Abstract
Mast cells are responsible for IgE-mediated allergic reactions. Phospholipase D1 (PLD1) and PLD2 regulate mast cell activation, but the mechanisms remain unclear. Here we show that PLD2 associates with and promotes activation of Syk, a key enzyme in mast cell activation. Antigen stimulation resulted in increased association and colocalization of Syk with PLD2 on the plasma membrane as indicated by coimmunoprecipitation and confocal microscopy. This association was dependent on tyrosine phosphorylation of Syk but not on PLD2 activity. In vitro, PLD2 interacted via its Phox homology (PX) domain with recombinant Syk to induce phosphorylation and activation of Syk. Furthermore, overexpression of PLD2 or catalytically inactive PLD2K758R enhanced antigen-induced phosphorylations of Syk and its downstream targets, the adaptor proteins LAT and SLP-76, while expression of a PLD2 siRNA blocked these phosphorylations. Apparently, the interaction of PLD2 with Syk is an early critical event in the activation of mast cells.
Introduction
In the allergic condition, mast cells are activated by multivalent binding of antigen to immunoglobulin E (IgE) that is bound to its multimeric receptor, FcϵRI.1 The ensuing aggregation of these receptors results in the rapid phosphorylation of tyrosine residues in the immunoreceptor tyrosine-based activation motifs (ITAMs) of the β and γ subunits of FcϵRI by Lyn kinase2 and, as a consequence, recruitment and activation of the protein tyrosine kinase, Syk.3,4 Syk is responsible for the activation of a large number of downstream signaling molecules, among them the linker of activated T cells (LAT) and SH2-containing leukocyte-specific protein of 76 kDa (SLP-76) adaptor proteins,5,6 and in this manner regulates degranulation and the generation of inflammatory mediators.1,7 Syk, a member of the Syk and ZAP70 protein tyrosine kinase family, contains tandem Src homology 2 (SH2) regions in its N-terminal domain that, by binding to the phosphorylated ITAMs, allow its subsequent activation whether by autophosphoylation, transphosphorylation, or phosphorylation by Lyn.8 The spatial orientation of the SH2 domains in Syk confers a conformational flexibility that permits binding to diphosphorylated ITAMs as well as the activation of Syk without additional stimulatory input.8 This flexibility may also account for its ability to initiate multiple signaling pathways.9 For example, the tyrosine phosphorylation of LAT and SLP-76 by Syk provides essential docking sites for the assembly of signaling molecules for further propagation of signals in activated mast cells.7
One enzyme that is actively engaged in the signaling processes in mast cells is phospholipase D (PLD), but its place and function in these processes is obscure. PLD is activated in isolated mast cells10 and cultured mast cell lines11-15 by antigen and other stimulants. The correlations between PLD activity and mast cell degranulation under a variety of experimental conditions as well as the fact that primary alcohols (which divert production of phosphatidic acid to the corresponding phosphatidyl alcohol) suppress degranulation suggest that PLD has a critical role in degranulation. Transient expression of either of the 2 known mammalian isoforms of PLD in the RBL-2H3 mast cell line has indicated that PLD1 and PLD2 associate with granule membranes and the plasma membrane, respectively.16,17 Also, both isoforms are activated upon antigen stimulation14,18 and appear to regulate different phases of degranulation in mast cells.17 However, the regulatory mechanisms are largely undefined.
PLD, which catalyzes the hydrolysis of phosphatidylcholine to form phosphatidic acid, is found in most mammalian cells where it is thought to regulate critical functions such as actin stress fiber formation, membrane trafficking, exocytosis, and endocytosis.19-22 These functions may be mediated through the production of phosphatidic acid, or its biologically active metabolites, or through direct interaction of PLD with other signaling molecules. Both PLD1 and PLD2 require phosphatidylinositol 4,5-bisphosphate (PIP2) for activity.23,24 In vitro, PLD1 is activated by direct interaction with small GTPases,23 Rho kinase,25 or protein kinase C (PKC)23,26-29 in the presence of PIP2, whereas PLD2 is activated by PIP2 alone.24,30,31
The PLDs contain lipid-binding Phox (PX) and pleckstrin homology (PH) domains in addition to other PLD signature domains.21,22 Although the PX and PH domains are thought to facilitate binding to polyphosphoinositides and proteins, the exact role of these domains in PLD function is still debated. Recently, the PLD PX and PH domains have been implicated in the direct interaction of PLD, particularly PLD2, with other proteins in a lipase-independent manner. These proteins include PLCγ,32 Munc-18-1,33 and PKCζ.34 It has also been reported that PLD and c-Src interact similarly but in a lipase-dependent manner to enable phosphorylation of PLD2 and activation of Src.35
The location of PLD2 on the plasma membrane of mast cells makes this isoform particularly accessible to FcϵRI-associated Src kinases. PLD2 is phosphorylated and activated by its interaction with the Src kinases, Fyn and Fgr, in RBL-2H3 mast cells.36 In this paper we have investigated whether PLD2 interacts also with downstream signaling molecules that are recruited through FcϵRI aggregation. Here, we demonstrate that PLD2 interacts in a catalytically independent manner with Syk, an interaction that is critical for optimal activation of Syk and distal signaling events in antigen-stimulated mast cells. The findings provide the first description of a requirement for PLD2 in the activation of Syk. This activation along with the production of phosphatidic acid through the catalytic activity of PLD defines a dual role for PLD in the activation of mast cells.
Materials and methods
Piceatannol was purchased from Alexis (San Diego, CA); PP2 from Calbiochem (La Jolla, CA); antibodies against phosphotyrosine (PY) (4G10), SLP-76, and LAT from Upstate Biotechnology (Lake Placid, NY); antibodies against Syk and GST and agarose-conjugated antibody against HA-tag from Santa Cruz Biotechnology (Santa Cruz, CA); ATP from ICN Biomedicals (Irvine, CA); cell culture reagents from GIBCO/Invitrogen (Carlsbad, CA); and dinitrophenyl (DNP)–specific monoclonal IgE and DNP-BSA from Sigma (St Louis, MO).
Extraction of RNA and reverse transcriptase–polymerase chain reaction (RT-PCR)
Total RNA was isolated from RBL-2H3 cells by use of TRIZol Reagent (Invitrogen, Carlsbad, CA) and was reverse transcribed with the Superscript first-strand synthesis system (Invitrogen) according to the manufacturer's protocol. PCR was performed at 94°C for 45 seconds, 55°C for 45 seconds, and 72°C for 60 seconds for 30 cycles. The following primers were used: rat PLD2 forward 5′-ATGACTGTAACCCAGACGGCACTC-3′, reverse 5′-CAGCTCCTGAAAGTGTCGGAATTT-3′; and rat GAPDH forward 5′-GTGGAGTCTACTGGCGTCTTC-3′, reverse 5′-CCAAGGCTGTGGGCAAGGTCA-3′.
Cloning of rat PLD2 and Syk from RBL-2H3 cells
Rat PLD2 cDNA was cloned into pCGN vector, and rat Syk cDNA was cloned into pCMV vector (Stratagene, La Jolla, CA) by PCR amplification using following primers: 5′-CCTGGAATCTGCTCTAGAATGACTGTAACCCAGACGGA-3′ and 5′-GGGCTATGTCCACACTTCTAAAG-3′ for PLD2; and 5′-GCTCTTGCATATGGATCCATGGCGGGCAATGCTGTGGA-3′ and 5′-CCTTAGTCAGATCTCGAGTTAGTTAACCACGTCGTAGT-3′ for Syk. Sequence and expression were confirmed by sequencing and Western blot analysis. Plasmids for murine HAPLD2K758R were kindly supplied by Dr Michael A. Frohman (Institute for Cell and Developmental Biology, State University of New York, Stony Brook). Full-length cDNA was excised from hemagglutinin-tagged plasmids by SmaI and XbaI for PLD2 and subcloned into a pEGFP-C expression vector (Clontech Laboratories, Palo Alto, CA). Sequences were confirmed by DNA sequence analysis.
Point mutation of myc-Syk
Mutations of tyrosine residues of myc-Syk were performed by use of the QuikChange site-directed mutagenesis kit (Stratagene) with the following primers: Syk Y130F, 5′-AACCTCATCAGGGAATTTGTGAAACAGACCTGG-3′; Syk Y290F, 5′-TCAAGAATCAAATCCTTCTCCTTCCCAAAGCCT-3′; SykY317F, 5′-GTGTCCTTCAATCCCTTTGAGCCAACGGGAGGG-3′; Syk Y342F, 5′-ATGGACACCGAGGTATTTGAGAGTCCTTACGCT-3′; Syk Y346F, 5′-GTATATGAGAGTCCTTTCGCTGACCCTGAAGAG-3′; Syk Y358F, 5′-CGGCCCAAAGAGGTCTTCCTGGACAGGAAACTG-3′; Syk Y519/520F, 5′-CTCCGTGCTGATGAAAACTTCTTCAAGGCCCAGACCCACGGG-3′; and Syk Y624/625F, 5′-CTGCGGCTTCGCAATTACTTCTTCGACGTGGTTAACTAAGAA-3′. Sequence and expression was confirmed by sequencing and Western blot analysis. Each of the mutated Syks were overexpressed in RBL-2H3 cells. Cell lysates (in lysis buffer with 1 M urea) were used as a source of mutated Syk for experiments in vitro.
Transient transfection of cells with rat PLD2 and Syk
RBL-2H3 cells were grown as monolayers in minimal essential medium with Earle salts supplemented with glutamine, antibiotics, and 15% fetal bovine serum.37 Cells were transiently transfected with each DNA preparation (25 μg/2 × 107 cells unless stated otherwise) by electroporation (EquiBio EasyjecT, 900 μF, 250 V; EquiBio, Kent, United Kingdom). Successful transfection was confirmed by Western blotting. Cells were used within 48 hours of transfection.
Synthesis and transfection of siRNA against PLD2
Short hairpin siRNA constructs were designed around a 21 nucleotide sequence in the rat PLD2 gene (open reading frame nucleotides 2053 to 2073). Sense and antisense RNA oligonucleotides that contained the loop sequence CCACC were synthesized by Lofstrand (Rockville, MD) and were cloned into the psiRNA-hH1zeo vector (Invivogen, San Diego, CA). The siRNA constructs (DNA 25 μg) were transfected into 2 × 107 cells that were then incubated in 100 μg/mL zeocin for selection. Two weeks later cells were harvested for the studies described.
Cell stimulation, immunoprecipitation, and immunoblotting
Transfected cells (about 1.0 × 106 cells per 10 cm2 Petri dishes) were washed with fresh growth medium 4 hours after transfection and incubated with 50 ng/mL IgE for 3 hours. The cells were washed, and medium was replaced with a PIPES-buffered medium (25 mM PIPES [pH 7.2], 159 mM NaCl, 5 mM KCl, 0.4 mM MgCl2, 1 mM CaCl2, 5.6 mM glucose, and 0.1% fatty acid–free fraction V from bovine serum). Cells were stimulated with 25 ng/mL DNP-BSA for 7 minutes or as indicated, chilled with ice to terminate stimulation, and then washed twice with ice-cold phosphate-buffered saline (PBS). Cells were lysed in 0.5 mL with ice-cold lysis buffer (20 mM HEPES [pH 7.5], 150 mM NaCl, 1% Nonidet P-40, 10% glycerol, 60 mM octyl β-glucoside, 10 mM NaF, 1 mM Na3VO4, 1 mM phenylmethylsulfonyl fluoride, 2.5 mM nitrophenylphosphate, 0.7 μg/mL pepstatin, and protease inhibitor cocktail tablet). Lysates were kept on ice for 30 minutes and then centrifuged 15 000g for 15 minutes at 4°C. The supernatant fraction was “precleared” by addition of 50 μL protein G–agarose followed by gentle rocking for 1 hour and centrifugation. Samples of the supernatant fraction of equal protein content were used for immunoprecipitation. HA-PLDs, Syk, and other proteins were immunoprecipitated by overnight incubation (at 4°C with gentle rocking) with specific antibodies and, in turn, protein G–agarose. The agarose was washed 5 times with a washing buffer (20 mM HEPES [pH 7.5], 150 mM NaCl, 0.1% Nonidet P-40, 10% glycerol, 10 mM NaF, 1 mM Na3VO4, 1 mM PMSF, 2.5 mM nitrophenylphosphate, 0.7 μg/mL pepstatin, and protease inhibitor cocktail tablet) and dissolved in 2 × Laemmli buffer.38 Proteins were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to nitrocellulose membranes (BA85; Schleicher and Schuell, Keene, NH). The immunoreactive proteins were detected by use of horseradish peroxidase–coupled secondary antibodies and enhanced chemiluminescence according to the manufacturer's instructions (Amersham Pharmacia Biotech, Piscataway, NJ).
Studies in cell-free systems with endogenous and recombinant Syk
Endogenous Syk was obtained from lysates of RBL-2H3 cells by immunoprecipitation with anti-Syk antibody. The immunoprecipitate was then washed with a kinase buffer (30 mM HEPES [pH 7.5], 10 mM MgCl2, and 2 mM MnCl2) and resuspended in 40 μL of the same buffer with or without 20 μM ATP and incubated for 90 minutes before addition of lysates of cells (2 mg protein) that had been transfected with HA-PLD2 cDNA or vector. The mixture was gently rocked overnight at 4°C and washed 5 times with washing buffer, and the precipitated proteins were subjected to immunoblot analysis for detection of HA-PLD2 with anti-HA antibody and phosphorylated Syk (pY-Syk) with antiphosphotyrosine antibody.
Studies of the in vitro phosphorylation and activation of Syk by PLD2 were performed with recombinant Syk (200 ng; Upstate Biotechnology) and HA-PLD2 that was obtained from HA-PLD2–transfected cells by use of the Catch and Release (Upstate Biotechnology) immunoprecipitation system according to the manufacturer's protocol. In this procedure, lysates of HA-PLD2–transfected cells were diluted to 1 mg protein per milliliter with a lysis/wash buffer (1 ×), and 500 μL of the diluted lysates were transferred to spin columns. A total of 4 μg of anti-HA antibody and 10 μL of the antibody capture affinity ligand were added to the diluted lysate. The spin columns were then gently rocked for 15 minutes at room temperature before centrifugation at 1500g for 10 minutes. The columns were washed twice with the lysis/wash buffer. HA-PLD2 was eluted from the columns and analyzed by Western blotting.
Free recombinant Syk (200 ng) was assayed for kinase activity in the presence or absence of immunoprecipitated HA-PLD2 as described above by use of an in vitro kinase assay kit (Tyrosine Kinase Assay Kit Chemiluminescence Detection from Upstate Biotechnology) according to manufacturer's instruction. For this assay, 54 residue synthetic peptide [GG (EEEEY)10 EE] was used as substrate and conditions were chosen such that phosphorylation was linear with respect to time and amount of recombinant Syk. Where indicated, an equal amount of bovine serum albumin (BSA) was used as the negative control for each assay. Because PLD2 is not phosphorylated by Syk,36 it would not act as a pseudosubstrate in this assay.
Studies in vitro with recombinant PLD2, glutathione S-transferase (GST) fusion proteins of PLD2 fragments, and Syk mutants
Recombinant human hexahistidine (His6) PLD2 was kindly supplied by S.H.R. (Pohang University of Science and Technology, Korea).39 His6-PLD2 (about 0.2 μg) was incubated (1.5 hours at 4°C) with 0.4 μg recombinant Syk in 50 μL lysis buffer.40 Syk was immunoprecipitated with anti-Syk antibody and, after separation of proteins by electrophoresis, immunoprecipitated His6-PLD2 and Syk were detected by immunoblotting.
GST fusion proteins of different domains of PLD2 were generated as previously described.40 The templates included the PX (65 to 192 [amino acid number from N-terminal sequence of human PLD2]) and PH (201 to 310) domains as well as fragments F1 (1 to 314), F2, (315 to 475) F3, (476 to 612) F4, (613 to 723) F5, (724 to 825) and F6 (826 to 934) of human PLD2. These mutants of PLD2 were amplified by PCR with the specified primers, digested with restriction enzymes EcoRI and XhoI, and ligated into pGEX-4T1 vector (Amersham Pharmacia Biotech). Escherichia coli BL21 cells were transformed with individual expression vectors encoding the GST fusion proteins and induced with 0.5 mM isopropyl-β-D-thiogalactopyranoside (IPTG) at 25°C for 4 hours. After harvesting the cells, GST fusion proteins of PLD2 were purified as previously described.40 The indicated GST fusion proteins (about 1 μg) on glutathione Sepharose 4B were incubated with 0.1 μg recombinant Syk, tyrosine-mutated Syk, or 1 mg RBL-2H3 cell lysate in 500 μL lysis buffer that contained 1 M urea to prevent nonspecific binding. After a brief centrifugation, the precipitated complexes were washed 5 times in the lysis buffer before separation and detection of proteins by electrophoresis and immunoblotting.
Measurement of degranulation
Secretion of granules was determined by measurement of release of the granule marker, β-hexosaminidase, by use of a colorimetric assay in which release of P-nitrophenol from P-nitrophenyl-N-acetyl-b-D-glucosaminide is measured.41 Values were expressed as the percent of intracellular β-hexosaminidase that was released into the medium.
Confocal microscopy
RBL-2H3 cells were transfected with the wild-type EGFP-PLD2 plasmid by electroporation as described above. The cells were then suspended in complete growth medium, transferred to Lab-Tek chambered coverslips (Nalge Nunc International, Naperville, IL), and then incubated overnight at 37°C. The cultures were washed 3 times with PBS. Cultures were fixed in 4% formaldehyde in PBS for 10 minutes, washed, and permeabilized by treatment with 0.5% Triton X-100 for 15 minutes. The fixed cells were washed again before incubation for 60 minutes with a blocking reagent, 1% BSA in PBS. The coverslips were incubated for 2 hours with a solution of the primary antibody in 1% BSA in PBS, washed, and then incubated with rhodamine-conjugated secondary antibody for 45 minutes. The coverslips were washed and mounts prepared by use of the Prolonged Antifade Kit (Molecular Probes, Eugene, OR). Confocal images were taken in a Bio-Rad MRC 1024 confocal laser scanning microscope with an Apochromat 60×/1.3 NA objective (Bio-Rad, Hercules, CA). The fluorescence images were acquired with Laser Sharp2000, v. 5.1 (Bio-Rad).
Results
PLD2 is associated with Syk in RBL-2H3 mast cells
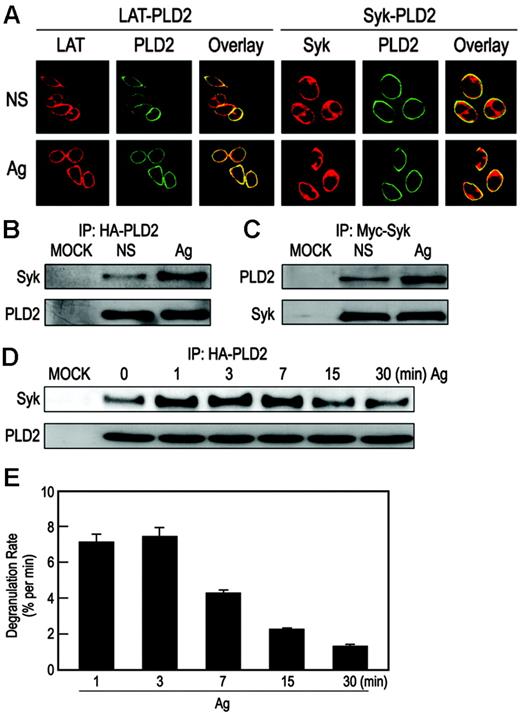
Examination of RBL-2H3 cells made to express EGFP-tagged PLD2 by confocal microscopy revealed that PLD2 was localized almost exclusively on the plasma membrane17 where it is colocalized with endogenous LAT (Figure 1A). The EGFP-tagged PLD2 was also colocalized with endogenous Syk although Syk itself was present mainly in the cytosol (Figure 1A). As in past studies,36,42 we were unable to reliably detect or immunoprecipitate endogenous PLD2 with any of the available antibodies to determine whether PLD2 associated with Syk. As an alternative strategy, cells were cotransfected with HA-PLD2 and myc-Syk plasmids, and the expressed proteins were immunoprecipitated with anti-HA or antimyc antibodies. As shown in Figure 1B-C, while HA-PLD2 and myc-Syk minimally coimmunoprecipitated with each other in the absence of stimulation, coimmunoprecipitation was substantially enhanced after antigen stimulation. The association was evident within 1 minute and reached a maximum over 3 minutes to 7 minutes (Figure 1D). In addition, the extent of this association appeared to correlate with the rate of degranulation: both events were maximal over the first 1 minute to 7 minutes, and then they gradually decreased with time (compare Figure 1D with Figure 1E).
The association of PLD2 and Syk is dependent on phosphorylation of Syk but not on PLD activity
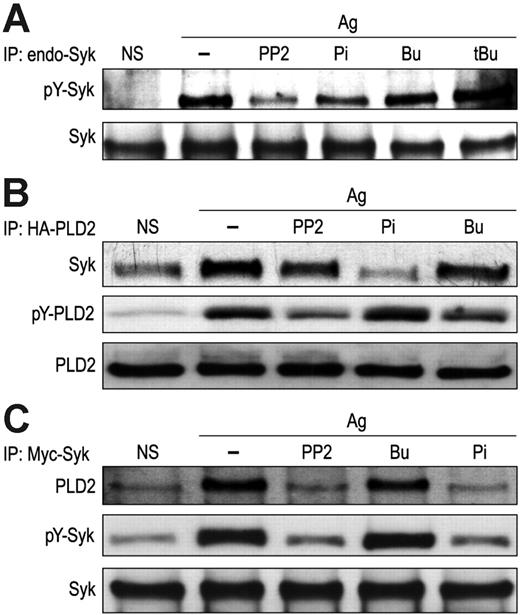
Inhibitors were tested to determine whether the antigen-induced association of PLD2 with Syk was dependent on activation of either molecule. The inhibitors included PP2 and piceatannol, which inhibit Src-family kinases and Syk, respectively, and 1-butanol, which has higher affinity for PLD than water and diverts production of phosphatidic acid by PLD to the relatively inert phosphatidylbutanol.43 The latter reaction is used widely to thwart PLD function and to measure PLD catalytic activity. Tertiary butanol, which is not a substrate for PLD, is used as a control to assess nonspecific actions of the alcohol. As shown in Figure 2A, antigen-induced phosphorylation of endogenous Syk was inhibited by PP2 and piceatannol but not by 50 mM 1-butanol or tertiary butanol. In cells transiently transfected with HA-PLD2 and Syk cDNA, the antigen-induced association of HA-PLD2 with Syk as determined by immunoprecipitation was similarly suppressed by PP2 and, to a larger extent, by piceatannol, while such association was minimally reduced by 1-butanol (Figure 2B-C). As previously reported,17 PP2 and 1-butanol suppressed tyrosine phosphorylation of HA-PLD2 (Figure 2B), while PP2 and piceatannol suppressed tyrosine phosphorylation of Syk (Figure 2C). These results indicated that the interaction of PLD2 and Syk was dependent on phosphorylation or activation of Syk but not on the activity or phosphorylation of PLD2.
Colocalization of PLD2 with LAT and Syk on the plasma membrane. (A) RBL-2H3 cells were transiently transfected with EGFP-PLD2 plasmid and then left unstimulated (NS) or stimulated with 25 ng/mL DNP-BSA (Ag) for 7 minutes for examination by confocal microscopy. Cells were counterstained with rhodamine-labeled antibodies against LAT or Syk. Representative photomicrographs are shown. (B-C) RBL-2H3 cells transiently cotransfected with HA-PLD2 and myc-Syk cDNA plasmids or mock transfected (MOCK) were stimulated or not with antigen as in panel A. Proteins were immunoprecipitated (IP) with anti-HA (B) or antimyc (C) antibodies and then subjected to immunoblot analysis with anti-Syk or anti-HA antibodies. (D) The period of stimulation of the cotransfected RBL-2H3 cells was also varied as indicated, and immunoprecipitates obtained with anti-HA antibody were subjected to immunoblot analysis with anti-Syk or anti-HA antibodies. (E) Cells were also stimulated with antigen to calculate the rate of degranulation at the given times as determined by the release of the granule marker, β-hexosaminidase. Values are expressed as percent of cellular β-hexosaminidase that was released into the medium per minute and are the mean ± SEM of values from 3 experiments.
Colocalization of PLD2 with LAT and Syk on the plasma membrane. (A) RBL-2H3 cells were transiently transfected with EGFP-PLD2 plasmid and then left unstimulated (NS) or stimulated with 25 ng/mL DNP-BSA (Ag) for 7 minutes for examination by confocal microscopy. Cells were counterstained with rhodamine-labeled antibodies against LAT or Syk. Representative photomicrographs are shown. (B-C) RBL-2H3 cells transiently cotransfected with HA-PLD2 and myc-Syk cDNA plasmids or mock transfected (MOCK) were stimulated or not with antigen as in panel A. Proteins were immunoprecipitated (IP) with anti-HA (B) or antimyc (C) antibodies and then subjected to immunoblot analysis with anti-Syk or anti-HA antibodies. (D) The period of stimulation of the cotransfected RBL-2H3 cells was also varied as indicated, and immunoprecipitates obtained with anti-HA antibody were subjected to immunoblot analysis with anti-Syk or anti-HA antibodies. (E) Cells were also stimulated with antigen to calculate the rate of degranulation at the given times as determined by the release of the granule marker, β-hexosaminidase. Values are expressed as percent of cellular β-hexosaminidase that was released into the medium per minute and are the mean ± SEM of values from 3 experiments.
Interaction of PLD2 with Syk enhances phosphorylation of Syk
Studies were conducted with cells transiently cotransfected with HA-PLD2 and myc-Syk cDNA to determine the consequences of this interaction. Of note, antigen-stimulated tyrosine phosphorylation of Syk was enhanced in cells transfected with either catalytically inactive PLD2K758R or the wild-type active PLD2 as compared with cells transfected with vector alone (Figure 3A). This enhancement was dependent on the amount of PLD2K758R or PLD2 in cells that had been transfected with increasing quantities of plasmids (Figure 3B-C). These results confirmed that the association of PLD2 with Syk was not dependent on activity of PLD2 (as in Figure 2) and, in addition, suggested that this association is critical for optimal phosphorylation of Syk in mast cells.
Synergistic phosphorylation of Syk by Lyn and PLD2
Lyn kinase is the primary kinase for phosphorylation of Syk.7 Accordingly, we examined the mutual effects of Lyn and PLD2 on the phosphorylation of Syk in combinatorial expression studies. Overexpression of Lyn increased the extent of phosphorylation of Syk in antigen-stimulated cells, and this increase was dependent on the amount of Lyn expressed (Figure 3D). Coexpression of Lyn with HA-PLD2 enhanced phosphorylation of Syk to a greater extent than expression of the Lyn or HA-PLD2 individually (Figure 3E). Data from all experiments indicated that this enhancement was synergistic rather than additive and showed a 3-fold increase over that expected from additive responses.
Association of PLD2 and Syk in a cell-free system enhances phosphorylation and activation of Syk
Studies were conducted with immunoprecipitated endogenous Syk and lysates of cells made to express HA-PLD2 to determine whether PLD2 interacted with Syk. It was found that provision of a phosphate donor, namely ATP, for autophosphorylation of Syk enhanced such interaction. For example, association of HA-PLD2 with Syk was increased more than 2-fold, as was the amount of phosphorylated Syk on prior exposure of Syk to 20 μM ATP (Figure 4A). This result verified that the association of PLD2 and Syk was dependent on the phosphorylation of Syk (as suggested by data in Figure 2). We next examined whether this association resulted in activation of Syk by incubating HA-PLD2 (immunoprecipitated by the Catch and Release system) with recombinant Syk. The presence of immunoprecipitated PLD2 not only increased the phosphorylation of Syk by ATP (Figure 4B) but also Syk activity in proportion to the amount of PLD2 (Figure 4C). In fact, the activation of Syk exhibited an almost absolute requirement for PLD2. These and previous results (Figure 2) clearly indicated that PLD2 is necessary for optimal phosphorylation and activation of Syk in mast cells.
Association of PLD2 with Syk is dependent on phosphorylation of Syk. (A) RBL-2H3 cells were exposed to 20 μM PP2, 120 μM piceatannol (Pi), 50 mM 1-butanol (Bu), or 50 mM tertiary butanol (tBu) for 10 minutes before stimulation with 25 ng/mL DNP-BSA (Ag) for 7 minutes or left unstimulated (NS). Endogenous Syk was immunoprecipitated with anti-Syk antibody for detection of tyrosine-phosphorylated Syk (pY-Syk) with antiphosphotyrosine antibody and Syk with anti-Syk antibody by immunoblotting. (B-C) RBL-2H3 cells transiently cotransfected with HA-PLD2 and myc-Syk plasmids were stimulated with antigen in the presence or absence of inhibitors as described for panel A. HA-PLD2 (B) and myc-Syk (C) were immunoprecipitated with agarose-conjugated antibody against HA-tag or antimyc antibody for immunoblotting and detection of HA-PLD2, myc-Syk, and their phosphorylated counterparts (pY-) with the appropriate antibody. Representative immunoblots from 3 experiments are shown.
Association of PLD2 with Syk is dependent on phosphorylation of Syk. (A) RBL-2H3 cells were exposed to 20 μM PP2, 120 μM piceatannol (Pi), 50 mM 1-butanol (Bu), or 50 mM tertiary butanol (tBu) for 10 minutes before stimulation with 25 ng/mL DNP-BSA (Ag) for 7 minutes or left unstimulated (NS). Endogenous Syk was immunoprecipitated with anti-Syk antibody for detection of tyrosine-phosphorylated Syk (pY-Syk) with antiphosphotyrosine antibody and Syk with anti-Syk antibody by immunoblotting. (B-C) RBL-2H3 cells transiently cotransfected with HA-PLD2 and myc-Syk plasmids were stimulated with antigen in the presence or absence of inhibitors as described for panel A. HA-PLD2 (B) and myc-Syk (C) were immunoprecipitated with agarose-conjugated antibody against HA-tag or antimyc antibody for immunoblotting and detection of HA-PLD2, myc-Syk, and their phosphorylated counterparts (pY-) with the appropriate antibody. Representative immunoblots from 3 experiments are shown.
Phosphorylation of Syk is enhanced by PLD2 and by Lyn. (A-C) RBL-2H3 cells were transiently cotransfected with myc-Syk along with HA-PLD2, catalytically inactive HA-PLD2K758R cDNA plasmids, or vector (V). Transfection was performed with 25 μg plasmid per 2 × 107 cells (A) or as indicated (B-C). Cells were stimulated for 7 minutes with 25 ng/mL antigen (Ag) or not stimulated (NS). Myc-Syk was immunoprecipitated (IP) from cell lystates with antimyc antibody, and precipitated proteins were subjected to immunoblot analysis for detection of tyrosine-phosphorylated Syk (pY-Syk) and myc-Syk with antibodies against phosphotyrosine and myc, respectively. (D-E) RBL-2H3 cells were cotransfected with cDNA constructs for myc-Syk along with vector (V), flag-Lyn, HA-PLD2, or the combination of flag-Lyn and HA-PLD2. Transfection was performed with 5 μg flag-Lyn and PLD2 DNA per 2 × 107 cells (E) or as indicated (D). Cells were stimulated with antigen or not for 7 minutes, and immunoblotting was performed for detection of phosphorylated Syk and myc-Syk. Representative immunoblots from 3 experiments are shown for all 5 panels, and relative densities are indicated in italics in panel E.
Phosphorylation of Syk is enhanced by PLD2 and by Lyn. (A-C) RBL-2H3 cells were transiently cotransfected with myc-Syk along with HA-PLD2, catalytically inactive HA-PLD2K758R cDNA plasmids, or vector (V). Transfection was performed with 25 μg plasmid per 2 × 107 cells (A) or as indicated (B-C). Cells were stimulated for 7 minutes with 25 ng/mL antigen (Ag) or not stimulated (NS). Myc-Syk was immunoprecipitated (IP) from cell lystates with antimyc antibody, and precipitated proteins were subjected to immunoblot analysis for detection of tyrosine-phosphorylated Syk (pY-Syk) and myc-Syk with antibodies against phosphotyrosine and myc, respectively. (D-E) RBL-2H3 cells were cotransfected with cDNA constructs for myc-Syk along with vector (V), flag-Lyn, HA-PLD2, or the combination of flag-Lyn and HA-PLD2. Transfection was performed with 5 μg flag-Lyn and PLD2 DNA per 2 × 107 cells (E) or as indicated (D). Cells were stimulated with antigen or not for 7 minutes, and immunoblotting was performed for detection of phosphorylated Syk and myc-Syk. Representative immunoblots from 3 experiments are shown for all 5 panels, and relative densities are indicated in italics in panel E.
The PX domain of PLD2 and tyrosine 317 of Syk are critical for PLD2/Syk interaction in vitro
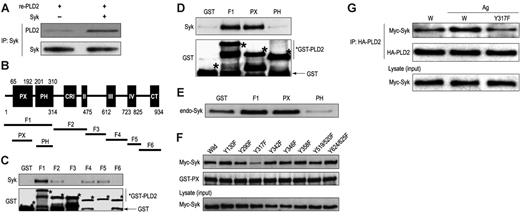
Studies were conducted with human recombinant His6-PLD2 and GST fusion proteins of fragments of PLD2 to determine whether these proteins interacted directly with Syk in vitro. Following incubation of full-length recombinant His6-PLD2 with Syk, His6-PLD2 was found to coimmunoprecipitate with Syk (Figure 5A). Of the various PLD2 fragments tested (F1 to F6 [Figure 5B]), only F1, which contained both the PX and PH domains of PLD2, coprecipitated with recombinant Syk (Figure 5C). Further studies with fragments restricted to the PX and PH domains (Figure 5B) clearly indicated that the PX domain specifically bound to recombinant Syk (Figure 5D) and endogenous Syk in RBL-2H3 cell lysates (Figure 5E) as did the F1 fragment (Figure 5D-E).
Association of PLD2 and Syk, although dependent on initial phosphorylation of Syk, results in additional phosphorylation and the activation of Syk. (A) Immunoprecipitated (IP) Syk from RBL-2H3 cells was incubated initially in the presence or absence of 20 μM ATP before addition of lysates of cells that had been transfected with HA-PLD2 cDNA or not (MOCK). The precipitated proteins were subjected to immunoblot analysis for detection of HA-PLD2 and phosphorylated Syk (pY-Syk) as described in “Materials and methods.” (B) Free recombinant Syk was phosphorylated with or without ATP and HA-PLD2 that had isolated by the Catch and Release system as described in “Materials and methods.” An equal amount of BSA was used as the negative control for HA-PLD2. The mixtures were subjected to immunoblot analysis for detection of phosphorylated Syk, HA-PLD2, and Syk. Representative immunoblots from 3 experiments are shown.(C) Free recombinant Syk was assayed for kinase activity in the absence or presence of the indicated amounts of HA-PLD2 as described for panel B by use of an in vitro kinase assay kit. An equal amount of BSA (B) was used as the negative control for each HA-PLD2 sample. Values are the mean ± SEM of 3 separate experiments.
Association of PLD2 and Syk, although dependent on initial phosphorylation of Syk, results in additional phosphorylation and the activation of Syk. (A) Immunoprecipitated (IP) Syk from RBL-2H3 cells was incubated initially in the presence or absence of 20 μM ATP before addition of lysates of cells that had been transfected with HA-PLD2 cDNA or not (MOCK). The precipitated proteins were subjected to immunoblot analysis for detection of HA-PLD2 and phosphorylated Syk (pY-Syk) as described in “Materials and methods.” (B) Free recombinant Syk was phosphorylated with or without ATP and HA-PLD2 that had isolated by the Catch and Release system as described in “Materials and methods.” An equal amount of BSA was used as the negative control for HA-PLD2. The mixtures were subjected to immunoblot analysis for detection of phosphorylated Syk, HA-PLD2, and Syk. Representative immunoblots from 3 experiments are shown.(C) Free recombinant Syk was assayed for kinase activity in the absence or presence of the indicated amounts of HA-PLD2 as described for panel B by use of an in vitro kinase assay kit. An equal amount of BSA (B) was used as the negative control for each HA-PLD2 sample. Values are the mean ± SEM of 3 separate experiments.
Point mutations were made of individual tyrosines of myc-Syk at amino acid numbers 130, 290, 317, 342, 346, 358, 519/520, and 624/625 (tyrosine to phenylalanine) to assess whether mutation of these sites disrupted binding to the PX domain of PLD2. As shown in Figure 5F, mutation of tyrosine 317 (Y317F) impaired binding to the GST-PX fusion protein while other mutations did not do so. This disruption was verified in vivo where the Y317F Myc-Syk mutant failed to exhibit increased binding to HA-PLD2 upon antigen stimulation in cells made to overexpress both proteins (Figure 5G).
Physiological significance of the interaction of PLD2 and Syk in mast cells
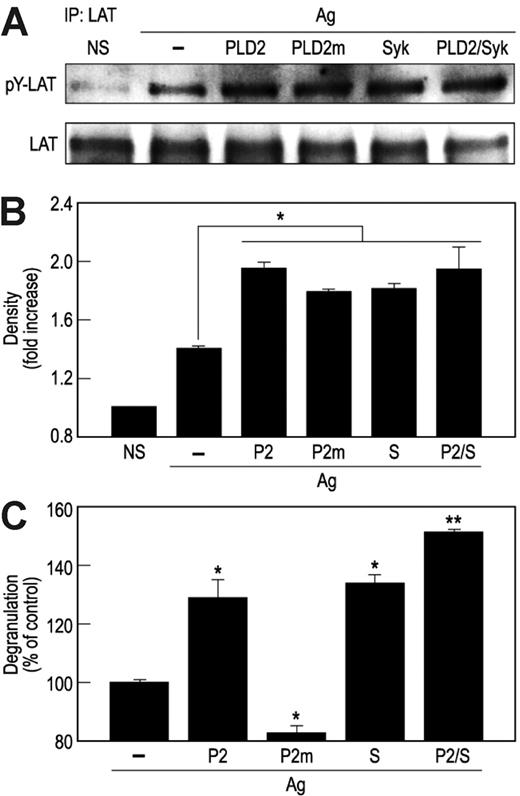
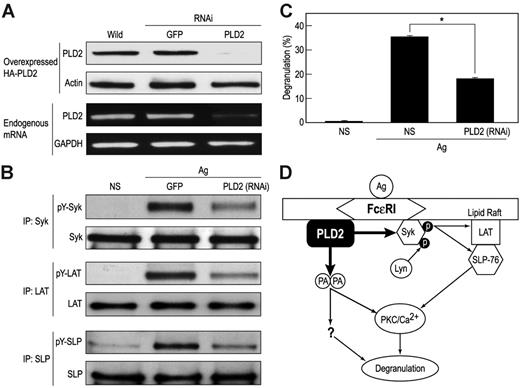
To assess the physiological relevance of our observations, cells were transfected with HA-PLD2, the catalytically inactive PLD2K758R, Syk, or the combination HA-PLD2 and Syk. All such transfections resulted in substantial augmentation of activating phosphorylations of LAT (Figure 6A-B) and SLP-76 (data not shown) as well as degranulation (Figure 6C) in antigen-stimulated cells. The ability of PLD2K758R to augment phosphorylation of LAT (Figure 6A) as well as Syk (Figure 3A) reaffirmed that PLD2 acted in a catalytically independent manner. To further verify that PLD2 regulated phosphorylation of Syk and downstream phosphorylations of LAT and SLP-76, cells were transfected with PLD2-specific siRNA. This transfection suppressed expression of PLD2 protein and mRNA (Figure 7A) and the activating phosphorylations of Syk, LAT, and SLP-76 (Figure 7B) in addition to degranulation (Figure 7C). It should be noted that for the data shown in Figure 7A, cells were made to express HA-PLD2 as a substitute for endogenous PLD2 because of the difficulty in detecting PLD2 with the available anti-PLD2 antibodies (see “PLD2 is associated with Syk in RBL-2H3 mast cells”). The results with siRNA together with those obtained by overexpression of PLD2 provided consistent evidence for the regulation of Syk activation and downstream events by PLD2.
PLD2 interacts via its PX domain with Syk in vitro, an interaction that is dependent on Syk tyrosine 317. (A) Following the incubation of recombinant hexahistidine (His6)–PLD2 with recombinant Syk, Syk was immunoprecipitated with anti-Syk antibody, and immunoblots were prepared for detection of His6-PLD2 and Syk as shown. (B) Structures of the individual fragments as well as the PX and PH domains of human PLD2 that were prepared as GST fusion proteins for studies shown in panels C-F. Numbers indicate the terminal amino acid of each nonoverlapping fragment (F) as described in “Materials and methods.” The initial and terminal amino acids of the PH and PX are also indicated (C-F). The GST fusion proteins were incubated with recombinant Syk (C-D), endogenous Syk (E), and the indicated mutants (tyrosine to phenylalanine) of myc-Syk (F). Lysates of RBL-2H3 cells and of cells made to overexpress the myc-Syk mutants were used as sources of endogenous Syk and mutated myc-Syk, respectively (see “Materials and methods”). Immunoblots of the protein precipitates were probed with antibodies against Syk, Myc, and GST as shown. (G) Cells were made to overexpress wild-type or the Y317F mutant of myc-Syk along with HA-PLD2. Cells were stimulated or not with antigen (Ag). HA-PLD2 was immunoprecipitated with anti-HA antibody, and HA-PLD2 and coimmunoprecipitated myc-Syk were detected by immunoblotting and use of antimyc and HA antibodies.
PLD2 interacts via its PX domain with Syk in vitro, an interaction that is dependent on Syk tyrosine 317. (A) Following the incubation of recombinant hexahistidine (His6)–PLD2 with recombinant Syk, Syk was immunoprecipitated with anti-Syk antibody, and immunoblots were prepared for detection of His6-PLD2 and Syk as shown. (B) Structures of the individual fragments as well as the PX and PH domains of human PLD2 that were prepared as GST fusion proteins for studies shown in panels C-F. Numbers indicate the terminal amino acid of each nonoverlapping fragment (F) as described in “Materials and methods.” The initial and terminal amino acids of the PH and PX are also indicated (C-F). The GST fusion proteins were incubated with recombinant Syk (C-D), endogenous Syk (E), and the indicated mutants (tyrosine to phenylalanine) of myc-Syk (F). Lysates of RBL-2H3 cells and of cells made to overexpress the myc-Syk mutants were used as sources of endogenous Syk and mutated myc-Syk, respectively (see “Materials and methods”). Immunoblots of the protein precipitates were probed with antibodies against Syk, Myc, and GST as shown. (G) Cells were made to overexpress wild-type or the Y317F mutant of myc-Syk along with HA-PLD2. Cells were stimulated or not with antigen (Ag). HA-PLD2 was immunoprecipitated with anti-HA antibody, and HA-PLD2 and coimmunoprecipitated myc-Syk were detected by immunoblotting and use of antimyc and HA antibodies.
Overexpression of HA-PLD2 or Syk enhances phosphorylation of LAT activation as well as degranulation. (A-B) RBL-2H3 cells were transfected with cDNA constructs for HA-PLD2 (PLD2 or P2), catalytically inactive PLD2K758R (PLD2m or P2m), Syk (S), or vector (–). Cells were stimulated for 7 minutes with antigen (Ag) or not stimulated (NS). LAT was immunoprecipitated from cell lysates with anti-LAT antibody, and precipitated proteins were subjected to immunoblot analysis for detection of LAT and phosphorylated LAT (pY-LAT) with anti-LAT and antiphosphotyrosine antibodies. Representative blots (A) and densitometric data (B) from 3 experiments are shown. (C) Cells were also stimulated with antigen for 7 minutes for measurement of release of the granule marker, β-hexosaminidase. Values are expressed as percent of release of β-hexosaminidase in vector-transfected cells (about 31% release) and are the mean ± SEM of values from 3 experiments. Significant increase or decrease in release: *P < .05; **P < .01.
Overexpression of HA-PLD2 or Syk enhances phosphorylation of LAT activation as well as degranulation. (A-B) RBL-2H3 cells were transfected with cDNA constructs for HA-PLD2 (PLD2 or P2), catalytically inactive PLD2K758R (PLD2m or P2m), Syk (S), or vector (–). Cells were stimulated for 7 minutes with antigen (Ag) or not stimulated (NS). LAT was immunoprecipitated from cell lysates with anti-LAT antibody, and precipitated proteins were subjected to immunoblot analysis for detection of LAT and phosphorylated LAT (pY-LAT) with anti-LAT and antiphosphotyrosine antibodies. Representative blots (A) and densitometric data (B) from 3 experiments are shown. (C) Cells were also stimulated with antigen for 7 minutes for measurement of release of the granule marker, β-hexosaminidase. Values are expressed as percent of release of β-hexosaminidase in vector-transfected cells (about 31% release) and are the mean ± SEM of values from 3 experiments. Significant increase or decrease in release: *P < .05; **P < .01.
Discussion
The mechanisms of receptor-mediated activation of PLD and its interactions with regulatory proteins and phospholipids have been investigated in some detail.23,32,33,44-48 However, less is known about the interactions of PLD with downstream signaling proteins that would account for its presumed roles in regulating cell function. Here we present evidence that PLD2 complexes with Syk and in doing so facilitates activation of mast cells in the manner depicted in Figure 7D. That is, the interaction of PLD2 with Syk in a catalytically independent manner enhances the activation of Syk and the downstream phosphorylation of the adaptor/docking proteins, LAT and SLP-76. The latter proteins are critical for correct assembly of signaling molecules and successful propagation of signals that lead to degranulation and de novo generation of inflammatory mediators in mast cells.7 In addition, the activation of PLD2 catalytic activity, whether by phosphorylation with Src kinases36 or by other mechanisms, results in production of phosphatidic acid, which is also required for degranulation.17 However, the role of phosphatidic acid in degranulation is unclear except that it may serve as a major source of diacylglycerol for sustained activation of PKC in mast cells.42
Suppression of PLD2 expression with siRNA impairs tyrosine phosphorylation of Syk, LAT, and SLP as well as degranulation. (A) RBL-2H3 cells were made to transiently express HA-PLD2 and siRNAs (RNAi) directed against PLD2 or as a control green fluorescent protein (GFP). Expression of HA-PLD2 and actin was determined by immunoblotting, and expression of PLD2 mRNA was determined by RT-PCR. (B) Cells made to express the PLD2 siRNA were also stimulated with 25 ng/mL antigen (Ag) or not (NS) for 5 minutes for detection of Syk, LAT, and SLP and their tyrosine phosphorylated (pY-) counterparts by immunoblotting after immunoprecipitation. (C) Cells were also stimulated with antigen for 15 minutes to measure release of the granule marker, β-hexosaminidase. Values are expressed as percent of cellular β-hexosaminidase that was released into the medium and are the mean ± SEM of values from 3 experiments. Significant decrease in release: *P < .01. (D) Model for the dual actions of PLD2. The data suggest that PLD2 acts in a catalytically independent and dependent manner to associate directly with Syk to enhance tyrosine phosphorylation and activation of Syk and downstream targets such as LAT and SLP-76 and form phosphatidic acid (PA), which may facilitate activation of PKC and other, as yet poorly defined, mechanisms that are essential for degranulation.
Suppression of PLD2 expression with siRNA impairs tyrosine phosphorylation of Syk, LAT, and SLP as well as degranulation. (A) RBL-2H3 cells were made to transiently express HA-PLD2 and siRNAs (RNAi) directed against PLD2 or as a control green fluorescent protein (GFP). Expression of HA-PLD2 and actin was determined by immunoblotting, and expression of PLD2 mRNA was determined by RT-PCR. (B) Cells made to express the PLD2 siRNA were also stimulated with 25 ng/mL antigen (Ag) or not (NS) for 5 minutes for detection of Syk, LAT, and SLP and their tyrosine phosphorylated (pY-) counterparts by immunoblotting after immunoprecipitation. (C) Cells were also stimulated with antigen for 15 minutes to measure release of the granule marker, β-hexosaminidase. Values are expressed as percent of cellular β-hexosaminidase that was released into the medium and are the mean ± SEM of values from 3 experiments. Significant decrease in release: *P < .01. (D) Model for the dual actions of PLD2. The data suggest that PLD2 acts in a catalytically independent and dependent manner to associate directly with Syk to enhance tyrosine phosphorylation and activation of Syk and downstream targets such as LAT and SLP-76 and form phosphatidic acid (PA), which may facilitate activation of PKC and other, as yet poorly defined, mechanisms that are essential for degranulation.
Activation of Syk is initially dependent on the phosphorylation of the 2 tyrosines in the FcϵRIγ chain ITAM by Lyn to enable the binding of Syk through its tandem SH2 domains to the phosphorylated ITAM (reviewed by Siraganian et al8 ). This binding promotes conformational changes in Syk and transphosphorylation or autophosphorylation of tyrosine residues at 519 and 520 (numbering based on the murine Syk sequence) in its putative activation loop. Additional phosphorylation of tyrosine residue 317 by Lyn49 and of tyrosines 342 and 346 by autophosphorylation50 within a linker B region provide docking sites for a variety of SH2 domain–containing proteins that include c-Cbl, PLCγ, the Src kinase Fgr, and Vav51-54 among others.55-62 Of these proteins, Vav and c-Cbl serve as, respectively, positive and negative regulators of Syk-mediated signaling processes.52,63 Mutational analysis has provided additional insight on the positive or negative roles that each of the B-linker tyrosines and their identified binding cohorts play in mast cell signaling.64 Our present studies suggest that PLD2 also may serve as a positive regulator of Syk (Figures 3, 4) and that phosphorylation of Syk might be facilitated by the interaction of Syk with PLD2 (Figures 3 and 4B) as well as with phosphorylated ITAM of FcϵRIγ.
Although the mechanism is not entirely clear, the association of PLD2 with Syk requires the phosphorylation of Syk (Figure 2), possibly by Lyn (Figure 3D), at tyrosine 317 (Figure 5F-G) and is dependent on the PX domain of PLD2 (Figure 5D) but not on PLD2 catalytic activity (Figure 2). Syk tyrosine 317 is reported to be a binding site for the negative regulator, Cbl, in RBL-2H3 cells,65,66 although it has been postulated that it may also have a positive role by recruiting the p85α subunit of phosphatidylinositol 3′-kinase to allow activation of Akt.64 Whether or how PLD2 competes with these molecules in binding to tyrosine 317 is unclear. With respect to PLD2, there are examples where the PX domain of PLD2 is known to enable direct interaction of PLD2 with other signaling proteins independently of the catalytic function of PLD2. One is the interaction of the PLD2 PX domain with the SH3 domain of PLC-γ1 that allows redistribution of PLC-γ1 to the membrane region in epidermal growth factor signaling.32 This interaction is dependent on tyrosine phosphorylation of PLC-γ1, analogous to the requirement for phosphorylation of Syk for its interaction with PLD2. PLD2 also interacts with the SH2 domain of the adaptor protein, Grb2,67 via its PX domain68 to allow recruitment of the Ras guanine-nucleotide exchange factor Sos and subsequent regulation of the p44/42erk pathway. Another example is that the direct interaction of PLD2-PX domain with PKCζ enhances stimulation and phosphorylation of the activation loop of PKCζ and downstream signals.34
As for PLC-γ1, Grb2, and PKCζ, the association of PLD2 with Syk regulates downstream signals. Syk directly phosphorylates LAT and SLP-76 during the activation of mast cells.7 These phosphorylations, in addition to that of Syk, are enhanced by overexpression of PLD2 (Figure 6) and inhibited by suppression of PLD2 expression with RNAi (Figure 7) to indicate that PLD2 is critical for stimulating the phosphorylation of Syk, LAT, and SLP-76 as well as degranulation (Figures 6, 7).
PLD2 is largely confined to the plasma membrane in RBL-2H3 mast cells where it is phosphorylated by FcϵRI-associated Src kinases such as Fyn and Fgr following antigen stimulation. This phosphorylation appears to be essential for the production of phosphatidic acid by PLD and degranulation.36 Here we show that PLD2 colocalizes with Syk on the plasma membrane (Figure 1). Like the Src kinases, Syk is normally located in the cytoplasm, but a minor fraction of this enzyme is recruited to FcϵRI in the vicinity of lipid rafts within the plasma membrane.69 Studies with confocal microscopy and cholesterol-chelating agents suggest that PLD2 is largely localized in lipid rafts in RBL-2H3 cells (W.S.C. and M.A.B., unpublished observations, June 2002). Therefore, it is possible that the interaction of PLD2 and Syk and the ensuing activation of Syk occur in conjunction with lipid rafts. However, whether this interaction also involves FcϵRI and other molecules is as yet undetermined.
In summary, our findings indicate that PLD2 is located primarily at the plasma membrane of mast cells where it can associate with Src kinases and undergo activation36 or, in a catalytically inert manner, with Syk to enhance or promote Syk activity (this paper). This is the first indication of a direct link between PLD2 and Syk. This interaction enhances the activation of Syk and the phosphorylation of Syk and its downstream targets, LAT and SLP-76. As a possible consequence, degranulation of mast cells is enhanced as well. Although the formation of phosphatidic acid through the catalytic action of PLD is necessary for degranulation of RBL-2H3 cells,17 it is not sufficient and the association of PLD2 with Syk appears equally critical. This dual role of PLD2 in the activation of mast cells has bearing on the pathology and treatment of mast cell–related allergic disease in view of the preeminent role of mast cells in allergic diseases.
Prepublished online as Blood First Edition Paper, March 30, 2006; DOI 10.1182/blood-2005-10-009159.
Supported by the Regional Research Centers Program of the Ministry of Education and Human Resources Development; the Ministry of Commerce, Industry and Energy through the Bio-Food and Drug Research Center at Konkuk University, Korea; and the Intramural Program of the National Institutes of Health (M.A.B.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal