Abstract
The FIP1L1-PDGFRA oncogene is a common cause of chronic eosinophilic leukemia (CEL), and encodes an activated tyrosine kinase that is inhibited by imatinib. FIP1L1-PDGFRA–positive patients with CEL respond to low-dose imatinib therapy, but resistance due to acquired T674I mutation has been observed. We report here the identification of sorafenib as a potent inhibitor of the FIP1 like 1–platelet-derived growth factor receptor alpha (FIP1L1-PDGFRα) (T674I) mutant. Sorafenib inhibited the proliferation of FIP1L1-PDGFRα and FIP1L1-PDGFRα(T674I)–transformed Ba/F3 cells and induced apoptosis of the EOL-1 cell line at a low nanomolar concentration. Western blot analysis confirmed that these effects were due to a direct effect on FIP1L1-PDGFRα and FIP1L1-PDGFRα(T674I). Sorafenib was recently approved for the treatment of renal cell carcinoma. Our data suggest that low doses of sorafenib could be efficient for the treatment of FIP1L1-PDGFRA–positive CEL and could be used to overcome resistance to imatinib associated with the T674I mutation.
Introduction
Idiopathic hypereosinophilic syndrome (HES) is a rare myeloproliferative disorder characterized by unexplained sustained high-grade eosinophilia (> 1500 eosinophils/μL blood). When clonality is demonstrated, the disease is reclassified as chronic eosinophilic leukemia (CEL).1,2 The imatinib-responsiveness of these malignancies led to the discovery of the FIP1L1-PDGFRA fusion gene in CEL, and the FIP1L1-PDGFRA fusion was also identified later in a subset of patients with systemic mastocytosis.3,4 FIP1L1-PDGFRA encodes a constitutively active tyrosine kinase that transforms hematopoietic cells in vitro and in vivo.3,5 FIP1L1-PDGFRα is more sensitive to imatinib than BCR-ABL, and patients respond well to low doses of imatinib.3,4,6,7
The development of resistance to small molecule kinase inhibitors has emerged as an important problem for targeted therapy of cancer, most often due to acquired mutations in the target kinase.8 These observations indicate that more than one inhibitor may be required for long-term treatment of patients with cancer. In the context of FIP1L1-PDGFRA–positive CEL, the development of resistance to imatinib due to a T674I point mutation in the ATP binding site of FIP1L1-PDGFRα that is homologous to the T315I mutation in BCR-ABL in chronic myeloid leukemia has been described.3,9-11 In this study we tested different small molecule inhibitors with known activity against PDGFR, KIT, or FLT3 for their inhibitory activity against FIP1L1-PDGFRα and its imatinib-resistant T674I mutant.
Study design
Inhibitors
PDGFR kinase inhibitor-I, -II, and -III, GTP-14 564, SU5614, AGL2043, VEGFR kinase inhibitor-IV, and K-252a were purchased from Calbiochem (San Diego, CA). Sorafenib (BAY43-9006, Nexavar) was custom synthesized. The inhibitors were stored in dimethyl sulfoxide (DMSO) at –20°C and diluted in RPMI-1640 medium.
Cell culture
Ba/F3 cells transformed by FIP1L1-PDGFRA variants or other activated tyrosine kinases were grown in RPMI-1640 medium supplemented with 10% fetal bovine serum. The EOL-1 (DSMZ, Braunschweig, Germany) and K562 cell lines were grown in RPMI-1640 medium supplemented with 20% fetal bovine serum. For dose-response curves, cells were seeded at 3 × 105 cells/mL, and viable cell numbers were determined at the beginning and after 24 hours (Ba/F3 cells) or 48 hours (EOL-1 cells) using the Celltiter AQueousOne Solution (Promega, Madison, WI) or trypan blue exclusion. Dose-response curves were fitted using Origin (OriginLab, Northampton, MA).
Western blotting
Cells were treated with kinase inhibitors for 90 minutes and then lysed in cold lysis buffer containing 1% Triton X-100 and phosphatase inhibitors. Samples were reduced and gel electrophoresis was performed using NuPage Bis-Tris 4% to 12% gels (Invitrogen, Carlsbad, CA). Standard Western blotting procedures were used with the polyclonal anti–phospho-(PDGFRα), polyclonal anti-PDGFRα, monoclonal anti-ERK2 (Santa Cruz Biotechnology, Santa Cruz, CA), monoclonal anti–phospho-ERK1/2 (Cell Signaling, Beverly, MA), and antimouse/antirabbit peroxidase-labeled antibodies (Amersham Biosciences, Freiburg, Germany).
Apoptosis assay
Apoptotic cells were detected by flow cytometric analysis, using Annexin-V and propidium iodide staining (Roche, Milan, Italy). Cells were analyzed on a FACScalibur cytometer (BectonDickinson, Mountain View, CA).
Results and discussion
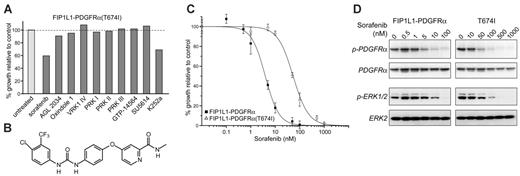
To identify novel potent inhibitors of FIP1L1-PDGFRα and its imatinib-resistant T674I mutant, we screened a variety of inhibitors with known activity against PDGFR, KIT, or FLT3, including sorafenib (BAY43-9006), a B-RAF inhibitor known to inhibit PDGFR.12 Although most of these inhibitors showed potent inhibition of FIP1L1-PDGFRα, only sorafenib and K-252a inhibited the growth of Ba/F3 cells transformed by FIP1L1-PDGFRα(T674I) at 100 nM (Figure 1A).
Further experiments were performed using concentrations of sorafenib (structure shown in Figure 1B) between 1 nM and 100 nM. Sorafenib induces a 50% inhibition of the growth of Ba/F3 cells transformed by FIP1L1-PDGFRα or its imatinib-resistant T674I mutant at 4 nM and 54 nM, respectively (Figure 1C). Western blotting analysis determining the phosphorylation status of FIP1L1-PDGFRα or FIP1L1-PDGFRα(T674I) confirmed that this inhibition was due to a direct effect on these kinases. In addition, the phosphorylation of ERK1/2, downstream effectors of FIP1L1-PDGFRα signaling, were also reduced upon treatment with sorafenib. Taken together, these results confirmed that sorafenib is a potent inhibitor of both FIP1L1-PDGFRα and FIP1L1-PDGFRα(T674I) (Figure 1D). In contrast, a direct inhibitory effect of K-252a on these kinases could not be confirmed, and thus K-252a is not a direct inhibitor of FIP1L1-PDGFRα(T674I) (data not shown).
Sorafenib inhibits FIP1L1-PDGFRα and FIP1L1-PDGFRα(T674I). (A) Initial screen of different PDGFR inhibitors (100 nM) using Ba/F3 cells expressing FIP1L1-PDGFRA(T674I). The inhibition by K252a was shown to be due to nonspecific toxicity. (B) Structure of sorafenib. (C) Dose-response curves showed inhibition of the growth of Ba/F3 cells expressing FIP1L1-PDGFRA or FIP1L1-PDGFRA(T674I) by sorafenib. Error bars show standard deviation. (D) Western blot analysis confirmed that sorafenib directly inhibits FIP1L1-PDGFRα and FIP1L1-PDGFRα(T674I). Phosphorylation of ERK1/2 was also decreased upon sorafenib treatment.
Sorafenib inhibits FIP1L1-PDGFRα and FIP1L1-PDGFRα(T674I). (A) Initial screen of different PDGFR inhibitors (100 nM) using Ba/F3 cells expressing FIP1L1-PDGFRA(T674I). The inhibition by K252a was shown to be due to nonspecific toxicity. (B) Structure of sorafenib. (C) Dose-response curves showed inhibition of the growth of Ba/F3 cells expressing FIP1L1-PDGFRA or FIP1L1-PDGFRA(T674I) by sorafenib. Error bars show standard deviation. (D) Western blot analysis confirmed that sorafenib directly inhibits FIP1L1-PDGFRα and FIP1L1-PDGFRα(T674I). Phosphorylation of ERK1/2 was also decreased upon sorafenib treatment.
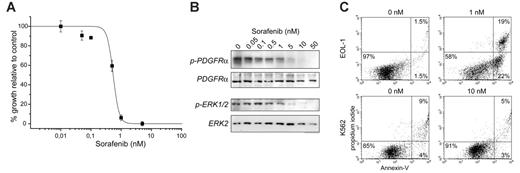
We next tested the activity of sorafenib in the EOL-1 cell line. EOL-1 cells were derived from a patient with FIP1L1-PDGFRA–positive CEL, and are a good in vitro model for the study of FIP1L1-PDGFRα inhibitors.13 The proliferation of EOL-1 cells was inhibited by sorafenib with an inhibitory concentration (IC50) value of 0.5 nM, and correlated with the inhibition of the phosphorylation of FIP1L1-PDGFRα and ERK1/2 (Figure 2A-B). In addition, 24 hours of treatment of EOL-1 cells with 1 nM sorafenib induced apoptotic cell death in more than 40% of the cells, whereas this treatment had no effect on BCR-ABL–expressing K562 cells (Figure 2C). The fact that EOL-1 cells are more sensitive to sorafenib than Ba/F3 cells is likely caused by the lower expression of the fusion gene and the difference in intracellular sorafenib concentrations.
We finally tested the specificity of sorafenib, by determining its inhibitory effect on a panel of activated tyrosine kinases expressed in Ba/F3 cells. At a concentration of 100 nM, sorafenib inhibited only PDGFRα, KIT, and FLT3, and showed no activity against the other tested tyrosine kinases (ABL1, ALK, AXL, EPHA2, EPHB4, FGFR1, JAK2, Lck, Mst1r, SRC, SYK, Tnk1, ZAP-70) at concentrations up to 1 μM (data not shown). These results, together with previously published data,14 confirm that sorafenib has no general inhibitory activity against tyrosine kinases at the concentration needed to inhibit FIP1L1-PDGFRα and FIP1L1-PDGFRα(T674I).
The development of potent inhibitors of BCR-ABL(T315I) or FIP1L1-PDGFRα(T674I) has been a challenge due to the “gatekeeper” function that was ascribed to this threonine residue in a variety of tyrosine kinases.3,11,15-17 We have previously reported that PKC412 has inhibitory activity against FIP1L1-PDGFRα(T674I), whereas there is relative resistance to AMN-107.5,18 Here we report that among 9 additional inhibitors with activity against FIP1L1-PDGFRα, only sorafenib had potent inhibitory activity for the T674I mutant. Although the development of resistance to imatinib due to the T674I mutation has been described in only 2 FIP1L1-PDGFRA–positive patients,3,9,10 this may become an important problem when more patients are treated for longer time.
Sorafenib potently inhibits EOL-1 cells and induces apoptosis. (A) Dose-response curve illustrating the inhibition of growth of EOL-1 cells by sorafenib. Error bars show standard deviation. (B) Western blot analysis confirmed that inhibition is due to a direct effect on FIP1L1-PDGFRα. Phosphorylation of ERK1/2 was similarly decreased upon sorafenib treatment. (C) Apoptosis assay of EOL-1 and K562 cells after 24 hours of treatment with sorafenib. Treatment of EOL-1 cells with 1 nM sorafenib clearly induces apoptosis (22% apoptotic cells and 19% dead cells), whereas treatment of BCR-ABL–expressing K562 cells with 10-fold higher concentrations had no apoptotic effect.
Sorafenib potently inhibits EOL-1 cells and induces apoptosis. (A) Dose-response curve illustrating the inhibition of growth of EOL-1 cells by sorafenib. Error bars show standard deviation. (B) Western blot analysis confirmed that inhibition is due to a direct effect on FIP1L1-PDGFRα. Phosphorylation of ERK1/2 was similarly decreased upon sorafenib treatment. (C) Apoptosis assay of EOL-1 and K562 cells after 24 hours of treatment with sorafenib. Treatment of EOL-1 cells with 1 nM sorafenib clearly induces apoptosis (22% apoptotic cells and 19% dead cells), whereas treatment of BCR-ABL–expressing K562 cells with 10-fold higher concentrations had no apoptotic effect.
Sorafenib was initially identified as a potent B-RAF and VEGFR inhibitor, and was subsequently shown to also inhibit the related receptor tyrosine kinases FLT3, KIT, and PDGFR.12,19 Sorafenib was recently approved for the treatment of advanced renal cell carcinoma, and is currently in clinical trials for the treatment of various solid tumors.20-22 These studies indicate that steady-state serum concentrations of up to 4 μM could be safely achieved in patients with a dose of 100 mg/d, with the maximum tolerated dose set at 400 mg/d.23,24 Since FIP1L1-PDGFRα and FIP1L1-PDGFRα(T674I) are completely inhibited at sorafenib concentrations of 100 nM to 1000 nM, an efficacious dose may be obtained with 100 mg/d or lower in FIP1L1-PDGFRA–positive patients with CEL. These data identify sorafenib as a potent inhibitor of FIP1L1-PDGFRα and its imatinib-resistant T674I mutant, and suggest that this inhibitor could be of value for the treatment of CEL and other cancers with activated PDGFRα.25 In addition, this study warrants further investigation of sorafenib as a potent inhibitor of oncogenic mutants of KIT and FLT3. Although PKC412 and AMN107 have been identified as potent FIP1L1-PDGFRα inhibitors, these drugs are not yet approved for clinical use. These results provide support for the evaluation of sorafenib in clinical trials as a potentially effective therapy for the treatment of FIP1L1-PDGFRα–positive CEL.
Prepublished online as Blood First Edition Paper, April 27, 2006; DOI 10.1182/blood-2006-02-004457.
Supported by grants from the Belgian Federation Against Cancer (J.C.), the “Fonds voor Wetenschappelijk Onderzoek-Vlaanderen” (P.M.), a Concerted Action Grant from the Katholieke Universiteit (KU) Leuven (P.M., J.C., P.V.), and the National Institutes of Health (E.H.S.). E.L. is an Aspirant, J.C. a postdoctoral researcher, and P.V. a clinical researcher of the “Fonds voor Wetenschappelijk Onderzoek-Vlaanderen.” This text presents research results of the Belgian program of Interuniversity Poles of attraction initiated by the Belgian State, Prime Minister's Office, Science Policy Programming. The scientific responsibility is assumed by the authors.
E.L., E.H.S., C.F., and J.C. designed the study, performed research, analyzed the data, and wrote the paper; N.M. and H.V.M. performed research; W.S. and M.B. contributed analytic tools; P.V. and P.M. designed the study, analyzed data, and wrote the paper.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal