complex where αMβ2 could serve as a signaling receptor.86 This interaction is likely to be mediated by both the kringle and proteolytic domains for uPA and the I-domain for αMβ2. This complex plays an essential role in the migration of inflammatory cells and vascular homeostasis. The uPA/uPAR complex was also found to be associated with the α5β1-integrin and capable of promoting adhesion and migration of Chinese hamster ovary cells as well as intracellular signal transduction through the integrin. In addition, a cyclic peptide DDGW discovered by phage display and an MMP-9-derived peptide motif HFDDDE both inhibited proMMP-9/αMβ2 complex formation and leukocyte migration in vitro and in vivo.47,87 However, this motif did not block leukocyte adhesion to ICAM-1 and fibrinogen, suggesting the integrin-bound MMP is essential for degradation of integrin-directed bonds to matrix proteins. Recently, proMMP-9 was found to be associated with ICAM-142 and DNA repair protein Ku44 on the surface of leukemic cells. ICAM-1 cleavage by MMP-9 resulted in tumor cell resistance to natural killer cell-mediated cytotoxicity. Also, a chaperone heat shock protein 90 (Hsp90) was found to interact with MMP-2 on the cell surface of fibrosarcoma cells, thus promoting MMP-2 activation, which is critical for tumor invasiveness.33 The binding mechanism of most of these interactions has not yet been elucidated.
Several cell-surface hyaluronan receptor CD44 isoforms, RECK, TSP-1, LRP, and cell-surface collagen IV chains also serve as MMP-9-docking molecules. The CD44/MMP-9 complex was found to be associated with invasiveness of mouse mammary carcinoma and human melanoma cells in vivo,41 suggesting that CD44 helps to localize MMP-9 activity to the cell surface. The GPI-linked proteins RECK and TSP-1 were not only identified as cell-surface receptors for MMP-9 but also were found to block their enzymatic activity.46,55 Interaction of MMPs with the cell surface not only may be needed for proenzyme activation and targeting at specific sites for degradation of cell-surface substrates, but also could promote intracellular degradation via receptor-mediated endocytosis (RME). Regulation of the cell-surface activity of proteolytic enzymes that are involved in cancer progression, including MMP-2, -9, -13, tPA, and uPA by endocytosis, has led to suppression of tumor cell invasion.88
A disintegrin and a metalloproteinase (ADAMs) and ADAM with a thrombospondin motif (ADAMTS) comprise a large family of proteins capable of interacting with integrins and involved in processes such as angiogenesis, fertilization, myogenesis, neurogenesis, and inflammation. Unlike the transmembrane proteins AD-AMs, ADAMTS proteins are soluble ECM proteases consisting of a prodomain, metalloprotease, and disintegrin domains, but devoid of ADAMs' cysteine-rich, EGF-like transmembrane and cytoplasmic domains.89 ADAM2 or fertilin β was one of the first disintegrins identified and found to interact with α6β1-integrin.73 To date, several other ADAM-integrin interactions have been identified: ADAM9 with αvβ5 and α6β1, ADAM12 and ADAM15 with α9β1, ADAM15 and ADAM23 with αvβ3, and ADAM15 with α5β1 (Table 1).90
Role of integrins and gelatinases in cancer progression
Early events in tumor progression are characterized by increases in cell proliferation, insensitivity to growth-inhibitory signals, reduced ability for differentiation, as well as the ability to escape from apoptosis and immune surveillance.91 Proteinases that degrade components of the ECM and are capable of processing nonmatrix substrates (eg, growth factors and their receptors, chemokines, adhesion molecules, and apoptotic mediators) have long been considered to be important at all stages of tumorigenesis.92 The combined participation of integrins and MMPs is required for invasion of tumor cells into surrounding connective tissues, intravasation and extravasation from blood vessels, and metastasis to distant organs.93 Indeed, studies on TIMPs have shown that overexpression or administration of these inhibitors as recombinant proteins inhibited experimental invasion and metastasis.94 In most cases, the stage of tumor progression correlates with the expression levels of gelatinases, as the invasive and metastatic potential of tumor cells is strongly affected by changes in gelatinase expression in animal models. Expression of MMP-2 and MMP-9 was found to be strongly up-regulated in cancers of lung, colon, breast, skin, and prostate, which correlated with increased tumor invasiveness and metastasis.22 Inhibition of MMP-9 expression in a model of experimental metastasis reduced the number of colonies formed in the lungs of mice.95 Further evidence supporting this hypothesis came from studies on MMP-2 and -9 null mice. These mice developed fewer tumors than the wild type.21
Integrins and gelatinases in invasion and metastasis
The initial step of tumor cell invasion is characterized by the breakdown of the basement membrane, a process known to be dependent on type IV collagen-degrading enzymes, mainly MMP-2 and MMP-9. Liotta et al obtained results where type IV gelatinase activity correlated with cancer metastasis.96 Endothelial cell proliferation and migration into the tumor tissue are mediated by angiogenic (eg, MMP-9, VEGF, and basic fibroblast growth factor [bFGF]) and lymphangiogenic factors that are released by tumor cells. Using DNA microarrays, primary tumor-gene expression profiles could be arranged in classes of “good” and “poor” prognosis. DNA-microarray analysis on human breast carcinoma cell lines that have metastasized to bone revealed some of the genes (eg, MMP-1, MMP-2, CXCR4, IL-11, and CTGF) responsible for the increased metastatic potential of breast cancer cells.97,98 Video-microscopy studies showed that MMPs play a significant role in tumor metastasis, as TIMP-1 and MMP inhibitor batimastat (BB-94) blocked the formation of tumors in secondary sites.99 The role of MMPs in tumor invasion and metastasis has also been studied using small-interfering RNAs and antisense technology.100,101 Gelatinases and MT-MMPs revealed a new mechanism to control metastasis by cleavage of the metastasis suppressor gene, KiSS-1.102 Finally, recent studies supporting the in vitro data from double MMP-2/MMP-9-deficient mice demonstrated that these enzymes cooperate in promoting the invasive phenotype of malignant keratinocytes in an experimental model in vivo.103
Changes in integrin expression and localization can also influence invasion and metastasis of tumor cells.104 Integrins were shown to be involved in the migration and liver metastasis of large cell lymphoma cells and angiogenesis, as αvβ3 antagonists induced apoptosis and blocked cancer cell invasion.105 α4β1-integrin has a dual role in cancer progression as it inhibited the initial invasive growth while promoting metastatic spread of melanoma cells. A different study showed that increased expression of this integrin could inhibit the invasive stage of metastasis formation.106 Blocking integrins with synthetic peptides containing an RGD sequence, antibodies, or disintegrins (integrin-binding proteins isolated from snake venom) has been demonstrated to interfere with tumor cell invasion and metastasis in vitro and in vivo.107 Of importance, cooperation between αvβ3 and MMP-9 increased migration of metastatic breast cancer cells.108 Also, several reports show that uPA binding to its receptor uPAR is a requirement for tumor cell invasion and metastasis, as this process is efficiently inhibited either by an amino-terminal fragment of urokinase or a mutant plasminogen activator inhibitor-2 (PAI-2).109 Finally, a recent study highlights the importance of chemokine receptors in breast cancer metastasis in vitro and in vivo.110
Integrins and gelatinases in cancer-associated inflammation
Chronic inflammation is also associated with a variety of cancers, including breast, liver, prostate, and skin.92 In human cancer, tumor cells are not the only source of MMPs. MMPs, mainly gelatinases, are predominantly produced by stromal cells, ranging from immune (lymphocytes and dendritic cells), inflammatory (granulocytes and monocytes), and vascular cells (vascular- and lymph-endothelial cells and pericytes). MMPs have been involved in the escape of cancer cells from immune surveillance. The escape mechanism occurs through MMP-9-induced cleavage of the interleukin-2 receptor (IL-2Rα),111 TGF-β activation,112 and ICAM-1 and ICAM-2 shedding,42,113 thus suppressing T-cell proliferation and immune response against tumors.
Chemokines play an essential role in regulating directional migration of leukocytes. Proteolytic cleavage of chemokines by MMPs can lead to enhanced or reduced leukocyte recruitment into tumors. For example, a cleaved form of MCP-3 produced by MMP-2 can bind to CC-chemokine receptors, and unlike intact MCP-3, it abrogates chemotaxis and suppresses inflammation.114 ET-1 processing by MMP-9 generates endothelin-1 (ET-1) that induces secretion of MMP-9 from neutrophils,115 suggesting that MMPs are both effectors of leukocyte migration and regulators of the inflammatory response. The importance of chemokine receptors in metastasis was demonstrated by inhibition of SDF-1 binding to its receptor. Dissociation of SDF-1/CXCR-4 complex by blocking antibodies strongly reduced breast cancer metastasis to lungs and lymph nodes in vivo.110 MMP-9 and VEGF are produced by mammary tumor-infiltrating immune cells.116 Expression of MMP-9 by tumor-infiltrating macrophages promotes angiogenesis as well as growth and invasion of xenografted ovarian cancer cells in vivo.117 Several studies show that cancer cells can promote the secretion of MMPs by stromal cells in a paracrine manner via secretion of growth factors, interleukins, and EMMPRIN.21 Recruitment of hematopoietic precursor cells is also required for tumor angiogenesis.118
Role of integrins and gelatinases in acute leukemias
Leukemia can be described as the uncontrolled proliferation of hematopoietic cells that lack the ability to differentiate into mature blood cells. The precise role of gelatinase expression in acute leukemias is not clear. So far, it is known that invasiveness of many hematologic malignancies, including myelo-monocytic leukemias, involves overexpression of proteolytic enzymes, such as MMP-2 and MMP-9.119 MMP-9 is induced and secreted in conditioned media of leukemic cell lines in response to extracellular stimuli, after pretreatment of cells with chemokines, and after cell adhesion to the ECM.119 Higher gelatinase expression levels were detected in the bone marrow plasma of patients with leukemia compared with healthy controls. After chemotherapy, the levels of TIMP-1 and TIMP-2 were significantly increased, whereas MMP-9 levels were lower in acute lymphoblastic leukemia (ALL) and acute myeloid leukemia (AML) patients. Accordingly, AML patients who achieved a complete remission showed significantly lower MMP-9 levels, suggesting that MMP-9 could be a surrogate marker of leukemic status in these patients. Also, the low MMP-9 expression levels in patients with leukemia correlated with increased survival.120
Several reports have demonstrated the involvement of both MMP-2/-9 gelatinases and β2-integrins in the growth and progression of myeloid and lymphoid neoplasms.121,122 Selective MMP-9 expression is induced as a result of αMβ2-integrin ligation in PMNs123 and αLβ2-integrin ligation in T-lymphoma cells.124 Also, studies from αM- and αL-integrin knock-out mice confirm the importance of β2-integrins in mediating leukocyte adhesion and migration.125 In accordance, high infiltration of leukemic blasts in patients with AML strongly correlated with increased expression of both αLβ2 - and αMβ2-integrins.121 AML cell adhesion to bone marrow fibroblast monolayers seems to require both β1- and β2-integrins, as antibodies against them inhibited the binding.126 Interaction between leukemic cells and bone marrow stroma cells has been shown to increase leukemic cell survival and chemotherapy-induced leukemia cell resistance.127
Increased vessel density was detected in the bone marrow of acute and chronic leukemia patients compared with normal bone marrow, and is known to be mediated by angiogenic factors such as VEGF and bFGF.128,129 Both increased plasma MMP-9 and VEGF correlated with high leukemia cell infiltration, suggesting that MMP-9 and VEGF act cooperatively in the process of leukemia cell invasion.122 Another study showed that increased vessel density was mediated by MMP-2 and MMP-9 overexpression in primary AML blasts by promoting endothelial cell migration.130 After achieving complete remission, the vessel number in AML patients was restored to normal levels. Furthermore, a gene therapy approach using a retroviral vector encoding for gelatinase inhibitors, endostatin and angiostatin, strongly inhibited bone marrow angiogenesis and leukemia tumor growth in vivo.131 These data suggest that gelatinases could be involved in leukemia progression. As a result, inhibitors of MMPs may be useful in treating hematologic malignancies.
Therapeutic intervention with MMP and integrin inhibitors
Due to the fact that integrins and MMPs are involved in tumor cell invasion and metastasis, over the past 20 years a lot of effort has been put into designing integrin and MMP inhibitors (MMPIs). Although endogenous inhibitors, such as TIMPs, inhibited tumor growth in transgenic mouse models, their use in cancer was limited due to poor pharmacokinetics, difficulties in protein administration, and broad spectrum of inhibition. To date, several synthetic MMPIs have been developed, tested widely in clinical trials, and classified into the following pharmacologic groups: collagen peptidomimetics, nonpeptidomimetics, tetracycline derivatives, and biphosphonates.132 The efficacy of these inhibitors in clinical trials is summarized in Table 2.
MMP and integrin antagonists in clinical trials
Inhibitors . | Structure . | Specificity . | Status/indication . |
|---|---|---|---|
| MMPs | |||
| Batimastat (BB-94) | Peptidomimetic | MMP-1, -2, -3, -7, -9 | Development halted |
| Marimastat (BB-2516) | Peptidomimetic | MMP-1, -2, -7, -9 | Phase 3/gastric cancer; phase 2/pancreatic cancer |
| BAY12-9566 | Nonpeptidomimetic | MMP-2, -3, -9 | Development halted |
| AG3340 | Nonpeptidomimetic | MMP-2, -3 | Phase 2/3/no benefit |
| BMS-275291 | Nonpeptidomimetic | Broad spectrum | Phase 1/2/NSCL |
| MMI270 | Nonpeptidomimetic | Broad spectrum | Phase 1/advanced cancer |
| Metastat (Col-3) | Tetracycline derivative | MMP-2, -9 | Phase 2/Kaposi sarcoma |
| Periostat | Tetracycline derivative | Broad spectrum | Phase M/periodontal disease |
| Neovastat (AE-941) | Shark cartilage extract | Broad spectrum | Phase 2/multiple myeloma; phase 3/NSCL |
| Not known | Green tea extract | MMP-2, -9 | Phase 3/cancer |
| Integrins | |||
| Efalizumab/Hu1124 | Humanized MAb | CD11α subunit | Phase 3/psoriasis |
| Anti-CD18 | Humanized MAb | CD18 | Phase 2/myocardial infarction |
| Anti-LFA1 | Murine | CD18 | Phase 3/allograft rejection |
| Hu23F2G | Humanized MAb | CD11/CD18 integrin | Phase 2/multiple sclerosis; phase 2/myocardial infarction; phase 3/stroke |
| LDP-01 | Humanized MAb | CD18 integrin subunit | Phase 2/allograft rejection/stroke |
| LDP-02 | Humanized MAb | α4β7-integrin | Phase 2/ulcerative colitis |
| Volociximab & erlotinnib | MAb | α5β1-integrin | Phase 2/metastatic NSCL |
| ATN-161 | PHSRN motif from FN | α5β1-integrin | Phase 1/NSCL |
| M200 | MAb | α5β1-integrin | Phase 2/kidney cancer |
| Vitaxin/LM609 | Humanized MAb | αVβ3-integrin | Phase 2/sarcoma |
| Antegren | Humanized MAb | α4β1-integrin | Phase 3/multiple sclerosis; phase 2/colitis, Crohn disease |
| Tysabri/natalizumab | MAb | α4β1-integrin | Phase M/multiple sclerosis |
| Abciximab | Chimeric Ab | αIIbβ3, αVβ3, αMβ2 | FDA approved |
| Eptifibatide | Cyclic heptapeptide | αIIbβ3-integrin | FDA approved |
| Tirofiban | Peptidomimetic | αIIbβ3-integrin | FDA approved/myocardial infarction |
| Cilengitide | Cyclic RGD peptide | αVβ3/αVβ5-integrin | Phase 2/GBM |
| Altocor/lovastatin | Chemical | αLβ2-integrin | FDA approval/atherosclerosis |
Inhibitors . | Structure . | Specificity . | Status/indication . |
|---|---|---|---|
| MMPs | |||
| Batimastat (BB-94) | Peptidomimetic | MMP-1, -2, -3, -7, -9 | Development halted |
| Marimastat (BB-2516) | Peptidomimetic | MMP-1, -2, -7, -9 | Phase 3/gastric cancer; phase 2/pancreatic cancer |
| BAY12-9566 | Nonpeptidomimetic | MMP-2, -3, -9 | Development halted |
| AG3340 | Nonpeptidomimetic | MMP-2, -3 | Phase 2/3/no benefit |
| BMS-275291 | Nonpeptidomimetic | Broad spectrum | Phase 1/2/NSCL |
| MMI270 | Nonpeptidomimetic | Broad spectrum | Phase 1/advanced cancer |
| Metastat (Col-3) | Tetracycline derivative | MMP-2, -9 | Phase 2/Kaposi sarcoma |
| Periostat | Tetracycline derivative | Broad spectrum | Phase M/periodontal disease |
| Neovastat (AE-941) | Shark cartilage extract | Broad spectrum | Phase 2/multiple myeloma; phase 3/NSCL |
| Not known | Green tea extract | MMP-2, -9 | Phase 3/cancer |
| Integrins | |||
| Efalizumab/Hu1124 | Humanized MAb | CD11α subunit | Phase 3/psoriasis |
| Anti-CD18 | Humanized MAb | CD18 | Phase 2/myocardial infarction |
| Anti-LFA1 | Murine | CD18 | Phase 3/allograft rejection |
| Hu23F2G | Humanized MAb | CD11/CD18 integrin | Phase 2/multiple sclerosis; phase 2/myocardial infarction; phase 3/stroke |
| LDP-01 | Humanized MAb | CD18 integrin subunit | Phase 2/allograft rejection/stroke |
| LDP-02 | Humanized MAb | α4β7-integrin | Phase 2/ulcerative colitis |
| Volociximab & erlotinnib | MAb | α5β1-integrin | Phase 2/metastatic NSCL |
| ATN-161 | PHSRN motif from FN | α5β1-integrin | Phase 1/NSCL |
| M200 | MAb | α5β1-integrin | Phase 2/kidney cancer |
| Vitaxin/LM609 | Humanized MAb | αVβ3-integrin | Phase 2/sarcoma |
| Antegren | Humanized MAb | α4β1-integrin | Phase 3/multiple sclerosis; phase 2/colitis, Crohn disease |
| Tysabri/natalizumab | MAb | α4β1-integrin | Phase M/multiple sclerosis |
| Abciximab | Chimeric Ab | αIIbβ3, αVβ3, αMβ2 | FDA approved |
| Eptifibatide | Cyclic heptapeptide | αIIbβ3-integrin | FDA approved |
| Tirofiban | Peptidomimetic | αIIbβ3-integrin | FDA approved/myocardial infarction |
| Cilengitide | Cyclic RGD peptide | αVβ3/αVβ5-integrin | Phase 2/GBM |
| Altocor/lovastatin | Chemical | αLβ2-integrin | FDA approval/atherosclerosis |
Phase M indicates on the market; NSCL, non-small-cell lung cancer; Mab, monoclonal antibody; FN, fibronectin; GBM, glioblastoma multiforme; and FDA, Food and Drug Administration
The design of collagen peptidomimetic MMPIs is based on the collagen-peptide backbone with zinc-binding hydroxamate moiety that coordinates the Zn2+ ion, thus inhibiting the MMP catalytic activity. An oral MMPI, marimastat, significantly increased survival of patients with gastric carcinoma. Treatment with marimastat was well tolerated by the patients, except for some minor side effects such as musculoskeletal pain, probably because of the need of MMPs in normal remodeling of the connective tissue of tendons and joints. In patients with advanced pancreatic cancer (a phase 2 study), marimastat showed comparable therapeutic effects as conventional therapy with gemcitabine that was used.133 The survival of patients suffering from glioblastoma multiforme was also improved by using marimastat in combination with temozolomide, a cytotoxic drug.134 Several nonpeptidomimetic MMP inhibitors, including BMS-275291, AG3340, and MMI270, have also been tested in clinical trials (Table 2).
Tetracyclines and biphosphonates have also been shown to block MMP activity.135 For example, a broad spectrum MMP inhibitor, metastat (or Col-3), showed increased tumor cell toxicity, reduced tumor-induced angiogenesis, as well as antimetastatic activity,136 and is currently being tested in patients with Kaposi sarcoma and brain cancer in a phase 2 clinical trial. Periostat, a tetracycline used for the treatment of periodontal diseases, is the only MMPI on the market. Of interest, compounds (TSRI265) capable of inhibiting interactions between MMPs and integrins showed promising results in animal experiments.85 Also, a cyclic peptide, CTTHWGFTLC, discovered by phage display technology as a selective gelatinase inhibitor, could block cell migration and tumor growth in a gelatinase-dependent manner.137
The involvement of integrins in tumor cell invasion and metastasis became clear after using αV-138 or β1-139 subunit-blocking antibodies or small synthetic antagonists generated from the ligand's recognition sequence. Humanized mAbs, vitaxin and efalizumab against αVβ3-140 and αL-subunit of αLβ2,141 respectively, and the synthetic, cyclic Arg-Gly-Asp (RGD) peptide motif142 present in many integrin ligands, were the 3 among many other integrin-binding agents that have entered cancer clinical trials (Table 2). Efalizumab and a recombinant mAb against α4β1, natalizumab, have shown a great promise in the treatment of psoriasis,141 as well as in multiple sclerosis and Crohn disease, respectively.143,144 However, β3- and β5-integrin knock-out mice showed increased expression of VEGFR-2 receptor, leading to enhanced tumor angiogenesis.145 Taken together, MMP and integrin knock-out models and inhibitors can increase our understanding of the multiple functions of these molecules in several diseases, including cancer. Such studies may be used to develop therapeutic agents that can interfere with the integrin and MMP function on invasive tumor cells and blood vessels.
Concluding remarks
The failure of MMPIs in several cancer clinical trials is not surprising.146 Most MMPIs were used to treat patients with late-stage tumors, whereas most results obtained from animal experiments show the need for targeting MMPs in early stages of cancer progression. Also, these inhibitors target all MMPs, many of which are needed for the processing of antiangiogenic factors, including angiostatin and endostatin. For that, increasing the selectivity of these compounds (for example, for gelatinases involved in metastasis) could solve the problem of side effects reported so far. MMPIs are known to target also ADAMTS, enzymes capable of reducing tumor growth by blocking tumor angiogenesis.147 It should be taken into consideration that other proteases are up-regulated during tumor progression that could compensate for the loss of MMPs. These proteases should be identified and targeted along with MMPs.
Extensive effort has been made in developing small molecules, peptides, and peptidomimetics capable of inhibiting interactions that occur on the cell surface. Several linear and cyclic peptides derived from sequences of β2-integrins, ICAMs, and ECM proteins have been shown to have inhibitory effects in vitro and in vivo. Indeed, ICAM-1-derived peptides can control immune responses in autoimmune diseases and allograft rejection by simply blocking ICAM-1 binding to αLβ2-integrin. Peptides derived from the sequence of β1- and β2-integrins have also been shown to be potent anti-inflammatory agents by blocking integrin-mediated adhesion of leukocytes.148 Furthermore, inhibition of an integrin-MMP cell-surface complex, αVβ3/MMP-2, dramatically suppressed tumor angiogenesis in vivo, suggesting that this interaction is essential for endothelial cell proliferation and migration.85 Such reagents were reported not only to interfere with ligand binding, but also could stabilize integrin conformations. Also, integrin-directed small molecules have entered phase 1 and 2 clinical cancer trials, as they showed strong inhibition of tumor angiogenesis.149 Peptides containing the RGD sequence have been demonstrated to inhibit experimental tumor metastasis in animal models.107
Studies on the role of MMP-9 in leukocyte migration have been controversial. For example, some reports have supported MMP-9 function in leukocyte migration,150,151 whereas others have not.152,153 These findings are not surprising as MMPs are known to have overlapping functions and other MMPs within the family could compensate for the loss of MMP-9.
The physiologic role of MMP-2 and -9 is not fully understood, but to our current knowledge they are involved in the processing of the extracellular matrix during growth and tissue differentiation, probably as critical factors for cell motility. Proteases and integrins for such a function have been expected to be colocalized at the surface of migrating leukocytes and other cells. Most MMPs, however, are secreted enzymes and the search for cell-surface receptors for MMPs has been going on for years. At the moment there are some hundred publications describing receptors, such as integrins for various MMPs, among them MMP-2 and -9. Like-wise, gelatinase activity has been found in the membrane of leukocytes, but the identification of the leukocyte integrins as gelatinase receptors is new to our knowledge47,87 and likely to extend our understanding of further mechanisms involved in leukocyte migration. Studies from knock-out models for integrins, including leukocyte β2-integrins, confirm their involvement in various steps of cancer development. Eventually, tumor growth and metastasis could be blocked by interfering with integrin function on tumor cells and blood vessels.
MMP-9 has been reported to cooperate with αVβ3- and α3β1-integrins. MMP-9 was able to stimulate αVβ3-integrin-dependent migration of metastatic breast cancer cells and α3β1-integrin-induced tumor invasion.108 MMP-9 and uPA have been also identified as critical players in the invasion of tumor cells to the blood circulation (a process called intravasation). These proteases may act in concert with integrins, such as αVβ5, which, in turn, is also required for tumor cell dissemination in a chicken chorionallantoic membrane assay.82
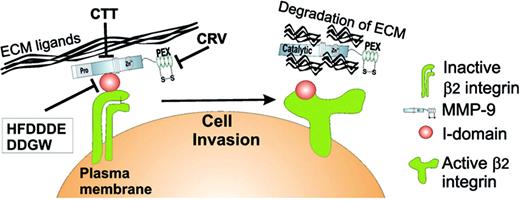
We have recently shown that proMMP-9/αMβ2 complex is stored within the intracellular granules in resting PMNs and translocated to the cell surface upon cell stimulation (Figure 2). This is a more plausible mechanism for the MMP/integrin complex formation than binding of a secreted MMP to an unoccupied integrin on the cell surface. Also, leukocyte integrins could play a role in targeting of proMMPs to a site where proteolytic activity is needed. However, it remains to be determined by which mechanism (pro)MMP-9 is located at the surface of cells lacking β2-integrins. Based on our findings, new and more effective cancer therapeutics could be achieved by blocking, not only MMPs alone but their association with integrins or other cell-surface receptors. A peptide motif derived from the MMP-9 catalytic domain, HFDDDE, and a cyclic peptide, DDGW, discovered by phage display both inhibited proMMP-9/αMβ2 complex formation and leukocyte migration in vitro and in a thioglycolate-induced peritonitis model in vivo.47,87 Also, a C-terminal domain MMP-9-binding peptide, CRVYGPYLLC (or CRV), inhibited the interaction between MMP-9 and αVβ5-integrin, resulting in reduced angiogenesis and tumor invasion.50 Finally, a cyclic peptide, CTTHWGFTLC, discovered by phage display technology as a selective gelatinase inhibitor, could block leukemia cell migration and tumor growth in a gelatinase-dependent manner.87,137 Selective antagonists of the MMP-9/αMβ2-integrin interaction may be therapeutic not only in leukemias but also in other types of malignancies where tumor-infiltrating leukocytes enhance tumor growth.
Schematic summary of the integrin/MMP complex in PMNs. The αMβ2/MMP-9 complex is formed in PMN intracellular granules and can be rapidly mobilized to the cell surface after exposure to degranulation stimuli, such as TNF-β, LPS, and fMLP. PMN degranulation can also be achieved when cells are in contact with ECM proteins. Upon PMN activation, cell-surface receptors are routinely shed from PMNs. Loss of these receptors is mainly due to PMN-derived MMP activity, a process that facilitates PMN rolling and migration via degradation of the vascular basement membrane during PMN extravasation. Disruption of the αMβ2-integrin/MMP-9 complex by integrin-(HFDDDE and DDGW) and MMP-9-(CTT and CRV) binding peptides strongly reduced PMN migration in vitro and in vivo.
Schematic summary of the integrin/MMP complex in PMNs. The αMβ2/MMP-9 complex is formed in PMN intracellular granules and can be rapidly mobilized to the cell surface after exposure to degranulation stimuli, such as TNF-β, LPS, and fMLP. PMN degranulation can also be achieved when cells are in contact with ECM proteins. Upon PMN activation, cell-surface receptors are routinely shed from PMNs. Loss of these receptors is mainly due to PMN-derived MMP activity, a process that facilitates PMN rolling and migration via degradation of the vascular basement membrane during PMN extravasation. Disruption of the αMβ2-integrin/MMP-9 complex by integrin-(HFDDDE and DDGW) and MMP-9-(CTT and CRV) binding peptides strongly reduced PMN migration in vitro and in vivo.
Prepublished online as Blood First Edition Paper, April 11, 2006; DOI 10.1182/blood-2006-02-005363.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal