Dendritic cells (DCs) include a heterogeneous family of professional APCs involved in initiation of immunity and in immunologic tolerance. Specifically, peripheral tolerance can be achieved and maintained by promoting regulatory T-cell (Treg) responses and/or T-cell anergy or deletion. Until recently, immature developmental stages of DC differentiation were believed to induce T-cell anergy or Treg cells, whereas DCs transformed into mature DCs by activation stimuli were thought to represent immunogenic DCs capable of inciting primary T-cell responses. This paradigm has been challenged by the demonstration of Treg-cell expansion by antigen-bearing, fully mature DCs. Similarly, semimature DCs with a distinctive interleukin 10 (IL-10)+IL-12- cytokine production profile might be endowed with tolerogenic functions, supporting the concept that DC maturation per se should no longer be considered as a distinguishing feature of immunogenic as opposed to tolerogenic DCs (TDCs). Cytokine-modulated TDCs reflect an incomplete or altered status of monocyte differentiation and promote in vitro induction of Treg cells and/or in vivo protection from autoimmune diseases. Several growth factors, including IL-10, transforming growth factor β (TGF-β), granulocyte colony-stimulating factor (G-CSF), hepatocyte growth factor (HGF), and vasoactive intestinal peptide (VIP), modulate DC maturation and favor the differentiation of TDCs. From a therapeutic standpoint, cytokine-modulated TDCs might be beneficial for prevention and/or treatment of posttransplantation graft-versus-host disease (GVHD) and autoimmunity.
Introduction
Dendritic cells (DCs) are highly specialized antigen (Ag)-presenting cells (APCs) that integrate a variety of incoming signals and orchestrate the immune response.1 Bidirectional interactions between DCs and Ag-experienced T cells initiate either an immunogenic or a tolerogenic pathway and are of crucial importance in autoimmune diseases and in transplantation medicine.2 Conventional DC subsets described in humans include myeloid DCs (mDCs) and plasmacytoid DCs (pDCs).3 Myeloid DCs develop from CD11c+HLA-DR+ blood precursors and undergo activation and maturation in response to triggering of toll-like receptors (TLRs), a class of pattern recognition receptors engaged by microbial products.1 DC maturation is paralleled by up-regulation of major histocompatibility complex (MHC) class II and costimulatory CD80/CD86 molecules and by production of interleukin (IL)-12. Plasmacytoid DCs might differentiate either from a common blood DC precursor or from a committed lymphoid progenitor; express CD123, CD4, and CD62L; and secrete type I interferon (IFN) in response to viruses and/or TLR9 ligands.3 In secondary lymphoid organs of mice, different subpopulations of DCs have been identified, namely, CD8α+ lymphoid DCs, CD8α- myeloid DCs, and Langerhans cell-derived DCs.4 In addition, expression of B220 on mouse DCs identifies a functional counter-part of human pDCs.4
DC ability to induce tolerance has been demonstrated initially by experiments on immature DCs residing in peripheral lymphoid tissues.5 Under steady-state conditions, immature DCs capture apoptotic bodies arising from cell turnover and, upon migration to draining lymph nodes, silence T cells to self-Ags.6 Short-lived, migratory DCs might transfer tissue-derived peptides to longerlived tolerogenic DCs (TDCs) upon reaching the lymph node. Interestingly, self-Ag transport, processing, and presentation for tolerance induction by DCs require partial maturation.7 In the absence of inflammation or TLR triggering, DCs will not produce IL-12, and DCs will be arrested at a semimature stage. Also, DC residence in a tolerizing milieu, for example, in mucosal or immune-privileged sites, affects DC capacity of priming Treg cells. DCs isolated from Peyer patches, lungs, or the anterior chamber of the eye display a mature phenotype, secrete IL-10 but not IL-12, and drive the development of IL-10-producing regulatory T (Treg) cells.7 Even more intriguing, fully mature, immunologically competent DCs can generate “tolerogenic” peptides upon processing of a self-Ag, thyroid peroxidase.8 Accordingly, Ag-loaded, mature DCs can expand CD4+CD25+ Treg cells with retained ability to suppress the proliferation of nonregulatory T cells.9 Thus, a growing body of experimental evidence indicates that DC maturation per se is neither a distinguishing feature of immunogenic as opposed to TDCs nor a control point for initiating immunity (Figure 1).
Given the remarkable functional plasticity of both mDCs and pDCs, it is presently believed that the net effect of Ag dose, DC lineage and maturational status, DC stimulation by pathogen-derived products, and cytokine milieu at sites of inflammation determines whether an immunogenic or a tolerogenic T-cell response will develop.1,5
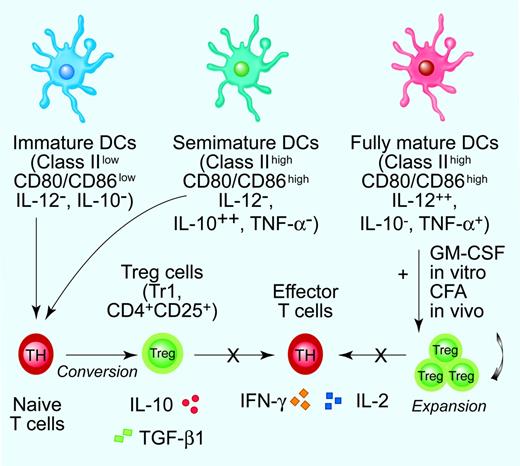
DC maturation stages in tolerance induction. The bimodal concept of fully mature DCs as inducers of immunity as opposed to immature DCs as promoters of T-cell tolerance has been recently revisited. It is presently believed that DCs integrate a variety of incoming signals and decide whether protective immunity or Ag-specific unresponsiveness develops.7 Current experimental evidence supports the concept that cytokine-modulated semimature DCs promote the conversion of naive T cells into Tr1 cells and/or CD4+CD25+ Treg cells that inhibit effector T-cell responses via secreted or membrane-bound immunosuppressive cytokines. The inability to produce detectable amounts of bioactive IL-12p70 coupled with enhanced IL-10 release are unique functional features of cytokine-modulated, semimature TDCs.7,34,39,41 On the contrary, mature DCs might be crucially involved in the expansion of preformed Treg cells, as highlighted by in vitro studies performed with BM-derived, GM-CSF-modulated DCs and by in vivo treatment with complete Freund adjuvant (CFA) and GM-CSF-differentiated DCs.9 Illustration by Paulette Dennis.
DC maturation stages in tolerance induction. The bimodal concept of fully mature DCs as inducers of immunity as opposed to immature DCs as promoters of T-cell tolerance has been recently revisited. It is presently believed that DCs integrate a variety of incoming signals and decide whether protective immunity or Ag-specific unresponsiveness develops.7 Current experimental evidence supports the concept that cytokine-modulated semimature DCs promote the conversion of naive T cells into Tr1 cells and/or CD4+CD25+ Treg cells that inhibit effector T-cell responses via secreted or membrane-bound immunosuppressive cytokines. The inability to produce detectable amounts of bioactive IL-12p70 coupled with enhanced IL-10 release are unique functional features of cytokine-modulated, semimature TDCs.7,34,39,41 On the contrary, mature DCs might be crucially involved in the expansion of preformed Treg cells, as highlighted by in vitro studies performed with BM-derived, GM-CSF-modulated DCs and by in vivo treatment with complete Freund adjuvant (CFA) and GM-CSF-differentiated DCs.9 Illustration by Paulette Dennis.
TDCs: a definition by function
Although expression of signaling lymphocyte activation molecule (SLAM), programmed cell death ligand-1 (PD-L1), DEC-205 (CD205), and inhibitory receptors of the immunoglobulin-like transcript (ILT) family (ILT3/ILT4) has been associated with DC tolerogenicity, TDCs conceivably correspond to functional subtypes rather than to unique DC lineages in vivo.10-13
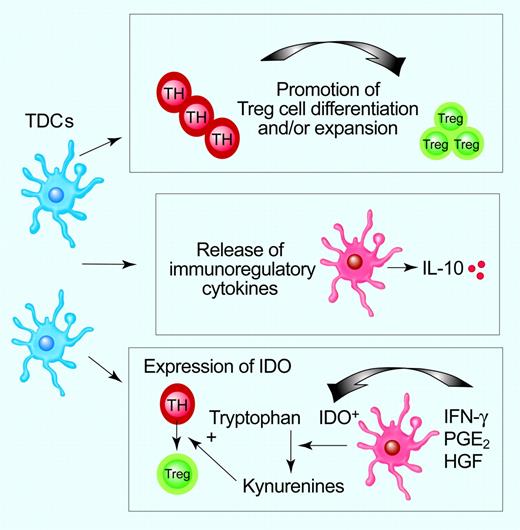
Promotion of Treg-cell conversion from naive T cells and/or expansion of pre-existing Treg cells, release of IL-10, and expression of indoleamine 2,3-dioxygenase (IDO) might underlie DC function in inducing peripheral tolerance (Figure 2).5,14,15 DCs, either at immature or at semimature stage, can differentiate both naturally occurring CD4+CD25+ Treg cells and adaptive, IL-10-secreting CD4+ type 1 (Tr1) cells either in vitro or in vivo,16,17 and overexpression of Jagged-1 on DCs induces Ag-specific, transforming growth factor (TGF)-β-producing Treg cells.18 Immature mDCs convert naive T cells into IL-10-producing Tr1 cells in vitro, whereas Ag-bearing TDCs injected subcutaneously in humans induce IL-10-producing, influenza-specific CD8+ T cells and mediate a decline of IFN-γ-producing T cells.19,20 Additionally, mature DCs expand CD4+CD25+ Tregs with retained suppressive activity and are capable of inhibiting diabetes development in NOD mice.9,21,22 These studies highlight that mature DCs might be critical for the restimulation and/or expansion of functional Treg cells (Figure 1).
IDO mediates consumption of tryptophan and induces T-cell suppression by activation of GCN2 kinase in T cells, altered APC function, bystander suppression, and/or generation of Treg cells.14,23 In mice, IDO expression segregates with B220+ pDCs and CD8α+ splenic DCs and with CD19+ pDCs contained within tumor-draining lymph nodes.24,25 In humans, CD123+CCR6+ DCs might acquire IDO functional activity after activation by IFN-γ or PGE2 or after CD80/CD86 ligation by cytotoxic T-lymphocyte Ag 4 (CTLA4)/CD28 on Treg cells.26,27
Intriguing evidence has been provided on the differential dynamics of DC-T-cell encounters in intact lymph nodes in the context of immunity and tolerance. During the course of tolerance induction by in vivo targeting of ovoalbumin to CD205 on DCs, T cells remain motile and establish transient interactions (lasting 30-90 seconds) with one or multiple Ag-bearing DCs (10-13 per hour).28 Conversely, stable and long-lasting DC-T-cell contacts culminate in productive T-cell responses. A functional consequence of the proposed model is that multiple brief signals delivered by a high proportion of DCs would be required to maintain peripheral T-cell tolerance.28
Cytokines as inducers of TDCs
DCs can be licensed to become tolerogenic by a variety of cytokines (Table 1). Conventionally, IL-10 and/or TGF-β have been implicated in the in vitro differentiation of TDCs. IL-10 interferes with the granulocyte macrophage (GM)-CSF/IL-13-induced differentiation of human monocytes to DCs but rather promotes the generation of macrophages with enhanced endocytic activity and poor Ag-presenting function.29 In mice, IL-10-elicited CD11clowCD45RBhigh TDCs acquire plasmacytoid morphology and immature phenotype and promote Tr1 differentiation in vitro and T-cell anergy in vivo.30 TGF-β-treated murine APCs induce CD8+ Treg cells, suppress de novo experimental autoimmune encephalomyelitis (EAE), and modulate ongoing EAE.31 Tumor necrosis factor α (TNF-α) differentiates murine semimature IL-12low DCs, expressing high levels of MHC class II and costimulatory molecules.32 Such thyroglobulin (TG)-pulsed, TNF-α-matured DCs induce IL-10-secreting CD4+CD25+FoxP3+ Treg cells, capable of suppressing TG-specific T cells in a cell contact-dependent manner.32 Similarly, GM-CSF induces murine semimature IL-12low DCs in vivo with high expression of MHC class II and costimulatory molecules.33
Cytokines involved in the in vitro or in vivo differentiation of TDCs
Cytokine, experimental model . | Additional stimuli applied during in vitro differentiation . | Sources . |
|---|---|---|
| TNF-α | Verginis et al,32, Menges et al43 | |
| Suppression of EAT | Supernatant of a GM-CSF–transfected cell line | |
| Suppression of EAE | Supernatant of a GM-CSF–transfected cell line or GM-CSF | |
| GM-CSF | Gangi et al33 | |
| Suppression of EAT after in vivo provision of GM-CSF | NA | |
| IL-10/TGF-β | Sato et al44 | |
| Protection from lethal GVHD | GM-CSF | |
| G-CSF | Rutella and colleagues,39,40,46 Kared et al,45 Morris et al50 | |
| Differentiation of human monocyte-derived DCs | Post–G-CSF serum containing high levels of IL-10/IFN-α | |
| Provision of G-CSF in vivo to bone marrow donor mice | NA | |
| Provision of G-CSF in vivo to mice with EAE, diabetes, and lupus nephritis | NA | |
| IFN-λ | Mennechet and Uzé37 | |
| Differentiation of human monocyte-derived DCs | GM-CSF/IL-4 | |
| VIP | Chorny and colleagues34,47 | |
| Differentiation of mouse bone marrow–derived DCs for subsequent use in EAE and rheumatoid arthritis | GM-CSF | |
| Differentiation of mouse bone marrow-derived DCs for subsequent use in posttransplantation GVHD | GM-CSF | |
| M-CSF | Li et al38 | |
| Differentiation of human monocyte-derived DCs | IL-4 | |
| TGF-β | Faunce et al31 | |
| Treatment of EAE mice with thioglycolate-elicited, mouse peritoneal exudate cells | Antigen (MBP) | |
| HGF | Rutella et al41 | |
| Differentiation of human monocyte-derived DCs | None or GM-CSF | |
| IL-16/TPO | Della Bella et al36 | |
| Differentiation of human CD34-derived DCs | GM-CSF/IL-4/Flt3L/TNF-α/SCF | |
| IL-21 | Brandt et al35 | |
| Differentiation of mouse bone marrow–derived DCs | GM-CSF | |
| IL-10 | Allavena et al,29 Wakkach et al30 | |
| Differentiation of human monocyte–derived DCs | GM-CSF/IL-13 | |
| Differentiation of mouse bone marrow–derived DCs; isolation of their natural in vivo counterpart | GM-CSF/TNF-α | |
| TSLP | Watanabe et al42 | |
| Differentiation of human CD4+ CD25+ FoxP3+ thymocytes by TSLP-treated CD11c+ thymic DCs | None |
Cytokine, experimental model . | Additional stimuli applied during in vitro differentiation . | Sources . |
|---|---|---|
| TNF-α | Verginis et al,32, Menges et al43 | |
| Suppression of EAT | Supernatant of a GM-CSF–transfected cell line | |
| Suppression of EAE | Supernatant of a GM-CSF–transfected cell line or GM-CSF | |
| GM-CSF | Gangi et al33 | |
| Suppression of EAT after in vivo provision of GM-CSF | NA | |
| IL-10/TGF-β | Sato et al44 | |
| Protection from lethal GVHD | GM-CSF | |
| G-CSF | Rutella and colleagues,39,40,46 Kared et al,45 Morris et al50 | |
| Differentiation of human monocyte-derived DCs | Post–G-CSF serum containing high levels of IL-10/IFN-α | |
| Provision of G-CSF in vivo to bone marrow donor mice | NA | |
| Provision of G-CSF in vivo to mice with EAE, diabetes, and lupus nephritis | NA | |
| IFN-λ | Mennechet and Uzé37 | |
| Differentiation of human monocyte-derived DCs | GM-CSF/IL-4 | |
| VIP | Chorny and colleagues34,47 | |
| Differentiation of mouse bone marrow–derived DCs for subsequent use in EAE and rheumatoid arthritis | GM-CSF | |
| Differentiation of mouse bone marrow-derived DCs for subsequent use in posttransplantation GVHD | GM-CSF | |
| M-CSF | Li et al38 | |
| Differentiation of human monocyte-derived DCs | IL-4 | |
| TGF-β | Faunce et al31 | |
| Treatment of EAE mice with thioglycolate-elicited, mouse peritoneal exudate cells | Antigen (MBP) | |
| HGF | Rutella et al41 | |
| Differentiation of human monocyte-derived DCs | None or GM-CSF | |
| IL-16/TPO | Della Bella et al36 | |
| Differentiation of human CD34-derived DCs | GM-CSF/IL-4/Flt3L/TNF-α/SCF | |
| IL-21 | Brandt et al35 | |
| Differentiation of mouse bone marrow–derived DCs | GM-CSF | |
| IL-10 | Allavena et al,29 Wakkach et al30 | |
| Differentiation of human monocyte–derived DCs | GM-CSF/IL-13 | |
| Differentiation of mouse bone marrow–derived DCs; isolation of their natural in vivo counterpart | GM-CSF/TNF-α | |
| TSLP | Watanabe et al42 | |
| Differentiation of human CD4+ CD25+ FoxP3+ thymocytes by TSLP-treated CD11c+ thymic DCs | None |
EAT indicates experimental autoimmune thyroiditis; EAE, experimental autoimmune encephalomyelitis; NA, not applicable; TSLP, thymic stromal lymphopoietin; and MBP, myelin basic protein
Mechanisms underlying the tolerogenic functions of DCs. TDCs might promote Ag-specific T-cell unresponsiveness by several mechanisms, including conversion of naive T cells into Treg cells, release of immunosuppressive cytokines, and expression of functional IDO with subsequent tryptophan depletion, accumulation of kynurenines, and enhancement of Treg-cell generation. Illustration by Paulette Dennis.
Mechanisms underlying the tolerogenic functions of DCs. TDCs might promote Ag-specific T-cell unresponsiveness by several mechanisms, including conversion of naive T cells into Treg cells, release of immunosuppressive cytokines, and expression of functional IDO with subsequent tryptophan depletion, accumulation of kynurenines, and enhancement of Treg-cell generation. Illustration by Paulette Dennis.
More recently, novel and unrecognized roles have been attributed to individual cytokines in the promotion of DC tolerogenicity (Table 1). Vasoactive intestinal peptide (VIP) is a neuropeptide released by immune cells in response to Ag stimulation and a potent anti-inflammatory agent. Exposure of murine BM-derived DCs to VIP translates into the differentiation of maturation-resistant, IL-10-secreting APCs.34 DCs modulated with VIP express high levels of MHC but low levels of costimulatory molecules and induce anergic Tr1-like cells.34 Also, IL-21 polarizes the GM-CSF-driven differentiation of murine BM-derived DCs toward immature DCs that inhibit Ag-specific T-cell proliferation.35
In humans, IL-16 and thrombopoietin govern the generation of CD34+ cell-derived IL-10+IL-12low TDCs, co-expressing ILTs, CD80/CD86, and MHC class II, and with impaired ability to present influenza virus to autologous CD4+ T cells.36 IFN-λ (IL-28/IL-29)-treated DCs acquire an MHC class I/II++ and costimulationlowIL-12p70low phenotype and induce an IL-2-dependent proliferation of CD4+CD25+FoxP3+ Treg cells.37 Macrophage (M)-CSF and IL-4 can induce monocyte-derived IL-10+IL-12neg TDCs.38 Granulocyte (G)-CSF indirectly favors the in vitro differentiation of peripheral blood monocytes into TDCs through the release of IL-10 and IFN-α.39 G-CSF-induced TDCs possess a mature, HLA-DRhighCD80/86high phenotype and release trace amounts of IL-12p70. Furthermore, G-CSF-driven TDCs activate the in vitro differentiation of IL-10/TGF-β-producing Tr1 cells, capable of suppressing bystander T cells in a cytokine-dependent manner.39 Similarly, naive CD4+ T cells exposed to G-CSF in vivo differentiate into IL-10-producing Tr1 cells after in vitro allo-Ag challenge.40 Hepatocyte growth factor (HGF) has been implicated in angiogenesis and promotion of tumor cell migration and invasiveness. HGF skews monocyte differentiation toward IL-10-producing, ILT3+CD209- costimulation-low TDCs.41 HGF-differentiated monocytes induce the in vitro development of CD4+CD25+FoxP3+ Treg cells that suppress conventional, nonregulatory T cells in a cell contact-dependent manner. HGF evokes a unique gene signature in monocytes, consisting of overexpression of genes potentially implicated in immune tolerance, for example, IDO, C5R1, CCL2, complement component C1q, and IL-10.41 Mechanistically, DC production of IL-10 and DC expression of ILT3 and IDO cooperate to the in vitro differentiation of Treg cells by HGF-modulated TDCs.41
Thymic stromal lymphopoietin (TSLP) is produced by epithelial cells of thymic Hassall's corpuscles. TSLP-treated thymic DCs express CD80/CD86 and MHC class II but release negligible amounts of IL-12p70 and promote the conversion of CD4+CD25- thymocytes into CD4+CD25+FoxP3+ Treg cells.42 Interestingly, TSLP-activated DCs exert limited effects on the expansion of preformed CD4+CD25+ thymocytes, supporting the notion that cytokine-modulated semimature TDCs might be preferentially implicated in the conversion of naive T cells into Tregs.42
TDCs in preclinical models of immune-mediated diseases and in organ transplantation
Because DCs play an undisputed role in inciting autoimmune diseases and in instigating transplant rejection, therapeutic harnessing of peripheral tolerance by TDCs represents an attractive avenue for future clinical applications. Animal models of autoimmunity have provided proof of principle in favor of therapeutic benefits of cytokine-modulated TDCs (Table 1).
In mice, Ag-pulsed, TGF-β2-treated TDCs induce regulatory CD8+ T cells and suppress ongoing EAE.31 TNF-α-differentiated, semimature DCs induce IL-10-secreting, Ag-specific Treg cells and protect from EAE.43 Host-matched TDCs differentiated with IL-10, TGF-β, and GM-CSF reduce serum levels of proinflammatory cytokines, induce mixed CD25+ and IL-10+ subpopulations of Tregs with retained graft-versus-leukemia (GVL) responses, and protect from lethal GVHD.44 Semimature DCs induced by GM-CSF expand TG-specific, IL-10-secreting CD4+CD25+ Treg cells, which suppress experimental autoimmune thyroiditis (EAT) upon adoptive transfer into TG-primed mice.33 Similarly, TNF-α-matured DCs pulsed with TG inhibit EAT by inducing CD4+CD25+ Treg cell activation and secretion of IL-10.32
Treatment with G-CSF is beneficial in spontaneous type 1 diabetes in NOD mice through reciprocal effects on TDCs and Treg cells.45 TDCs in protected mice are enriched in CD11c+B220+Gr-1+ cells producing IFN-α in the absence of measurable IL-12p70 release.45 G-CSF-recruited pDCs exhibit a semimature phenotype with reduced expression of MHC molecules and CD80, but normal levels of CD86 and CD40, and trigger the accumulation of CD4+CD25+ Treg cells upon adoptive transfer to secondary NOD recipients.45,46
VIP-differentiated TDCs possess therapeutic effect in murine EAE and rheumatoid arthritis.34 CD4+ T cells isolated from TDC-treated animals are enriched in CD4+CD25+FoxP3+ Treg cells and IL-10+ Tr1 cells. Additionally, host-matched, VIP-generated TDCs with an in vivo half-life of 17 days induce the differentiation of CD4+ Treg cells, protecting mice from acute GVHD in a haplotype- and TGF-β/IL-10-dependent manner and, remarkably, leave GVL responses unaffected.47
DCs participate in active regulation of allogeneic graft rejection. In rodents, administration of host-type immature DCs promotes Treg responses and prolongs graft survival.48 Although most attempts to induce donor Ag-specific tolerance have employed donor-derived DCs to interfere with the direct pathway of allorecognition, several techniques have been developed to load recipient DCs with donor MHC allo-Ags, thus modulating the indirect pathway of allorecognition. In this respect, in vivo targeting of recipient-type DCs with intravenous administration of donor MHC+ apoptotic cells prolongs the survival of vascularized heart allografts in the absence of concomitant immunosuppression.48
Permanent acceptance of organ allografts has not been generally achieved with TDC therapy alone. Blockade of the CD40-CD154 signaling pathway might be synergistic with DC therapy, thus promoting the survival of skin allografts.48 Pharmacologically treated DCs that are decommissioned from full maturation might also contribute to the maintenance of transplantation tolerance through the promotion of Treg-cell differentiation.48 Simultaneous targeting of DCs and Treg cells might be a desirable approach to induce transplantation tolerance, and a self-maintaining regulatory loop has been recently described in a murine model of cardiac transplantation, where TDCs induce Treg cells and Treg cells, in turn, program the generation of TDCs.49 Importantly, inclusion of TDCs in future therapeutic regimens might minimize dependence on nonspecific immunosuppressive drugs currently administered for transplant rejection.
Conclusions and future perspectives
Theoretically, TDCs for clinical application might be obtained after in vivo cytokine administration or might be generated ex vivo from monocyte populations with good manufacturing practice (GMP)-grade cytokine cocktails. Cytokines possess druglike properties, such as potency, but also have disadvantages, such as a short half-life. Considerable effort has been devoted to engineering cytokines with enhanced half-lives and, for instance, pegylated G-CSF manifests a remarkable potency in inducing the differentiation of Treg cells that protect against mouse acute GVHD.50
Pharmacologic arrest of DC maturation or use of genetically engineered DCs expressing immunosuppressive molecules might selectively enhance DC tolerogenicity. Among the various drugs implicated in the promotion of DC tolerogenicity, 1α,25-dihydroxivitamin D3 induces the differentiation of TDCs with up-regulated expression of ILT3, low IL-10, and enhanced IL-12 secretion.51 The armamentarium of inhibitory pharmacologic agents is expected to increase as more compounds are evaluated for the ability to affect DC functions.
Transfection of DCs with a gene construct encoding a modified CTLA4 molecule translates into deficient expression of CD80/CD86 and induction of T-cell anergy and might represent an attractive means of restoring tolerance in autoimmune diseases.52 A human HGF expression vector administered in liposomal formulation decreases IL-12, IFN-γ, and TNF-α expression in tissues, thus improving mice survival from acute GVHD.53 It is tempting to speculate that the beneficial effects of HGF treatment on GVHD reported in this study might be, at least in part, attributed to the cytokine-driven promotion of DC tolerogenicity.41
An important issue to be considered when designing DC-based immunotherapy protocols is whether TDCs might inadvertently receive in vivo maturation signals in an inflammatory microenvironment and incite unwanted T-cell responses as fully mature DCs. To date, several reports have demonstrated a stable phenotype of cytokine-modulated DC preparations, indicating that TDCs differentiated in the presence of G-CSF, IL-21, VIP, or low-dose GM-CSF might be resistant to further maturation-inducing stimuli.7,34,39 This concern is further mitigated by recent findings that endogenous modulators produced at sites of inflammation, for example, PGE2 and histamine, might interfere with DC maturation and promote DC tolerogenic functions.54,55
GVHD represents a privileged clinical setting for human trials of TDCs/Treg cells because of predictable time of onset, profound host lymphopenia favoring homeostatic proliferation of infused and/or in vivo-generated Tregs, broad alloreactive repertoire, and relative ease of procurement of donor-type monocytes and/or CD4+CD25+ Treg cells. Theoretically, TDCs might be differentiated from host-type monocytes challenged with donor-derived cells in the presence of immunoregulatory cytokines and/or drugs. It is conceivable that TDCs must be administered repeatedly after disease onset, as TDC half-life approaches 17 to 18 days in animal models of GVHD.44,47 Ongoing clinical trials in haploidentical stem cell transplantation will determine whether donor cells cultured ex vivo with IL-10 in the presence of irradiated host cells provide immune reconstitution with anergic, host-specific Tr1 cells.17
It remains to be determined whether TDCs induce undesired in vivo systemic immunosuppression through the generation of Treg cells. Whereas activation of Treg cells is Ag-specific, activated Treg cells might induce Ag-nonspecific suppression.16 Importantly, TDC therapy of GVHD is associated with maintenance of GVL activity, thus re-assuring of preserved antitumor T-cell responses in TDC-treated animals.44 In vitro experimental evidence further indicates that the tolerizing capacity of semimature TDCs might be restricted to Ag-specific CD4+ T cells and leave CD8+ T-cell effector functions unaffected.56 Collectively, basic findings on DC functional plasticity provide grounds for optimism in clinical translation of TDCs to human immune-mediated disorders. Manipulation of DC effector functions by external stimuli warrants further investigations and will hopefully lead to safe and efficacious TDC-based therapies in the near future.
Supported by Fondazione Cassa di Risparmio di Roma, Rome, Italy, and by the Italian Ministry for University and Research (MIUR).
Prepublished online as Blood First Edition Paper, May 9, 2006; DOI 10.1182/blood-2006-03-006403.
We apologize to colleagues whose studies could not be cited because of space restrictions. We thank Professor Claudio Fiocchi (The Cleveland Clinic Foundation, Lerner Research Institute, Cleveland, OH) for helpful comments.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal