Abstract
Regulatory T cells (Tregs) that constitutively express FOXP3 are instrumental to the maintenance of tolerance and may suppress graft-versus-host disease (GVHD) in humans. To determine whether regulatory T cells in allogeneic stem cell transplants (SCTs) ameliorate GVHD after transplantation, we quantitated the coexpression of FOXP3 on CD4+ T cells in 32 donor SCTs infused into HLA-matched siblings and examined GVHD incidence in respective recipients. High CD4+FOXP3+ T-cell count in the donor was associated with a reduced risk of GVHD. We monitored Tregs during immune reconstitution in 21 patients with leukemia undergoing a T-cell–depleted allogeneic SCT. Early after SCT, there was a significant expansion in the CD4+FOXP3+ T-cell compartment. A low CD4+FOXP3+ T-cell count early after SCT (day 30) was associated with an increased risk of GVHD, and the ratio of CD4+FOXP3+ T cells to CD4+CD25+FOXP3– T cells was significantly reduced in patients with GVHD, suggesting diminished control of effector T cells. Our findings suggest that graft Treg content may predict for risk of GVHD after SCT. Determining the Treg levels in the donor and manipulating Tregs early after transplantation may provide a new approach to controlling GVHD.
Introduction
Following allogeneic stem cell transplantation (SCT) in adults, T cells reconstitute from expanding mature T cells contained within the stem cell allograft and from a residual population of recipient lymphocytes, which survive the preparative regimen. Early after transplantation, donor T cells responding to the recipient environment undergo clonal expansions causing graft-versus-host disease (GVHD) and exerting a graft-versus-leukemia (GVL) effect. GVHD and GVL reactions are driven by antigens present in the recipient and favored by the unique lymphocyte-depleted immune environment created by the preparative regimen, which may stimulate homeostatic T-cell expansion.1,2
Prior clinical studies have identified donor graft characteristics associated with an increased risk for GVHD in patients who have undergone SCT, including the infusion of increased numbers of total nucleated cells (TNCs), CD34+ cells, or CD3+ T cells.3-5 Recently, attention has been directed toward regulatory T cells (Tregs) and their potential to attenuate GVHD and autoimmunity in murine models, reviewed in Bach, Shevach, and Wood and Sakaguchi.6-8 Tregs, phenotypically characterized by coexpression of CD4 and CD25 (the interleukin-2 [IL-2] receptor α chain) both in mice and humans, can inhibit activation of autoreactive T cells in an antigen-specific, cell-contact–dependent manner.9-15 CD25 also is expressed by recently activated T cells and is therefore an unreliable marker for Tregs. FOXP3, which encodes a forkhead/winged helix transcription factor also known as Scurfin, was identified as a key regulatory gene required for the development and functional activity of CD4+CD25+ regulatory T cells.16-18 Unlike CD25, Foxp3 is exclusively expressed by Tregs16,17 and not by activated T cells19 and is thus a reliable surrogate molecular marker for quantifying Tregs in peripheral blood.
Several studies have demonstrated that CD4+CD25+ Tregs suppress GVHD in animal models.20-24 Human and murine Tregs share many characteristics, but in humans, data on the impact of Tregs on GVHD following SCT are conflicting, possibly due to differences in surface phenotyping techniques used to characterize these cells.25-29 Stanzani et al28 reported that patients who experienced GVHD after SCT had significantly higher frequencies and absolute numbers of CD4+ and CD8+ T cells coexpressing CD25 in their donor grafts than those who did not. They suggested that the coexpression of CD4 and CD25 may be insufficient to identify Treg in humans and that these cells may represent an activated T-cell phenotype.28
In this study we sought to further clarify these findings by examining the immunophenotypic composition of donor peripheral blood stem cell (PBSC) grafts and the association between CD4+FOXP3+ T cells in donor grafts and the incidence of GVHD in 32 HLA-matched sibling transplant recipients of these grafts. We hypothesized that the increasing frequencies of CD4+FOXP3+ T cells will be associated with a lower incidence of GVHD in patients who have undergone SCT. We focused on the incidence of acute GVHD as the primary end point of the study. We found that patients who received SCT with lower absolute numbers of FOXP3+CD4+ T cells were associated with a greater risk of developing GVHD. We also monitored Treg reconstitution by phenotyping and FOXP3 gene expression in 21 patients with hematologic malignancies following T-cell–depleted allogeneic SCT. We found a significant expansion of the FOXP3+CD4+ T-cell compartment early after SCT, compared to healthy donors; however, patients with GVHD had significantly fewer FOXP3+CD4+ T cells. Importantly, the frequency of FOXP3+CD4+ in donor T cells may be a predictor of GVHD incidence in recipients after HLA-matched SCT.
Patients, materials, and methods
Patients and donors
Patients and their HLA-identical sibling donors were treated according to National Institutes of Health (NIH) protocols approved by the National Heart, Lung and Blood Institute's Review Board. After written informed consent, 32 patients with leukemia and their respective stem cell donors were analyzed to study the relationship between donor Tregs and GVHD. We selected 14 donors of patients developing grade 0 to 1 acute GVHD (aGVHD) and 18 donors of patients developing grade 2 to 4 aGVHD. In addition, samples were analyzed at regular intervals (days +30, +45, +60, +90, and +120) following transplantation to study reconstitution of Tregs. Due to sample limitations, Treg reconstitutions could be analyzed in only 21 patients. Clinical characteristics of these 21 patients and their respective donors are presented in Table 1. Patients were excluded if they had intercurrent illness, relapsed leukemia, or infection at the time of sample collection.
Characteristics of 21 patients with leukemia and their respective donors
Patient no. . | Age, y . | Sex . | Disease . | Prior therapy . | Disease risk . | Donor age, y . | Donor sex . | CSA . | CD34 dose, × 106 cells/kg . | CD3 dose, × 104 cells/kg . | DLI . | Max grade of GVHD . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 44 | F | AML | Y | HR | 44 | F | N | 5.82 | 2 | 45 | 2 |
| 2 | 31 | M | AML | Y | HR | 20 | F | N | 4.78 | 2 | 45 | 2 |
| 3 | 16 | F | AML | Y | LR | 15 | F | N | 3.339 | 2 | 45 | 2 |
| 4 | 29 | M | AML | Y | LR | 28 | F | Y | 5.98 | 2 | 60 | 2 |
| 5 | 36 | F | AML | Y | HR | 33 | F | Y | 4.98 | 2 | 60 | 1 |
| 6 | 32 | F | AML | Y | LR | 34 | M | Y | 6.59 | 2 | 100 | 2 |
| 7 | 45 | M | CLL | Y | HR | 46 | M | Y | 7.04 | 2 | 60 | 2 |
| 8 | 10 | M | ALL | Y | HR | 17 | F | Y | 5.26 | 2 | 60 | 0 |
| 9 | 16 | M | AML | Y | HR | 18 | F | Y | 10.05 | 2 | No | 4 |
| 10 | 38 | F | AML | Y | LR | 42 | F | Y | 3.2 | 10 | 100 | 3 |
| 11 | 30 | M | CML | Y | HR | 37 | M | N | 6.62 | 2 | No | 4 |
| 12 | 26 | F | AML | Y | HR | 32 | M | N | 5.9 | 2 | No | 4 |
| 13 | 37 | M | CLL | Y | HR | 45 | M | Y | 15.9 | 2 | No | 0 |
| 14 | 42 | F | CML | Y | HR | 47 | M | N | 5.65 | 2 | No | 4 |
| 15 | 27 | F | ALL | Y | HR | 25 | F | Y | 4.95 | 2 | 45 | 0 |
| 16 | 35 | F | AML | N | HR | 31 | F | N | 7.65 | 2 | No | 3 |
| 17 | 18 | F | CML | Y | LR | 27 | F | N | 7.22 | 2 | No | 3 |
| 18 | 19 | M | ALL | Y | HR | 19 | M | Y | 5.03 | 2 | 63 | 2 |
| 19 | 40 | M | CML | Y | LR | 44 | F | N | 6.94 | 2 | 48 | 0 |
| 20 | 17 | M | ALL | Y | HR | 18 | F | N | 7.2 | 2 | 45 | 1 |
| 21 | 32 | M | AML | Y | LR | 30 | F | N | 4.36 | 2 | 45 | 2 |
Patient no. . | Age, y . | Sex . | Disease . | Prior therapy . | Disease risk . | Donor age, y . | Donor sex . | CSA . | CD34 dose, × 106 cells/kg . | CD3 dose, × 104 cells/kg . | DLI . | Max grade of GVHD . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 44 | F | AML | Y | HR | 44 | F | N | 5.82 | 2 | 45 | 2 |
| 2 | 31 | M | AML | Y | HR | 20 | F | N | 4.78 | 2 | 45 | 2 |
| 3 | 16 | F | AML | Y | LR | 15 | F | N | 3.339 | 2 | 45 | 2 |
| 4 | 29 | M | AML | Y | LR | 28 | F | Y | 5.98 | 2 | 60 | 2 |
| 5 | 36 | F | AML | Y | HR | 33 | F | Y | 4.98 | 2 | 60 | 1 |
| 6 | 32 | F | AML | Y | LR | 34 | M | Y | 6.59 | 2 | 100 | 2 |
| 7 | 45 | M | CLL | Y | HR | 46 | M | Y | 7.04 | 2 | 60 | 2 |
| 8 | 10 | M | ALL | Y | HR | 17 | F | Y | 5.26 | 2 | 60 | 0 |
| 9 | 16 | M | AML | Y | HR | 18 | F | Y | 10.05 | 2 | No | 4 |
| 10 | 38 | F | AML | Y | LR | 42 | F | Y | 3.2 | 10 | 100 | 3 |
| 11 | 30 | M | CML | Y | HR | 37 | M | N | 6.62 | 2 | No | 4 |
| 12 | 26 | F | AML | Y | HR | 32 | M | N | 5.9 | 2 | No | 4 |
| 13 | 37 | M | CLL | Y | HR | 45 | M | Y | 15.9 | 2 | No | 0 |
| 14 | 42 | F | CML | Y | HR | 47 | M | N | 5.65 | 2 | No | 4 |
| 15 | 27 | F | ALL | Y | HR | 25 | F | Y | 4.95 | 2 | 45 | 0 |
| 16 | 35 | F | AML | N | HR | 31 | F | N | 7.65 | 2 | No | 3 |
| 17 | 18 | F | CML | Y | LR | 27 | F | N | 7.22 | 2 | No | 3 |
| 18 | 19 | M | ALL | Y | HR | 19 | M | Y | 5.03 | 2 | 63 | 2 |
| 19 | 40 | M | CML | Y | LR | 44 | F | N | 6.94 | 2 | 48 | 0 |
| 20 | 17 | M | ALL | Y | HR | 18 | F | N | 7.2 | 2 | 45 | 1 |
| 21 | 32 | M | AML | Y | LR | 30 | F | N | 4.36 | 2 | 45 | 2 |
DLI indicates donor lymphocyte infusion; F, female; HR, high risk; M, male; LR, low risk; Cy, cyclophosphamide; CSA, cyclosporine A.
Transplant approach
All patients and donors gave written informed consent on treatment or cell collection protocols approved by the NIH Institutional Review Board. Eleven patients with acute myeloblastic leukemia (AML), 4 patients with acute lymphoblastic leukemia (ALL), 4 patients with chronic myeloid leukemia (CML), and 2 patients with chronic lymphocytic leukemia (CLL) received a T-cell–depleted granulocyte colony-stimulating factor (G-CSF)–mobilized peripheral blood stem cell transplant (PBSCT). The conditioning regimen was 12 Gy total body irradiation (TBI), fludarabine (Flu) 125 mg/m2, and cyclophosphamide (Cy). CD34 cells were positively selected using anti-CD34 beads, and residual T cells were removed with a cocktail of anti-CD2, anti-CD6, and anti-CD7 antibody-coated beads. The CD34 cell dose ranged from 3.20 to 15.90 (median, 6.44) × 106/kg; T-cell dose was fixed at 2 × 104 CD3 cells/kg recipient weight by adding back lymphocytes where necessary. Eleven patients received no (N) cyclosporine (CSA) during the first 6 weeks following transplantation, and 10 received low-dose (LD) CSA (target plasma level, 100-200 μg/mL), starting on day –4. CSA was continued for at least 6 months if indicated for chronic GVHD (cGVHD). In the absence of GVHD, donor T cells (1 × 107/kg) were added back preemptively in 14 patients on days 45 to 100 after transplantation. Standard prophylaxis against infection included fluconazole and bactrim for at least 6 months after transplantation and weekly surveillance for cytomegalovirus (CMV) antigenemia as described previously.30,31
Flow cytometry
Cells were phenotypically analyzed by 4- or 5-color flow cytometry using a titrated panel of directly conjugated antibodies to CD3, CD4, CD25 (M-A251), CD27, and CD45RO (all Beckman Coulter, Miami, FL). Fluorescein isothiocyanate (FITC), phycoerythrin (PE), PercP, Cy5PE, Cy7PE, and allophycocyanin (APC) were used as fluorophores. Intracellular analysis of FoxP3 (eBioscience, San Diego, CA) and CTLA-4 (Beckman Coulter) was performed after fixation and permeabilization according to the manufacturer's recommendation. Flow cytometry was performed on an LSR II flow cytometer (BD Biosciences, San Jose, CA) using BD FacsDiva software (BD Biosciences).
Cell isolation
Peripheral mononuclear cells (PBMCs) were separated using Ficoll-Hypaque density gradient centrifugation (Organon Teknika, Durham, NC) and subsequently frozen in RPMI 1640 complete medium (CM) (Life Technologies, Gaithersburg, MD) supplemented with 20% heat-inactivated fetal calf serum (FCS) and 10% dimethyl sulfoxide (DMSO) according to standard protocols. Before use, frozen cells were thawed, washed, and suspended in RPMI-CM + 10% pooled AB serum (Sigma Chemical, St Louis, MO). CD4+ T-cell populations were purified with immunomagnetic beads (Dynal Biotech, Oslo, Norway). Immunomagnetic beads were detached from isolated cells by using DetachaBead (Dynal) with high purity (> 97%) and viability (> 99%). The purity of positively and negatively selected cells was checked by flow cytometry.
RNA extraction and cDNA synthesis
RNA isolation on test samples was performed using RNeasy mini kits (Qiagen, Valencia, CA). Total RNA was eluted with water and stored at –80°C. For reverse transcription of mRNA and cDNA synthesis, 1 μg total RNA was reverse transcribed and stored at –20°C until quantitative real-time polymerase chain reaction (qPCR) was performed.
qPCR
Gene expression was measured using an ABI Prism 7900 Sequence Detection System (Applied Biosystems, Foster City, CA) as described previously.32,33 Threshold cycle (CT) during the exponential phase of amplification was determined by real-time monitoring of fluorescent emission after cleavage of sequence-specific probes by nuclease activity of Taq polymerase. Beta-actin (ACTB) was used as an internal control gene for mRNA expression. Primer and probe sequences for ACTB were as follows: 5′-GGCACCCAGCACAATGAAG (forward), 5′-GCCGATCCACACGGAGTACT (reverse), FAM-TCAAGATCATTGCTCCTCCTGAGCGCTAMRA (probe). To detect FOXP3, Assays-on-Demand Gene Expression probes for FOXP3 (Hs 00203958; Applied Biosystems) were used according to manufacturer's guidelines. To create a standard curve, ACTB and FOXP3 cDNA were amplified by PCR using the same primers designed for qPCR, purified, and quantified by UV spectrophotometry. The number of cDNA copies was calculated by using the molecular weight of each gene amplicon. Serial dilutions of the amplified genes at known concentrations were tested by qPCR. qPCR reactions of cDNA specimens, cDNA standards, and water as negative control (NTC) were conducted in a total volume of 20 μL with TaqMan MasterMix (Applied Biosystems), 400 nM primers, and 200 nM probe. Thermal cycler parameters included 10 minutes at 95°C and 40 cycles of 95°C for 15 seconds and 60°C for 1 minute. Standard curve extrapolation of copy number was performed for both FOXP3 and ACTB. Sample data were normalized by dividing the number of copies of FOXP3 transcripts by the number of copies of ACTB transcripts. All PCR assays were performed in duplicate and reported as the mean.
Statistical analysis
Data were analyzed by Pearson correlation and Mann-Whitney test with the use of Prism 4.00 for Windows software (Graph Pad Software, San Diego, CA). P values less than .05 were considered statistically significant.
Results
Phenotypic and molecular characterization of Treg cells
The population of Tregs within the CD4+ subset was more precisely delineated using cell surface and intracellular phenotyping for coexpression of Foxp3, CTLA-4, and CD27, markers shown to be associated with regulatory activity10,34,35 as well as quantitative RT-PCR for FOXP3 gene expression. Representative data on one individual are shown in Figure 1A-F, but results were confirmed in samples from 32 healthy donors and 21 patients. Of the total CD4+ T-cell population, a median of 4.6% (0.1%-24.3%) were CD25+ (Figure 1A), and a median of 3.7% (0.3%-17.5%) were FOXP3+ (Figure 1B). Of note, a proportion of CD4+ T cells were FOXP3+ but CD25low (median, 2.5%; 0.4%-11.3%) (Figure 1C), confirming murine data that FOXP3 can be expressed in CD25low T-cell populations.16,19,36 A median of 35% (4.5%-81.8%) of CD4+CD25+ T cells also coexpressed CTLA-4 (Figure 1D-F).
To validate the assumption that FOXP3 mRNA transcription reflects FOXP3 protein expression and the frequency of CD4+FOXP3+ expressing regulatory T cells, we compared FOXP3 mRNA expression by qPCR with flow cytometric quantification of CD4+FOXP3+ T cells. Whereas qPCR analysis gives a quantitative measure of FOXP3 transcripts in the CD4 population and is limited to quantifying mRNA levels per population, intracellular staining for FOXP3 allows for the detection of protein per cell and estimation of the frequency of FOXP3-expressing CD4+ T cells. A significant correlation was seen between FOXP3 gene expression and CD4+FOXP3+ T cells by flow cytometry (r = 0.7, P < .001; Figure 1G), confirming that the 2 assays measure the same T-cell population.
Phenotypic characterization of regulatory T cells. Phenotypic analysis was performed on healthy donors (n = 32) and patients with leukemia (n = 21) followed longitudinally from baseline (before SCT), days +30, +45, +60, +90, and +120 after SCT. Representative data on one individual are presented here. (A) PBMCs were stained initially with anti-CD3, anti-CD4, and anti-CD25 antibodies followed by intracellular staining with anti–CTLA-4 and anti-FOXP3. (B) The histogram was gated on CD4+CD25+ T cells and represents the proportion of CD4+CD25+ T cells that are FOXP3+. (C) The dot plot was gated on CD4+ T cells: the upper right quadrant represents CD25+FOXP3+ T-cell population, and the lower right quadrant, the CD25–FOXP3+ population. (D-F) CTLA-4 and FOXP3: histograms are gated on CD4+FOXP3+ and CD4+FOXP3– populations. Whereas CD4+FOXP3+ T cells also co-express CTLA-4 (E), CD4+FOXP3– T cells were essentially CTLA-4 negative (F). (G) Correlation between FOXP3 mRNA copies and CD4+FOXP3+ (%). Dots correspond to individual samples tested from healthy donors and patients with leukemia. A significant positive correlation was seen between the frequency of CD4+FOXP3+ T cells and FOXP3 mRNA copies, as shown by the trendline.
Phenotypic characterization of regulatory T cells. Phenotypic analysis was performed on healthy donors (n = 32) and patients with leukemia (n = 21) followed longitudinally from baseline (before SCT), days +30, +45, +60, +90, and +120 after SCT. Representative data on one individual are presented here. (A) PBMCs were stained initially with anti-CD3, anti-CD4, and anti-CD25 antibodies followed by intracellular staining with anti–CTLA-4 and anti-FOXP3. (B) The histogram was gated on CD4+CD25+ T cells and represents the proportion of CD4+CD25+ T cells that are FOXP3+. (C) The dot plot was gated on CD4+ T cells: the upper right quadrant represents CD25+FOXP3+ T-cell population, and the lower right quadrant, the CD25–FOXP3+ population. (D-F) CTLA-4 and FOXP3: histograms are gated on CD4+FOXP3+ and CD4+FOXP3– populations. Whereas CD4+FOXP3+ T cells also co-express CTLA-4 (E), CD4+FOXP3– T cells were essentially CTLA-4 negative (F). (G) Correlation between FOXP3 mRNA copies and CD4+FOXP3+ (%). Dots correspond to individual samples tested from healthy donors and patients with leukemia. A significant positive correlation was seen between the frequency of CD4+FOXP3+ T cells and FOXP3 mRNA copies, as shown by the trendline.
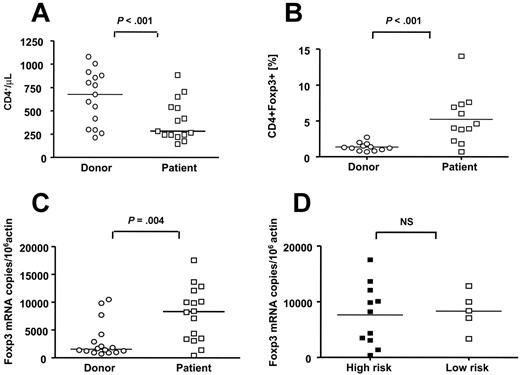
Compared to healthy donors, leukemic patients have increased numbers of FOXP3+ Tregs
We compared the frequency of Treg cells in patients with leukemia prior to SCT (n = 16; median, 31 years of age; range, 10-44 years) and their respective donors (n = 16, median, 32.5 years of age; range, 15-58 years) using flow cytometry and real-time PCR for FOXP3 gene expression. Nine patients with AML, 2 patients with ALL, 3 patients with CML, and 2 patients with CLL were studied. All patients except for one patient with CML had received prior standard chemotherapy for their underlying leukemia. PBMCs from donors used in this study were obtained prior to G-CSF administration for stem cell mobilization. Characteristics of patients and donors are presented in Table 1. Patients with leukemia analyzed prior to initiation of conditioning had significantly fewer CD4+ cells/μL (median, 282 cells/μL; range, 143-882 cells/μL) compared to healthy donors (median, 676 cells/μL; range, 212-1080 cells/μL), P < .001) (Figure 2A). However, the Treg compartment was significantly expanded in patients compared with healthy donors as determined by a greater proportion of CD4+FOXP3+ T cells in CD4+ T cells (median, 4.3%; range, 0.7%-14.0% compared to median 1.8%; range, 0.7%-4.1%, respectively; P < .001), and FOXP3 mRNA copy numbers (median, 8285 FOXP3 copies/106 actin; range, 399-17 512 FOXP3 copies/106 actin compared to 1535 FOXP3 copies/106 actin; range, 686-10 466 FOXP3 copies/106 actin, respectively; P = .004) (Figure 2B,C). To further investigate if Tregs have an impact on the disease status prior to transplantation, patients were stratified into high-risk and low-risk groups as defined in Table 1. No significant difference was seen in the relative and absolute numbers of Tregs in patients with high-risk disease (median, 3.9%; range, 0.7%-14.0% and 11 cells/μL; range, 2-4 cells/μL) compared with patients with low-risk disease (median, 4.6%; range, 3.8%-6.9% and 12 cells/μL; range, 9-17 cells/μL). Similarly, disease status did not significantly affect FOXP3 gene expression (median, 8193 FOXP3 mRNA copies/106 actin copies; range, 399-17 512 FOXP3 mRNA copies/106 actin copies compared to median, 8376 FOXP3 mRNA copies/106 actin copies; range, 3344-12 801 FOXP3 mRNA copies/106 actin copies, respectively) (Figure 2D).
Kinetics of Treg reconstitution after SCT
We examined Treg reconstitution at regular intervals (day [D] +30, D +45, D +60, D +90, and D +120) following SCT in 21 patients (Table 1). Immune reconstitution of CD4+CD3+ T cells, CD4+FOXP3–, and CD4+FOXP3+ T cells is shown in Figure 3. In the early period after SCT (D 30), there was a profound depletion in relative and absolute numbers of CD4+ T cells compared with healthy donors (median, 18.1%; range, 1.6%-65% and 51 cells/μL; range, 11-571 cells/μL compared to median, 54.4%; range, 24%-75% and 282 cells/μL; range, 140-882 cells/μL, respectively; P < .001) (Figure 3A,D). Furthermore, there was a significant reduction in relative and absolute numbers of CD4+FOXP3– T cells (non-Treg fraction) compared with the T-cell subsets of the peripheral blood stem cell product obtained from healthy donors before T-cell depletion (median, 10.9%; range, 1.0%-61.5% and median, 13 cells/μL; range, 0.2-121 cells/μL compared to median, 55.0%; range, 24%-70% and 416 cells/μL; range, 50-794 cells/μL, respectively; P < .01) (Figure 3B,E). On the other hand, the proportion of CD4+FOXP3+ T cells was significantly higher at D 30 after SCT compared with the healthy donors (median, 6.2%; range, 2.4%-13%, compared to 1.3%; range, 0.7%-2.7%), P = .004 (Figure 3C), with an absolute number not significantly different from that of the healthy donors (median, 8 cells/μL; range, 1-25 cells/μL, compared to median, 16 cells/μL; range, 3-77 cells/μL; P = .09) (Figure 3F). Thus, in these patients, early after SCT the recovery of CD4+ T-cell subsets was not uniform and was associated with preferential expansion and relative enrichment of CD4+FOXP3+ T-cell subsets. Of 21 patients studied, 14 received preemptive donor lymphocyte infusion (DLI) 45 to 100 days after SCT. We studied the influence of DLI on Treg reconstitution by examining the CD4+FOXP3+ T-cell content in samples available after DLI. Of note, none of the patients studied received DLI prior to day 45 after SCT; therefore, data analyzed from days 30 to 45 after SCT are not influenced by DLI. Infusion of donor lymphocytes does not appear to affect CD4+, CD4+FOXP3–, and CD4+FOXP3+ T-cell reconstitution (Figure 3).
The Treg compartment is expanded in patients with leukemia prior to SCT compared to healthy donors. PBMCs were stained with monoclonal antibodies to CD3, CD4, and FOXP3, and the absolute and relative frequencies of CD4+ and CD4+FOXP3+ T cells were studied in patients with leukemia before SCT (□) and their respective donors (○) (A-B). PBMCs were obtained from donors prior to G-CSF administration for stem cell mobilization. In addition, we examined FOXP3 gene expression in purified CD4+ T cells from patients and donors (C). Patients with leukemia had fewer CD4+ T cells compared to healthy donors (n = 16) (A); however, the Treg compartment of patients with leukemia was relatively expanded as demonstrated by increased percentage of CD4+FOXP3+ T cells (n = 12) (B) and increased numbers of FOXP3 mRNA copies (n = 5) (C). (D) Patients were further stratified into high-risk (n = 11) (▪) and low-risk (n = 5) (□) disease as defined in Table 1. There was no significant association between FOXP3 mRNA expression and disease status. Bars represent median values. NS indicates not significant.
The Treg compartment is expanded in patients with leukemia prior to SCT compared to healthy donors. PBMCs were stained with monoclonal antibodies to CD3, CD4, and FOXP3, and the absolute and relative frequencies of CD4+ and CD4+FOXP3+ T cells were studied in patients with leukemia before SCT (□) and their respective donors (○) (A-B). PBMCs were obtained from donors prior to G-CSF administration for stem cell mobilization. In addition, we examined FOXP3 gene expression in purified CD4+ T cells from patients and donors (C). Patients with leukemia had fewer CD4+ T cells compared to healthy donors (n = 16) (A); however, the Treg compartment of patients with leukemia was relatively expanded as demonstrated by increased percentage of CD4+FOXP3+ T cells (n = 12) (B) and increased numbers of FOXP3 mRNA copies (n = 5) (C). (D) Patients were further stratified into high-risk (n = 11) (▪) and low-risk (n = 5) (□) disease as defined in Table 1. There was no significant association between FOXP3 mRNA expression and disease status. Bars represent median values. NS indicates not significant.
Treg reconstitution after SCT. Using flow-based frequency enumeration and absolute lymphocyte counts acquired from a complete blood count obtained on the same day, relative and absolute numbers of CD4+CD3+, CD4+FOXP3–, and CD4+FOXP3+ were calculated for healthy donors (n = 21) (○), patients with leukemia at baseline, prior to allogeneic transplantation (n = 21) (pre; □), and patients after SCT (n = 21) at regular intervals: days +30 (▾), +45 (♦), +60 (•), +90 (▪), and +120 (▴). Bars indicate median values. Samples obtained following DLI are presented as symbols in red. Compared to healthy donors, on day 30 after SCT, circulating CD4+ and CD4+FOXP3– T-cell compartments were significantly reduced (A-B, D-E), whereas the percent of CD4+ subset composed of FOXP3+ cells was significantly increased (C). Bars represent median values.
Treg reconstitution after SCT. Using flow-based frequency enumeration and absolute lymphocyte counts acquired from a complete blood count obtained on the same day, relative and absolute numbers of CD4+CD3+, CD4+FOXP3–, and CD4+FOXP3+ were calculated for healthy donors (n = 21) (○), patients with leukemia at baseline, prior to allogeneic transplantation (n = 21) (pre; □), and patients after SCT (n = 21) at regular intervals: days +30 (▾), +45 (♦), +60 (•), +90 (▪), and +120 (▴). Bars indicate median values. Samples obtained following DLI are presented as symbols in red. Compared to healthy donors, on day 30 after SCT, circulating CD4+ and CD4+FOXP3– T-cell compartments were significantly reduced (A-B, D-E), whereas the percent of CD4+ subset composed of FOXP3+ cells was significantly increased (C). Bars represent median values.
CSA does not affect recovery of CD4+FOXP3+ T cells
We examined the influence of CSA on Treg recovery. Eleven patients received no (N) CSA during the first 6 weeks following transplantation, and 10 patients received low-dose (LD) CSA (target plasma level, 100-200 μg/mL), starting on day –4. Treg cell development in the first 30 to 45 days after SCT as determined by proportion of CD4+ T cells that were FOXP3+ and FOXP3 gene expression was not significantly different in patients who did not receive CSA compared with those who received low-dose CSA; median, 7.3% (range, 0.5%-12.9%) compared with 5.0% (0.7-11) and 4215 FOXP3 mRNA copies/106 actin copies (738-7821) compared with 4318 FOXP3 mRNA copies/106 actin copies (728-16 951) (Figure 4B,C). Of note, samples were analyzed from days 30 and 45 after SCT, and in a number of patients repetitive measurements from both days 30 and 45 are presented here.
The donor CD4+FOXP3+ T-cell content correlates with the risk of acute GVHD
We compared the absolute number of CD4+ and CD4+FOXP3+ T cells in donors of patients who developed grade 2-4 acute GVHD and donors of patients who developed grade 0-1 GVHD (Figure 5). The donor absolute CD4+ T-cell count was not statistically different in the 2 groups (median, 636 cells/μL; range, 210-1275 cells/μL compared to 805 cells/μL; range, 420-1261 cells/μL; P = .09) (Figure 5A). However, the donor CD4+FOXP3+ T-cell count was significantly higher for patients who did not develop GVHD compared with patients who developed GVHD (median, 13 cells/μL; range, 8-39 cells/μL compared with 8 cells/μL; range, 3-19 cells/μL, respectively) (Figure 5B). In contrast there was no correlation between donor absolute CD4+CD25+ T-cell count and the subsequent development of GVHD (median, 29 cells/μL, no GVHD; range, 8.5-91 cells/μL compared with 33 cells/μL; range, 8-70 cells/μL, GVHD). In this study group donor age and sex were not found to significantly affect the risk of GVHD (data not shown).
Effect of low-dose cyclosporine A (CSA) on D 30 CD4+ and CD4+FOXP3+ T-cell recovery. Patients who received low-dose CSA (▪) were compared to those who did not receive CSA (•) as immunoprophylaxis for the first 4 weeks after SCT. (A) Low-dose CSA had no significant effect on CD4+ T-cell and (B) CD4+FOXP3+ T-cell recovery. (C) Similarly, FOXP3 mRNA gene expression was not significantly different (NS) in the 2 groups. Bars represent median values.
Effect of low-dose cyclosporine A (CSA) on D 30 CD4+ and CD4+FOXP3+ T-cell recovery. Patients who received low-dose CSA (▪) were compared to those who did not receive CSA (•) as immunoprophylaxis for the first 4 weeks after SCT. (A) Low-dose CSA had no significant effect on CD4+ T-cell and (B) CD4+FOXP3+ T-cell recovery. (C) Similarly, FOXP3 mRNA gene expression was not significantly different (NS) in the 2 groups. Bars represent median values.
Inverse relationship between CD4+FOXP3+ and CD4+CD25+ T cells and acute GVHD. We compared CD4+, CD4+FOXP3+, and CD4+CD25+ T cells in donors of patients with no or grade 1 GVHD (n = 12) (○) and donors of patients with GVHD (n = 18) (•). We also examined CD4+, CD4+FOXP3+, and CD4+CD25+ T cells at days 30 to 45 after SCT in patients with grade 0 to 1 GVHD (n = 6) (□) and patients with grade 2 to 4 GVHD (n = 13) (▪). (A) The absolute CD4+ T-cell count was not significantly different in donors and patients with or without GVHD. (B) Donors of patients who did not develop GVHD had increased numbers of CD4+FOXP3+ T cells. Similarly, in patients with GVHD, after SCT, the absolute CD4+FOXP3+ T-cell count was significantly lower, whereas the absolute CD4+CD25+ T-cell count was significantly higher than those who did not develop GVHD (B-C). In addition, the proportion of CD4+CD25+ T cells that were FOXP3+ was significantly lower in patients with GVHD, indicating expansion of CD4+CD25+FOXP3– effector T cells (D). Bars indicate median values. Symbols for patient-donor pairs are color matched.
Inverse relationship between CD4+FOXP3+ and CD4+CD25+ T cells and acute GVHD. We compared CD4+, CD4+FOXP3+, and CD4+CD25+ T cells in donors of patients with no or grade 1 GVHD (n = 12) (○) and donors of patients with GVHD (n = 18) (•). We also examined CD4+, CD4+FOXP3+, and CD4+CD25+ T cells at days 30 to 45 after SCT in patients with grade 0 to 1 GVHD (n = 6) (□) and patients with grade 2 to 4 GVHD (n = 13) (▪). (A) The absolute CD4+ T-cell count was not significantly different in donors and patients with or without GVHD. (B) Donors of patients who did not develop GVHD had increased numbers of CD4+FOXP3+ T cells. Similarly, in patients with GVHD, after SCT, the absolute CD4+FOXP3+ T-cell count was significantly lower, whereas the absolute CD4+CD25+ T-cell count was significantly higher than those who did not develop GVHD (B-C). In addition, the proportion of CD4+CD25+ T cells that were FOXP3+ was significantly lower in patients with GVHD, indicating expansion of CD4+CD25+FOXP3– effector T cells (D). Bars indicate median values. Symbols for patient-donor pairs are color matched.
Inverse relationship between CD4+CD25+ T cells and CD4+FOXP3+ T cells and acute GVHD
We also compared the absolute number of CD4+ and CD4+FOXP3+ T cells at days 30 to 45 after SCT in patients with acute GVHD (grade 2-4) and patients with no GVHD (grade 0-1) (Figure 5). The absolute CD4+ T-cell count was not statistically different between the 2 groups (Figure 5A). However, the CD4+FOXP3+ T-cell count was significantly higher in the early period after SCT in patients with no or mild GVHD (median, 4 cells/μL; range, 4-8 cells/μL) compared with patients with GVHD (median, 0.5 cellsμL; range, 0-5 cells/μL; P < .05) (Figure 5B). In contrast, patients with acute GVHD had significantly more CD4+CD25+ T cells early after SCT (D 30-45) (median, 43 cells/μL; range, 5-819 cells/μL) compared to patients with no or only grade 1 acute GVHD (median, 7 cells/μL; range, 1-33 cells/μL; P < .01) (Figure 5C). This could be due to expansion of CD25+ effector T cells in acute GVHD. This possibility was further supported by the observation that the proportion of FOXP3+CD4+CD25+ T cells was significantly lower in patients with GVHD (median, 34.9%; range, 4.5%-49% for GVHD compared with 52.5%; range, 39.6%-86.3% for no GVHD; P = .019) (Figure 5D).
Discussion
In this study we examined the role of Treg cells in the donor graft in the maintenance of tolerance to host after SCT. Because human SCT grafts vary in composition with respect to the numbers and functions of T cells, we sought to determine the association between CD4+FOXP3+ and CD4+CD25+ T cells in donor grafts and the incidence of GVHD in our patient population. It was shown recently that CD25 expression on donor CD4+ or CD8+ T cells is associated with increased risk of GVHD in patients following allogeneic stem cell transplantation.28 We found that patients who experienced GVHD had significantly fewer numbers of CD4+ FOXP3+ T cells in their donor grafts than those who did not. Furthermore, after SCT, recipients whose donors had increased numbers of CD4+FOXP3+ T cells were less likely to develop GVHD. Unlike Stanzani et al28 we failed to show a correlation between donor CD4+CD25+ T cells and GVHD. This may be related to different patient populations and transplant protocols.
We also determined CD4+CD25+ and CD4+FOXP3+ T-cell numbers in patients early after SCT. Several studies in animal models showed that CD4+CD25+ Treg cells suppress GVHD.20-24 Whether the findings from murine bone marrow transplantation models hold true for human allogeneic SCT is as yet unknown, since comparable clinical trials using Treg infusions have not yet been reported. Recent studies in patients after SCT explored the relationship between putative regulatory T cells with GVHD.25,26,37,38 Results and conclusions vary, probably reflecting difficulties to reliably identify and quantify Treg cells in patients with GVHD. The lack of specific surface markers for Treg cells until recently represented a major obstacle for such studies. Nevertheless, 2 groups have found an inverse correlation between FOXP3 mRNA levels and GVHD in patients.25,29 Using phenotypic criteria for Treg (high expression levels of CD25), a similar trend was reported by Sanchez et al.38 Conversely, Clark et al26 detected increased numbers of CD4+CD25+ T cells in patients with chronic GVHD.26,38 Our data reconcile these findings.37 By using intracellular staining for FOXP3 protein, we were able to directly enumerate CD4+FOXP3+ regulatory T cells. A proportion of CD4+FOXP3+ T cells were found to be CD25low, further emphasizing the shortcomings of CD25 as a marker of Treg activity. We found higher numbers of CD4+ T cells expressing CD25 in patients with GVHD compared to those who did not experience GVHD. The proportion of CD4+CD25+ T cells that were FOXP3+ in this patient population was significantly lower, indicating that most CD25 expressing T cells in patients with GVHD were activated, not regulatory, T cells. Conversely, patients with increased numbers of CD4+FOXP3+ T cells early after SCT (D 30-45) were less likely to develop GVHD. The fact that one third of patients did not receive DLI and the remainder received DLI only after day 45 after SCT provided us with a unique opportunity to study the influence of DLI on Treg reconstitution. Examining CD4+ and CD4+FOXP3+ T-cell numbers in patients who received DLI compared to those who did not indicates that infusion of donor lymphocytes does not influence Treg reconstitution.
All patients in this study received a T-cell–depleted SCT with a fixed CD3+ T-cell dose of 2 × 104/kg, clearly demonstrating that Treg reconstitution can take place from a small inoculum of CD3+ T cells. In addition, it is important to note that despite the low T-cell numbers infused with the graft, significant GVHD was observed in a number of patients. This further emphasizes the possible role of the Treg content of the graft and the risk of GVHD in a T-depleted setting. It is conceivable, however, that Treg reconstitution may have a different kinetic in T-replete allografts. To address this point, the association between the Treg content of the graft and GVHD in the T-replete setting will have to be further studied in patients receiving conventional allografts.
About 50% of patients in our study were on protocols that did not include CSA for GVHD prophylaxis in the first 6 weeks after SCT. The remainder received low dose CSA, aiming for a target plasma level of 100 to 200 μg/mL, allowing us to study the effect of low-dose CSA treatment on Treg recovery. It has been proposed that signaling by the T-cell growth factor, IL-2, is crucial for the functional activity of Tregs.39,40 In addition, IL-2 administration in cancer patients leads to expansion of Tregs in vivo.41,42 CSA inhibits T-cell receptor–induced activation and IL-2 production and may interfere with Treg function and maintenance. Indeed, it was reported that CSA blocks the generation of Treg cells after pretransplantation donor-specific blood transfusion in rats.43 Interestingly, we observed that Treg recovery early after SCT was not significantly affected by CSA. This is consistent with a recent observation that CSA was shown not to significantly interfere with the activation or suppressor function of freshly isolated human CD4+CD25+ Tregs in vitro.44
In this study, we also monitored recovery of Tregs during lymphocyte reconstitution in patients who underwent T-depleted allogeneic SCT for a variety of hematologic malignancies. Somewhat paradoxically, lymphopenia can induce heightened immunity,45 which, among other factors, has been attributed to a depletion of Tregs. However, we found that after SCT, lymphopenia was associated with a relative preponderance of Treg cells. By intracellular staining for FOXP3 protein and real-time PCR for gene expression, significantly higher proportions of FOXP3-positive CD4+ T cells were found to occur in samples obtained between 30 and 120 days after SCT. Furthermore, FOXP3 gene expression in CD4+ T cells confirmed significantly higher FOXP3 mRNA copy numbers in CD4+ T cells from post-SCT samples compared to the donor graft. These findings are consistent with other reports showing an important role for lymphopenia in expansion of Treg cells,41,46,47 thus suggesting that recovery of Tregs may help in the maintenance of self-tolerance. Treg expansion in vivo appears to occur through peripheral expansion rather than through thymopoiesis.41 It is also likely that the relative increase in CD4+FOXP3+ T cells seen in patients with leukemia prior to transplantation is similarly related to lymphopenia-driven Treg expansion following chemotherapy-induced cytopenia.
Our data suggest that the Treg content of the donor can predict the risk of GVHD after SCT. Therefore, determining the donor Treg content pre-SCT may improve donor selection. Furthermore, graft manipulation to selectively expand and infuse donor Tregs at the time of transplantation could further reduce the risk of GVHD. In addition, the posttransplantation period represents a unique and transient stage of immune recovery with a relative expansion of Treg cells. We found an inverse relationship between the day-30 Treg content and the ratio of CD4+FOXP3+ T cells to CD4+CD25+ T cells and risk of acute GVHD after SCT. Determining the Treg content in patients early after SCT may help identify patients at risk of developing acute GVHD, and ex vivo expansion and activation of regulatory T cells could be developed as a cell-based therapy to prevent or treat GVHD.
Prepublished online as Blood First Edition Paper, April 20, 2006; DOI 10.1182/blood-2006-02-003996.
K.R. and S.M. contributed equally to this work.
Supported by a Dr-Mildred-Scheel-Stiftung grant, Germany (S.M.).
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal