Two significant and disparate advances in the quest to therapeutically up-regulate fetal hemoglobin (HbF) in the beta globin disorders are reported in this issue. Franco and colleagues define highly significant differential survival of sickle red cells as a direct function of their cellular fraction of HbF, a new benchmark of severity. Jiang and colleagues identify an allele outside the globin gene locus, c-myb, suppression of which enhances fetal globin through acceleration of erythroid cell maturation, without general marrow suppression.
HbF reduces hematopathology in sickle cell disease and beta thalassemia; high total HbF levels (> 20%) and more than 75% of F cells generally correlate with mild clinical courses in the sickle syndromes.1-4 However, many patients do not achieve such levels with currently available therapy,3 and precisely how much HbF per red blood cell is required to reduce hemolysis, and related serious complications, is not well characterized. Better measures for efficacy of HbF-stimulating therapies at the cellular level are needed.FIG1
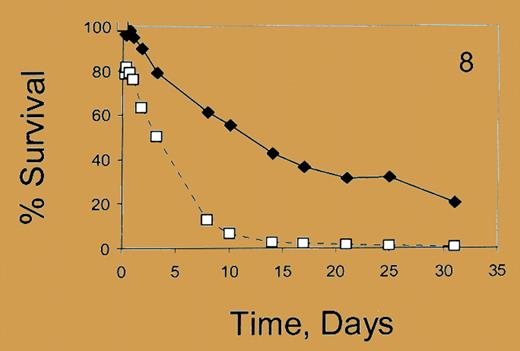
Percent survival. See the complete figure in the article beginning on page 1073.
Percent survival. See the complete figure in the article beginning on page 1073.
Franco and colleagues now report quantitative differences in the in vivo survival of red cells containing high, low, and no HbF proportions/cell in sickle cell patients. Survival studies with ex vivo–labeled, reinfused red cells demonstrated that high HbF–containing cells survive 6 weeks, while non–F cells survive 2 weeks. Cells with higher proportions of HbF/cell survived longer than those with lower HbF content. An intriguing observation was that red cells with no HbF have shorter survival in patients with high (> 88%) F-cell proportions compared with patients with lower F-cell proportions (< 16%). These measures of cellular HbF content provide a new metric for assessing cellular pathology related to hemolysis, potentially to assess responses to HbF-stimulating therapies and to predict when combinations of therapies are likely required in an individual patient.FIG2
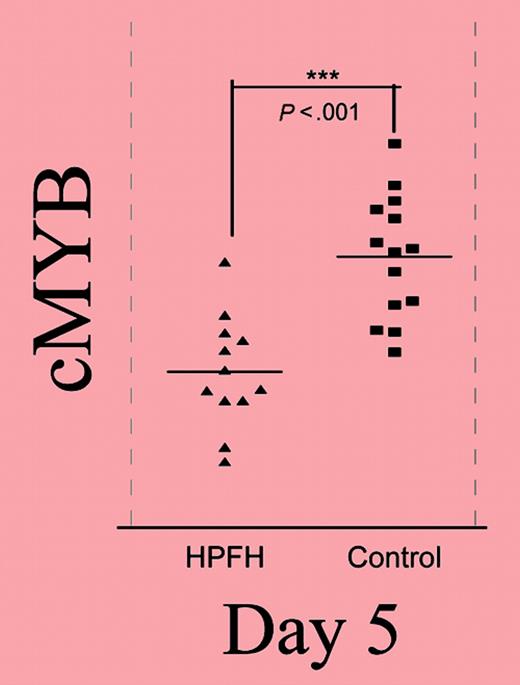
Relative expression of the gene in the 6q23 candidate interval throughout phase II. See the complete figure in the article beginning on page 1077.
Relative expression of the gene in the 6q23 candidate interval throughout phase II. See the complete figure in the article beginning on page 1077.
New molecular approaches to therapeutic HbF induction are particularly needed for the beta thalassemias. After mapping a modifier (QTL) of HbF expression to chromosome 6q23 in a kindred with heterocellular HPFH, Jiang and colleagues investigated the relationship between HbF levels and expression of the 5 genes in the region linked to that HPFH mutation, using erythroid cultures from individuals with low and high HbF levels. One gene, c-myb, was found to have an expression pattern that correlated (inversely) with HbF levels, and enforced overexpression of c-myb suppressed HbF production. In elegant developmental studies of globin expression during culture, the authors demonstrate an increase in HbF expression and a higher ratio of mature (glycophorin-expressing) cells earlier in the HPFH cells than in normal cells. Erythropoietic maturation is accurately recapitulated in their culture system. Normal and altered c-myb expression, target genes that interact with c-myb, and potential mechanisms of the inverse association between c-myb and HbF are reviewed.
This discovery of a novel modifying allele outside the globin gene locus offers an entirely new avenue to fetal globin induction, operating through accelerated maturation of erythroid cells, but without the generalized marrow suppression of chemotherapeutic agents. c-myb and the genes it regulates provide new targets for exploration and potential exploitation in therapeutic approaches. ▪

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal