Comment on Datta et al, page 1021
Prolonged treatment of HTLV-I–infected cells with AZT results in the inhibition of telomerase activity and reactivation of the p53 tumor-suppressor functions, eventually inducing HTLV-I cell senescence and death. Therefore, p53 status is a predictive marker of the AZT response in ATLL patients.
Adult T-cell leukemia/lymphoma (ATLL) is a malignant lymphoproliferation of mature activated T cells that develops in a subset of human T-cell leukemia virus type I (HTLV-I)–infected individuals after a long period of latency. It is characterized by the clonal integration of one or more HTLV-I proviruses in the tumor cells. The survival rate of ATLL patients, especially those who develop the acute leukemic or lymphoma forms, is very poor, and ATLL remains therefore one of the most severe lymphoproliferations.FIG1
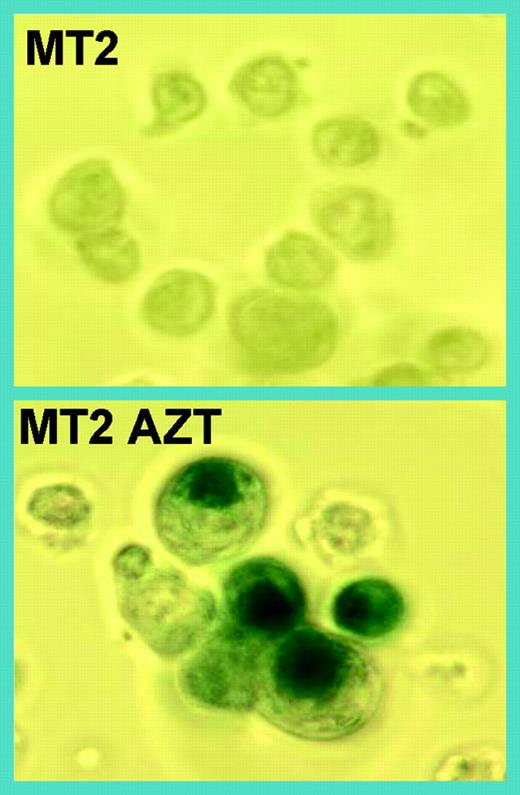
HTLV-I–infected MT2 cells undergo senescence after long-term AZT treatment, as determined by senescence β gal assay. See the complete figure in the article beginning on page 1021.
HTLV-I–infected MT2 cells undergo senescence after long-term AZT treatment, as determined by senescence β gal assay. See the complete figure in the article beginning on page 1021.
Because HTLV-I–transformed cells are resistant to nearly all apoptosis-inducing agents, the treatment of ATLL patients using conventional chemotherapy has very limited advantage. Zidovudine (AZT), which was initially synthesized as part of an anticancer drug discovery program, has been shown to inhibit HTLV-I transmission in vitro. More recently, antiretroviral therapy using the combination of AZT and interferon alpha (IFN-α) has also been shown to induce a high rate of complete remission and to prolong the survival of some ATLL patients.1,2
But until the report by Datta and colleagues in this issue of Blood, the mechanism of action of AZT and IFN-α was controversial. Some studies argued in favor of an antiviral effect through the inhibition of HTLV-I replication, while others proposed a direct antiproliferative effect of the drugs on the leukemic cells.3-5 For these reasons, the predictive markers for choosing AZT rather than another drug for treating ATLL patients were unknown.
Here, instead of treating the HTLV-I–infected cells for a short period of time, as is usually the case in vitro, Datta and colleagues performed a long-term treatment with AZT. AZT can act as an inhibitor of the telomerase functions, and indeed, under these circumstances all HTLV-I cell lines tested entered senescence rather than apoptosis. This was concomitant with a reduction of the telomerase activity and a decrease in telomere length.
Of interest, the levels of the p53 tumor suppressor were much higher in cells that were exposed to AZT for several weeks, but no effect was seen after 24 hours of treatment. Because p53 protein is inactive but wild type in sequence in most HTLV-I–infected cells,6 Datta and colleagues determined whether AZT prolonged treatment-reactivated p53 functions. Following ionization-radiation of AZT-treated cells, the mRNA levels of p21waf and Bax (2 p53-dependent genes) increased and confirm the working model. The required duration of AZT treatment in vitro is consistent with the slow kinetic that is observed in vivo in ATLL-treated patients. Datta and colleagues also demonstrate that AZT treatment induces telomere shortening of fresh ATLL cells. The percentage of ATLL patients who are refractory to AZT is very similar to that of the patients whose p53 gene is mutated. For this reason, the authors investigate whether there was a correlation between these 2 observations. In a retrospective analysis, they demonstrate that there was a perfect parallel between the p53 status and the response to AZT treatment: all patients carrying a wt-p53 gene responded to AZT and went into partial or complete remission. By contrast, AZT had no effect in ATLL patients carrying a mutated p53 sequence.
It is estimated that several thousand ATLL patients pass away each year. Until this work, it was unclear why some AZT-treated patients enter remission while others die. This very nice study provides for the first time a rationale for treating or not treating HTLV-I ATLL patients with AZT. Patients with a mutated p53 gene might therefore be treated with alternative drugs, such as arsenic trioxide, which target both the Tax protein and the NF-κb pathway.7 ▪

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal